Abstract
Background
Stevens–Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are dermatologic emergencies with high morbidity and mortality risk. Cyclosporine, an immunomodulatory agent, is sometimes used off-label, and its role continues to be debated. This meta-analysis aimed to provide an update of current evidence and to clarify the role of cyclosporine in SJS/TEN treatment better.
Methods
Using the keywords [cyclosporine OR cyclosporine OR ciclosporin OR CsA] AND [Steven-Johnson OR SJS OR toxic epidermal OR epidermal necrolysis OR TEN OR hypersensitivity OR dermatologic OR burns], a preliminary search on the PubMed, Ovid, Web of Science, and Google Scholar Database yielded 615 papers published in English between January1, 1960 and July 1, 2017. The inclusion criteria for this review were: 1) published retrospective or prospective study (excluding single case reports); 2) patients with clinical diagnosis of SJS or TEN; 3) trial of cyclosporine treatment; and 4) available survival/mortality data.
Results
A total of 12 studies, with a total of 358 SJS/TEN patients were reviewed. Two studies were excluded from the meta-analysis as they did not report SCORe of toxic epidermal necrosis/predicted mortality data; one was excluded because of possible data irregularities. Meta-analysis of nine studies revealed a significant reduction in mortality risk with cyclosporine therapy (standardized mortality ratio 0.320; 95% CI: 0.119–0.522; P=0.002). Cyclosporine was also generally well tolerated with little adverse effects or increased infection, albeit the patients tended to be critically ill. Publication bias was observed in the funnel plot and Egger test (P=0.0467).
Conclusion
Currently available evidence are predominantly open trials and retrospective studies with a significant risk of bias, perhaps owing to the rarity and life-threatening nature of the condition. Given its immunomodulatory actions, cyclosporine could be a potential treatment option for SJS/TEN in addition to best supportive measures. Further confirmation with robust randomized, controlled trials or larger case series is necessary and should be encouraged.
Keywords: SJS, TEN, epidermal necrolysis, cyclosporine, CsA, meta-analysis
Introduction
Stevens–Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are rare dermatologic emergencies with excessive morbidity and mortality risk. The incidence rate is 0.5–1.4 per million per year, and the average mortality rate is estimated to be 25%–35%.1 SJS and TEN are severe mucocutaneous adverse reactions, most commonly triggered by medications, including antibiotics, anticonvulsants, and nonsteroidal anti-inflammatory drugs.2 SJS and TEN are considered a disease continuum and involve skin detachment of <10% and >30% of body surface area, respectively.3 SJS/TEN overlap describes patients with skin detachment of 10%–30% of body surface area. Patients are often critically ill and are presented with fever (prodromal) and widespread necrosis and detachment of the epidermis.4 Atypical targetoid macules appear on the skin, which eventually blister and slough off as areas of (full thickness) epidermal necrosis.
Beyond supportive care, there are no established systemic therapies for SJS and TEN. Supportive care is the cornerstone of treatment. Systemic treatment of SJS/TEN remains a matter of debate and contention.5 Several well-characterized human leukocyte antigen (HLA) associations, for example, HLA-B*1502,6 strongly predispose patients to specific delayed-type drug hypersensitivity reactions, including SJS/TEN. Most of the known HLA associations are class I, lending further support for the postulated role of CD8+ T cells in the pathogenesis of SJS/TEN.7 Adjunctive therapies include systemic corticosteroids, intravenous immune globulin (IVIg), cyclosporine (CsA), plasmapheresis, and anti-TNF monoclonal antibodies.8 All of these carry limitations, potential adverse effects, and risks.
