Abstract
Rationale: Isoniazid-monoresistant tuberculosis (INH-monoresistant TB) is the most common drug-resistant TB type in the United States; however, its impact on TB treatment outcomes is not clear.
Objectives: This study aims to understand 1) factors associated with INH-monoresistant TB and 2) the association between INH monoresistance and response to TB treatment.
Methods: We studied all patients with TB (age, ≥15 yr) reported to the Georgia State Electronic Notifiable Disease Surveillance System (SENDSS) from 2009 to 2014. INH-monoresistant TB was defined as a Mycobacterium tuberculosis isolate resistant to isoniazid only. Time to sputum culture conversion was defined as the time (measured in days) from TB treatment initiation to the date of the first consistently negative culture result reported to the SENDSS. Logistic regression and Cox proportional hazard models were used to estimate the odds and hazard rate of sputum culture conversion, all-cause mortality, and poor TB outcome among patients with INH-monoresistant TB.
Results: Among 1,141 culture-confirmed patients with available drug susceptibility testing results, 998 (87.5%) were susceptible to TB first-line drugs, and 143 (12.5%) were patients with INH-monoresistant TB. In multivariable analysis, male sex (adjusted odds ratio [aOR], 1.62; 95% confidence interval [CI], 1.01–2.67) and homelessness (aOR, 5.55; 95% CI, 3.38–9.17) were associated with higher odds of INH-monoresistant TB. In the same multivariable model, older age (≥65 yr old) (aOR, 0.21; 95% CI, 0.07–0.55) and miliary disease (aOR, 0.19; 95% CI, 0.01–0.96) were associated with lower odds of INH-monoresistant TB. Among 1,116 patients with pulmonary TB, the median time to sputum culture conversion was 30 days (interquartile range, 13–58). The rate of culture conversion was similar among patients with and without INH monoresistance (adjusted cause-specific hazard ratio, 1.15; 95% CI, 0.95–1.40). INH-monoresistant TB was not significantly associated with poor TB treatment outcomes (aOR, 1.61; 95% CI, 0.67–3.70) or mortality during TB treatment (aOR, 1.72; 95% CI, 0.58–4.94).
Conclusions: Our findings suggest that compared with drug-susceptible TB, patients in Georgia with INH-monoresistant TB have a similar response to TB treatment including culture conversion rate, final TB treatment outcome, and all-cause mortality.
Keywords: tuberculosis, isoniazid monoresistant, homeless, HIV, culture conversion
Tuberculosis (TB) is a major global health problem with 10.4 million incident cases and 1.4 million deaths among HIV-negative cases annually (1). In 2016 the World Health Organization (WHO) reported 580,000 cases of rifampin-resistant (including multidrug-resistant [MDR]) TB (1). Nearly 10% of TB cases are isoniazid resistant (i.e., TB strains resistant to isoniazid without rifampin resistance) (2–4), and the global burden of drug-resistant TB (DRTB) will likely reach 1 million incident cases annually. Global TB control is severely inhibited by DRTB, which requires substantial resources to diagnose and treat (5). In 2015, 11% of patients in the United States with TB were resistant to at least isoniazid (including MDR TB) (6). During 2009–2014, the state of Georgia reported an average of two MDR TB cases annually, but the rate of isoniazid resistance (INH-R) ranged from 7 to 22% (7). Isoniazid (INH)-monoresistant TB (i.e., TB strains resistant to INH only) is the most common type of drug-resistant TB among those with monoresistant TB (8), but evidence about whether INH-monoresistant TB is associated with clinical characteristics and immunosuppressive comorbidities or impacts TB treatment outcomes is limited.
Previous studies reported associations between INH-monoresistant TB and smoking (9), history of incarceration (10, 11), positive tuberculin skin test result (10), foreign-born status (12), drug use (13), and previous TB treatment (for active and latent TB) (12). Other studies reported that patients with INH-monoresistant TB have similar clinical presentation (i.e., presence of radiographic cavities) compared with patients with drug-susceptible TB (14). But the majority of these previous studies were conducted with macrolevel data (i.e., Israel, United States, and India), and little is known about clinical characteristics of patients in local settings with INH-monoresistant TB. Among patients in Georgia with TB, the prevalence of immunosuppressive conditions is high (6), but the distribution of characteristics among patients with INH-monoresistant TB is unknown. Identifying subgroup populations in which INH-monoresistant TB is prevalent is critical to monitor epidemiologic trends in TB transmission and may inform TB prevention strategies.
The 2016 WHO guidelines for treatment of DRTB highlight the need for additional evidence regarding the impact of INH resistance on TB treatment outcomes (15). Drug resistance that is not MDR TB may increase the risk of poor TB treatment outcomes (10, 16) such as treatment failure (2, 17), TB relapse (2), acquisition of additional resistance (17), and subsequent development of acquired MDR TB (2, 18, 19). A study conducted among a cohort of patients with tuberculous meningitis also reported that a higher proportion of patients with INH resistance died during treatment when compared with those without (20). Two meta-analyses reported that patients with INH-R treated according to standard TB regimens had increased risk of poor outcomes (21, 22), but these meta-analyses did not assess the effect of INH-R on culture conversion. Another previous study reported similar TB treatment success rates among patients with and without INH monoresistance (12). Using data from the state of Georgia, our study aimed to 1) determine patient characteristics associated with INH-monoresistant TB and 2) estimate the relationship between INH monoresistance and response to TB treatment (including rate of sputum culture conversion, final treatment outcome, and mortality).
Methods
Setting and Design
We performed a retrospective cohort study of TB cases in Georgia during 2009–2014. In Georgia, all TB cases are reported to the Georgia Department of Public Health (GDPH) at the county or state level (23). Study eligibility criteria included patients with culture-confirmed TB (age, ≥15 yr) with known drug susceptibility testing (DST) results for at least INH and rifampin (RIF) reported to the GDPH State Electronic Notifiable Disease Surveillance System (SENDSS) during 2009–2014. We excluded patients with clinical TB (i.e., without culture-positive confirmation). All patients were initially treated with a four-drug regimen (INH, RIF, pyrazinamide [PZA], and ethambutol [EMB]) according to state guidelines (24).
Study information was collected from the SENDSS. Based on the U.S. Centers for Disease Control and Prevention (CDC)’s Report of Verified Case of Tuberculosis (RVCT), the SENDSS routinely collects medical record and laboratory information on patients with TB including demographics and clinical information. DST results for INH, RIF, and EMB were performed by the GDPH, using mycobacterial growth indicator tubes (BACTEC, BD Dickinson) as previously described (25). If resistance to any first-line TB drugs was detected, samples were sent to the CDC for second-line DST (26).
