Abstract
Background
Biologic factors guide treatment decisions and have a significant impact on prognosis for breast cancer patients. This study was undertaken to develop a staging system incorporating biologic factors in addition to standard anatomic factors in the American Joint Committee on Cancer (AJCC) pathologic stage (PS) to assess disease-specific survival (DSS).
Methods
3,327 patients treated with surgery as an initial intervention at MD Anderson from 2007-2013 were identified. Multivariate analyses of factors including PS, T stage (T), nodal stage (N), grade (G), estrogen receptor (ER) status (E) and HER2 status (H) were performed to identify associations with DSS. A score of 0 to 4 was assigned for each factor by considering the hazard ratio magnitude. Multiple staging system models were then constructed: PS, PS+G, PS+G+E, PS+G+E+H, T+N, T+N+G, T+N+G+E, and T+N+G+E+H. Model performance was quantified using Harrell’s concordance index and Akaike Information Criterion (AIC) were used to compare model fits. Comparable cases from California (n=67,944) were used for validation.
Results
Median follow-up was 5.0 years (range, 0.1–8.8). Five-year DSS was 97.9% (95%CI:97.3%-98.4%). Models incorporating grade, ER status and HER2 status were most precise with identical C-index (0.81) and comparable AIC (994.9 for PS+G+E+H and 987.8 for T+N+G+E+H). Both models were externally validated.
Conclusion
These results confirm the importance of biologic factors in determining prognosis for breast cancer patients. We propose the Bioscore which incorporates grade, ER and HER2 status with AJCC PS to provide more refined stratification of breast cancer patients undergoing surgery as an initial intervention with respect to DSS.
Introduction
The goal of cancer staging systems is to inform prognosis and guide clinicians in designing an individual patient’s treatment plan. One staging system used for breast cancer patients is the American Joint Committee on Cancer (AJCC) system which is based on the tumor size (T), the presence or absence of lymph node involvement (N), and the presence or absence of distant metastasis (M). The TNM status for a patient is determined and this corresponds with a specific disease stage.1 Breast cancer patients are assigned a clinical stage at the time of diagnosis, then after surgery, the pathologic stage is determined by evaluation of the resected tumor and regional lymph nodes.
It is well accepted that tumor biologic features including grade, hormone receptor (HR) status, and HER2 status have predictive and prognostic value in breast cancer patients. Treatment recommendations are made largely based on HR and HER2 expression.2–5 Patient outcomes within each TNM stage therefore have wide variation with respect to survival based on biologic features. Recognizing this, the expert panel that was convened to develop the 8th edition of the AJCC staging system sought to incorporate biologic factors. The expert panel, which included one author of the current study (EAM), found that there are limited published data quantifying the impact of biologic factors on prognosis making it difficult to incorporate biologic factors into the staging system based on a lack of evidence.
Our group had previously recognized the limitations of a staging system based only on anatomy and had reported a novel staging system for predicting disease-specific survival (DSS) for patients treated with surgery as the initial intervention. This staging system incorporated grade and estrogen receptor (ER) status with pathologic stage to facilitate improved stratification with respect to DSS when compared to pathologic stage alone.6 The development of this staging system predated the routine use of trastuzumab in the adjuvant setting for HER2-positive patients which began in 2006. Because this staging system does not account for the favorable response of HER2-positive tumors to trastuzumab, it cannot be used to provide prognostic information for patients with HER2-positive breast cancer. The current study was therefore undertaken to update the staging system with a more contemporary cohort of patients treated at MD Anderson to include those with HER2-positive disease receiving trastuzumab, and to validate this staging system using a large cohort of patients reported to the population-based California Cancer Registry (CCR).
