Abstract
CD8+ T cells and NK cells are both cytotoxic effector cells of the immune system, but the recognition, specificity, sensitivity, and memory mechanisms are drastically different. While many of these topics have been extensively studied in CD8+ T cells, very little is known about NK cells. Current cancer immunotherapies mainly focus on CD8+ T cells, but have many issues of toxicity and efficacy. Given the heterogeneous nature of cancer, personalized cancer immunotherapy that integrates the power of both CD8+ T cells in adaptive immunity and NK cells in innate immunity might be the future direction, along with precision targeting and effective delivery of tumor-specific, memory CD8+ T cells and NK cells.
Graphical Abstract
Introduction
With cancer incidence rates at an all-time high[1] and immunology research booming, the prospect of cancer immunotherapies is becoming a major topic of interest in biological and chemical engineering fields. The most widely studied cell type for cellular immunotherapy is the T cell, a central component of adaptive immunity. The advent of T-cell checkpoint inhibitors, such as anti-PD-1 and anti-CTLA4 therapies [2], and chimeric antigen receptor (CAR) T-cells, such as the recently FDA-approved CD19 CAR-T cell [3], has shifted the paradigm of cancer treatment to widely applicable therapy options. However, these therapeutic strategies may precipitate autoreactive T cell responses: checkpoint inhibitors override peripheral tolerance mechanisms, and CARs cross-react with healthy tissues. Many clinical studies have unfortunately fallen short of expectations; the nature of cancer causes it to generate large heterogeneities among patients and to mutate away from its immune attackers, resulting in non-response or relapse [4–6]. This has lead researchers to investigate the use of natural killer (NK) cells, another cytotoxic immune cell, for cancer therapy. In contrast to the single dominant T cell receptor (TCR) on T cells, NK cells have a wide array of activating and inhibitory receptors that act as a balance to determine functional activity, presenting an equally large collection of potential targets. Some of these receptors, such as Ly49C and KIR2DL1, recognize a “missing-self” status: the expression of appropriate number of major histocompatibility complex class I (MHC-1) molecules represents normal self-cells and elicits an inhibitory signal to NK cells. Downregulation of MHC-1 is often evolved in tumor cells as a mechanism of immune-evasion from T cells, which require MHC-1 signaling for activation, and therefore NK cell intervention could be used as a potent relapse therapy [7]. NK cells are now considered a bridge between innate and adaptive immunity, as it was discovered that NK cells gain memory functional phenotypes after encountering target cells [8–10], similar to T cells. In this review, we will compare and contrast two cytotoxic cells, CD8+ T cells in adaptive immunity and NK cells in innate immunity, and further discuss recent advances in cancer immunotherapy involving these two cells.
CD8+ T cells versus NK cells in Basic Immunology
Recognition
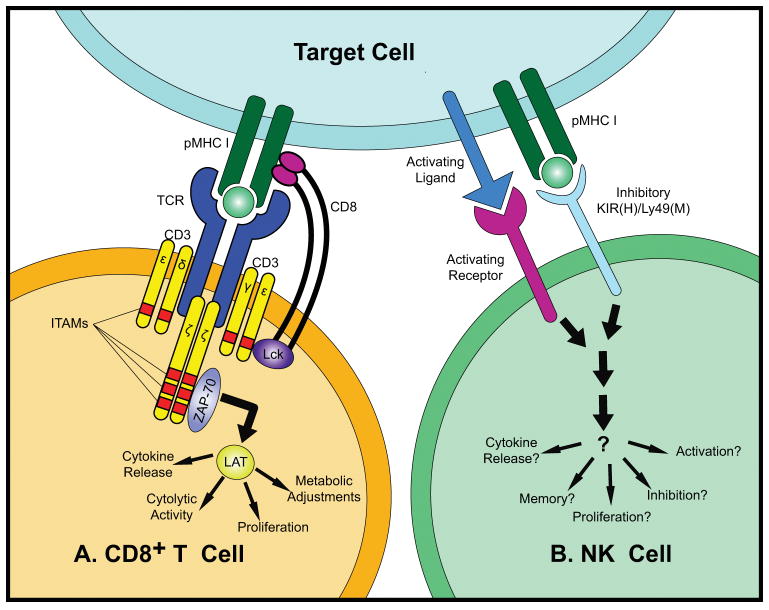
CD8+ T cells and NK cells have different mechanisms of target recognition and signaling cascades to achieve very similar goals: to kill infected and transformed cells. The antigen recognition by T cells has been extensively studied (Fig. 1A). CD8+ T cells use their T cell antigen receptors (TCRs) to recognize peptide-major histocompatibility complexes (pMHC) presented on the antigen-presenting cell surface [11]. The coreceptor CD8 assists the TCR recognition by binding to the same MHC-I molecule [12,13]. The association of TCR and CD8 with the pMHC triggers the phosphorylation of CD3 immunoreceptor tyrosine-based activation motifs (ITAMs) by Lck, a tyrosine kinase associated with the cytoplasmic region of CD8 [14]. The phosphorylated CD3 results in the recruitment and activation of ZAP-70, which in turn phosphorylates LAT. LAT kinase concatenates with TCR to facilitate signaling during activation [15]. LAT has a quite extensive signalosome, and transmits a myriad of cellular responses, including cytokine release and metabolic adjustments [14]. In addition to the TCR, a T cell has a number of accessory molecules including co-stimulatory and co- inhibitory receptors (Fig. 2A) [16]. These receptors together control the activation, differentiation and function of the T cell.
