Abstract
Rationale: Studies suggest that adults with bronchiectasis are at increased risk of cardiovascular comorbidities.
Objectives: We aimed to quantify the relative risk of incident cardiovascular events after a respiratory tract infection among adults with bronchiectasis.
Methods: Using UK electronic primary care records, we conducted a within-person comparison using the self-controlled case series method. We calculated the relative risk of first-time cardiovascular events (either first myocardial infarction or stroke) after a respiratory tract infection compared with the individual’s baseline risk.
Results: Our cohort consisted of 895 adult men and women with non–cystic fibrosis bronchiectasis with a first myocardial infarction or stroke and at least one respiratory tract infection. There was an increased rate of first-time cardiovascular events in the 91-day period after a respiratory tract infection (incidence rate ratio, 1.56; 95% confidence interval, 1.20–2.02). The rate of a first cardiovascular event was highest in the first 3 days after a respiratory tract infection (incidence rate ratio, 2.73; 95% confidence interval, 1.41–5.27).
Conclusions: These data suggest that respiratory tract infections are strongly associated with a transient increased risk of first-time myocardial infarction or stroke among people with bronchiectasis. As respiratory tract infections are six times more common in people with bronchiectasis than the general population, the increased risk has a disproportionately greater impact in these individuals. These findings may have implications for including cardiovascular risk modifications in airway infection treatment pathways in this population.
Keywords: bronchiectasis, cardiovascular disease, myocardial infarction, stroke, self-controlled case series
Bronchiectasis is a suppurative chronic lung disease characterized by repeated infections, and recent studies have shown that the incidence and prevalence of bronchiectasis in the United Kingdom (1) and United States (2) is increasing. Previous studies in the general population (3–5) and in people with chronic obstructive pulmonary disease (COPD) (6) have established an association between respiratory tract infections and cardiovascular events, and in previous work we have shown that people with bronchiectasis experience more cardiovascular comorbidities than the general population (7). It has been suggested that repeated lower respiratory tract infections result in an acute phase response and increased systemic inflammation, which contribute to the increased cardiovascular risk seen. Although it is likely this association could be generalized to individuals with bronchiectasis, the occurrence of respiratory tract infections among people with bronchiectasis is far higher and results in substantial morbidity and mortality. Hence, quantifying the effect and magnitude of cardiovascular events after a respiratory tract infection is important.
Using routinely collected, anonymized UK primary care data, we conducted a within-person comparison to assess the impact of respiratory tract infections on the risk of incident cardiovascular events among people with non–cystic fibrosis (CF) bronchiectasis.
Methods
Data Source
We used anonymized electronic primary care data from the Clinical Practice Research Datalink (CPRD; www.cprd.com). Information is recorded as part of routine care and includes diagnostic and prescribing information. Studies have previously demonstrated that individuals who contribute to CPRD are broadly representative of the UK general population (8). Approval was obtained from the Independent Scientific Advisory Committee, which oversees research involving CPRD data (protocol ref: 13_030R; available on request)
Study Population
Our study population consisted of a dynamic cohort of individuals with a recorded diagnosis of bronchiectasis who were alive and contributing to CPRD at any point between January 1, 2000, and December 31, 2013. We identified individuals with a record of bronchiectasis using predefined Read codes (H34.00, H34z.00, H340.00, A115.00, H341.00, P86.100) that have been previously been used in epidemiological studies (1, 7). We excluded people with a coexisting diagnosis of CF, anyone younger than 18 years of age at time of diagnosis, and individuals who only had a record of bronchiectasis at time of death. All individuals had at least 12 months of electronic records that were of research quality before entry into the study. The start of our observation period was the latest of study start date or date of diagnosis of bronchiectasis. The end of the study observation period was the earliest of date of death, transfer out of practice, last date of data collection or study end date.