Patients and Methods
Patient population
A prospectively maintained database was used to identify 3,327 patients with non-metastatic invasive breast cancer who underwent surgery as a first intervention at MD Anderson from January 2007 through December 2013. None of these patients had been included in the development of the initial staging system incorporating biologic factors. Clinicopathologic data were recorded including: age, modified Black’s nuclear grade, ER status, HER2 status, and pathologic stage determined according to the seventh edition of the AJCC staging guidelines. Patients with incomplete data were excluded. Prior to 2010, tumors were classified as ER positive if there was >10% staining. A cut-off of 1% was used for patients treated after 2010, consistent with the change in American Society of Clinical Oncology (ASCO) guidelines.7 HER2 status was defined as positive if 3+ on immunohistochemistry or gene amplification was shown on fluorescence in situ hybridization. An external cohort was identified from the CCR including 67,944 patients diagnosed between 2005 and 2010 with a first primary non-metastatic breast cancer who underwent surgery as a first intervention with known grade, ER status, and HER2 status. CCR patients were followed for vital status through December 31, 2013.
Model Building
The clinical endpoint was DSS calculated from the date of diagnosis to the date of death from breast cancer. Patients not experiencing this endpoint were censored at last follow-up. Univariate and two multivariate analyses were performed to identify factors associated with DSS. The first multivariate analysis included pathologic T stage and pathologic N stage as separate variables. The second analysis used the AJCC pathologic stage, which takes into account the combined T and N stage, as a variable. A prognostic score of 0 to 4 was then assigned to each factor by considering the magnitude of the hazard ratio and defining cut-offs. Only independent predictors of DSS (P<.05) were assigned a score. For binary variables, the comparison group with a significant impact on DSS was assigned 1 point. For ordinal variables, the comparison groups which were determined to have a significant impact on DSS with a hazard ratio between 1.1 and 3 were assigned 1 point, variables determined to have a hazard ratio between 3.1 and 6 were assigned 2 points, variables with a hazard ratio between 6.1 and 10 were assigned 3 points, and variables with a hazard ratio more than 10 were assigned 4 points. An overall staging score was calculated by summing scores for the individual predictors of DSS.
Models were built to determine the utility of combining variables including T stage (T), N stage (N), pathologic stage (PS), grade (G), ER status (E) and HER2 status (H) in determining DSS. The first set of models used pathologic T and N stage as the backbone and included: T+N, T+N+G, T+N+G+E and T+N+G+E+H. The second set used PS as the backbone and included: PS, PS+G, PS+G+E and PS+G+E+H. Model performance was quantified using Harrell’s concordance index (C-index) which can range from perfect discordance (0.0) to perfect concordance (1.0).8 Akaike’s information criterion (AIC) was determined.9 AIC takes into account how well the model fits the data and the complexity of the model, thereby decreasing the risk of overfitting.
Applying the point values described above, prognostic scores and an overall staging score were calculated for the CCR data. Disease-specific survival was then modeled in the CCR validation data using the same combinations of prognostic variables.
Statistical analyses were performed using R 3.2.1 (http://www.r-project.org/). The institutional review boards at MD Anderson and the Cancer Prevention Institute of California approved this study.
Results
Clinicopathologic characteristics of the MD Anderson cohort are shown in table 1. Median follow-up time for the cohort was 5.0 years (range, 0.1–8.8 years). The estimated 5-year DSS rate for the entire cohort was 97.9% (95% CI:97.3%-98.4%). The results of univariate and multivariate analyses for clinicopathologic factors associated with DSS as well as points assigned for each predictor are shown in table 2. DSS, the C-index, and AIC for each proposed staging system are shown in figure 1. The two staging systems that included grade, ER status and HER2 status had the highest C-indexes (0.813 for PS+G+E+H and 0.811 for T+N+G+E+H) and the lowest AIC (993.9 for PS+G+E+H and 986.6 for T+N+G+E+H) indicating that the addition of the biologic factors of grade, ER status and HER2 status to anatomic factors facilitates improved stratification with respect to DSS. The estimated 5-year DSS determined using the AJCC PS ranged from 79.5% to 99.1%. In comparison, the estimated 5-year DSS rates determined using the PS+G+E+H system (Bioscore) ranged from 33.3% to 100%. The 5-year DSS outcomes by pathologic stage and Bioscore are shown in table 3.
