Abstract
Background
Premedication is the most common way to minimize distress in children entering the operating room and to facilitate the smooth induction of anesthesia and is accomplished using various sedative drugs before the children are being transferred to the operating room. The aim of this study was to compare the effect of oral dexmedetomidine (DEX) and oral midazolam (MID) on preoperative cooperation and emergence delirium (ED) among children who underwent dental procedures at our hospital between 2016 and 2017.
Patients and methods
The medical records of 52 children, who were American Society of Anesthesiologists I, aged between 3 and 7 years, and who underwent full-mouth dental rehabilitation under general anesthesia (GA), were evaluated. Twenty-six patients were given 2 µg/kg of DEX, while another 26 patients were given 0.5 mg/kg of MID in apple juice as premedication agents. The patients’ scores on the Ramsay Sedation Scale (RSS), Parental Separation Anxiety Scale (PSAS), Mask Acceptance Scale, Pediatric Anesthesia Emergence Delirium Scale (PAEDS), and hemodynamic parameters were recorded from patients’ files. The level of sedation of children had been observed just before premedication and at 15, 30, and 45 min after premedication. The data were analyzed using a chi-square test, Fisher’s exact test, Student’s t-test, and analysis of variance in SPSS.
Results
The Mask Acceptance Scale and PSAS scores and RSS scores at 15, 30, and 45 min after premedication were not statistically different (p>0.05) in both groups, whereas the PAEDS scores were significantly lower in the DEX group (p<0.05).
Conclusion
Oral DEX provided satisfactory sedation levels, ease of parental separation, and mask acceptance in children in a manner similar to MID. Moreover, children premedicated with DEX experienced lesser ED than those premedicated with MID.
Keywords: dexmedetomidine, midazolam, emergence delirium, dental treatment, child
Background
General anesthesia (GA) is an advanced behavioral management technique frequently used by dentists to provide quality dental care for young children who are unable to tolerate dentistry in a routine clinical setting.1 Pediatric patients are usually uncooperative, fearful, anxious, or physically resistant, especially during times of parental separation, venepuncture, or mask application.2 Premedication is the most common way to minimize distress for children entering the operating room and to facilitate the smooth induction of anesthesia and is accomplished using various sedative drugs before they are being transferred to an operating room.3
Midazolam (MID), which is an anxiolytic, sedative, hypnotic, and amnesic drug, has been widely used for premedication via several routes.2 Cox et al4 reported that 0.5 mg/kg of oral MID was effective in reducing separation and induction anxiety in children. Although MID is effective for reducing anxiety, there are adverse effects associated with its use, such as postoperative behavioral changes, cognitive impairment, paradoxical reactions, and respiratory depression.5 Additionally, studies have shown that MID was ineffective in preventing emergence delirium (ED) when compared to other drugs such as propofol, ketamine, α-2 agonist, and fentanyl.6 Therefore, different drugs, including α-2 adrenoceptor agonists, which produce a type of sedation known as “cooperative” or “arousable” that is different from the “clouding of consciousness” sedation, are considered as alternatives for premedication in pediatric anesthesia.7
Dexmedetomidine (DEX) is a highly selective α-2 adrenoceptor agonist that provides sedation, anxiolysis, and analgesic effects without causing deleterious respiratory depression. Recently, it has been extensively explored in pediatric populations for premedication.8
ED refers to behaviors, such as inconsolable crying, thrashing, kicking, disorientation, hallucinations, and cognitive and memory impairment, during the recovery period following GA.9 Untreated ED may lead to additional nursing care, the need for use of analgesics or sedatives, and delayed discharge from the hospital. There are many studies that have evaluated ED in children undergoing various types of surgical procedures.10–12 However, there are limited studies evaluating ED after dental treatment with GA.
This study was designed with an aim to compare the effects of oral DEX and oral MID on preoperative cooperation and ED in children aged 3–7 years who underwent dental procedures under GA.
Patients and methods
The study included records of children who underwent full-mouth dental rehabilitation due to lack of chairside cooperation in the dental clinic between November 2016 and April 2017. This retrospective study was approved by the Research Ethics Committee of the Adnan Menderes University Faculty of Dentistry (2017/2018). Obtaining written informed consent for this study was not necessary, as the study involved retrospective review of patients’ data. Records that identified the subject of the study were kept confidential during data collection. The study was registered (Protocol Registration Receipt NCT03357718) at https://www.ClinicalTrials.gov.