Figure 1. (A). T Cell Recognition and Signaling.
The TCR and CD8 bind a pMHC presented on the antigen-presenting cell surface, causing the phosphorylation of the ITAMs of the CD3 (γ, δ, ε and ζ) chains by Lck, a tyrosine kinase associated with the coreceptor CD8. The tyrosine kinase ZAP-70 is then recruited to CD3 by binding to the phosphorylated ITAMs, leading to the phosphorylation of ZAP-70 by Lck. The activated ZAP-70 then phosphorylates LAT. Activation of LAT leads to extensive cellular adjustments, including proliferation, metabolic changes, cytolytic activity, cytokine release, and others. (B). NK Cell Recognition and Signaling. NK cell surface activating and inhibitory receptor-ligand interactions mediate the recognition and signaling of an NK cell. Some receptors present on each NK cell are stochastic, whereas others such as NKp46 and NKG2D are constitutive. The combinatorial threshold that must be reached to activate or inactivate the NK cell is largely unknown.
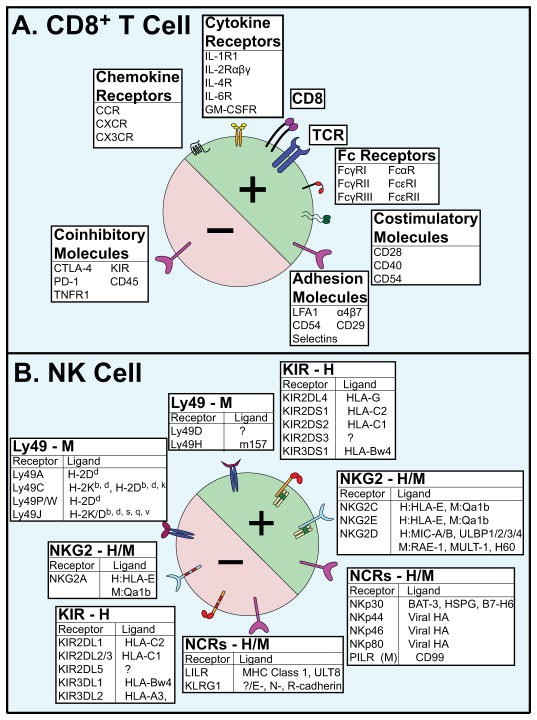
Figure 2. (A). Activating (+) and inhibitory (−) Cell Surface Molecules of CD8+ T Cells.