Definitions
Primary outcomes in this study were 1) INH monoresistance at time of TB diagnosis, 2) rate of sputum culture conversion, and 3) TB treatment outcomes (final treatment outcome and mortality). First, INH monoresistance was defined for strains of Mycobacterium tuberculosis (MTB) resistant to INH only with recorded DST results available for at least both INH and RIF. Patients were classified as having drug-susceptible TB if they were susceptible to at least INH and RIF and had no reported resistance to other drugs (PZA and EMB). Other types of drug resistance (non–INH-monoresistant TB, polydrug-resistant TB [i.e., resistance to two or more TB drugs but not MDR TB], and MDR TB) were excluded. Second, time to sputum culture conversion was defined as the time (in days) from TB treatment initiation to date of the first of two consecutively negative sputum cultures reported to the SENDSS. Third, final TB treatment outcome was defined as favorable or poor. Patients who were cured or completed TB treatment were categorized as having a favorable outcome (27). Patients with TB who died during treatment, were lost to follow-up, or stopped/refused TB treatment were categorized as having a poor outcome (28). We also examined all-cause mortality during TB treatment separately.
Other patient characteristics used in the study included demographic information, prior TB history, illicit drug use, alcohol abuse, history of latent TB infection prophylaxis, acid-fast bacilli smear status, cavitary disease, and site of disease. HIV and diabetes were defined by the SENDSS. Age was categorized according to standard WHO classifications (1). We used GDPH categories for race/ethnicity, which included non-Hispanic white, non-Hispanic black, Hispanic all races, and non-Hispanic Asian. Treatment duration was defined as number of days between TB treatment initiation and treatment completion and classified into three categories: “≤6 months,” “7–12 months,” and “≥12 months.”
Spoligotyping and mycobacterial interspersed repetitive unit analysis were used to identify TB genotypes and were categorized dichotomously according to the largest Georgia MTB cluster, classified as “GENType G05625” and “other” (7). GENType G05625 was associated with a recent TB outbreak among homeless shelter residents in the Atlanta area (7).
Statistical Analyses
We used χ2 and Fisher exact tests to assess bivariate associations between characteristics of patients and prevalent INH monoresistance. Multivariable logistic regression was used to determine the adjusted association between patient characteristics and INH monoresistance. We used cumulative incidence curves and cause-specific proportional hazard models to evaluate whether INH-monoresistant TB was associated with rate of culture conversion in the presence of a competing risk (i.e., all-cause mortality during TB treatment). Patients with TB were censored if they were lost to follow-up, stopped/refused TB treatment (with no prior documentation of culture conversion), or did not convert sputum cultures to negative by time of treatment completion. Proportional hazard assumptions were assessed by three methods: 1) graphically, 2) goodness-of-fit tests, and 3) time-dependent models (29). Multivariable logistic regression was also used to estimate the association between INH monoresistance with final TB treatment outcome and all-cause mortality. To include all eligible study participants in the multivariable models, persons with missing covariate values were coded as a separate category. Purposeful selection of covariates included in all multivariable models was based on observed bivariate associations between patient characteristics with both INH monoresistance and treatment outcomes, suspected confounders according to previous published literature, and directed acyclic graph theory (30). Effect modification between INH monoresistance and culture conversion was assessed by likelihood ratio test. Sensitivity analyses were used to assess model misspecification by fitting multiple covariate subsets, including logistic models with GENType G05625 information to determine the adjusted association between HIV and INH-monoresistant TB. An additional sensitivity analysis was performed to assess error from unmeasured confounding due to treatment regimen change in estimating associations between INH monoresistance and TB treatment outcomes (31). Analyses were performed with SAS version 9.4 (SAS Institute), with a two-sided P value less than 0.05 considered significant.
Institutional Review Board
This study was reviewed and approved by the institutional review boards (IRBs) at Georgia State University, Emory University, and the GDPH (Atlanta, GA). All IRBs granted a waiver of informed consent for the use of TB surveillance data.
Results
Baseline Characteristics and INH-Monoresistant TB
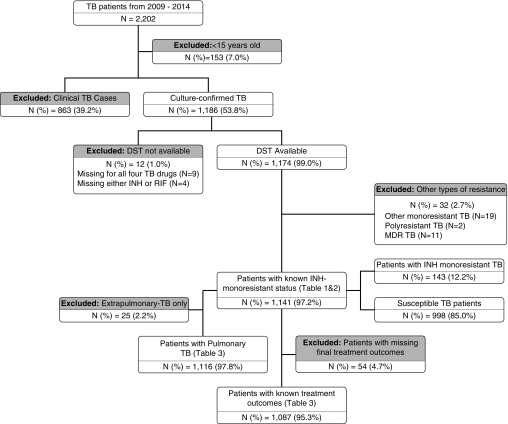
From 2009 to 2014, 2,202 patients with TB were reported to the SENDSS. Of these 2,202 patients, 153 (7.0%) were less than 15 years old and excluded from the analyses; an additional 863 (39.2%) patients were excluded due to a negative (665 of 863, 77.1%) or missing (198 of 863, 22.9%) culture at baseline (Figure 1). Among the 1,186 patients with culture-confirmed TB in the state of Georgia, 12 (1.0%) had missing DST results at baseline and were excluded. Of eligible patients with available DST results, 1,141 (97.2%) were included in the final analyses. We did not observe differences in birthplace, occupation, or HIV or diabetes status among patients with culture-confirmed TB and patients with clinical TB (Table E1 in the online supplement). Compared with patients with clinical TB, patients with culture-confirmed TB were more likely to be male (70.7 vs. 61.8%), homeless (14.5 vs. 6.3%), and illicit drug users (12.2 vs. 6.4%) (P value for each comparison, <0.05).
Figure 1.
Study flowchart and number of patients with tuberculosis (TB) included in the analyses and reasons for exclusion. DST = drug susceptibility testing; INH = isoniazid; MDR = multidrug resistant; RIF = rifampin.