We manually checked the records of patients who were premedicated by MID or DEX before dental procedures. The inclusion criteria were as follows: age between 3 and 7 years, American Society of Anesthesiologists I, induced and maintained with sevoflurane as the general anesthetic, and lack of chairside cooperation in the dental clinic. The exclusion criteria included congenital disease; DEX, MID, or propofol allergy; asthma; mental retardation; and those whose dental records were incomplete. Thirteen records were excluded from the DEX group due to the presence of congenital diseases and mental retardation; 10 records were excluded from the MID group due to the lack of the data after a manual search.
After the patients’ files were retrieved, two groups were created. The DEX group (n=26) included patients who received 2 µg/kg of oral DEX in apple juice 45 min before the induction of anesthesia. The MID group (n=26) received 0.5 mg/kg of MID in apple juice 45 min before the induction of anesthesia.
Scales used in the study
The Ramsay Sedation Scale (RSS), the Parental Separation Anxiety Scale (PSAS), the Mask Acceptance Scale, and the Pediatric Anesthesia Emergence Delirium Scale (PAEDS) records from the patients’ files were extracted. PSAS (at separation), Mask Acceptance Scale (at anesthesia induction), and PAEDS score (just before premedication and at 15, 30, and 45 min after premedication) of the children were recorded.
RSS
The scoring is done as follows: 1=patient is anxious and agitated or restless or both; 2=patient is cooperative, oriented, and tranquil; 3=patient responds to commands only; 4=patient exhibits a brisk response to a light glabellar tap; 5=patient exhibits a sluggish response to a light glabellar tap; and 6=patient exhibits no response.13 A Ramsay sedation score of “1” was considered as an unsatisfactory level of sedation and “≥2” as satisfactory sedation in this study.
PSAS
The behavior score of the child on separation from their parents is evaluated according to a 4-point scale, that is, 1=easy separation; 2=whimpers, but is easily reassured; 3=cries and cannot be easily reassured, but is not clinging to parents; and 4=cries and is clinging to parents. PSAS scores of 1 and 2 were considered as “successful parental separation” in this study.14
Mask Acceptance Scale
This scale is used to evaluate mask acceptance behavior of patients by a 4-point scale during the mask application by the anesthetist: 1=excellent (unafraid, cooperative, and accepts the mask easily); 2=good (slight fear of mask, easily reassured); 3=fair (moderate fear of mask, not calmed with reassurance); and 4=poor (terrified, crying, or combative). Scores of “1” and “2” were considered as “satisfactory” mask acceptance15,16 in this study.
PAEDS
The PAEDS is used to assess patients on five psychometric items:
The child makes eye contact with the caregiver.
The child’s actions are purposeful.
The child is aware of his or her surroundings.
The child is restless.
The child is inconsolable.
Items 1, 2, and 3 are reversed scored as follows: 4=not at all; 3=just a little; 2=quite a bit; 1=very much; and 0=extremely. Items 4 and 5 are scored as follows: 0=not at all; 1=just a little; 2=quite a bit; 3=very much; and 4=extremely. The scores of each item are summed to obtain a total PAEDS score. ED increases directly with the total score. A score ≥10 was considered as the presence of ED.16,17 This scale is used to evaluate the ED for all patients at postanesthesia care unit in our department. Duration of operation was defined as time between beginning of dental procedure and completion of the procedure. Duration of anesthesia was defined as the period from induction of anesthesia to extubation.
Hemodynamic parameters including heart rate (HR), respiratory rate (RR), and peripheral capillary oxygen saturation (SpO2) at baseline and at 15, 30, and 45 min after premedication were also noted from the files.18
Statistical analyses
The results are presented as mean±SD for quantitative variables and are summarized as absolute frequencies and percentages for categorical variables. Categorical variables were compared using a chi-square test or Fisher’s exact test. Quantitative variables were also compared with a Student’s t-test. The variations in baseline hemodynamic variables, including HR, RR, and SpO2, between the groups, were analyzed using a repeated-measures analysis of variance. Statistical analyses were carried out using SPSS software version 20 (IBM Corporation, Armonk, NY, USA). A p-value of <0.05 was considered statistically significant.