There have been some case reports of the positive efficacy of CsA (in doses of 3–5 mg/kg/d) to retard the progression of SJS/TEN and promote rapid re-epithelization.9–11 Although this is the most widely used dose, there is no consensus on its role in SJS/TEN or the appropriate duration of therapy.12
CsA is a cyclic peptide of 11 amino acids isolated from the soil fungus Tolypocladium inflatum Gams. It is hydrophobic and lipophilic and shows high interindividual and intraindividual variation in terms of pharmacokinetics.13 CsA may have an ameliorative effect in SJS/TEN patients by opposing the apoptotic pathway in nonlesional skin.12 Given its immunomodulatory actions, CsA could be a potential treatment option for SJS/TEN patients. A recent meta-analysis on systemic immunomodulating therapies for SJS and TEN analyzed only one trial pertaining to the use of CsA.14 Therefore, this meta-analytic review aims to clarify better the role of CsA in SJS/TEN treatment and generate directions for future research.
Methods
Literature search was done in accordance with preferred reporting items for systematic reviews and meta-analysis guidelines. Using the keywords [cyclosporine OR cyclosporine OR ciclosporin OR CsA] AND [Steven–Johnson OR SJS OR toxic epidermal OR epidermal necrolysis OR TEN OR hypersensitivity OR dermatologic OR burns], a preliminary search on the PubMed, Ovid, Web of Science, and Google Scholar Database yielded 615 papers published in English between January1, 1960 and July 1, 2017. Grey literature was not searched. Title/abstract screening was performed independently by (QX Ng and MLZQ De Deyn) to identify articles of interest. For relevant abstracts, full articles were obtained, reviewed, and also checked for references of interest. The authors of the articles were not contacted to provide additional data.
Full articles were obtained for all selected abstracts and reviewed by three researchers (QX Ng, MLZQ De Deyn, and N Venkatanarayanan) for inclusion. Any disagreement was resolved by discussion and consensus among the three researchers. The inclusion criteria for this review were: 1) published retrospective or prospective study (excluding single case reports); 2) patients with clinical diagnosis of SJS or TEN; 3) trial of CsA treatment; and 4) available survival/mortality data. Data such as study design, study population, and demographics, SCORe of toxic epidermal necrosis (SCORTEN), a severity-of-illness score for toxic epidermal necrolysis, and outcome measure were extracted. The primary outcome measure of interest was the mortality benefit with CsA treatment. Standardized mortality ratio (SMR) of observed to the predicted number of deaths was calculated for each study. SCORTEN is a well-validated tool for predicting mortality in SJS/TEN patients.15,16 Because the normal distribution can take all real numbers (is continuous), but the binomial distribution can only take integer values (is discrete), for studies with no observed deaths, a continuity correction factor of 1 was added to both the observed and expected deaths for purposes of calculating SMR. SMRs were pooled, and where appropriate, 95% CI and P-values were calculated.
Heterogeneity among the different studies pooled was examined using the I2 statistic and Cochran’s Q test. Publication bias was assessed using a funnel plot and Egger test. All analyses were done using MedCalc statistical software version 14.8.1 (MedCalc Software BVBA, Ostend, Belgium; http://www.medcalc.org; 2014) and STATA version 13.0 (STATA Corp., College Station, TX, USA).
Results
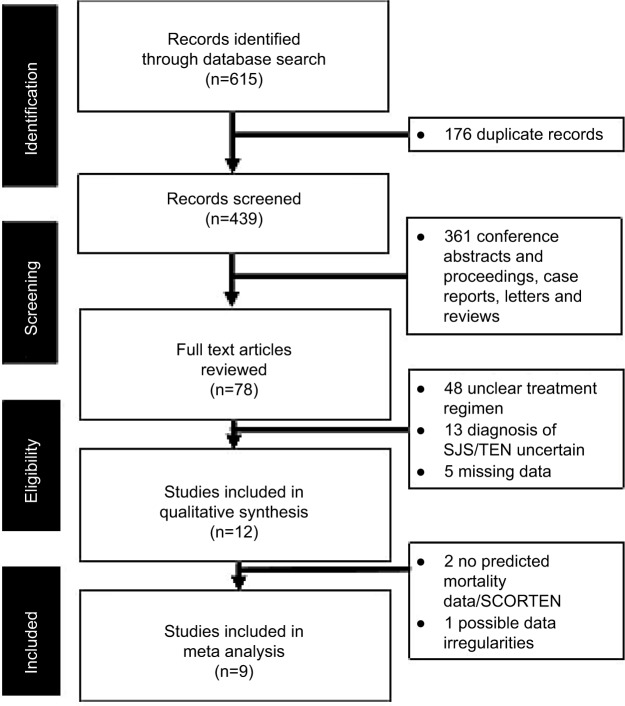
The abstraction process (and reasons for exclusion) was detailed in Figure 1. The key details of each study were extracted and summarized in Table 1. Of the 12 studies reviewed, two were excluded from the final meta-analysis as they did not report SCORTEN/predicted mortality data, and one was excluded on the basis of possible data irregularities, which may unduly affect the reliability of the meta-analysis.