Definition of Outcomes
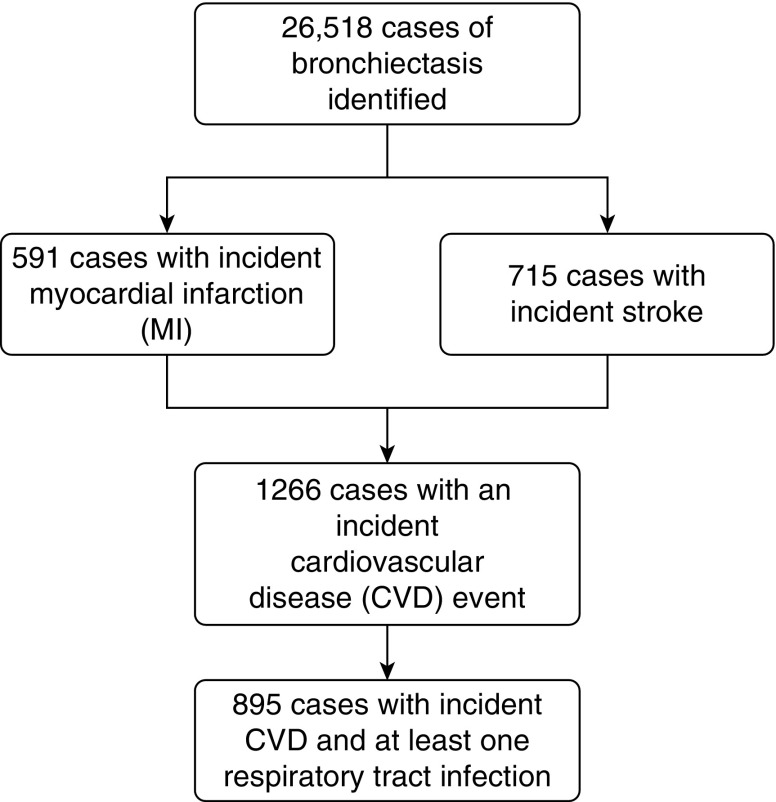
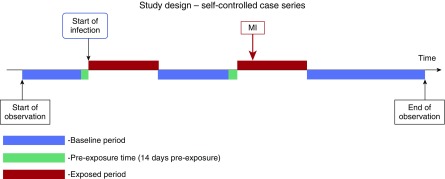
Our primary outcome was first record of a cardiovascular event, a composite outcome of first recorded diagnosis of myocardial infarction (MI) or stroke that occurred during the study observation period. Individuals with diagnoses of MI or stroke before the start of the study period were excluded (Figure 1). The validity of recording of MI (9, 10) and stroke (10, 11) in electronic primary care records has previously been shown to be high. MI and stroke events that coincided with date of death were also excluded. One of the assumptions of the self-controlled case series method is that the outcome of interest must not censor the observation period (in our case, if a cardiovascular event increases the likelihood of death). However, there is evidence that the method is robust to this assumption (12). We restricted our outcomes events to non-fatal MI and strokes to overcome this potential bias. Our secondary outcomes were incident MI and stroke events analyzed separately (Figure 2).
Figure 1.
Identification of study population.
Figure 2.
Pictorial representation of the self-controlled case series. MI, myocardial infarction.
Definition of Respiratory Tract Infection
We searched medical records for diagnoses of respiratory tract infections. The medical Read codes used to identify respiratory tract infections were developed by two clinical epidemiologists, one of whom was a consultant respiratory physician (13), and were consistent with the UK’s National Institute of Health and Care Excellence guidelines for the diagnosis of lower respiratory tract infection and clinical diagnosis of community-acquired pneumonia (14). We excluded Read codes suggestive of an exacerbation of COPD (15), as we have previously demonstrated that approximately one-third of individuals with bronchiectasis also have a coexisting diagnosis of COPD (1). Codes used to define respiratory tract infection can be found in the Appendix (online supplement) and included report of these codes with and without treatment with antibiotics either by the general practitioner (i.e., treated at home) or in the hospital. Date of the respiratory tract infection was deemed to be the date the patient visited the general practitioner or hospital as was recorded in the electronic medical record.
Statistical Analysis
We undertook within-person comparisons in our population of individuals with bronchiectasis who had both a first cardiovascular outcome and at least one respiratory tract infection during our study observation period. The self-controlled case series study design is similar to cohort methodology and is adapted from rate modeling using a Poisson distribution. This study design relies on within-person comparisons in a population with both the exposure and outcome of interest (12, 16). An advantage of the within-person comparison method is that possible confounders, such as smoking habit, hypertension, hyperlipidemia, and diabetes mellitus, are removed.