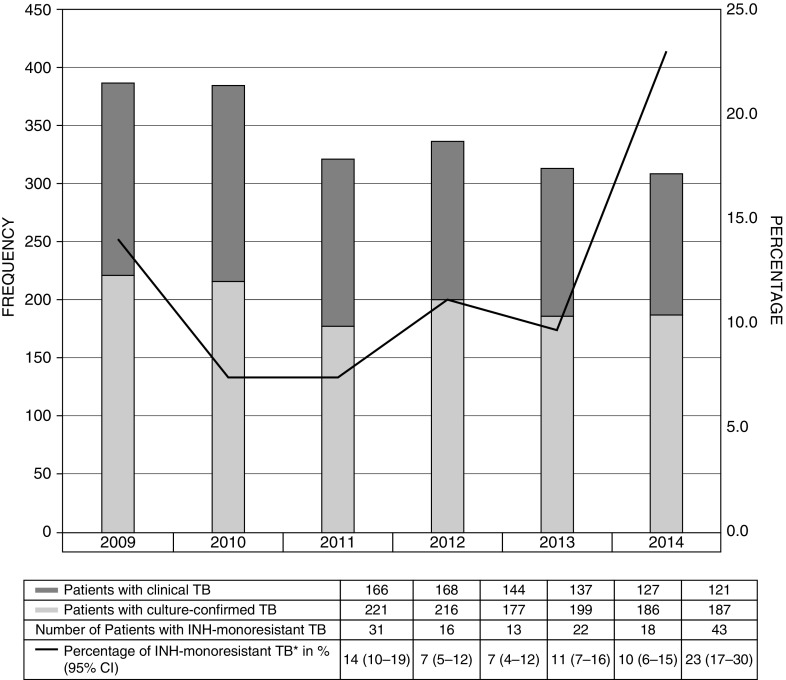
Among patients included in the final analyses, 143 of 1,141 (12.5%; 95% confidence interval [CI], 10.7–14.6%) had INH-monoresistant TB during the study period. From 2009 to 2014, the proportion of INH-monoresistant TB ranged from 7.3 to 23.0% (Figure 2). The majority of patients in our cohort were male (70.8%), non-Hispanic black (50.1%), and born in the United States (55.3%) (Table 1). Among study patients, 13.4% had HIV infection and 13.3% had diabetes. GENType G05625 was the most common identified genotype: 5.0% of all patients (54 of 1,083) with known genotype and 39.7% of patients with INH-monoresistant TB.
Figure 2.
Number of patients with tuberculosis (TB) and percentage of isoniazid (INH)-monoresistant TB in the state of Georgia, 2009–2014. *Percentage of INH-monoresistant TB among patients with culture-confirmed TB. CI = confidence interval.
Table 1.
Baseline characteristics of patients in the state of Georgia with culture-confirmed isoniazid-monoresistant tuberculosis, 2009–2014 (N = 1,141)
| Variable | Type of Resistance* |
Total: N = 1,141 | P Value (χ2) | |
|---|---|---|---|---|
| Susceptible†: N (%) = 998 (87.5) |
INH-Monoresistant‡: N (%) = 143 (12.5) |
[n (%)] |
||
| [n (%)] | [n (%)] | |||
| Age group, yr | 0.01§ | |||
| 15–24 | 117 (11.7) | 17 (11.9) | 134 (11.7) | |
| 25–44 | 367 (36.8) | 52 (36.4) | 419 (36.7) | |
| 45–64 | 365 (36.6) | 67 (47.9) | 432 (37.9) | |
| ≥65 | 149 (14.9) | 7 (4.9) | 156 (13.7) | |
| Sex, male | 692 (69.3) | 116 (81.1) | 808 (70.8) | 0.004§ |
| Race/ethnicity | ||||
| Non-Hispanic white | 146 (14.8) | 20 (14.0) | 166 (14.7) | 0.01§ |
| Non-Hispanic black | 479 (48.5) | 88 (61.5) | 567 (50.1) | |
| Hispanic | 200 (20.2) | 14 (9.8) | 214 (18.9) | |
| Non-Hispanic Asian | 163 (16.5) | 21 (14.7) | 184 (16.3) | |
| Missing | 10 | 0 | 10 | |
| Foreign born | 465 (46.6) | 45 (31.5) | 510 (44.7) | <0.001§ |
| Occupation, employed | 355 (36.0) | 79 (55.6) | 434 (38.4) | <0.001§ |
| Resident of Fulton County | 153 (15.3) | 59 (41.3) | 212 (18.6) | <0.001§ |
| Homelessness | 104 (10.4) | 64 (44.8) | 168 (14.7) | <0.001§ |
| History of imprisonment | 45 (4.5) | 10 (7.0) | 55 (4.8) | 0.22 |
| Resident of long-term care facility | 14 (1.4) | 1 (0.7) | 15 (1.3) | 0.71|| |
| Contact with patient with TB | 120 (12.4) | 17 (11.9) | 137 (12.4) | 0.85 |
| Prior history of TB | 55 (5.5) | 9 (6.3) | 64 (5.6) | 0.70 |
| Illicit drug user | 106 (10.7) | 35 (24.5) | 141 (12.4) | <0.001§ |
| Alcohol abuser | 180 (18.2) | 30 (21.0) | 210 (18.5) | 0.42 |
| HIV status, positive | 107 (11.3) | 39 (28.5) | 146 (13.4) | <0.001§ |
| Patients with diabetes | 134 (13.9) | 13 (9.1) | 147 (13.3) | 0.11 |
| History of LTBI prophylaxis | 37 (3.8) | 6 (4.2) | 43 (3.9) | 0.83 |
| Baseline TST, positive | 474 (69.9) | 61 (62.2) | 535 (68.9) | 0.13 |
| Baseline smear, positive | 673 (67.8) | 95 (66.4) | 768 (67.7) | 0.74 |
| Baseline CXR reading, abnormal | 868 (93.1) | 130 (92.9) | 998 (93.1) | 0.90 |
| Cavitary disease | 336 (36.3) | 38 (27.3) | 374 (35.1) | 0.04§ |
| Miliary disease | 31 (3.4) | 1 (0.7) | 32 (3.0) | 0.11 |
| TB genotype, G05625 | 0 (0.0) | 54 (39.7) | 54 (5.2) | <0.001§ |
| EMB, missing¶ | 14 (1.4) | 0 (0.0) | 14 (1.2) | 0.24|| |
| PZA, missing¶ | 746 (74.8) | 69 (48.3) | 815 (71.4) | <0.001§|| |
| Site of disease | ||||
| Pulmonary | 893 (89.5) | 123 (86.0) | 1016 (89.0) | 0.37 |
| Pulmonary + extrapulmonary | 83 (8.3) | 17 (11.9) | 100 (8.8) | |
| Extrapulmonary only | 22 (2.2) | 3 (2.1) | 25 (2.2) | |
| TB treatment duration | ||||
| ≤6 mo | 174 (17.4) | 16 (11.2) | 190 (16.7) | 0.13 |
| 7–12 mo | 725 (72.7) | 109 (76.2) | 834 (73.1) | |
| >12 mo | 99 (9.9) | 18 (12.6) | 117 (10.2) | |
| TB treatment outcome | ||||
| Completed | 844 (89.1) | 124 (88.6) | 968 (89.1) | 0.87 |
| Died | 75 (7.9) | 12 (8.6) | 87 (8.0) | |
| Lost to follow-up | 21 (2.2) | 3 (2.1) | 24 (2.2) | |
| Uncooperative/refused to continue treatment | 3 (0.3) | 0 (0.0) | 3 (0.3) | |
| Stopped due to adverse reaction | 4 (0.4) | 1 (0.7) | 5 (0.5) | |
| Missing | 51 | 3 | 54 | |
Definition of abbreviations: CXR = chest X-ray; DST = drug susceptibility testing; EMB = ethambutol; INH = isoniazid; LTBI = latent TB infection; PZA = pyrazinamide; RIF = rifampin; TB = tuberculosis; TST = tuberculin skin test.