Table 1.
Clinicopathologic factors for the MD Anderson Cohort (N=3,327)
| Variable | No. of Patients (%) |
|---|---|
|
| |
| Age year | |
| Mean | 58 |
| Median (range) | 57 (25-99) |
|
| |
| Pathologic T stage | |
| T1 | 2,013 (60.5%) |
| T2 | 1,125 (33.8%) |
| T3 | 189 (5.7%) |
|
| |
| Pathologic N stage | |
| N0 | 2,230 (67.1%) |
| N1mic | 180 (5.4%) |
| N1 | 726 (21.8%) |
| N2 | 123 (3.7%) |
| N3 | 65 (2.0%) |
|
| |
| Pathologic Stage | |
| I (A and B) | 1602 (48.1%) |
| IIA | 999 (30.0%) |
| IIB | 467 (14.1%) |
| IIIA | 194 (5.8%) |
| IIIC | 65 (2.0%) |
|
| |
| ER status | |
| Positive | 2,901 (87.2%) |
| Negative | 426 (12.8%) |
|
| |
| PR status | |
| Positive | 2491 (74.9%) |
| Negative | 836 (25.1%) |
|
| |
| HER2 status | |
| Positive | 306 (9.2%) |
| Negative | 3,021 (90.8%) |
|
| |
| Nuclear Grade | |
| 1 | 482 (14.5%) |
| 2 | 1,815 (54.5%) |
| 3 | 1,030 (31.0%) |
|
| |
| Adjuvant chemotherapy | |
| No | 1,651 (50.4%) |
| Yes | 1,624 (49.6%) |
|
| |
| Adjuvant hormonal therapy | |
| No | 612 (18.8%) |
| Yes | 2,648 (81.2%) |
Abbreviations: ER, estrogen receptor; PR, progesterone receptor
Table 2.
Univariate and Multivariate Analyses for Clinicopathologic Factors Associated with DSS in the MD Anderson Cohort
| Factor | 5-yr DSS (%) | Univariate Analysis | Multivariate Analysis 1 | Multivariate Analysis 2 | Points Assigned | |||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| HR | P | HR | P | HR | P | |||
|
| ||||||||
| Pathologic T Stage | ||||||||
| T1 | 98.9 | Referent | Referent | 0 | ||||
| T2 | 95.9 | 4.3 | <.0001 | 2.5 | .001 | 1 | ||
| T3 | 95.5 | 4.3 | .001 | 2.5 | .04 | 1 | ||
|
| ||||||||
| Pathologic N Stage | ||||||||
| N0 | 98.7 | Referent | Referent | 0 | ||||
| N1mic | 98.9 | 0.4 | .4 | .4 | 0.3 | 0 | ||
| N1 | 97.1 | 1.9 | .03 | 1.4 | 0.3 | 0 | ||
| N2 | 93.7 | 5.8 | <.0001 | 4.6 | <.0001 | 2 | ||
| N3 | 79.5 | 16.2 | <.0001 | 8.6 | <.0001 | 3 | ||
|
| ||||||||
| Pathologic Stage | ||||||||
| I (A and B) | 99.1 | Referent | Referent | 0 | ||||
| IIA | 98.0 | 2.8 | .002 | 2.3 | .01 | 1 | ||
| IIB | 95.6 | 4.8 | <.0001 | 4.0 | <.0001 | 2 | ||
| IIIA | 95.4 | 6.8 | <.0001 | 7.2 | <.0001 | 3 | ||
| IIIC | 79.5 | 26.6 | <.0001 | 19.9 | <.0001 | 4 | ||
|
| ||||||||
| ER Status | ||||||||
| Pos | 98.8 | Referent | Referent | Referent | 0 | |||
| Neg | 92.9 | 4.9 | <.0001 | 2.5 | .001 | 2.5 | .001 | 1 |
|
| ||||||||
| PR Status | ||||||||
| Pos | 98.8 | Referent | Referent | Referent | ||||
| Neg | 95.2 | 4.0 | <.0001 | NS | NS | |||
|
| ||||||||
| HER2 Status | ||||||||
| Pos | 97.5 | Referent | Referent | Referent | 0 | |||
| Neg | 98.0 | 0.8 | .5 | 2.2 | .03 | 2.2 | .04 | 1 |
|
| ||||||||
| Nuclear Grade | Referent | Referent | Referent | |||||
| 1 | 99.8 | 5.0 | 4.2 | 4.0 | 0 | |||
| 2 | 98.9 | 25.0 | .1 | 13.8 | .2 | 13.0 | .2 | 0 |
| 3 | 95.3 | .001 | .01 | .01 | 1 | |||
Abbreviations: DSS, disease-specific survival; ER, estrogen receptor; PR, progesterone receptor
Figure 1.