The self-controlled case series method relies on several assumptions, an important one being that the occurrence of the outcome event should not alter the probability of subsequent exposure. In this study, a cardiovascular event should not change the subsequent probability of developing a respiratory tract infection. Such a change would alter the baseline rate of events, and this may result in an over- or underestimate of the relative rate of events occurring in exposed periods compared with baseline periods. If there is a transient change in the probability of postoutcome exposures, this bias can be overcome by removing a predefined period of time before exposure from all other baseline periods (17). In our study, a 2-week period before first record of a respiratory tract infection was removed from the baseline period. All assumptions of the self-controlled case series method are detailed in the Appendix (online supplement).
Fixed-effects conditional Poisson regression was used to generate incidence rate ratios comparing the rate of incident cardiovascular events during our prespecified high-risk intervals after a respiratory tract infection compared with all other observed periods for each individual. Our high-risk interval was defined as extending up to 91 days after a respiratory tract infection, subdivided into smaller periods of 1 to 3 days, 4 to 7 days, 8 to 14 days, 15 to 29 days, and 29 to 91 days after an infection. These risk windows have previously been used to evaluate the risk of MI and stroke after acute infections in the general population (4). For people who had repeated respiratory tract infections during the study observation period, each episode of infection was followed by a 91-day interval, subdivided into the smaller periods mentioned above, where individuals were considered at high risk. For those who had multiple records of respiratory tract infections within the high-risk interval, only the first record of infection was used to define the start and end of the high-risk interval. Because age varies with time, we adjusted for age in 5-year age bands over the age of 45 years. As seasonal patterns are seen in both cardiovascular events and respiratory tract infections, we then repeated the analyses controlling for the effect of seasonal variation by dividing the study observation period into warmer months (April to September) and cooler months (October to March).
Secondary Analyses
We repeated the analyses for the individual outcomes of first MI and stroke, in turn. Finally, we also explored the impact of coexisting COPD in the association of cardiovascular outcomes after a respiratory tract infection. The main analyses were repeated after excluding people with bronchiectasis who also had a diagnosis of COPD.
Power Calculation
Before undertaking the analyses, we expected to identify approximately 800 individuals with bronchiectasis who had an incident cardiovascular event and at least one respiratory tract infection. With this sample size we calculated that we would have more than 80% power at 5% significance level to detect an incidence rate ratio of 1.5 or greater in the first 2 weeks after an infection. All statistical analyses were conducted using Stata version 14.
Results
From a cohort of 26,518 individuals with bronchiectasis, our final study population consisted of 895 individuals with bronchiectasis who had both a first cardiovascular event and at least one respiratory tract infection during the study observation period (Figure 1). A total of 468 (52.9%) were women, the median age at time of diagnosis of bronchiectasis was 63.7 years (interquartile range, 50.0–72.1), and 235 (26.3%) were current smokers (Table 1). The mean observation period for the study population was 6.2 years (standard deviation, 4.5). After controlling for the effects of age, the rate of incident cardiovascular events was 56% higher in the 91-day period after a record of respiratory tract infection (incidence rate ratio, 1.56; 95% confidence interval [CI], 1.20–2.02). The rate of a first cardiovascular event was highest in the first 3 days after a respiratory tract infection (incidence rate ratio, 2.73; 95% CI, 1.41–5.27) and fell after the first 14 days (Table 2). The rate of incident cardiovascular events remained higher after a respiratory tract infection after controlling for the effects on seasonality (Table 2).
Table 1.
Baseline characteristics of the bronchiectasis cohort (N = 895)
| Characteristic | |
|---|---|
| Sex | |
| Male | 427 (47.1) |
| Female | 468 (52.9) |
| Age category, years | |
| <45 | 146 (16.3) |
| 45–55 | 114 (12.7) |
| 56–65 | 187 (20.9) |
| 66–75 | 248 (27.7) |
| >75 | 200 (22.4) |
| Mean (SD) observation period, years | 6.2 (4.5) |
| Median (IQR) number of respiratory tract infections during the study period | 5 (2–10) |
| Smoking habit | |
| Never smoked | 165 (18.4) |
| Ex-smoker | 478 (53.4) |
| Current smoker | 235 (26.3) |
| Missing information | 17 (1.9) |
Definition of abbreviations: IQR = interquartile range; SD = standard deviation.
Data presented as n (%) unless otherwise noted.
Table 2.