Drug resistance patterns of patients with culture-confirmed TB excluding other patients with monoresistant, polyresistant, and multidrug-resistant (MDR) TB.
Patients susceptible to all of the first-line TB drugs used in the state of Georgia (isoniazid, rifampin, ethambutol), sensitivity test for pyrazinamide will be performed if MDR TB is detected.
Patients resistant only to isoniazid (at least tested for INH and RIF).
The finding is statistically significant at a level of confidence of 5% (P < 0.05).
P values from Fisher’s exact test.
χ2/Fisher’s exact test comparing patient with missing and sensitive DST.
Factors Associated with INH-Monoresistant TB
In bivariate analyses, male sex (crude odds ratio [cOR], 1.90; 95% CI, 1.24–3.00), unemployment (cOR, 2.12; 95% CI, 1.44–3.17), homelessness (cOR, 6.96; 95% CI, 4.72–10.25), use of illicit drugs (cOR, 2.72; 95% CI, 1.75–4.15), and HIV infection (cOR, 3.14; 95% CI, 2.04–4.76) were associated with increased odds of INH-monoresistant TB. Older age (≥65 yr) (cOR, 0.32; 95% CI, 0.12–0.78), foreign born (cOR, 0.53; 95% CI, 0.36–0.76), cavitary disease (cOR, 0.66; 95% CI, 0.44–0.97), and miliary disease (cOR, 0.21; 95% CI, 0.01–0.97) were associated with lower odds of INH monoresistance at the time of TB diagnosis.
In multivariable analysis, male sex (adjusted odds ratio [aOR], 1.62; 95% CI, 1.01–2.67), homelessness (aOR, 5.55; 95% CI, 3.38–9.17), and HIV infection (aOR, 1.85; 95% CI, 1.08–3.13) were associated with significantly increased odds of INH monoresistance at the time of TB diagnosis (Table 2). The odds of INH-monoresistant TB were significantly lower among patients at least 65 years of age (aOR, 0.21; 95% CI, 0.07–0.55) and those with miliary disease (aOR, 0.19; 95% CI, 0.01–0.96). The odds of INH-monoresistant TB were similar among patients with diabetes compared with those without diabetes (aOR, 1.03; 95% CI, 0.51–1.94). In a separate model adjusted for GENType G05625, HIV infection was not significantly associated with INH-monoresistant TB (aOR, 1.57; 95% CI, 0.75–3.13) (Table E2).
Table 2.
Unadjusted and adjusted odds ratios for isoniazid monoresistance at time of TB diagnosis among patients in the state of Georgia with culture-confirmed TB, 2009–2014 (N = 1,141)
| Variable | Odds Ratio |
|
|---|---|---|
| cOR (95% CI) | aOR (95% CI)* | |
| Age, yr | ||
| 15–24 | 1.00 | 1.00 |
| 25–44 | 0.98 (0.55–1.80) | 0.69 (0.36–1.36) |
| 45–64 | 1.26 (0.73–2.30) | 0.55 (0.29–1.09) |
| ≥65 | 0.32 (0.12–0.78)† | 0.21 (0.07–0.55)† |
| Sex | ||
| Female | 1.00 | 1.00 |
| Male | 1.90 (1.24–3.00)† | 1.62 (1.01–2.67)† |
| Race/ethnicity | ||
| Non-Hispanic white | 1.00 | 1.00 |
| Non-Hispanic black | 1.34 (0.81–2.31) | 0.80 (0.45–1.47) |
| Hispanic, all races | 0.51 (0.25–1.04) | 0.44 (0.17–1.13) |
| Non-Hispanic Asian | 0.94 (0.49–1.81) | 1.31 (0.55–3.20) |
| Other | NA | NA |
| Foreign born | ||
| No | 1.00 | 1.00 |
| Yes | 0.53 (0.36–0.76) | 0.85 (0.44–1.59) |
| Occupation | ||
| Employed | 1.00 | 1.00 |
| Unemployed | 2.12 (1.44–3.17)† | 1.15 (0.72–1.84) |
| Ineligible for employment, student, retired | 0.86 (0.48–1.48) | 1.25 (0.64–2.36) |
| Homelessness | ||
| No | 1.00 | 1.00 |
| Yes | 6.96 (4.72–10.25)† | 5.55 (3.38–9.17)† |
| History of imprisonment | ||
| No | 1.00 | |
| Yes | 1.57 (0.73–3.06) | |
| Resident of long-term care facility | ||
| No | 1.00 | |
| Yes | 0.49 (0.03–2.49) | |
| Contact with patient with TB | ||
| No | 1.00 | |
| Yes | 0.95 (0.54–1.59) | |
| TB history | ||
| No | 1.00 | 1.00 |
| Yes | 1.15 (0.52–2.27) | 1.08 (0.46–2.31) |
| Illicit drug use | ||
| No | 1.00 | 1.00 |
| Yes | 2.72 (1.75–4.15)† | 1.17 (0.68–1.97) |
| Alcohol abuse | ||
| No | 1.00 | |
| Yes | 1.20 (0.77–1.83) | |
| HIV status | ||
| Negative | 1.00 | 1.00 |
| Positive | 3.14 (2.04–4.76)† | 1.85 (1.08–3.13)† |
| Diabetes status | ||
| No | 1.00 | 1.00 |
| Yes | 0.62 (0.33–1.09) | 1.03 (0.51–1.94) |
| History of LTBI prophylaxis | ||
| No | 1.00 | |
| Yes | 1.10 (0.41–2.47) | |
| Baseline TST | ||
| Negative | 1.00 | |
| Positive | 0.71 (0.46–1.11) | |
| Baseline smear | ||
| Negative | 1.00 | |
| Positive | 0.94 (0.65–1.37) | |
| Baseline CXR reading | ||
| Normal | 1.00 | |
| Abnormal | 0.96 (0.50–2.03) | |
| Cavitary disease | ||
| No | 1.00 | 1.00 |
| Yes | 0.66 (0.44–0.97)† | 0.72 (0.46–1.12) |
| Miliary disease | ||
| No | 1.00 | 1.00 |
| Yes | 0.21 (0.01–0.97)† | 0.19 (0.01–0.96)† |
| TB treatment duration | ||
| ≤6 mo | 1.00 | |
| 7–12 mo | 1.64 (0.97–2.93) | |
| >12 mo | 1.98 (0.96–4.09) | |
| Site of disease | ||
| Pulmonary | 1.00 | |
| Pulmonary + extrapulmonary | 1.49 (0.83–2.53) | |
| Extrapulmonary only | 0.99 (0.23–2.91) | |
Definition of abbreviations: aOR = adjusted odd ratio; CI = confidence interval; cOR = crude odds ratio; CXR = chest X-ray; LTBI = latent TB infection; NA = not available; TB = tuberculosis; TST = tuberculin skin test.