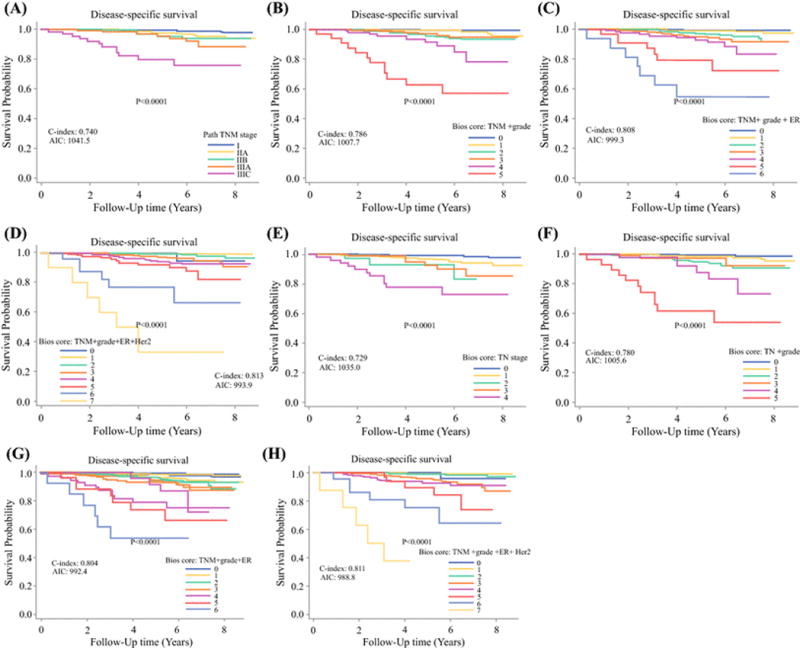
Kaplan-Meier survival plots with risk tables showing association between different staging systems and disease-specific survival in breast cancer patients treated at the MD Anderson Cancer Center with surgery as the first intervention. (A) American Joint Committee on Cancer pathologic stage (PS); (B) pathologic stage plus grade (PS+G); (C) pathologic stage plus grade plus estrogen receptor status (PS+G+E); (D) pathologic stage plus grade plus estrogen receptor status plus HER2 status (PS+E+G+H); (E) T stage (T) + N stage (N); (F) T stage plus N stage plus grade (T+N+G); (G) T stage plus N stage plus grade plus estrogen receptor status (T+N+G+E); (H) T stage plus N stage plus grade plus estrogen receptor status plus HER2 status (T+N+G+E+H). Log-rank test is shown for each comparison. C-index, Harrell’s concordance index; AIC, Akaike’s information criterion.
Table 3.