Results
There were no statistically significant differences between the groups regarding demographics, duration of operation, and duration of anesthesia (p>0.05). The mean age of the patients was 5.2±1.9 years. Demographic data of the patients are shown in Table 1.
Table 1.
Comparison of demographic information, duration of operation, and duration of anesthesia between the groups
| MID group, n=26 | DEX group, n=26 | p-value | |
|---|---|---|---|
| Age (years) | 5.1±1.4 | 5.3±2.3 | 0.72 |
| Gender (male/female) | 18/8 | 15/11 | 0.56 |
| Weight (kg) | 19.4±5.9 | 18.8±2.9 | 0.65 |
| Duration of operation (min) | 54.6±20.7 | 45.5±17.6 | 0.09 |
| Duration of anesthesia (min) | 67.8±21.7 | 65.7±23.9 | 0.74 |
Notes: Data are expressed as mean±SD or the number of children. Significant differences are at p<0.05.
Abbreviations: DEX, dexmedetomidine; MID, midazolam.
The baseline RSS score was comparable in both groups (p>0.05). The value of the RSS score was not significantly different in the DEX and MID groups at 15, 30, and 45 min (p>0.05). There were no patients with RSS scores higher than 2.
Analyses of the PSAS scores demonstrated that most of the children in both groups showed a satisfactory response during parental separation, and there was no statistically significant difference between the groups (p=1.00).
In terms of mask acceptance behavior, both groups performed satisfactory mask acceptance (p=1.00). Table 2 shows the distribution and comparison of sedation satisfaction percentages of the groups in terms of time.
Table 2.
Ramsay sedation levels of the groups and comparison of the groups in terms of preoperative cooperation
| Time interval since premedication | MID group, n (%) | DEX group, n (%) | χ2/p-value |
|---|---|---|---|
| Ramsay baseline | 0.00/1.00 | ||
| Unsatisfactory | 26 (100) | 26 (100) | |
| Satisfactory | 0 (0) | 0 (0) | |
| Ramsay 15 min | 0.07/0.78 | ||
| Unsatisfactory | 12 (46.2) | 11 (42.3) | |
| Satisfactory | 14 (53.2) | 15 (57.7) | |
| Ramsay 30 min | 1.08/0.29 | ||
| Unsatisfactory | 3 (11.5) | 1 (3.8) | |
| Satisfactory | 23 (88.5) | 25 (96.2) | |
| Ramsay 45 min | 1.02/0.31 | ||
| Unsatisfactory | 1 (3.8) | 0 (0) | |
| Satisfactory | 25 (96.2) | 26 (100) | |
| Successful parental separation | 0.00/1.00 | ||
| Yes | 24 (92.3) | 24 (92.3) | |
| No | 2 (7.7) | 2 (7.7) | |
| Mask acceptance | 0.00/1.00 | ||
| Satisfactory | 24 (92.3) | 24 (92.3) | |
| Unsatisfactory | 2 (7.7) | 2 (7.7) | |
| Emergence delirium | 5.53/0.01a | ||
| Present | 58 (19.2) | 0 (0) | |
| Absent | 21 (80.8) | 26 (100) |
Notes: Values in number (%).
Significant differences between groups at the 0.05 level.
Abbreviations: DEX, dexmedetomidine; MID, midazolam.