Adjusted odds ratio after controlling for age, sex, race, foreign born, occupation, homelessness, TB history, illicit drug use, HIV status, diabetes, cavitary disease, and miliary disease (N = 1,141).
The finding is statistically significant at level of confidence of 5% (P < 0.05).
INH-Monoresistant TB and Rate of Sputum Culture Conversion
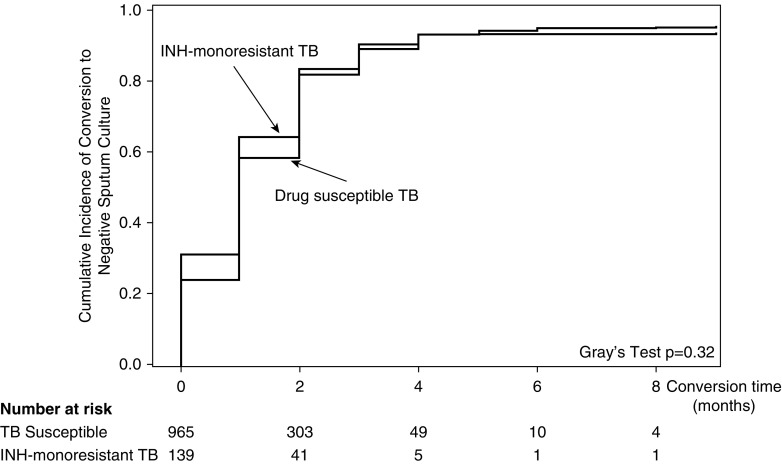
After excluding patients with extrapulmonary TB only (25 of 1,141, 2.2%), most patients with pulmonary TB (956 of 1,116, 85.7%) converted sputum cultures from positive to negative. Of 160 patients who did not convert cultures to negative, 62 (38.8%) completed TB treatment, 10 (6.2%) had an adverse reaction, 52 (32.5%) died, and 36 (22.5%) had missing treatment outcome information. Among patients who converted, the median time to culture conversion was 30 days (interquartile range [IQR], 13–58) (Table E3). In unadjusted analysis, the time to sputum culture conversion among patients with INH-monoresistant TB was similar compared with patients with drug-susceptible TB (median, 27 d [IQR, 10–51] vs. 32 d [IQR, 13–59]) (P = 0.32) (Figure 3).
Figure 3.
Cumulative incidence curve for sputum culture conversion among adults with and without isoniazid-monoresistant tuberculosis, accounting for death as a competing risk factor. INH = isoniazid; TB = tuberculosis.
In multivariable proportional hazard analyses, we found similar rates of culture conversion among patients with INH-monoresistant TB compared with patients with drug-susceptible TB (adjusted hazard ratio [aHR], 1.15; 95% CI, 0.95–1.39) (Table E3). Using a cause-specific proportional hazard model, we observed similar hazard rates among patients with or without INH-monoresistant TB (aHR, 1.15; 95% CI, 0.95–1.40) (Table 3). In the same cause-specific multivariable model, the rate of sputum culture conversion was significantly lower among patients with a positive smear (aHR, 0.60; 95% CI, 0.52–0.69) (Table E3). No effect modification was detected between INH monoresistance and other clinical characteristics with rate of culture conversion.
Table 3.
Univariate and multivariable odds and hazard rate ratio analyses for TB treatment outcomes among patients in the state of Georgia with culture-confirmed TB, 2009–2014 (N = 1,116)
| Variable | n (%) | Unadjusted Estimation | Adjusted Estimation |
|---|---|---|---|
|
Sputum Conversion*(N = 1,116) | |||
| INH-monoresistant TB | HR (95% CI) | aHR (95% CI) | |
| No | 826/976 (84.6)† | 1.00 | 1.00 |
| Yes | 130/140 (92.9)† | 1.17 (0.97–1.41)‡ | 1.15 (0.95–1.40)§ |
|
Poor TB Treatment Outcome||(N = 1,087) | |||
| INH-monoresistant TB | OR (95% CI) | aOR (95% CI) | |
| No | 103/947 (10.9) | 1.00 | 1.00 |
| Yes | 16/140 (11.4) | 1.06 (0.59–1.80)¶ | 1.61 (0.67–3.70)** |
|
All-Cause Mortality (N = 1,087) | |||
| INH-monoresistant TB | OR (95% CI) | aOR (95% CI) | |
| No | 75/947 (7.9) | 1.00 | 1.00 |
| Yes | 12/140 (8.6) | 1.09 (0.55–1.99)¶ | 1.72 (0.58–4.94)** |
Definition of abbreviations: aHR = adjusted hazard ratio; aOR = adjusted odds ratio; CI = confidence interval; HR = hazard ratio; INH = isoniazid; OR = odds ratio; TB = tuberculosis.
Proportional hazard analysis was used to estimate the crude and adjusted hazard rate of sputum culture conversion.
Culture conversion by INH-monoresistant status among patients with culture-confirmed TB.
Crude hazard ratio for sputum culture conversion from the conventional Cox proportional hazard model.
Cause-specific Cox proportional hazard model accounting for death as a competing risk. Model was adjusted for age, sex, HIV status, diabetes status, tuberculin skin test at baseline, and smear status.
Included in poor TB treatment outcomes were patients who died during TB treatment, were lost to follow-up, or who stopped/refused TB treatment.
Odds ratios were estimated from the logistic regression model.
Model adjusted for age, sex, HIV status, smear, and TB treatment duration.
INH-Monoresistant TB and Poor TB Treatment Outcome
Among patients included in the final analyses, 1,087 of 1,141 patients (95.3%) had TB treatment outcome information. After excluding 54 patients (4.7%) with missing treatment outcomes, the overall risk of poor TB treatment outcome was 11.0% (119 of 1,087) and the risk of all-cause mortality was 8.0% (87 of 1,087). The risk of poor TB treatment outcome was similar among those with (11.4%) and without (10.9%) INH-monoresistant TB (P = 0.85). After adjusting for potential confounders, the odds of poor TB treatment outcome among patients with and without INH-monoresistant TB were not significantly different (aOR, 1.61; 95% CI, 0.67–3.70) (Table 3). The risk of all-cause mortality was similar among those with (8.6%) and without (7.9%) INH-monoresistant TB (P = 0.85). In a multivariable model, the adjusted odds of all-cause mortality were similar in patients with and without INH-monoresistant TB (aOR, 1.72; 95% CI, 0.58–4.94). In sensitivity analyses that externally adjusted for the unmeasured confounder TB regimen change, the range of odds for poor TB treatment was 0.71–1.15, and 0.73–1.18 for all-cause mortality during TB treatment (data not shown).