Five-year DSS outcomes by pathologic stage and Bioscore
| Pathologic Stage | DSS (95% CI) |
Bioscore | DSS (95% CI) |
|---|---|---|---|
| I(A and B) (n=1,602) |
99.1% (98.5%-99.5%) |
0 (n=36) |
100% |
| IIA (n=999) |
98.0% (96.5%-98.8%) |
1 (n=1,204) |
99.4% (98.8%-99.8% |
| IIB (n=467) |
95.6% (92.3%-97.5%) |
2 (n=919) |
99.2% (98.0%-99.7%) |
| IIIA (n=194) |
95.4% (89.7%-98.0%) |
3 (n=667) |
97.2% (95.2%-98.4%) |
| IIIC (n=65) |
79.5% (65.6%-88.2%) |
4 (n=339) |
94.2% (90.1%-96.7%) |
| 5 (n=129) |
92.0% (84.5%-96.0%) |
||
| 6 (n=23) |
77.3% (53.6%-89.9%) |
||
| 7 (n=10) |
33.3% (6.3%-64.6%) |
Abbreviations: DSS, disease-specific survival
Supplemental table 1 shows clinicopathologic characteristics of the California breast cancer patient cohort. The median follow-up time for this cohort was 5.3 years (range, 0.0-9.0 years), and the estimated 5-year DSS was 94.9% (95% CI: 94.7%-95.1%). In this external cohort, the addition of biologic tumor characteristics again facilitated stratification with respect to DSS compared to pathologic stage alone (Figure 2, supplemental table 2).
Figure 2.
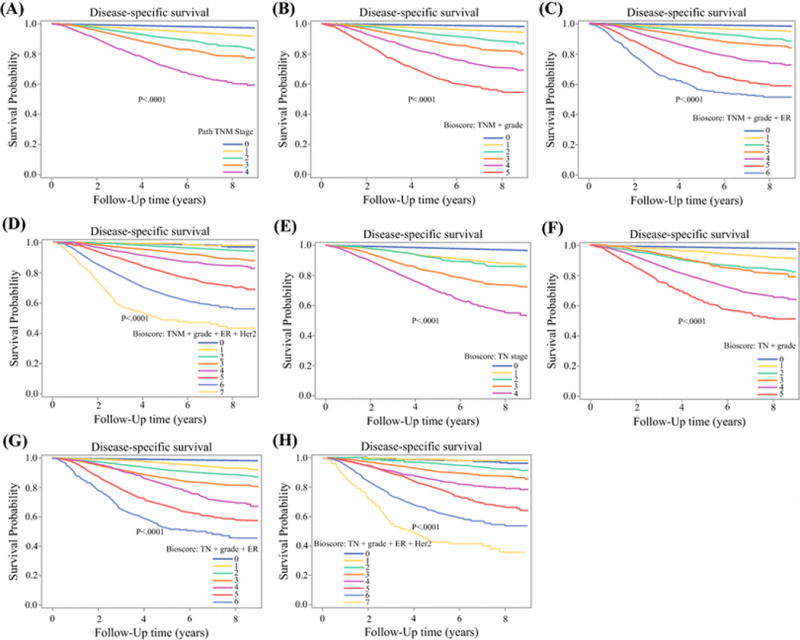
Kaplan-Meier survival plots with risk tables of disease-specific survival for patients in the external validation cohort from the California Cancer Registry. (A) American Joint Committee on Cancer pathologic stage (PS); (B) pathologic stage plus grade (PS+G); (C) pathologic stage plus grade plus estrogen receptor status (PS+G+E); (D) pathologic stage plus grade plus estrogen receptor status plus HER2 status (PS+E+G+H); (E) T stage (T) + N stage (N); (F) T stage plus N stage plus grade (T+N+G); (G) T stage plus N stage plus grade plus estrogen receptor status (T+N+G+E); (H) T stage plus N stage plus grade plus estrogen receptor status plus HER2 status (T+N+G+E+H). Log-rank test is shown for each comparison.