The TCR is responsible for antigen recognition. CD8 is a co-receptor to aid TCR antigen recognition. Fc Receptors (FcRs) are so named for being glycoproteins that bind the constant (Fc) region of immunoglobulins and transducing a signal. The Greek nomenclature denotes the class of immunoglobulin (α, γ, and ε,). Costimulatory and coinhibitory molecules are accessory molecules that enhance or diminish, respectively, the signal of the TCR. Adhesion molecules assist in bringing the target cell into tight contact with the CD8+ T cell. Chemokine receptors are G-protein coupled receptors (GPCRs) involved in chemotactic pathways such as migration and adhesion. These receptors are so named based on how many non-conserved residues separate the binding cysteines: CCR have two adjacent cysteines, whereas CX3CR have three residues between the two cysteines. Many of the receptors indicated here denote an entire family (e.g. CCR represents CCR1-8, differentially expressed on CD8+ subsets); all of these receptors have multiple possible ligands. Abbreviations: CD (Cluster of Differentiation); IL-_R (Interleukin _ Receptor); LFA (Leukocyte Function-Associated Antigen); CTLA (Cytotoxic T-Lymphocyte-Associated); KIR (Killer Immunoglobulin-like Receptor); PD (Programmed Death); TNFR (Tumor Necrosis Factor Receptor). (B). Activating (+) and Inhibitory (−) Cell Surface Molecules of NK Cells. NK cells express an array of activating and inhibitory receptors for recognition. Ly49 and KIR receptors are hypothesized to be a result of convergent evolution due to the presence of immunoreceptor tyrosine-based activation/inhibitor motifs (ITAMs/ITIMs, respectively). The KIR ligands are particular HLA molecules only expressed on distressed cells. Abbreviations: H (Human); M (Murine); GM-CSFR (Granulocyte Macrophage Colony Stimulating Factor Receptor); HLA (Human Leukocyte Antigen); MIC (MHC Class I Chain- related); HA (Hemagluttinin); PILR (Paired Ig-like Receptor); LILR (Leukocyte Immunoglobulin-like Receptor); KLRG1 (Killer Cell Lectin-like Receptor G1).
NK cell recognition is much less understood (Fig. 1B). In stark contrast to T cells, there is no single dominant receptor to mediate NK cell recognition. Rather, NK cells express an array of innate activating and inhibitory receptors (Fig. 2B) to sense their environment and respond to alterations caused by infections, stress and transformation [17]. Although it is generally believed that the balance between activating and inhibitory receptor engagements determines the activation of an NK cell, the molecular mechanism of NK cell recognition remains unclear and different models have been proposed [18–22]. The most well characterized model is the ‘missing-self’ mechanism, proposed by Klas Kärre in 1985 [7,23,24]. This mechanism describes NK cells recognition of the self-identifying MHC-I molecules by Ly49 family receptors to inhibit NK cell activation in mice. In primates, Ly49 is replaced by killer-cell immunoglobulin-like receptors (KIRs), which bind to HLA molecules to transduce inhibitory signal. The reduction or loss of MHC or HLA expression may lead to NK cell activation in a missing-self manner. In another recognition mechanism, antibody-dependent cellular cytotoxicity (ADCC), NK cell receptors FcγRIIIA and/or FcγRIIIC bind to the Fc portions of antibodies bound to a target cell, transduce an activating signals, and lead to NK cell activation [25]. Finally, the effector functions of NK cells can be enhanced by cytokines [17,26–29], and certain cytokine stimulations alone are sufficient to activate NK cells [30]. Other receptors involved in NK cell recognition include the NKG2 family, thought to regulate activity, and natural cytotoxicity receptors (NCRs), which are expressed in both humans and mice and may be regulated by cytokines. Therefore, while, many models of NK cell recognition have been proposed, these mechanisms most likely function synchronously, and more comprehensive research is needed to elucidate the recognition mechanism of NK cells.
Specificity, Sensitivity, and Speed
Antigen-specific targeting is essential for adaptive immunity. TCRs can discriminate closely related peptides, and even one amino acid change in the peptide can lead to distinct T cell responses [31–33]. T cells are incredibly sensitive in antigen detection – even a single pMHC is sufficient to trigger T cell calcium signaling and three pMHCs can lead to CD8+ T cells killing [34–36]. There are many models of T cell activation to explain the high specificity and sensitivity of T cells [37,38]. Part of this specificity and sensitivity arises from the binding structures of the pMHC-TCR interactions itself and the resulting kinetics, forces, and signals. The structure and signaling of the TCR and accessory molecules is shown in Figure 1A. It is not entirely clear why T cells have such a high specificity and sensitivity. In our opinion, the binding of a TCR to the pMHC may lead to a conformational change within the TCR. The conformational change enables the TCR to precisely decipher subtle structural differences among peptides and proportionally propagate the recognition signals to CD3 cytoplasmic domains via mechanical force, ensuring the extraordinary specificity [39–46]. Meanwhile, multiple TCRs serially engage with a single pMHC, which allows accumulation of enough stimulatory signals to reach the threshold of T-cell activation, accounting for the exquisite sensitivity [31,47–50]. However, direct experimental evidences with enough spatiotemporal resolution are needed to test such a hypothesis, and other mechanisms may be involved [37].