Adjusted incidence rate ratios of a first cardiovascular event in risk periods after a respiratory tract infection
| Risk Period (d) | No. of Events | IRR (95% CI)* | IRR (95% CI)† |
|---|---|---|---|
| 1–91 | 125 | 1.56 (1.20–2.02) | 1.25 (1.02–1.53) |
| 1–3 | 82 | 2.73 (1.41–5.27) | 2.39 (1.21–5.62) |
| 4–7 | 21 | 2.13 (1.46–2.74) | 2.01 (1.22–2.78) |
| 8–14 | 9 | 1.93 (1.11–2.08) | 1.73 (1.09–2.13) |
| 15–28 | 8 | 1.36 (0.87–2.11) | 1.16 (0.77–2.19) |
| 29–91 | 5 | 1.19 (0.73–1.51) | 1.08 (0.69–1.53) |
| Baseline period | 706 | 1.00 | 1.00 |
Definition of abbreviations: CI = confidence interval; IRR = incidence rate ratio.
IRR adjusted for age.
IRR adjusted for age and season.
Secondary Analyses
A total of 443 people with bronchiectasis had an incident MI and at least one episode of a respiratory tract infection during our study period. The age-adjusted rate ratio for first MI was substantially higher in the 14 days after a respiratory tract infection than in periods without a respiratory tract infection. The rate ratio for first MI peaked in the first 3 days after a respiratory tract infection, where it was more than four times higher than baseline (Table 3). A total of 479 individuals with bronchiectasis and at least one respiratory tract infection had an incident stroke. The age-adjusted rate ratio for first stroke was also higher after a respiratory tract infection and was highest in the first 3 days (incidence rate ratio, 1.79; 95% CI, 1.20–3.53; Table 4). Additional adjustment for seasonality had little impact on the rate ratios for first MI and stroke (Tables 3 and 4). After excluding individuals with coexisting COPD (n = 464), we found marginal change to the age-adjusted rate of incident cardiovascular events after a respiratory tract infection (Table 5). Controlling for the effects of season in addition to age did not alter the rate ratios for incident cardiovascular event in this subset.
Table 3.
Adjusted incidence rate ratios of first myocardial infarction in risk periods after a respiratory tract infection
| Risk Period (d) | No. of Events | IRR (95% CI)* | IRR (95% CI)† |
|---|---|---|---|
| 1–3 | 36 | 4.26 (2.00–9.06) | 4.25 (1.99–9.04) |
| 4–7 | 10 | 2.28 (1.03–5.54) | 2.27 (1.03–5.55) |
| 8–14 | 7 | 1.30 (0.78–2.46) | 1.29 (0.76–2.49) |
| 15–28 | 5 | 1.04 (0.69–2.81) | 1.05 (0.68–2.46) |
| 29–91 | 4 | 1.05 (0.73–1.50) | 1.03 (0.71–1.52) |
| Baseline period | 347 | 1.00 | 1.00 |
Definition of abbreviations: CI = confidence interval; IRR = incidence rate ratio.
IRR adjusted for age.
IRR adjusted for age and season.
Table 4.
Adjusted incidence rate ratios of first stroke in risk periods after respiratory tract infection
| Risk Period (d) | No. of Events | IRR (95% CI)* | IRR (95% CI)† |
|---|---|---|---|
| 1–3 | 48 | 1.79 (1.20–3.53) | 1.77 (1.15–3.56) |
| 4–7 | 13 | 1.59 (1.12–4.75) | 1.58 (1.12–4.76) |
| 8–14 | 10 | 1.32 (0.96–3.09) | 1.29 (0.94–3.09) |
| 15–28 | 3 | 1.14 (0.79–4.15) | 1.14 (0.77–4.17) |
| 29–91 | 4 | 0.98 (0.62–3.31) | 0.95 (0.60–3.32) |
| Baseline period | 383 | 1.00 | 1.00 |
Definition of abbreviations: CI = confidence interval; IRR = incidence rate ratio.
IRR adjusted for age.
IRR adjusted for age and season.
Table 5.
Adjusted incidence rate ratios of a first cardiovascular event in risk periods after a respiratory tract infection excluding people with a coexisting diagnosis of COPD
| Risk Period (d) | No. of Events | IRR (95% CI)* |
|---|---|---|
| 1–3 | 42 | 2.38 (1.77–4.76) |
| 4–7 | 17 | 2.30 (1.46–3.89) |
| 8–14 | 5 | 1.61 (1.15–2.24) |
| 15–28 | 4 | 1.36 (0.67–2.76) |
| 29–91 | 2 | 0.97 (0.55–2.16) |
| Baseline period | 352 | 1.00 |
Definition of abbreviations: CI = confidence interval; COPD = chronic obstructive pulmonary disease; IRR = incidence rate ratio.