Discussion
The goals of the AJCC staging system include providing prognostic information for patients and facilitating a common language for physicians to communicate regarding a patient’s disease. The 7th edition of the AJCC system relied only on anatomic factors including primary tumor size and the presence or absence of lymph node or distant metastases. While anatomic factors are important in informing prognosis and guiding treatment options for breast cancer patients, biologic factors are routinely assessed and used to guide treatment decisions. Despite the widespread acceptance that biologic factors therefore impact prognosis, the expert panel convened to develop the AJCC 8th edition found limited published data quantifying that impact thus making it difficult to incorporate biologic factors into the staging system based on a lack of evidence. To address that, here we have shown that incorporation of the biologic factors of grade, ER status and HER2 status allows for refined stratification with respect to DSS for breast cancer patients treated with surgery as an initial intervention. Although the 8th edition of the AJCC staging did not formally incorporate the Bioscore, based on these and other data, the 8th edition of the breast cancer staging system, published in October 2016, includes an unchanged anatomic stage as well as a prognostic stage that incorporates biologic factors.1,10
In a previous study defining a staging system incorporating biologic factors in patients who underwent surgery as the initial intervention, we evaluated 3,728 patients treated between 1997 and 2006. We showed that incorporating grade and ER status along with pathologic stage defined a staging system that was more precise with respect to determining DSS than pathologic stage alone.6 A limitation of this previous work was that it predated the routine use of trastuzumab in patients with HER2-positive breast cancer. Multiple studies have shown that overexpression or amplification of HER2 in the primary tumor is associated with a worse prognosis in untreated patients11,12 and that treatment with trastuzumab improves outcomes in the metastatic, adjuvant and neoadjuvant settings.13–21 The Bioscore can range from 0 to 7 points with points assigned based on AJCC pathologic stage, grade, ER status and HER2 status. A lower score is associated with better DSS. Patients with HER2-positive tumors do not receive any points assigned for that variable whereas patients with HER2-negative disease have one point assigned reflecting that they are not expected to benefit from trastuzumab therapy. Similarly, patients with ER-negative tumors receive one point reflecting that they are not expected to benefit from administration of adjuvant endocrine therapy. Current ASCO guidelines recommend using biomarkers, specifically ER and HER2, to guide adjuvant therapy decisions.4 The Bioscore accounts for this clinical management of breast cancer to provide accurate prognostic information for patients treated with regimens targeting the underlying biology of their breast cancer.
The cohort used to define the Bioscore was from a single academic center and, although not all patients adhere to treatment recommendations, all patients with HER2-positive breast cancer received trastuzumab and all patients with HR-positive disease were advised to take endocrine therapy with over 90% accepting that recommendation. The fact that the Bioscore was developed at a single center however does represent one limitation of this study. Specifically, the majority of patients seen at MD Anderson during the study period with higher stage (stage III) disease, triple negative breast cancer, or HER2-positive breast cancer received neoadjuvant chemotherapy therefore are not included in the current cohort. This is reflected in the very favorable 5-year DSS rate of 98% for the entire cohort. In addition, 85% of patients in the MD Anderson cohort had a Bioscore of 0-3 with a corresponding 5-year DSS of greater than 97%, and 65% had a Bioscore ranging from 0-2 with a corresponding 5-year DSS of greater than 99%. Fewer patients had higher stage disease or triple negative tumors. However those patients did have higher Bioscores with worse prognosis, further supporting the concept of biologic factors providing additional, important information with respect to prognosis. Our group has also published a staging system, the Neo-Bioscore, which incorporates presenting clinical stage, final pathologic stage, grade, ER status and HER2 status to provide prognostic information for patients receiving neoadjuvant chemotherapy.22
Importantly, the Bioscore was validated with a cohort of 67,944 patients from the CCR suggesting broad applicability. When compared to the MD Anderson cohort, the CCR cohort had a higher percentage of patients with ER-positive and HER2-negative tumors. These patients would be more likely to have a lower bioscore. Therefore, in addition to confirming excellent separation of the curves for high risk patients with bioscores of 5, 6 or 7 as was seen in the MD Anderson cohort, the data from the California Cancer Registry also showed excellent separation of the curves for bioscores of 0 to 4 suggesting utility in stratifying these lower risk patients as well.