Figure 1.
PRISMA flowchart showing the studies identified during the literature search and abstraction process.
Abbreviations: SJS, Stevens–Johnson syndrome; TEN, toxic epidermal necrolysis; SCORTEN, SCORe of toxic epidermal necrosis; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Table 1.
Characteristics of all studies included in this review
| Study | Study design | Study sample (N) | Country | Total BSA and SCORTEN | Treatment regimen (n) | Conclusion | |||
|---|---|---|---|---|---|---|---|---|---|
| Arévalo et al, 200017 | Retrospective case series | 17 | Spain | Mean total BSA 83±17%; SCORTEN not specified | • CsA 3 mg/kg/d enterally every 12 hours, for 2 weeks and then tapered gradually (11) • Cyclophosphamide (150 mg IV every 12 hours) and different doses of corticosteroids (≥1 mg/kg/d of 6-methyl-prednisolone) (6) |
CsA is safe and is associated with rapid re-epithelization and a lower rate of multi-organ failure, severe leukopenia, and death than treatment with cyclophosphamide and corticosteroids in patients with severe TEN | |||
| Firoz et al, 201218 | Prospective | 82* | USA | Mean total BSA 34.8±26.1%; mean SCORTEN 2.17 |
• CsA (regimen not specified) only used in patients with low BSA and SCORTEN of 0–1 (8) • IVIg 4 g/kg divided over 3 days if patients presented within 72 hours of blistering (23) • Supportive care for patients who presented ≥3 days of blistering (51) |
No significant difference in survival among all three treatment options (P=0.15, log-rank test). IVIg did not significantly alter mortality | |||
| Giudice et al, 201719 | Retrospective case series | 12 | Italy | Mean total BSA 76.7±12.3%;mean SCORTEN 4.3 |
Standardized treatment protocol: CsA IV 250 mg/d or 4 mg/kg/d in pediatric patients on day one, at day three, daptomycin and plasmapheresis were introduced. CsA continued for 15 days, daptomycin for 10 days, plasmapheresis consisted of 7 cycles spaced by 2 days each (12) | Standardized treatment protocol consisting of CsA and plasmapheresis is safe and efficacious in patients with severe TEN | |||
| González-Herrada et al, 201720 | Retrospective and prospective cases | 42 | Spain | Mean total BSA 43.5±26.9%; mean SCORTEN 2.39 |
• CsA 3 mg/kg/d until complete re-epithelialization and then gradual taper (26) • IVIg 0.75 g/kg/d for 4 days (11) • Prednisone-equivalent 37.5–100 mg/d for 9–12 days (2) • Supportive care (3) |
CsA offers mortality benefit for SJS/TEN patients | |||
| Kirchhof et al, 201410 | Retrospective case series | 64 | Canada | Mean total BSA 28.7±26.6%; mean SCORTEN 1.65 |
• Supportive care (12) • IVIg 1 g/kg/d for 3 days (35) • CsA 3–5 mg/kg/d orally or IV for an average of 7 days (15) • IVIg and CsA (2) |
Relative mortality benefit of CsA (SMR 0.42) over IVIg (SMR 1.43) in patients with SJS/TEN | |||
| Lee et al, 201721 | Retrospective case series | 44 | Singapore | Mean total BSA 29±25%; mean SCORTEN 2.5 |