IRR adjusted for age.
Because the self-controlled case series is usually undertaken in studies of shorter follow-up, we stratified the analysis on people with less than and more than 6 years of follow-up, because this is the mean. We found the incidence rate ratio is similar between the two, suggesting the result is not being driven by unadjusted confounding among people with longer follow-up (see Table E1b in the online supplement). We also repeated the analysis using calendar year, 2-year age bands, and season (Table E1a).
Discussion
Our population-based study found that among people with non–cystic fibrosis bronchiectasis, a respiratory tract infection is strongly associated with a transient increase in the rate of incident cardiovascular events. The rate of first cardiovascular event was more than twofold higher in the first 3 days after a respiratory tract infection and remained almost double up to 2 weeks after, compared with periods without infection. Although the rates of both first myocardial infarction and stroke were elevated after a respiratory tract infection, the effect was most pronounced with first myocardial infarction. The impact of respiratory tract infection on cardiovascular risk was greatest in the first week after the infection, suggesting a narrow window for risk factor modification. Although a substantial number of individuals with bronchiectasis in our population also had a coexisting record of chronic obstructive pulmonary disease, our secondary analyses showed that the association persists after excluding this subset of people. The age-standardized (standardized to the 2004 UK population) rate of respiratory tract infections in the entire bronchiectasis cohort (n = 26,415) was 632.7 per 1,000 person-years. This was approximately six times higher than the age-standardized rate of community-acquired lower respiratory tract infections in the general population (13). Hence, the relative risk of a cardiovascular event occurring after a respiratory tract infection in a patient with bronchiectasis is sixfold higher than the relative risk of a cardiovascular event occurring after a systemic respiratory tract infection in the general population.
One of the strengths of our study is the large population of people with bronchiectasis and the length of our observation period, which enabled us to quantify the risk of incident cardiovascular events after a respiratory tract infection. An advantage of the self-controlled case series method is that it eliminates confounding by factors that vary between individuals, such as smoking, hypertension, hyperlipidemia, and diabetes mellitus, because each person acts as their own control (18). Furthermore, within-comparison between time periods relative to the exposure also removes the possibility of reverse causation. It is possible that there is some misclassification between cardiovascular and infective events; however, this is unlikely to account for the relationship we have seen in this study. In addition to the above, all key assumptions of the self-controlled case series methodology were met. We only used first-time diagnoses of cardiovascular events, as the risk of a subsequent event is likely to be different from the first. Studies have established the validity of medical diagnoses within CPRD to be high (10, 19), and the use of prospectively collected information from electronic primary care records minimizes the potential of recall or observer bias.
Our study has a number of limitations that need to be taken into consideration. Although we have studied a UK bronchiectasis population, the results are likely to be generalizable to other bronchiectasis populations worldwide, particularly in countries where the etiology of bronchiectasis is similar to the United Kingdom (20). A potential limitation is the validity of diagnosis of bronchiectasis. Although we were unable to validate the diagnosis of bronchiectasis, only a small number of Read codes that have previously been published (1) were used to identify people with bronchiectasis. A total of 70.7% of patients with bronchiectasis who had a cardiovascular event also had a respiratory tract infection, suggesting that around one-third of the initial cohort did not have clinically significant bronchiectasis. This is in keeping with previous studies and likely due to the increase in computed tomography scans occurring in older individuals leading to a diagnosis of non–clinically significant disease.
To improve the specificity of our bronchiectasis population, we excluded anyone who was younger than 18 years of age at time of diagnosis of bronchiectasis and those who also had a recorded diagnosis of cystic fibrosis. As we did not have access to radiological information from our cohort, it is not possible to confirm if the diagnosis of bronchiectasis was made according to current guidelines. However, bronchiectasis is usually diagnosed in a secondary care setting after patients have had radiological confirmation with a computed tomography scan, as demonstrated by the recent British Thoracic Society bronchiectasis audit (20). Therefore, it is unlikely that a diagnosis of bronchiectasis would present in primary care records without confirmation from healthcare providers in secondary care. Further reassurance of the external validity of diagnosis of bronchiectasis is supported by the fact that the demographic features of our cohort are consistent with the UK population of patients with bronchiectasis (21–23). Our bronchiectasis cohort is also likely to represent individuals with a diagnosis of bronchiectasis in their primary care records across the disease spectrum. It is possible that by excluding people with bronchiectasis before the age of 18 years we have disproportionately excluded those with primary ciliary dyskinesia, making the results less applicable to that group; however, we are not able to test this.