Like other aspects of NK cells, the specificity and sensitivity of NK cells are much less studied than those of T cells. The main theory for NK cell specificity is the sheer number of receptors on the NK cell surface, as NK cells transmit a range of responses rather than a simple dichotomy for specific recognition. This is hypothesized to be due to the integration of multiple activating and inhibitory signals received from target cells (Fig. 1B) [19,20]. In addition, NK cells are known to react to the environment; if a signal persists, NK cells will downregulate the response for that interaction. Thus far, no studies are able to determine the exact threshold of activation versus inhibition when an NK cell comes in contact with its target cell. For example, if a virus-infected cell is down-regulating its MHC I expression rather than ablating it, this is sufficient to activate an NK cell. How this changes if other inhibitory or activating ligands are also present has not yet been deciphered, nor how this response differs between NK cell subtypes. Quantitative analysis of NK cell specificity and sensitivity, though important, has not been established.
CD8+ T cells and NK cells response rates are also quite different. As one of the hallmarks of innate immunity, innate immune cells are the first responders to sites of infection. Therefore, NK cells are much quicker to establish a robust response than CD8+ T cells[8].
This is also exemplified by the NK cell ability to recruit T cells and other adaptive responders to sites of infection[51].
Memory
Once effector T cells and NK cells come in contact with a target cell that elicits a response, alterations must be made to this signal such that a secondary encounter with the same target cell may elicit a faster response [52]. This memory response is an evolutionary advantage, and works in different ways between T cells and NK cells.
Following exposure to target cells, CD8+ T cells undergo massive clonal expansion, followed by a contraction of about 95% [53]. The 5% of remaining CD8+ T cells then become memory T cells. Reinfection causes these memory cells to proliferate more rapidly than the initial naïve T cells [54]. Memory T cells are known to secrete more cytokines than effector T cells, and they also display different surface markers [52,55]. Memory T cells are further divided into central memory and effector memory based on the presence or absence of the CCR7 marker; CCR7− T cells are effector memory T cells, which have receptors to migrate to inflamed tissues and display immediate effector capabilities, whereas CCR7+ T cells are central memory T cells which instead stimulate dendritic cells before differentiating into CCR7− cells [56]. These subsets are further divided based on specific surface markers [57]. There are also evidences suggesting that cytokines and chemokines are required for the reactivation of memory cells, which may be secreted by supporting innate cells [58].
NK cells had been previously classified as innate immunity due to the quick response time and lack of somatic rearrangement of receptor genes, but they have since been found to possess certain qualities of adaptive immunity [8,29,59–64]. In a seminal study by the Lanier group, it was found that NK cells undergo phases of expansion, contraction, and retention similar to that of T cells [59]. This study also found that the retained cells at the end of the contraction resulted in a robust response following a secondary exposure to infection, indicating that these cells represent NK cell memory. Mechanistically, NK cell memory has been studied mostly in the murine cytomegalovirus (MCMV) model. One of these studies found that pro-inflammatory cytokines, like IL-12, IL-18, type I IFNs, and IFN-γ, are produced upon acute infection [65]. NK cells will then undergo a proliferation phase, although it is unclear whether this phase results in a heterogeneous population [61]. The contraction phase then results in long-lasting memory NK cells that have a profound response following a secondary encounter. It is not entirely clear whether NK cell memory is antigen-specific, though Lanier group has elegantly shown that NK cells can generate antigen-specific memory using the MCMV mouse model [59]. In addition, significant data have been collected suggesting a critical role of cytokines in the generation of memory cells [62]. Studies have shown that even cytokine activated NK cells can generate memory cells that remember polarizing cytokine signals [18]. However, further studies in real diseases such as cancer, as well as in humans, are needed to fully understand NK cell memory.
CD8+ T cells versus NK cells in Cancer Immunotherapies
Current Immunotherapies
CD8+ T cells play a critical role in current cancer immunotherapies (Table 1). For example, as tumors evolve, some cancer cells upregulate the expression of PD-L1, interacting with PD-1 on CD8+ T cells, suppressing T cell function and proliferation.
Table 1. Current Cancer Immunotherapies for CD8+ T Cells and NK Cells.