• CsA 3 mg/kg/d for 10 days, then 2 mg/kg/d for 10 days, and finally 1 mg/kg/d for 10 days (24) • Supportive care (20) |
Relative mortality benefit of CsA (SMR 0.42) over supportive care (SMR 1.02) | |||
| Mohanty et al, 201722 | Retrospective case series | 28 | India | Mean total BSA 35.95±20.33%; mean SCORTEN 2.05 | • CsA 5 mg/kg/d in three divided doses for 10 days, along with supportivecare (19) • Supportive care (9) |
SMR of CsA group (0.32) nearly 3.3 times lower than the only supportive treatment group (1.06) | |||
| Rajaratnam et al, 201023 | Retrospective case series | 21 | UK | Mean total BSA 44%; mean SCORTEN 3.1 |
• CsA IV 2.5–4 mg/kg/d for 3–5 days (3) • IVIg 0.4–1.0 g/kg/d for 3–7 days (14) • Cyclophosphamide IV 2.5 mg/kg/for 3 days (2) |
Corticosteroids did not appear beneficial compared to IVIg or CsA | |||
| Reese et al, 20119 | Retrospective case series | 4 | USA | Mean total BSA 35.8%; mean SCORTEN 1.25 |
• CsA 5 mg/kg/d in two divided doses for 5 days to a month (4) | CsA is efficacious with rapid response and re-epithelization. Short-term use of CsA did not have adverse reactions or increased infections | |||
| Singh et al, 201311 | Retrospective case series | 11 | India | Mean total BSA 23.4±16.3%; mean SCORTEN 1.45 |
• CsA 3 mg/kg/d in three divided doses for 7 days, then 2 mg/kg/d in two divided doses for another 7 days (11) | Faster re-epithelization, shorter hospital stay and relative mortality benefit of CsA over corticosteroids. CsA was also well tolerated by all the patients | |||
| Szepietowski et al, 199724 | Retrospective case series | 3 | Poland | Not specified | • CsA 8–10 mg/kg/d for 10–21 days and corticosteroids (3) | Combined and monotherapy with CsA appear superior to monotherapy with corticosteroids. CsA is beneficial for TEN patients | |||
| Valeyrie-Allanore et al, 201025 | Open, Phase II trial | 29 | France | Mean total BSA 12.2±8.2%; mean SCORTEN 1.27 |
• CsA orally through NG tube, 1.5 mg/kg twice daily for 10 days, then 1 mg/kg twice daily for 10 days, and finally 0.5 mg/kg twice daily for 10 days (29) | CsA was well tolerated; 26 out of 29 patients completed the 1-month treatment. Lower than expected mortality and disease progression observed | |||
Note:
Expression of concern by journal editor and staff over possible data irregularities.
Abbreviations: BSA, body surface area; CsA, cyclosporine; IV, intravenous; IVIg, intravenous immunoglobulin; NG, nasogastric; SCORTEN, SCORe of toxic epidermal necrosis; SJS, Stevens–Johnson syndrome; SMR, standardized mortality ratio; TEN, toxic epidermal necrolysis.
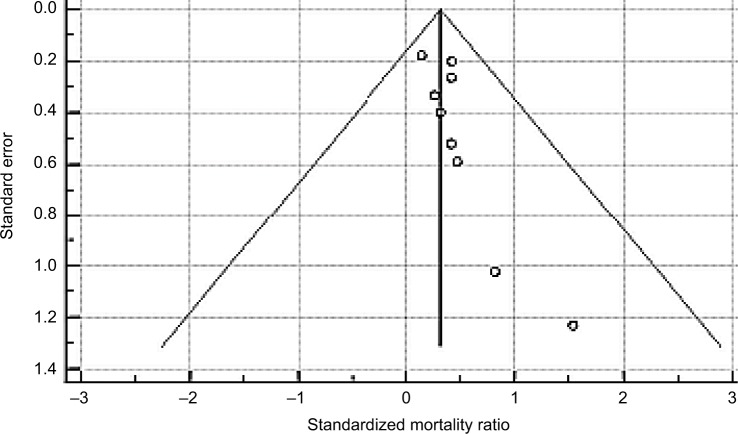
With regard to the possibility of publication bias, visual inspection of the funnel plot revealed a slight asymmetrical distribution of studies (Figure 2) and Egger test was significant for publication bias (P=0.0467).