Another potential limitation of our study is that we used date of diagnosis of respiratory tract infection rather than date of onset, which could not be determined accurately. This is likely to result in underestimation of the duration of the increased risk of first myocardial infarction or stroke. Furthermore, we will not have captured all respiratory tract infections in people with bronchiectasis, as some patients may have rescue antibiotics at home to self-medicate at onset of symptoms. It is important to note that the exposure in our study was a respiratory tract infection that precipitated consultation with primary care, the severity of which will vary between individuals. We were unable to use the British Thoracic Society guideline definition of infective exacerbation of bronchiectasis (24) as our exposure definition because of the small number of people who met these criteria. We also did not have information on disease severity or secondary care records to assess if disease severity or hospitalization influenced the effect of respiratory tract infections on the risk of incident cardiovascular outcomes. Therefore, it is likely that our findings are actually an underestimate of the risk of cardiovascular disease after airway infection in people with bronchiectasis.
To our knowledge, this is the first study to demonstrate that, among a population of people with bronchiectasis, an acute respiratory tract infection is associated with a transient increased risk of incident cardiovascular outcomes. Our findings are consistent with studies showing that in the general population (3–5), and among people with chronic obstructive pulmonary disease (6), the risk of myocardial infarction and stroke are higher after a respiratory tract infection. Using routine primary care data, studies have shown that the relative risk for both myocardial infarction (3, 4) and stroke (4) were substantially higher in the first few days after a respiratory tract infection, especially after influenza infection (25). More recently, a study using two separate community-based cohorts demonstrated that an episode of pneumonia that resulted in a hospital admission was associated with a short- and longer-term risk of cardiovascular disease (26). It has also been demonstrated that the risk of myocardial infarction and stroke is higher after an exacerbation of chronic obstructive pulmonary disease (6).
The increased risk of cardiovascular outcomes after respiratory tract infections in bronchiectasis may be due to a number of reasons, one of which is shared risk factors, such as hypoxia and transient increase in systemic inflammation. It has been hypothesized that respiratory tract infections contribute to progression of atherosclerotic plaques (27), which, coupled with elevated levels of inflammatory cytokines (28), precipitate cardiovascular events. Arterial stiffness has been shown to increase acutely during infective exacerbations of chronic obstructive pulmonary disease (29), increasing the risk of myocardial infarction and stroke after airway infection in this population. Aortic stiffness has previously been shown to be elevated in people with bronchiectasis (30), raising the possibility that this mechanism contributes to the increased risk in cardiovascular outcomes seen in our study.
Fifty-one percent of people with bronchiectasis in our cohort also had a diagnosis of chronic obstructive pulmonary disease, which is also associated with increased risk of cardiovascular disease. However, our secondary analyses, excluding individuals with coexisting diagnoses of chronic obstructive pulmonary disease, suggest that the increased cardiovascular risk after infection still persists.
In conclusion, our study has quantified the increased risk of incident cardiovascular outcomes, which among people with bronchiectasis is more than double in the first 7 days after a respiratory tract infection compared with periods without an infection. This increased relative cardiovascular risk in people with bronchiectasis is substantially higher than the risk of myocardial infarction or stroke after community-acquired respiratory tract infections in the general population. These data suggest recurrent respiratory tract infections contribute to the increased cardiovascular comorbidities in people with bronchiectasis, and clinical trials of targeted antiplatelets or statins for prevention of respiratory infection–related vascular events are warranted.
Supplementary Material
Acknowledgments
Acknowledgment
This paper is dedicated to the memory of Dr. Adrian Root, a brilliant epidemiologist, wonderful colleague, and friend.
Footnotes
Supported by a National Institute for Health Research Academic Clinical Lectureship (V.N.) and the GlaxoSmithKline/British Lung Foundation chair of Epidemiological Respiratory Research (R.B.H.).