Discussion
During the 6-year study period, more than 10% of patients with TB in the state of Georgia were reported to have INH monoresistance, and nearly one-quarter of all patients with TB had INH monoresistance in 2014. Our study reported that HIV infection and homelessness were significantly associated with INH monoresistance in Georgia, likely due to a recent outbreak cluster. Despite the high prevalence of INH monoresistance, we found that this DST pattern did not significantly affect TB treatment outcomes including rate of sputum culture conversion, final TB treatment outcome, and odds of mortality.
In the United States more than 1 in 10 patients with TB had INH resistance in 2014 (6); however, few studies have reported the clinical characteristics of patients with INH-monoresistant TB (10, 12). A previous study of a nationwide cohort of patients with TB, conducted from 1993 to 2003 in the United States, reported that the odds of INH-monoresistant TB were similar among patients with HIV infection compared with patients who were HIV negative (aOR, 1.0; 95% CI, 0.9–1.1) (10). A retrospective study of a cohort from San Francisco reported a lower proportion of INH monoresistance among TB patients with HIV infection compared with those who were HIV negative (aOR, 0.5; 95% CI, 0.2–1.2) (12). Unlike these two previous studies, our findings suggest that TB patients with HIV infection had almost twice the odds of INH-monoresistant TB compared with HIV-negative subjects. The association between HIV status and INH-monoresistant TB we reported is likely due to the clustering of patients with HIV infection in the setting where an INH-monoresistant TB strain is actively circulating (32). In 2014, the GDPH reported a large TB genotype cluster that was 100% resistant to INH (including 27 patients from the recent homeless shelter outbreak in Fulton County), of which 41% had HIV infection (7). Our findings suggest a relationship between HIV infection with this recent TB outbreak among the homeless population in metropolitan Atlanta, indirectly leading to the observed association between HIV infection and INH-monoresistant TB. After accounting for the clustering of the outbreak genotype, we do not believe that HIV is an independent risk factor for increased risk of INH-monoresistant TB.
Time to sputum culture conversion is an established metric for clinical management of patients with TB, is approved by the U.S. Food and Drug Administration (FDA) as an endpoint for phase 2 trials for TB drugs (33), and is a predictor of final TB treatment outcome (34) correlated with regimen effectiveness (35). However, there are limited data on the relationship between INH monoresistance and time required to achieve sputum culture conversion (12, 16). Our findings indicated that patients with INH-monoresistant TB had a similar rate of sputum culture conversion compared with patients with drug-susceptible TB; therefore, altered treatment might not be required in similar clinical settings. Similar to our finding, a study of a smaller cohort from Atlanta also reported that INH-monoresistant TB was not associated with time to sputum culture conversion (36). Similar to a retrospective cohort study conducted among children at three hospitals in the Western Cape, South Africa (37), we also found that INH-monoresistant TB was not significantly associated with either poor treatment outcome or all-cause mortality during TB treatment. Our findings contrast with a retrospective longitudinal study conducted in South Africa and reporting that INH-monoresistant TB was associated with increased risk of treatment failure (aOR, 6.84; 95% CI, 4.29–10.89) and mortality during TB treatment (aOR, 1.81; 95% CI, 1.11–2.95) (38). Another prospective cohort study conducted in southern Mexico reported an increased risk of TB treatment failure (aHR, 12.35; 95% CI, 3.38–45.15) and death (aHR, 3.30; 95% CI, 1.00–10.84) among patients with INH-monoresistant TB (39). However, unlike our study, the studies from South Africa and Mexico did not adjust for baseline smear status or duration of TB treatment, and both had a higher proportion of patients with previous TB treatment (60 and 10%, respectively). In general, our findings contrast with a meta-analysis reporting that current WHO treatment guidelines might be insufficient to treat patients with INH-R, demonstrated by a significantly higher cumulative incidence of failure and relapse combined among INH-R compared with drug-susceptible patients (21). However, unlike most previous studies, we specifically studied patients with INH-monoresistant TB in a population with infrequent TB retreatment; we thoroughly evaluated confounder adjustment methods with sensitivity analyses; and our primary outcomes included rate of sputum culture conversion, poor TB treatment outcome, and all-cause mortality.
INH has high early bactericidal activity (EBA) (40, 41), defined as the ability to reduce MTB concentration during the early phase of TB treatment (42). In our study, the similarity of culture conversion and TB treatment outcomes between patients with INH-monoresistant and drug-susceptible TB suggests that despite INH monoresistance, some bactericidal activity likely remains. This finding is supported by an EBA study conducted in Cape Town, reporting that patients with INH resistance still had positive EBAs (ranging from 0.005 to 0.09 log10 cfu/ml sputum/d) (43).
Our study was subject to limitations. First, our study only included a cohort of patients with TB who had a positive culture result at baseline and available DST results. DST was not performed uniformly among patients; some patients did not have DST results for EMB and PZA. Therefore, the generalizability of our findings to clinically defined (culture-negative) patients with TB may be limited. However, we performed additional analyses (data not shown) categorizing those with pending/missing diagnostic culture results as culture-confirmed; these analyses resulted in no substantial differences in the adjusted HR for culture conversion (aHR, 1.25 vs. 1.27) when comparing patients with INH-monoresistant TB with those with drug-susceptible TB. Furthermore, patients with culture-confirmed and clinical TB in our study were similar with respect to birthplace, occupation, HIV status, and diabetes status. Second, our study used surveillance data only, and did not include information on contact tracing to analyze epidemiologic links for the INH-monoresistant TB transmission in Georgia. We also did not have information on the level of INH resistance (i.e., high- vs. low-level resistance) among patients with INH-monoresistant TB. However, 41.3% of patients with INH-monoresistant TB in our study were from Fulton County, where a recent TB outbreak occurred, suggesting that the observed relationship was more likely to be associated with TB clustering. Third, we did not have direct measures of the extent of immunosuppression among patients with HIV or diabetes. For example, we did not have CD4 count or viral load information on patients with HIV infection. We also did not have a measure of blood glucose control among patients with TB and diabetes. In addition, as we relied on surveillance records, some misclassification of HIV status, diabetes status, and illicit drug and alcohol use was possible. Nonetheless, previous studies have demonstrated that TB notification and surveillance systems in the United States, similar to the one used in these analyses, have high accuracy and reliability rates (44). Fourth, we did not have information on treatment regimen change or adherence during TB treatment for patients in the cohort. However, patients in Georgia are treated according to U.S. CDC guidelines, and the TB treatment regimens for each type of TB likely included little variation (36, 45). Moreover, our sensitivity analysis for unmeasured confounding by treatment regimen change suggested this error may be away from the null. Despite limitations, the present study included a large and well-characterized cohort of patients with culture-confirmed TB. Our study also had a high coverage of genotyping among patients with culture-positive TB.