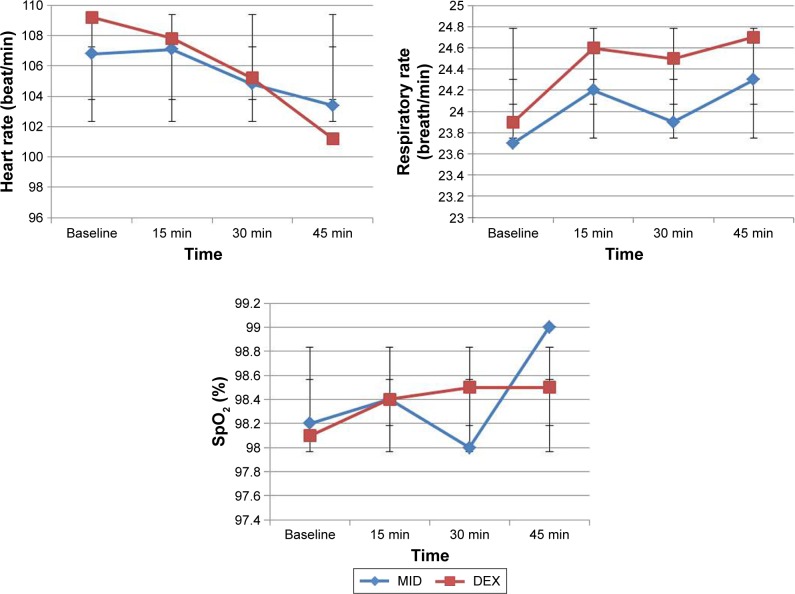
Overall, we did not observe any clinically significant effects of the study drug on SpO2 and no patient had a reduction in SpO2 below 95% during the observation period after premedication. There were no significant differences in the mean HRs, SpO2, and RR of both groups at baseline and at 15, 30, and 45 min (p>0.05; Figure 1).
Figure 1.
Mean heart rate, respiration rate, and SpO2 levels of the groups during the premedication period.
Abbreviations: DEX, dexmedetomidine; MID, midazolam; SpO2, peripheral capillary oxygen saturation.
In the postanesthesia care unit, children in the DEX group showed a significantly lower ED score compared to those in the MID group (p<0.05).
There were significant time effects (p<0.05) and group time interactions on HR (p<0.05) in the DEX group only. HR was reduced significantly from baseline at 15, 30, and 45 min after drug administration in the DEX group (p<0.05).
There were no statistically significant group and time effects and group × time interactions (p>0.05) on RR and SpO2.
Discussion
This retrospective study demonstrated that 2 µg/kg of oral DEX and 0.5 mg/kg of MID provided effective sedation, satisfactory separation from parents, and satisfactory mask acceptance in children between 3 and 7 years of age who underwent dental surgery under GA. Additionally, a lower incidence of ED was observed in the DEX group compared to the MID group.
Patients, and particularly those in younger age groups, who generally have low levels of cooperation in the dental clinic, also show uncooperative behavior in the perioperative period for dental GA.19,20 Therefore, it is considered that the need for premedication is increased in patients who require dental treatment under GA. Studies have concluded that the use of sedative premedication may help to reduce anxiety in children, minimize emotional trauma, and facilitate a smooth induction of anesthesia in children undergoing full-mouth rehabilitation.2,21 Moreover, the use of DEX and MID for premedication reduced the requirement of anesthetics during GA.22 Our study included young children who could not be treated in a routine clinical setting with sedation and non-pharmacologic behavior control techniques, such as voice control, hand over mouth, and intimidation. Yao et al23 showed that intranasal DEX premedication of 1 and 2 µg/kg was associated with a dose-dependent reduction in sevoflurane from 1.92% to 1.53% and 1.23%, corresponding to decreases of 20% and 36%, respectively. MID administered in a premedication setting often increases the potency of inhalational anesthetic agents.22
MID is a common and routinely used drug for oral premedication in children at a dose of 0.5 mg/kg.11 MID has a rapid onset and short duration of action and provides reliable sedation and anxiolysis. Beyond these advantages, there are some disadvantages such as bitter taste, cognitive impairment, long-term behavioral disturbances, paradoxical reactions, hiccups, and respiratory depression.5 The bioavailability of oral MID varies from 15% to 27% in children.24 Fabre et al25 reported that the maximum concentration (Cmax) of 0.6 mg/kg of MID rectally was calculated as 147±58 µg/L, median tmax was 31.5 min (range: 18–38 min), and the half-life was 1.3±0.3 h in a pediatric population.