Author Contributions: V.N., I.D., and J.K.Q. conceived and designed the study. V.N. and J.K.Q. were involved in the acquisition of the data. V.N., A.A.R., and J.K.Q. were involved in the analyses of the data. V.N., A.A.R., I.D., L.S., R.B.H., and J.K.Q. were involved in the interpretation of the data and in writing or revising the manuscript before submission. V.N. takes responsibility for the integrity of the work in this manuscript and is the guarantor of the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Quint JK, Millett ER, Joshi M, Navaratnam V, Thomas SL, Hurst JR, et al. Changes in the incidence, prevalence and mortality of bronchiectasis in the UK from 2004 to 2013: a population-based cohort study. Eur Respir J. 2016;47:186–193. doi: 10.1183/13993003.01033-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seitz AE, Olivier KN, Adjemian J, Holland SM, Prevots DR. Trends in bronchiectasis among Medicare beneficiaries in the United States, 2000 to 2007. Chest. 2012;142:432–439. doi: 10.1378/chest.11-2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meier CR, Jick SS, Derby LE, Vasilakis C, Jick H, Meier CR. Acute respiratory-tract infections and risk of first-time acute myocardial infarction. Lancet. 1998;351:1467–1471. doi: 10.1016/s0140-6736(97)11084-4. [DOI] [PubMed] [Google Scholar]
- 4.Smeeth L, Thomas SL, Hall AJ, Hubbard R, Farrington P, Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med. 2004;351:2611–2618. doi: 10.1056/NEJMoa041747. [DOI] [PubMed] [Google Scholar]
- 5.Clayton TC, Capps NE, Stephens NG, Wedzicha JA, Meade TW. Recent respiratory infection and the risk of myocardial infarction. Heart. 2005;91:1601–1602. doi: 10.1136/hrt.2004.046920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donaldson GC, Hurst JR, Smith CJ, Hubbard RB, Wedzicha JA. Increased risk of myocardial infarction and stroke following exacerbation of COPD. Chest. 2010;137:1091–1097. doi: 10.1378/chest.09-2029. [DOI] [PubMed] [Google Scholar]
- 7.Navaratnam V, Millett ER, Hurst JR, Thomas SL, Smeeth L, Hubbard RB, et al. Bronchiectasis and the risk of cardiovascular disease: a population-based study. Thorax. 2017;72:161–166. doi: 10.1136/thoraxjnl-2015-208188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herrett E, Gallagher AM, Bhaskaran K, Forbes H, Mathur R, van Staa T, et al. Data resource profile: Clinical Practice Research Datalink (CPRD) Int J Epidemiol. 2015;44:827–836. doi: 10.1093/ije/dyv098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhattarai N, Charlton J, Rudisill C, Gulliford MC. Coding, recording and incidence of different forms of coronary heart disease in primary care. PLoS One. 2012;7:e29776. doi: 10.1371/journal.pone.0029776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herrett E, Thomas SL, Schoonen WM, Smeeth L, Hall AJ. Validation and validity of diagnoses in the General Practice Research Database: a systematic review. Br J Clin Pharmacol. 2010;69:4–14. doi: 10.1111/j.1365-2125.2009.03537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou EHGK, Graham DJ, Ding Y, Levenson M, Rose M, Davillier SF, et al. Validation of stroke in the Clinical Practice Research Datalink (CPRD) [abstract] Pharmacoepidemiol Drug Saf. 2013;22:234. [Google Scholar]
- 12.Whitaker HJ, Hocine MN, Farrington CP. The methodology of self-controlled case series studies. Stat Methods Med Res. 2009;18:7–26. doi: 10.1177/0962280208092342. [DOI] [PubMed] [Google Scholar]
- 13.Millett ER, Quint JK, Smeeth L, Daniel RM, Thomas SL. Incidence of community-acquired lower respiratory tract infections and pneumonia among older adults in the United Kingdom: a population-based study. PLoS One. 2013;8:e75131. doi: 10.1371/journal.pone.0075131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Institute for Health and Care Excellence Pneumonia in adults: diagnosis and management 2014[accessed 2017 Jan 5]. Available from: https://www.nice.org.uk/guidance/cg191. [PubMed] [Google Scholar]