Figure 2.
Funnel plot (with pseudo 95% confidence limits) to assess publication bias; Egger test for publication bias=0.751, 95% CI=0.0146–1.488, P=0.0467.
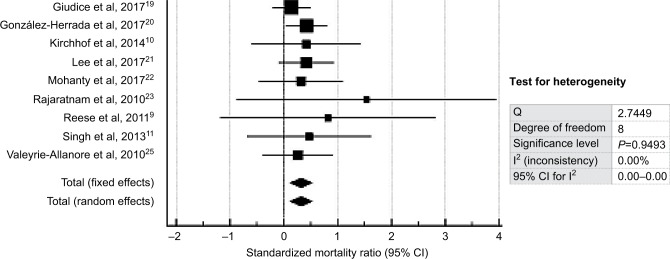
The meta-analysis found that CsA use was associated with improved mortality in SJS/TEN patients as the pooled SMR was 0.320 (95% CI: 0.119–0.522; P=0.002). The forest plot is shown in Figure 3. Fixed- and random-effects analyses yielded similar results.
Figure 3.
Forest plot showing the standardized mortality ratio and 95% CI of studies on cyclosporine therapy.
Discussion
Almost all of the studies reviewed, support the mortality benefits of CsA therapy for SJS/TEN patients. The pooled SMR was 0.320 (95% CI: 0.119–0.522; P=0.002), indicating the mortality benefit of CsA in SJS/TEN treatment. In our meta-analysis, only one study23 found slightly increased mortality risk with CsA treatment (SMR 1.538, 95% CI: 1.30–5.08). In this study, the sample that received CsA was limited to three patients. One patient survived and there was an initial favorable effect with evidence of re-epithelialization of the skin in one of the patients who died. It is worth mentioning that the patient had a background medical history of alcoholic hepatitis and encephalopathy. SCORTEN predicted mortality rate was 1.3 deaths, whereas the actual mortality rate after 3–5 days of IV CsA 2.5–4 mg/kg/d was two deaths.
Our findings were concordant with the results of an earlier meta-analysis,14 which reported mortality benefit for CsA when compared to supportive care (OR: 0.1, 95% CI: 0.0–0.4). However, the OR was based on a single trial of CsA25 and calculated using an exact logistic regression model. Our meta-analysis thus significantly strengthens the evidence supporting the use of CsA in SJS/TEN patients.
Current understanding of the pathophysiology and mechanisms underlying SJS/TEN remains incomplete, due to the rarity of the condition and lack of a reliable animal model for investigation.26 It is presumed that apoptosis is the key mechanism underlying keratinocyte death in SJS/TEN. Cytotoxic T cells are activated by a culprit drug, which releases granulysin.27
The best rationale for using CsA in SJS/TEN is that cytotoxic T cells destroying epithelial cells are drug-specific and HLA class I restricted, like effector cells in acute graft rejection or acute graft-versus-host disease.7 Fas ligand is unlikely a pathogenic mechanism. If it were, IVIg would be useful and would be considered instead of CsA. It is predominantly T/NK with granulysin.27
The observed clinical benefits of CsA for SJS/TEN patients could stem from its mechanism of action. It is an immunosuppressive agent targeting the calcineurin complex. The activation of the calcineurin complex, following T-cell receptor activation, results in dephosphorylation of the nuclear factor of activated T cells (NFAT), leading to its migration into the nucleus and binding with its intranuclear counterpart. The resultant complex is a transcription factor for inflammatory cytokines such as interleukin (IL-2). CsA binds to cyclophilin thereby, preventing the dephosphorylation of NFAT and subsequent downregulation of IL-2.28 This leads to a consequent decrease in the number of CD4+ and CD8+ (cytotoxic) T cells in the epidermis, which are key mediators involved in the pathogenesis of SJS/TEN.29 CsA has also been shown to inhibit TNF-α production.29 TNF-α is another key cytokine involved in the amplification of apoptotic pathways implicated in SJS/TEN.