DEX has become more frequently used as a drug for pre-medication in children.2,10,26 It is a new α2 agonist with a more selective action on α2-adrenergic receptors and a short half-life. Studies showed that DEX is an effective and safe sedative in children. Moreover, DEX has analgesic and anti-shivering properties and does not cause respiratory depression. The bioavailability of oral DEX following the orogastric route is 16%, as compared to 65% for intranasal, 82% for buccal, and 104% for intramuscular (IM) routes.24 Anttila et al27 stated that the maximum serum concentration of 2 µg/kg of oral DEX was achieved to be 0.11±0.04 µg/L in 2.2±0.5 h, after a lag time of 0.6±0.3 h, in an adult study. Additionally, the terminal half-life was estimated to be 1.2±0.3 h in their study.
Absorption of orally administered drugs is affected by factors such as the form and physicochemical features of the drug, its lipophilic properties, the pH of the digestive tract, fullness of the stomach, length of time the drug is in contact with the mucosa, and the flow of saliva. A higher pH promotes lipid solubility and accelerates absorption across mucosal membranes.28 Fruit juices with an acidic pH may inhibit cytochrome P4503A4 activity and slow catabolism.29 Therefore, the duration of the sedative effect and the efficacy of MID may be increased.
In previous studies, there was a wide variation in the dosage and the application route of drugs for premedication. DEX and MID are only available as an intravenous (IV) formulation. MID is the most commonly used drug for premedication through IV, IM, oral, rectal, and nasal routes.30 Many studies have shown that 0.5 mg/kg of oral MID was effective in providing preoperative cooperation.11,31 DEX may be administered through IV, oral, buccal, and IM routes.32 In previously conducted studies, the oral route was reported to be more acceptable than the nasal route in children aged 2–6 years.32 Faritus et al10 concluded that 2 µg/kg of oral DEX and 0.5 mg/kg of MID as premedication, 45 min pre-operatively in children undergoing congenital heart surgery, provided ease with parental separation and mask acceptance behavior without any side effects on hemodynamic variables. Therefore, we used 2 µg/kg of DEX and 0.5 mg/kg of MID orally in this study. The risk of aspiration increases when the gastric fluid volume is above 0.4 mL/kg. The total volume of the oral premedication was kept at 0.2 mL/kg in this study.
In this study, we evaluated the sedation levels of patients via RSS, and no patient had an RSS higher than “2” in either group. We considered an RSS of 2 and above as “adequate sedation”, meaning that the patients were cooperative, awake, oriented, and calm. We observed that an RSS of 2 was sufficient to provide satisfactory preoperative cooperation in children who underwent dental procedures. Mountain et al33 concluded that 4 µg/kg of oral DEX and 0.5 mg/kg of MID 30 min before an operation provided a satisfactory mask acceptance behavior and ease of parental separation. Additionally, they found no statistically significant differences between the groups in terms of ED without side effects (blood pressure and HR fluctuation). Contrary to their findings, previous studies noted that DEX might cause negative effects on respiration and cardiovascular stability, such as hypotension, bradycardia, and hypoxia, depending on the dose and route of administration.26,34
Faritus et al10 administered 2 µg/kg of oral DEX and 0.5 mg/kg of MID as premedication 45 min preoperatively in children, and this provided ease for parental separation and mask acceptance behavior, without any side effects on hemodynamic variables. Similarly, we obtained satisfactory outcomes in terms of sedation level, parental separation, and mask acceptance in both groups 45 min after the same doses and routes for premedication. In addition, we evaluated the ED levels in the children, and significantly lower levels of ED were observed in the DEX group in this study.
Reducing preoperative anxiety is important, not only to improve preoperative cooperation for patients and families but also for immediate postoperative outcomes. Aono et al35 found that high levels of preoperative anxiety were associated with an increased incidence of ED. The incidence of ED was 60% in preschool children undergoing anesthesia with sevoflurane without premedication.36 Özcengiz et al12 showed that ED of children premedicated orally 45 min preoperatively with 2.5 mg/kg of DEX, 0.5 mg/kg of MID, and 0.1 mg/kg of melatonin was significantly lower than that of a placebo group. In this study, the overall incidences of ED were 0% and 19.2% in the DEX and MID groups, respectively. Jannu et al37 reported that 4 µg/kg of oral DEX vs 0.75 mg/kg of oral MID as premedication provided a lower incidence of ED in children aged 1–7 years. Prabhu and Mehandale38 compared the effect of 4 µg/kg of oral DEX vs 0.5 mg/kg of oral MID as premedication and concluded that oral DEX is superior to oral MID for reducing the incidence (from 40% to 4.4%) and severity of ED. Similar to their study, the incidence of ED in the DEX group was significantly lower than that in the MID group. Moreover, the dose of DEX was lower in our study.