Conclusions
Our evaluation of the impact of INH monoresistance on three clinical metrics of response to TB treatment did not find evidence to suggest that patients with INH-monoresistant TB required altered treatment considerations. Patients with and without INH-monoresistant TB had similar sputum culture conversion rates, final TB treatment outcomes, and mortality risk. This study adds important information regarding the association between clinical epidemiologic factors and INH-monoresistant TB. The association between HIV status, homelessness, and INH-monoresistant TB reported in our study may mirror the recent TB outbreak among the homeless population in the Atlanta metropolitan area. Expanding our current understanding of the relationship between INH-monoresistant TB and response to TB treatment is needed to inform TB treatment guidelines.
Supplementary Material
Footnotes
Supported in part by the National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases (K23AI103044 and R21AI122001) (Bethesda, MD); and by the Atlanta Clinical and Translational Science Institute (NIH/NCATS UL1TR000454) and the Georgia Tuberculosis Elimination and Laboratory Cooperative Agreement (CDC-RFA-PS15-1501) (Atlanta, GA). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author Contributions: A.D.S. and M.J.M. conceived the design, performed the analyses, and wrote the initial draft. A.D.S., R.-M.F.S., and L.D. acquired the data. All authors contributed to interpretation of the data, revised the manuscript, and approved the final version.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.World Health Organization. Geneva: World Health Organization; 2016. Global tuberculosis report 2016. [Google Scholar]
- 2.Stagg HR, Lipman MC, McHugh TD, Jenkins HE. Isoniazid-resistant tuberculosis: a cause for concern? Int J Tuberc Lung Dis. 2017;21:129–139. doi: 10.5588/ijtld.16.0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Geneva: World Health Organization; 2014. Global tuberculosis report 2014. [Google Scholar]
- 4.Jenkins HE, Zignol M, Cohen T. Quantifying the burden and trends of isoniazid resistant tuberculosis, 1994–2009. PLoS One. 2011;6:e22927. doi: 10.1371/journal.pone.0022927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shah NS, Auld SC, Brust JC, Mathema B, Ismail N, Moodley P, et al. Transmission of extensively drug-resistant tuberculosis in South Africa. N Engl J Med. 2017;376:243–253. doi: 10.1056/NEJMoa1604544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Atlanta, GA: Centers for Disease Control and Prevention; 2015. Reported tuberculosis in the United States, 2015. [Google Scholar]
- 7.Georgia Department of Public Health. Atlanta, GA: Georgia Department of Public Health; 2014. 2014 Georgia tuberculosis report. [Google Scholar]
- 8.Gegia M, Cohen T, Kalandadze I, Vashakidze L, Furin J. Outcomes among tuberculosis patients with isoniazid resistance in Georgia, 2007–2009. Int J Tuberc Lung Dis. 2012;16:812–816. doi: 10.5588/ijtld.11.0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fox L, Kramer MR, Haim I, Priess R, Metvachuk A, Shitrit D. Comparison of isoniazid monoresistant tuberculosis with drug-susceptible tuberculosis and multidrug-resistant tuberculosis. Eur J Clin Microbiol Infect Dis. 2011;30:863–867. doi: 10.1007/s10096-011-1167-4. [DOI] [PubMed] [Google Scholar]
- 10.Hoopes AJ, Kammerer JS, Harrington TA, Ijaz K, Armstrong LR. Isoniazid-monoresistant tuberculosis in the United States, 1993 to 2003. Arch Intern Med. 2008;168:1984–1992. doi: 10.1001/archinte.168.18.1984. [DOI] [PubMed] [Google Scholar]
- 11.Smith CM, Trienekens SC, Anderson C, Lalor MK, Brown T, Story A, et al. Twenty years and counting: epidemiology of an outbreak of isoniazid-resistant tuberculosis in England and Wales, 1995 to 2014. Euro Surveill. 2017;22(8) doi: 10.2807/1560-7917.ES.2017.22.8.30467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cattamanchi A, Dantes RB, Metcalfe JZ, Jarlsberg LG, Grinsdale J, Kawamura LM, et al. Clinical characteristics and treatment outcomes of patients with isoniazid-monoresistant tuberculosis. Clin Infect Dis. 2009;48:179–185. doi: 10.1086/595689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Villegas L, Otero L, Sterling TR, Huaman MA, Van der Stuyft P, Gotuzzo E, et al. Prevalence, risk factors, and treatment outcomes of isoniazid- and rifampicin-mono-resistant pulmonary tuberculosis in Lima, Peru. PLoS One. 2016;11:e0152933. doi: 10.1371/journal.pone.0152933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vadwai V, Shetty A, Soman R, Rodrigues C. Determination of risk factors for isoniazid monoresistance and multidrug-resistant tuberculosis in treatment failure patients. Scand J Infect Dis. 2012;44:48–50. doi: 10.3109/00365548.2011.611169. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. Geneva: World Health Organization; 2016. WHO treatment guidelines for drug-resistant tuberculosis 2016 update. [Google Scholar]
- 16.Wang TY, Lin SM, Shie SS, Chou PC, Huang CD, Chung FT, et al. Clinical characteristics and treatment outcomes of patients with low- and high-concentration isoniazid-monoresistant tuberculosis. PLoS One. 2014;9:e86316. doi: 10.1371/journal.pone.0086316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Menzies D, Benedetti A, Paydar A, Martin I, Royce S, Pai M, et al. Effect of duration and intermittency of rifampin on tuberculosis treatment outcomes: a systematic review and meta-analysis. PLoS Med. 2009;6:e1000146. doi: 10.1371/journal.pmed.1000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hazbon MH, Brimacombe M, Bobadilla del Valle M, Cavatore M, Guerrero MI, Varma-Basil M, et al. Population genetics study of isoniazid resistance mutations and evolution of multidrug-resistant Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2006;50:2640–2649. doi: 10.1128/AAC.00112-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu Y, Hoffner S, Jiang W, Wang W, Xu B. Extensive transmission of isoniazid resistant M. tuberculosis and its association with increased multidrug-resistant TB in two rural counties of eastern China: a molecular epidemiological study. BMC Infect Dis. 2010;10:43. doi: 10.1186/1471-2334-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vinnard C, Winston CA, Wileyto EP, Macgregor RR, Bisson GP. Isoniazid resistance and death in patients with tuberculous meningitis: retrospective cohort study. BMJ. 2010;341:c4451. doi: 10.1136/bmj.c4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gegia M, Winters N, Benedetti A, van Soolingen D, Menzies D. Treatment of isoniazid-resistant tuberculosis with first-line drugs: a systematic review and meta-analysis. Lancet Infect Dis. 2017;17:223–234. doi: 10.1016/S1473-3099(16)30407-8. [DOI] [PubMed] [Google Scholar]