While the Bioscore represents a significant improvement over pathologic stage alone with respect to providing prognostic information, it too has limitations. In patients with the most favorable tumor biology, ER-positive, HER2-negative tumors, that are node negative, the most recent ASCO guidelines state that there is sufficient evidence of clinical utility for biomarker assays including Oncotype DX, EndoPredict, PAM50, Breast Cancer Index, and urokinase plasminogen activator and plasminogen activator inhibitor type 1.4 Using Oncotype DX as an example, studies performed on archival tumor samples showed that this assay provides prognostic information independent of other clinicopathologic features and that the Oncotype-DX recurrence score predicts benefit from chemotherapy.23–25 More recently, an initial report from a prospective trial evaluating the assay in HR-positive, HER2-negative, node negative patients, showed that in patients with a recurrence score of less than 11 who received endocrine therapy alone, the 5-year invasive DFS rate was 93.8%, thereby providing additional evidence of the clinical utility of the assay.26 Based on these data, the 8th edition prognostic stage categorizes any patient with a T1-2N0, ER-positive, HER2-negative and a recurrence score less than 11 as stage IA disease. With respect to the Bioscore, it is possible that two patients with ER+ tumors that are the same grade and pathologic stage could have different Oncotype DX recurrence scores could suggest different treatment recommendations as well as a different prognosis despite the same Bioscore. Data regarding the Oncotype DX score for patients included in the current study was not available for analysis therefore it is uncertain whether that data was used to inform adjuvant chemotherapy decisions. Future work could address the use of Oncotype DX, or other genomic assays, to further refine the Bioscore for patients with ER-positive, HER2-negative, node negative breast cancer.
In conclusion, we would suggest that data from the current study supports the recent modification of the AJCC staging system for breast cancer to include biologic features. The addition of grade, ER status and HER2 status as biologic modifiers to the AJCC staging system for breast cancer will facilitate more refined information regarding prognosis therefore will allow the staging system to retain its utility in clinical practice. A staging system based on anatomic factors alone limits the ability to fully understand prognosis or make treatment decisions, therefore failure to modify by incorporating biologic factors risks would have rendered the AJCC staging system obsolete.
Supplementary Material
Synopsis.
The Bioscore incorporates pathologic stage and biologic factors to stratify patients with respect to disease-specific survival (DSS). Increased discriminatory ability was demonstrated by variation in 5-year DSS from 33.3%-100% by Bioscore versus 79.5%–99.1% for pathologic stage. The model was validated using 67,944 cases from California.
Acknowledgments
The collection of cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract HHSN261201000140C awarded to the Cancer Prevention Institute of California, contract HHSN261201000035C awarded to the University of Southern California, and contract HHSN261201000034C awarded to the Public Health Institute; and the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under agreement U58dP003862-01 awarded to the California Department of Public Health. The ideas and opinions expressed herein are those of the authors and endorsement by the State of California, Department of Public Health and Nation Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors is not intended nor should be inferred.
This work was supported in part by a cancer center support grant from the National Cancer Institute to the University of Texas MD Anderson Cancer Center (CA016672). Analysis of cancer registry data was supported by the National Cancer Institute under contract HHSN261201000140C awarded to the Cancer Prevention Institute of California. Elizabeth A. Mittendorf is an R. Lee Clark Fellow of The University of Texas MD Anderson Cancer Center supported by the Jeanne F. Shelby Scholarship Fund. Sharon H. Giordano is supported by grants from the Cancer Prevention Research Institute of Texas (CPRIT RP140020) and the Komen for the Cure Foundation (SA150061).