Drugs used for premedication may cause changes in vital signs.3,11 In our study, there were no significant differences in the mean HR, RR, and SpO2 values after the administration of DEX and MID. However, HR in the DEX group decreased significantly 30 min after drug administration in comparison with the MID group. Despite this decrease, hemodynamic variables remained within normal limits and did not differ from the MID group.
This clinical study was not free of limitations. First, due to the retrospective nature of the study, the results depended on the records of patients’ files. Second, IV formulations of DEX and MID were used as an oral preparation. Third, mixing of DEX and MID with apple juice may have affected the pH of the drug and its absorption. Regardless of these limitations, there is a strong need for prospective randomized studies with larger sample sizes to find the optimum doses of DEX and to evaluate its safety and efficacy in children undergoing dental procedures under GA.
Conclusion
We conclude that 2 µg/kg of oral DEX provided satisfactory sedation levels, separation from parents, and mask acceptance in children, with a similar effect to 0.5 mg/kg of oral MID as premedication before comprehensive dental treatment. Additionally, DEX was more effective than MID in preventing ED.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Dziedzic A. The role of general anesthesia in special care and pediatric dentistry; inclusion criteria and clinical indications. SAAD Dig. 2017;30:47–53. [PubMed] [Google Scholar]
- 2.Sheta SA, Al-Sarheed MA, Abdelhalim AA. Intranasal dexmedetomidine vs midazolam for premedication in children undergoing complete dental rehabilitation: a double-blinded randomized controlled trial. Paediatr Anaesth. 2014;24(2):181–189. doi: 10.1111/pan.12287. [DOI] [PubMed] [Google Scholar]
- 3.Yuen VM, Hui TW, Irwin MG, Yuen MK. A comparison of intranasal dexmedetomidine and oral midazolam for premedication in pediatric anesthesia: a double-blinded randomized controlled trial. Anesth Analg. 2008;106(6):1715–1721. doi: 10.1213/ane.0b013e31816c8929. [DOI] [PubMed] [Google Scholar]
- 4.Cox RG, Nemish U, Ewen A, Crowe MJ. Evidence-based clinical update: does premedication with oral midazolam lead to improved behavioural outcomes in children? Can J Anaesth. 2006;53(12):1213–1219. doi: 10.1007/BF03021583. [DOI] [PubMed] [Google Scholar]
- 5.Bergendahl H, Lönnqvist PA, Eksborg S. Clonidine in pediatric anesthesia: review of the literature and comparison with benzodiazepines for premedication. Acta Anaesthesiol Scand. 2006;50(2):135–143. doi: 10.1111/j.1399-6576.2006.00940.x. [DOI] [PubMed] [Google Scholar]
- 6.Dahmani S, Stany I, Brasher C, et al. Pharmacological prevention of sevoflurane- and desflurane-related emergence agitation in children: a meta-analysis of published studies. Br J Anaesth. 2010;104(2):216–223. doi: 10.1093/bja/aep376. [DOI] [PubMed] [Google Scholar]
- 7.Pasin L, Febres D, Testa V, et al. Dexmedetomidine vs midazolam as preanesthetic medication in children: a meta-analysis of randomized controlled trials. Paediatr Anaesth. 2015;25(5):468–476. doi: 10.1111/pan.12587. [DOI] [PubMed] [Google Scholar]
- 8.Peng K, Wu SR, Ji FH, Li J. Premedication with dexmedetomidine in pediatric patients: a systematic review and meta-analysis. Clinics (Sao Paulo) 2014;69(11):777–786. doi: 10.6061/clinics/2014(11)12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vlajkovic GP, Sindjelic RP. Emergence delirium in children: many questions, few answers. Anesth Analg. 2007;104(1):84–91. doi: 10.1213/01.ane.0000250914.91881.a8. [DOI] [PubMed] [Google Scholar]