CsA, however, is not without its own inherent set of drawbacks. Contraindications to its use include patients with severe renal disease, severe infections, or internal malignancy.30 While common adverse effects of CsA are nausea, hypertension, nonmelanoma skin cancer, renal dysfunction, hyperlipidemia, headache, and tremors.31 However, it was extremely encouraging that cyclosporine was generally well tolerated in most of the trials and case series, even though these patients were often critically ill.9,11,12
The limitations of the current meta-analysis should also be discussed. First, the funnel plot and Egger test (P=0.0467) showed some likelihood of publication bias. Future meta-analysis should include non-English studies and grey literature as some trials may have been missed in the search process. There is also a risk of bias inherent to the reanalyses of the same case series examined in earlier meta-analyses.14,20 Second, there was a paucity of studies examining the use of cyclosporine in SJS/TEN patients, and no randomized controlled trials on the subject exists. This is perhaps not unexpected given the rarity and life-threatening nature of the SJS/TEN. Practically, the possibility of conducting a rigorous double-blind, placebo-controlled trial to study the use of CsA in SJS/TEN patients is remote. Larger case series may be the feasible step forward to help support or refute current evidence. This could be achieved through collaborative effort by tertiary hospitals in keeping a registry of SJS/TEN cases treated with CsA, meticulously documenting the clinical course (including adverse effects) of these patients. Last but not least, another important area for future research concerns the use of cyclosporine in special patient populations, for example, those with HIV/AIDS, preexisting renal or liver disease, and the elderly (especially those with polypharmacy). The use of CsA in patients with HIV infections is still debated and limited data exist.32
Conclusion
CsA use was associated with improved mortality in SJS/TEN patients, with a pooled SMR of 0.320 (95% CI: 0.119–0.522, P=0.002) based on nine studies and a total of 256 SJS/TEN patients. Case series, retrospective studies, and an open, Phase II trial have documented its efficacy, safety, and beneficial effects in SJS/TEN patients. A rigorous double-blind, randomized trial would be necessary to confirm its efficacy. However, this may be challenging to achieve given the rarity and life-threatening nature of SJS/TEN. International effort in collating larger case series may be the feasible step forward to help support or refute current evidence.
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Author contributions
Qin Xiang Ng conceived, designed, and carried out the study and the relevant data analysis and interpretation. Michelle Lee Zhi Qing De Deyn, Nandini Venkatanarayanan, and Collin Yih Xian Ho carried out the study and the relevant data
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Harr T, French LE. Toxic epidermal necrolysis and Stevens-Johnson syndrome. Orphanet J Rare Dis. 2010;5(1):39. doi: 10.1186/1750-1172-5-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abe J, Umetsu R, Mataki K, et al. Analysis of Stevens-Johnson syndrome and toxic epidermal necrolysis using the Japanese adverse drug event report database. J Pharm Health Care Sci. 2016;2(1):14. doi: 10.1186/s40780-016-0048-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bastuji-Garin S, Rzany B, Stern RS, et al. Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol. 1993;129(1):92–96. [PubMed] [Google Scholar]