- 22.Stagg HR, Harris RJ, Hatherell H-A, Obach D, Zhao H, Tsuchiya N, et al. What are the most efficacious treatment regimens for isoniazid-resistant tuberculosis? A systematic review and network meta-analysis. Thorax. 2016;71:940–949. doi: 10.1136/thoraxjnl-2015-208262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Georgia Department of Public Health. Atlanta, GA: Georgia Department of Public Health; 2013. 2013 Georgia tuberculosis report. [Google Scholar]
- 24.Georgia Department of Public Health. Atlanta, GA: Georgia Department of Public Health; 2014. Georgia tuberculosis reference guide. [Google Scholar]
- 25.Huebner RE, Good RC, Tokars JI. Current practices in mycobacteriology: results of a survey of state public health laboratories. J Clin Microbiol. 1993;31:771–775. doi: 10.1128/jcm.31.4.771-775.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Georgia Department of Public Health. Atlanta, GA: Georgia Department of Public Health; 2004. Mycobacteriology lab tests: quick reference sheets. [Google Scholar]
- 27.Orenstein EW, Basu S, Shah NS, Andrews JR, Friedland GH, Moll AP, et al. Treatment outcomes among patients with multidrug-resistant tuberculosis: systematic review and meta-analysis. Lancet Infect Dis. 2009;9:153–161. doi: 10.1016/S1473-3099(09)70041-6. [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization. Geneva: World Health Organization; 2013. Definitions and reporting framework for tuberculosis—2013 revision. [Google Scholar]
- 29.Kleinbaum DG, Klein M. New York: Springer; 2011. Survival analysis: a self-learning text, 3rd ed. [Google Scholar]
- 30.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10:37–48. [PubMed] [Google Scholar]
- 31.Greenland S. Basic methods for sensitivity analysis of biases. Int J Epidemiol. 1996;25:1107–1116. [PubMed] [Google Scholar]
- 32.Escombe AR, Moore DA, Gilman RH, Pan W, Navincopa M, Ticona E, et al. The infectiousness of tuberculosis patients coinfected with HIV. PLoS Med. 2008;5:e188. doi: 10.1371/journal.pmed.0050188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Food and Drug Administration. Rockville, MD: Food and Drug Administration; 2013. Guidance for Industry: Pulmonary tuberculosis: developing drugs for treatment. [Google Scholar]
- 34.World Health Organization. Treatment of tuberculosis: guidelines for national programmes. 2003. Contract No. WHO/CDS/TB/2003.313. Geneva: World Health Organization.
- 35.Holtz TH, Sternberg M, Kammerer S, Laserson KF, Riekstina V, Zarovska E, et al. Time to sputum culture conversion in multidrug-resistant tuberculosis: predictors and relationship to treatment outcome. Ann Intern Med. 2006;144:650–659. doi: 10.7326/0003-4819-144-9-200605020-00008. [DOI] [PubMed] [Google Scholar]
- 36.Schechter MC, Bizune D, Kagei M, Machaidze M, Holland DP, Oladele A, et al. Time to sputum culture conversion and treatment outcomes among patients with isoniazid-resistant tuberculosis in Atlanta, Georgia. Clin Infect Dis. 2017;65:1862–1871. doi: 10.1093/cid/cix686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garcia-Prats AJ, du Plessis L, Draper HR, Burger A, Seddon JA, Zimri K, et al. Outcome of culture-confirmed isoniazid-resistant rifampicin-susceptible tuberculosis in children. Int J Tuberc Lung Dis. 2016;20:1469–1476. doi: 10.5588/ijtld.16.0293. [DOI] [PubMed] [Google Scholar]
- 38.van der Heijden YF, Karim F, Mufamadi G, Zako L, Chinappa T, Shepherd BE, et al. Isoniazid-monoresistant tuberculosis is associated with poor treatment outcomes in Durban, South Africa. Int J Tuberc Lung Dis. 2017;21:670–676. doi: 10.5588/ijtld.16.0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baez-Saldana R, Delgado-Sanchez G, Garcia-Garcia L, Cruz-Hervert LP, Montesinos-Castillo M, Ferreyra-Reyes L, et al. Isoniazid mono-resistant tuberculosis: impact on treatment outcome and survival of pulmonary tuberculosis patients in southern Mexico 1995–2010. PLoS One. 2016;11:e0168955. doi: 10.1371/journal.pone.0168955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lalande L, Bourguignon L, Bihari S, Maire P, Neely M, Jelliffe R, et al. Population modeling and simulation study of the pharmacokinetics and antituberculosis pharmacodynamics of isoniazid in lungs. Antimicrob Agents Chemother. 2015;59:5181–5189. doi: 10.1128/AAC.00462-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wallis RS, Phillips M, Johnson JL, Teixeira L, Rocha LM, Maciel E, et al. Inhibition of isoniazid-induced expression of Mycobacterium tuberculosis antigen 85 in sputum: potential surrogate marker in tuberculosis chemotherapy trials. Antimicrob Agents Chemother. 2001;45:1302–1304. doi: 10.1128/AAC.45.4.1302-1304.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hafner R, Cohn JA, Wright DJ, Dunlap NE, Egorin MJ, Enama ME, et al. DATRI 008 Study Group. Early bactericidal activity of isoniazid in pulmonary tuberculosis: optimization of methodology. Am J Respir Crit Care Med. 1997;156:918–923. doi: 10.1164/ajrccm.156.3.9612016. [DOI] [PubMed] [Google Scholar]
- 43.Donald PR, Sirgel FA, Botha FJ, Seifart HI, Parkin DP, Vandenplas ML, et al. The early bactericidal activity of isoniazid related to its dose size in pulmonary tuberculosis. Am J Respir Crit Care Med. 1997;156:895–900. doi: 10.1164/ajrccm.156.3.9609132. [DOI] [PubMed] [Google Scholar]
- 44.Doyle TJ, Glynn MK, Groseclose SL. Completeness of notifiable infectious disease reporting in the United States: an analytical literature review. Am J Epidemiol. 2002;155:866–874. doi: 10.1093/aje/155.9.866. [DOI] [PubMed] [Google Scholar]
- 45.Blumberg HM, Burman WJ, Chaisson RE, Daley CL, Etkind SC, Friedman LN, et al. American Thoracic Society; Centers for Disease Control and Prevention; Infectious Diseases Society. Treatment of tuberculosis. Am J Respir Crit Care Med. 2003;167:603–662. doi: 10.1164/rccm.167.4.603. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.