- 10.Faritus SZ, Khazaee-Koohpar M, Ziyaeifard M, Mehrabanian MJ. Oral dexmedetomidine versus midazolam as anesthetic premedication in children undergoing congenital heart surgery. Anesth Pain Med. 2015;5(3):e25032. doi: 10.5812/aapm.5(3)2015.25032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumari S, Agrawal N, Usha G, Talwar V, Gupta P. Comparison of oral clonidine, oral dexmedetomidine, and oral midazolam for premedication in pediatric patients undergoing elective surgery. Anesth Essays Res. 2017;11(1):185–191. doi: 10.4103/0259-1162.194586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Özcengiz D, Gunes Y, Ozmete O. Oral melatonin, dexmedetomidine, and midazolam for prevention of postoperative agitation in children. J Anesth. 2011;25(2):184–188. doi: 10.1007/s00540-011-1099-2. [DOI] [PubMed] [Google Scholar]
- 13.Ramsay MA, Savege TM, Simpson BR, Goodwin R. Controlled sedation with alphaxalone-alphadolone. Br Med J. 1974;2(5920):656–659. doi: 10.1136/bmj.2.5920.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dashiff CJ, Weaver M. Development and testing of a scale to measure separation anxiety of parents of adolescents. J Nurs Meas. 2008;16(1):61–80. doi: 10.1891/1061-3749.16.1.61. [DOI] [PubMed] [Google Scholar]
- 15.Weldon BC, Watcha MF, White PF. Oral midazolam in children: effect of time and adjunctive therapy. Anesth Analg. 1992;75(1):51–55. doi: 10.1213/00000539-199207000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Shukry M, Clyde MC, Kalarickal PL, Ramadhyani U. Does dexmedetomidine prevent emergence delirium in children after sevoflurane-based general anesthesia? Pediatr Anesth. 2005;15(12):1098–1104. doi: 10.1111/j.1460-9592.2005.01660.x. [DOI] [PubMed] [Google Scholar]
- 17.Sikich N, Lerman J. Development and psychometric evaluation of the pediatric anesthesia emergence delirium scale. Anesthesiology. 2004;100(5):1138–1145. doi: 10.1097/00000542-200405000-00015. [DOI] [PubMed] [Google Scholar]
- 18.Aldrete JA, Kroulik D. A postanesthetic recovery score. Anesth Analg. 1970;49(6):924–934. [PubMed] [Google Scholar]
- 19.Knapp R, Gilchrist F, Rodd HD, Marshman Z. Change in children’s oral health-related quality of life following dental treatment under general anesthesia for the management of dental caries: a systematic review. Int J Paediatr Dent. 2017;27(4):302–312. doi: 10.1111/ipd.12259. [DOI] [PubMed] [Google Scholar]
- 20.Jankauskiene B, Virtanen JI, Kubilius R, Narbutaite J. Oral health-related quality of life after dental general anesthesia treatment among children: a follow-up study. BMC Oral Health. 2014;14(1):81. doi: 10.1186/1472-6831-14-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.El Batawi HY. Effect of intraoperative analgesia on children’s pain perception during recovery after painful dental procedures performed under general anesthesia. Eur Arch Paediatr Dent. 2015;16(1):35–41. doi: 10.1007/s40368-014-0143-y. [DOI] [PubMed] [Google Scholar]
- 22.Aranake A, Mashour GA, Avidan MS. Minimum alveolar concentration: ongoing relevance and clinical utility. Anesthesia. 2013;68(5):512–522. doi: 10.1111/anae.12168. [DOI] [PubMed] [Google Scholar]
- 23.Yao Y, Qian B, Lin Y, Wu W, Ye H, Chen Y. Intranasal dexmedetomidine premedication reduces minimum alveolar concentration of sevoflurane for laryngeal mask airway insertion and emergence delirium in children: a prospective, randomized, double-blind, placebo-controlled trial. Paediatr Anaesth. 2015;25(5):492–498. doi: 10.1111/pan.12574. [DOI] [PubMed] [Google Scholar]