- 4.Schwartz RA, McDonough PH, Lee BW. Toxic epidermal necrolysis: Part I. Introduction, history, classification, clinical features, systemic manifestations, etiology, and immunopathogenesis. J Am Acad Dermatol. 2013;69(2):173–e1. doi: 10.1016/j.jaad.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Schneider JA, Cohen PR. Stevens-Johnson syndrome and toxic epidermal necrolysis: a concise review with a comprehensive summary of therapeutic interventions emphasizing supportive measures. Adv Ther. 2017;34(6):1235–1244. doi: 10.1007/s12325-017-0530-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Locharernkul C, Loplumlert J, Limotai C, et al. Carbamazepine and phenytoin induced Stevens-Johnson syndrome is associated with HLA-B* 1502 allele in Thai population. Epilepsia. 2008;49(12):2087–2091. doi: 10.1111/j.1528-1167.2008.01719.x. [DOI] [PubMed] [Google Scholar]
- 7.White KD, Chung WH, Hung SI, et al. Evolving models of the immunopathogenesis of T cell–mediated drug allergy: the role of host, pathogens, and drug response. J Allergy Clin Immunol. 2015;136(2):219–234. doi: 10.1016/j.jaci.2015.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwartz RA, McDonough PH, Lee BW. Toxic epidermal necrolysis: Part II. Prognosis, sequelae, diagnosis, differential diagnosis, prevention, and treatment. J Am Acad Dermatol. 2013;69(2):187–e1. doi: 10.1016/j.jaad.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Reese D, Henning JS, Rockers K, et al. Cyclosporine for SJS/TEN: a case series and review of the literature. Cutis. 87(1):24–29. 201. [PubMed] [Google Scholar]
- 10.Kirchhof MG, Miliszewski MA, Sikora S, et al. Retrospective review of Stevens-Johnson syndrome/toxic epidermal necrolysis treatment comparing intravenous immunoglobulin with cyclosporine. J Am Acad Dermatol. 2014;71(5):941–947. doi: 10.1016/j.jaad.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 11.Singh GK, Chatterjee M, Verma R. Cyclosporine in Stevens-Johnson syndrome and toxic epidermal necrolysis and retrospective comparison with systemic corticosteroid. Indian J Dermatol Venereol Leprol. 2013;79(5):686. doi: 10.4103/0378-6323.116738. [DOI] [PubMed] [Google Scholar]
- 12.Kuma P, Kanti Das N. Cyclosporine in toxic epidermal necrolysis: a brief review of the emerging therapeutic modality. Dermatol Online J. 2016;22(10) [PubMed] [Google Scholar]
- 13.Méndez A, Monforte V, Berastegui C, et al. High intra-individual variability of cyclosporine pharmacokinetics in lung transplant recipients without cystic fibrosis. Clin Transplant. 2014;28(6):743–748. doi: 10.1111/ctr.12371. [DOI] [PubMed] [Google Scholar]
- 14.Zimmermann S, Sekula P, Venhoff M, et al. Systemic immunomodulating therapies for Stevens-Johnson syndrome and toxic epidermal necrolysis: a systematic review and meta-analysis. JAMA Dermatol. 2017;153(6):514–522. doi: 10.1001/jamadermatol.2016.5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fouchard N, Bertocchi M, Roujeau JC, et al. SCORTEN: a severity-of-illness score for toxic epidermal necrolysis. J Invest Dermatol. 2000;115(2):149–153. doi: 10.1046/j.1523-1747.2000.00061.x. [DOI] [PubMed] [Google Scholar]
- 16.Guegan S, Bastuji-Garin S, Poszepczynska-Guigné E, et al. Performance of the SCORTEN during the first five days of hospitalization to predict the prognosis of epidermal necrolysis. J Invest Dermatol. 2006;126(2):272–276. doi: 10.1038/sj.jid.5700068. [DOI] [PubMed] [Google Scholar]
- 17.Arévalo JM, Lorente JA, González-Herrada C, Jiménez-Reyes J. Treatment of toxic epidermal necrolysis with cyclosporin A. J Trauma Acute Care Surg. 2000;48(3):473–478. doi: 10.1097/00005373-200003000-00017. [DOI] [PubMed] [Google Scholar]