- 15.Rothnie KJ, Müllerová H, Hurst JR, Smeeth L, Davis K, Thomas SL, et al. Validation of the recording of acute exacerbations of COPD in UK primary care electronic healthcare records. PLoS One. 2016;11:e0151357. doi: 10.1371/journal.pone.0151357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petersen I, Douglas I, Whitaker H. Self controlled case series methods: an alternative to standard epidemiological study designs. BMJ. 2016;354:i4515. doi: 10.1136/bmj.i4515. [DOI] [PubMed] [Google Scholar]
- 17.Pratt NL, Roughead EE, Ramsay E, Salter A, Ryan P. Risk of hospitalization for stroke associated with antipsychotic use in the elderly: a self-controlled case series. Drugs Aging. 2010;27:885–893. doi: 10.2165/11584490-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 18.Farrington CP. Relative incidence estimation from case series for vaccine safety evaluation. Biometrics. 1995;51:228–235. [PubMed] [Google Scholar]
- 19.Hansell A, Hollowell J, Nichols T, McNiece R, Strachan D. Use of the General Practice Research Database (GPRD) for respiratory epidemiology: a comparison with the 4th Morbidity Survey in General Practice (MSGP4) Thorax. 1999;54:413–419. doi: 10.1136/thx.54.5.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lonni S, Chalmers JD, Goeminne PC, McDonnell MJ, Dimakou K, De Soyza A, et al. Etiology of non-cystic fibrosis bronchiectasis in adults and its correlation to disease severity. Ann Am Thorac Soc. 2015;12:1764–1770. doi: 10.1513/AnnalsATS.201507-472OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hill AT, Routh C, Welham S. National BTS bronchiectasis audit 2012: is the quality standard being adhered to in adult secondary care? Thorax. 2014;69:292–294. doi: 10.1136/thoraxjnl-2013-203739. [DOI] [PubMed] [Google Scholar]
- 22.Hill AT, Welham S, Reid K, Bucknall CE British Thoracic Society. British Thoracic Society national bronchiectasis audit 2010 and 2011. Thorax. 2012;67:928–930. doi: 10.1136/thoraxjnl-2012-201983. [DOI] [PubMed] [Google Scholar]
- 23.Evans IE, Bedi P, Quinn TM, Hill AT. Bronchiectasis severity is an independent risk factor for vascular disease in a bronchiectasis cohort. Chest. 2017;151:383–388. doi: 10.1016/j.chest.2016.09.022. [DOI] [PubMed] [Google Scholar]
- 24.Pasteur MC, Bilton D, Hill AT British Thoracic Society Bronchiectasis non-CF Guideline Group. British Thoracic Society guideline for non-CF bronchiectasis. Thorax. 2010;65:i1–i58. doi: 10.1136/thx.2010.142778. [DOI] [PubMed] [Google Scholar]
- 25.Warren-Gash C, Hayward AC, Hemingway H, Denaxas S, Thomas SL, Timmis AD, et al. Influenza infection and risk of acute myocardial infarction in England and Wales: a CALIBER self-controlled case series study. J Infect Dis. 2012;206:1652–1659. doi: 10.1093/infdis/jis597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corrales-Medina VF, Alvarez KN, Weissfeld LA, Angus DC, Chirinos JA, Chang C-CH, et al. Association between hospitalization for pneumonia and subsequent risk of cardiovascular disease. JAMA. 2015;313:264–274. doi: 10.1001/jama.2014.18229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harskamp RE, van Ginkel MW. Acute respiratory tract infections: a potential trigger for the acute coronary syndrome. Ann Med. 2008;40:121–128. doi: 10.1080/07853890701753672. [DOI] [PubMed] [Google Scholar]
- 28.Wedzicha JA, Seemungal TA, MacCallum PK, Paul EA, Donaldson GC, Bhowmik A, et al. Acute exacerbations of chronic obstructive pulmonary disease are accompanied by elevations of plasma fibrinogen and serum IL-6 levels. Thromb Haemost. 2000;84:210–215. [PubMed] [Google Scholar]
- 29.Patel ARC, Kowlessar BS, Donaldson GC, Mackay AJ, Singh R, George SN, et al. Cardiovascular risk, myocardial injury, and exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;188:1091–1099. doi: 10.1164/rccm.201306-1170OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gale NS, Bolton CE, Duckers JM, Enright S, Cockcroft JR, Shale DJ. Systemic comorbidities in bronchiectasis. Chron Respir Dis. 2012;9:231–238. doi: 10.1177/1479972312459973. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.