Footnotes
Disclosures: The authors have no relevant financial disclosures
References
- 1.Amin MB, Edge SB, Greene F. AJCC Cancer Staging Manual. (8th) 2016 [Google Scholar]
- 2.Clinical Practice Guidelines in Oncology: Breast. National Comprehensive Cancer Network; 2016. [Google Scholar]
- 3.Coates AS, Winer EP, Goldhirsch A, et al. Tailoring therapies-improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol. 2015 Aug;26(8):1533–1546. doi: 10.1093/annonc/mdv221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harris LN, Ismaila N, McShane LM, et al. Use of Biomarkers to Guide Decisions on Adjuvant Systemic Therapy for Women With Early-Stage Invasive Breast Cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2016;34(10):1134–1150. doi: 10.1200/JCO.2015.65.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Poznak C, Somerfield MR, Bast RC, et al. Use of Biomarkers to Guide Decisions on Systemic Therapy for Women With Metastatic Breast Cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2015;33(24):2695–2704. doi: 10.1200/JCO.2015.61.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yi M, Mittendorf EA, Cormier JN, et al. Novel staging system for predicting disease-specific survival in patients with breast cancer treated with surgery as the first intervention: time to modify the current American Joint Committee on Cancer staging system. J Clin Oncol. 2011;29(35):4654–4661. doi: 10.1200/JCO.2011.38.3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28(16):2784–2795. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harrell FE, Jr, Califf RM, Pryor DB, Lee KL, Rosati RA. Evaluating the yield of medical tests. JAMA. 1982 May 14;247(18):2543–2546. [PubMed] [Google Scholar]
- 9.Akaike H. New look at statistical-model identification. IEEE Trans Automat Contr. 1974;19:716–723. [Google Scholar]
- 10.Giuliano AE, Connolly JL, Edge SB, et al. Updates to the AJCC Breast TNM Staging System: The 8th Edition. CA: A Cancer Journal for Clinicians. in press. [Google Scholar]
- 11.Paik S, Hazan R, Fisher ER, et al. Pathologic findings from the National Surgical Adjuvant Breast and Bowel Project: prognostic significance of erbB-2 protein overexpression in primary breast cancer. J Clin Oncol. 1990;8(1):103–112. doi: 10.1200/JCO.1990.8.1.103. [DOI] [PubMed] [Google Scholar]
- 12.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 13.Buzdar AU, Ibrahim NK, Francis D, et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol. 2005;23(16):3676–3685. doi: 10.1200/JCO.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 14.Buzdar AU, Suman VJ, Meric-Bernstam F, et al. Fluorouracil, epirubicin, and cyclophosphamide (FEC-75) followed by paclitaxel plus trastuzumab versus paclitaxel plus trastuzumab followed by FEC-75 plus trastuzumab as neoadjuvant treatment for patients with HER2-positive breast cancer (Z1041): a randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14(13):1317–1325. doi: 10.1016/S1470-2045(13)70502-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cobleigh MA, Vogel CL, Tripathy D, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17(9):2639–2648. doi: 10.1200/JCO.1999.17.9.2639. [DOI] [PubMed] [Google Scholar]
- 16.Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164–172. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 17.Gianni L, Eiermann W, Semiglazov V, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet J. 2010;375(9712):377–384. doi: 10.1016/S0140-6736(09)61964-4. [DOI] [PubMed] [Google Scholar]
- 18.Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(1):25–32. doi: 10.1016/S1470-2045(11)70336-9. [DOI] [PubMed] [Google Scholar]
- 19.Perez EA, Romond EH, Suman VJ, et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol. 2014;32(33):3744–3752. doi: 10.1200/JCO.2014.55.5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353(16):1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 21.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353(16):1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 22.Mittendorf EA, Vila J, Tucker SL, et al. The Neo-Bioscore Update for Staging Breast Cancer Treated With Neoadjuvant Chemotherapy: Incorporation of Prognostic Biologic Factors Into Staging After Treatment. JAMA Oncol. 2016;2(7):929–936. doi: 10.1001/jamaoncol.2015.6478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Albain KS, Barlow WE, Shak S, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol. 2010;11(1):55–65. doi: 10.1016/S1470-2045(09)70314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351(27):2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 25.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351(27):2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 26.Sparano JA, Gray RJ, Makower DF, et al. Prospective Validation of a 21-Gene Expression Assay in Breast Cancer. N Engl J Med. 2015;373(21):2005–2014. doi: 10.1056/NEJMoa1510764. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