- 18.Firoz BF, Henning JS, Zarzabal LA, Pollock BH. Toxic epidermal necrolysis: five years of treatment experience from a burn unit. J Am Acad Dermatol. 2012;67(4):630–635. doi: 10.1016/j.jaad.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 19.Giudice G, Maggio G, Bufano L, et al. Management of toxic epidermal necrolysis with plasmapheresis and cyclosporine A: our 10 years’ experience. Plast Reconstr Surg Glob Open. 2017;5(2):e1221. doi: 10.1097/GOX.0000000000001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.González-Herrada C, Rodríguez-Martín S, Cachafeiro L, et al. Ciclosporin use in epidermal necrolysis is associated with an important mortality reduction: evidence from three different approaches. J Invest Dermatol. 2017;137(10):2092–2100. doi: 10.1016/j.jid.2017.05.022. [DOI] [PubMed] [Google Scholar]
- 21.Lee HY, Fook-Chong S, Koh HY, et al. Cyclosporine treatment for Stevens-Johnson syndrome/toxic epidermal necrolysis: retrospective analysis of a cohort treated in a specialized referral center. J Am Acad Dermatol. 2017;76(1):106–113. doi: 10.1016/j.jaad.2016.07.048. [DOI] [PubMed] [Google Scholar]
- 22.Mohanty S, Das A, Ghosh A, et al. Effectiveness, safety and tolerability of cyclosporine versus supportive treatment in Stevens-Johnson sndrome/toxic epidermal necrolysis: a record-based study. Indian J Dermatol Venereol Leprol. 2017;83(3):312. doi: 10.4103/ijdvl.IJDVL_201_16. [DOI] [PubMed] [Google Scholar]
- 23.Rajaratnam R, Mann C, Balasubramaniam P, et al. Toxic epidermal necrolysis: retrospective analysis of 21 consecutive cases managed at a tertiary centre. Clin Exp Dermatol. 2010;35(8):853–862. doi: 10.1111/j.1365-2230.2010.03826.x. [DOI] [PubMed] [Google Scholar]
- 24.Szepietowski J, Wa̧sik F, Szybejko-Machaj G, Maj J. Toxic epidermal necrolysis successfully treated with cyclosporin. Report of three cases. J Eur Acad Dermatol Venereol. 1997;9(2):169–172. [Google Scholar]
- 25.Valeyrie-Allanore L, Wolkenstein P, Brochard L, et al. Open trial of ciclosporin treatment for Stevens-Johnson syndrome and toxic epidermal necrolysis. Br J Dermatol. 2010;163(4):847–853. doi: 10.1111/j.1365-2133.2010.09863.x. [DOI] [PubMed] [Google Scholar]
- 26.Stern RS, Divito SJ. Stevens-Johnson syndrome and toxic epidermal necrolysis: associations, outcomes, and pathobiology – thirty years of progress but still much to be done. J Invest Dermatol. 2017;137(5):1004–1008. doi: 10.1016/j.jid.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chung WH, Hung SI, Yang JY, et al. Granulysin is a key mediator for disseminated keratinocyte death in Stevens-Johnson syndrome and toxic epidermal necrolysis. Nat Med. 2008;14(12):1343–1350. doi: 10.1038/nm.1884. [DOI] [PubMed] [Google Scholar]
- 28.Amor KT, Ryan C, Menter A. The use of cyclosporine in dermatology: part I. J Am Acad Dermatol. 2010;63(6):925–946. doi: 10.1016/j.jaad.2010.02.063. [DOI] [PubMed] [Google Scholar]
- 29.Remick DG, Nguyen DT, Eskandari MK, et al. Cyclosporine A inhibits TNF production without decreasing TNF mRNA levels. Biochem Biophys Res Commun. 1989;161(2):551–555. doi: 10.1016/0006-291x(89)92634-x. [DOI] [PubMed] [Google Scholar]
- 30.Lebwohl M, Ellis C, Gottlieb A, et al. Cyclosporine consensus conference: with emphasis on the treatment of psoriasis. J Am Acad Dermatol. 1998;39(3):464–475. doi: 10.1016/s0190-9622(98)70325-1. [DOI] [PubMed] [Google Scholar]
- 31.Palestine AG, Nussenblatt RB, Chan CC. Side effects of systemic cyclosporine in patients not undergoing transplantation. Am J Med. 1984;77(4):652–656. doi: 10.1016/0002-9343(84)90356-5. [DOI] [PubMed] [Google Scholar]
- 32.Jacobson SK, Calne RY, Wreghitt TG. Outcome of HIV infection in transplant patient on cyclosporin. Lancet. 1991;337(8744):794. doi: 10.1016/0140-6736(91)91414-p. [DOI] [PubMed] [Google Scholar]