- 24.Kumari S, Agrawal N, Usha G, Talwar V, Gupta P. Comparison of oral clonidine, oral dexmedetomidine, and oral midazolam for premedication in pediatric patients undergoing elective surgery. Anesth Essays Res. 2017;11(1):185–191. doi: 10.4103/0259-1162.194586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fabre E, Chevret S, Piechaud JF, et al. An approach for dose finding of drugs in infants: Sedation by midazolam studied using the continual reassessment method. Br J Clin Pharmacol. 1998;46(4):395–401. doi: 10.1046/j.1365-2125.1998.00788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berkenbosch JW, Tobias JD. Development of bradycardia during sedation with dexmedetomidine in an infant concurrently receiving digoxin. Pediatr Crit Care Med. 2003;4(2):203–205. doi: 10.1097/01.PCC.0000059737.86673.28. [DOI] [PubMed] [Google Scholar]
- 27.Anttila M, Penttilä J, Helminen A, Vuorilehto L, Scheinin H. Bioavailability of dexmedetomidine after extravascular doses in healthy subjects. Br J Clin Pharmacol. 2003;56(6):691–693. doi: 10.1046/j.1365-2125.2003.01944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karl HW, Rosenberger JL, Larach MG, Ruffle JM. Transmucosal administration of midazolam for premedication of pediatric patients. Comparison of the nasal and sublingual routes. Anesthesiology. 1993;78(5):885–891. doi: 10.1097/00000542-199305000-00013. [DOI] [PubMed] [Google Scholar]
- 29.Kim H, Yoon YJ, Shon JH, Cha IJ, Shin JG, Liu KH. Inhibitory effects of fruit juices on CYP3A activity. Drug Metab Dispos. 2006;34(4):521–523. doi: 10.1124/dmd.105.007930. [DOI] [PubMed] [Google Scholar]
- 30.Kupietzky A, Houpt MI. Midazolam: a review of its use for conscious sedation in children. Pediatr Dent. 1993;15(4):237–241. [PubMed] [Google Scholar]
- 31.Kaviani N, Shahtusi M, Haj Norousali Tehrani M, Nazari S. Effect of oral midazolam premedication on children’s co-operation before general anesthesia in pediatric dentistry. J Dent (Shiraz) 2014;15(3):123–128. [PMC free article] [PubMed] [Google Scholar]
- 32.Verma RK, Paswan A, De A, Gupta S. Premedication with midazolam nasal spray: an alternative to oral midazolam in children. Anesth Pain Med. 2012;1(4):248–251. doi: 10.5812/aapm.4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mountain BW, Smithson L, Cramolini M, Wyatt TH, Newman M. Dexmedetomidine as a pediatric anesthetic premedication to reduce anxiety and to deter emergence delirium. AANA J. 2011;79(3):219–224. [PubMed] [Google Scholar]
- 34.Santos LC, Ludders JW, Erb HN, Basher KL, Kirch P, Gleed RD. Sedative and cardiorespiratory effects of dexmedetomidine and buprenorphine administered to cats via oral transmucosal or intramuscular routes. Vet Anaesth Analg. 2010;37(5):417–424. doi: 10.1111/j.1467-2995.2010.00555.x. [DOI] [PubMed] [Google Scholar]
- 35.Aono J, Mamiya K, Manabe M. Preoperative anxiety is associated with a high incidence of problematic behavior on emergence after halothane anesthesia in boys. Acta Anaesthesiol Scand. 1999;43(5):542–544. doi: 10.1034/j.1399-6576.1999.430509.x. [DOI] [PubMed] [Google Scholar]
- 36.Son JS, Jang E, Oh MW, Lee JH, Han YJ, Ko S. A comparison of postoperative emergence agitation between sevoflurane and thiopental anesthesia induction in pediatric patients. Korean J Anesthesiol. 2015;68(4):373–378. doi: 10.4097/kjae.2015.68.4.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jannu V, Mane RS, Dhorigol MG, Sanikop CS. A comparison of oral midazolam and oral dexmedetomidine as premedication in pediatric anesthesia. Saudi J Anaesth. 2016;10(4):390–394. doi: 10.4103/1658-354X.177333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prabhu MK, Mehandale SG. Comparison of oral dexmedetomidine versus oral midazolam as premedication to prevent emergence agitation after sevoflurane anesthesia in Pediatric patients. Indian J Anaesth. 2017;61(2):131–136. doi: 10.4103/0019-5049.199852. [DOI] [PMC free article] [PubMed] [Google Scholar]