Abbreviations: NSCLC (Non-Small Cell Lung Cancer), SCLC (Small Cell Lung Cancer), RCC (Renal Cell Carcinoma), HNSCC (Head and Neck Squamous Cell Carcinoma), MCC (Merkel Cell Carcinoma), CLL (Chronic Lymphoblastic Leukemia), ALL (Acute Lymphoblastic Leukemia), AML (Acute Myeloid Lymphoma). [5,66,73,78,91,98,106,107,109,126,139–151].
| CD8+ Cell | NK Cell | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Cancer Type Targeted | Development Stage | Ref. | Cancer Type Targeted | Development Stage Ref. | |||
| Checkpoint Inhibitor | Checkpoint Inhibitor | ||||||
|
| |||||||
| Anti-PD-1 | Anti-KIR2D | ||||||
| Nivulumab | Melanoma, NSCLC, RCC | FDA-Approved | Sgambato 2016 | IPH2101 mAb | Myeloma | Pre-Clinical Research Carlsten 2016 | |
| Permbrolizumab | NSCLC, HNSCC, cHL | FDA-Approved | Sgambato 2016 | Others | Various | Phase I/II | Muntasell |
| Others | Various | Under Development | Kyi 2016 | Anti-NKG2D | 2017 | ||
| Anti-PD-L1 | Monalizumab | CLL, HNSCC | |||||
| Avelumab | MCC | FDA-Approved | Kaufman 2016 | Others | Various | Phase I/II | McWiliams |
| Atezolizumab | NSCLC | FDA-Approved | Fehrenbacher 2016 | Anti-KIR | 2016 Under Development Muntasell | ||
| Others | Various | Under Development | Swaika 2015 | Lirilumab | AML | 2017 | |
| Anti-CTLA-4 | Others | Various | |||||
| Ipilumumab | Melanoma (NSCLC, SCLC, bladder in trials) | FDA-Approved | Larkin 2015 | Anti-CD137 | Phase II | Alici 2010 | |
| Others | Various | Under Development | Kyi 2016 | Various | Various | Under Development | Muntasell 2017 |
| Phase I/II | Yonezawa 2015 | ||||||
|
| |||||||
| Cytokine Therapy | Cytokine Therapy | ||||||
|
| |||||||
| IL-2 Agonists | ALL, AML, Melanoma | FDA-Approved | Wu 2013 | Same as CD8+ T cells; these therapies affect both cell types | Guillerey 2016 | ||
| IL-15 Agonists | AML, RCC, Melanoma | Phase III | Pilipow 2015 | ||||
|
| |||||||
| CAR | CAR | ||||||
|
| |||||||
| CD-19 | CD-19 | ||||||
| Tisangenleceucel | B-Cell Lymphoma | FDA-Approved | Mueller 2017 | Cord Blood Derived | B-Cell Lymphoma | Phase I/II | Liu 2017 |
| Others | B-Cell Lymphoma | Under Development | Frigault 2016 | Others | ALL, CLL | Phase I/II | Rezvani 2017 |
| Universal CAR | Multiple | Pre-Clinical Research | Ren 2017 | CD33 | AML | Phase I/II | Rezvani 2017 |
| Other CARs | Various | Pre-Clinical Research | Frigault 2016 | Others | Various | Pre-Clinical | Rezvani 2017 |
|
| |||||||
| BiTEs | BiTEs | ||||||
|
| |||||||
| Blinatumomab | B-Cell Lymphoma | FDA-Approved | Topp 2015 | ? | |||
| BCMA/CD3 | Multiple Myeloma | Pre-Clinical Research | Hipp 2017 | ||||
| Others | Various | Under Development | Huehls 2015 | ||||
|
| |||||||
| MESA | MESA | ||||||
|
| |||||||
| Theoretical | Various | Pre-Clinical Research | Daringer 2014 | ? | |||
Question marks denote research yet to be done.
Checkpoint inhibitor anti-PD-1/PD-L1 therapy blocks this interaction, reinvigorating the killing function of CD8+ T cells [66–72]. Another checkpoint inhibitor, anti-CTLA4 antibody, prevents tumor induced T cell anergy [70,73–76]. Cytokine therapy, such as treatment with IL-2 or IFN-α, is another CD8+ T cell dependent treatment, which enhances local T cell activity [18,26,77,78]. Studies showed a positive correlation between the amount of CD8+ tumor infiltrating lymphocytes and progression-free survival with immunotherapy [2,66,68,69,72–74,79–87]. CAR-T cells utilize the cytotoxicity of CD8+ T cells to eradicate cancer [3–5,88]. CARs incorporate an extracellular programmable antigen-specific binding region with activating intracellular signaling components, such that recognition of a particular antigen expressed on a tumor cell will lead the killing by the CAR-T cell. CD19 CAR-T cell therapy has achieved gratifying success in hematological malignancies, including a recent FDA approval (FDA website: Kymriah) [3]. Other directions currently being explored are the following: include bi-specific T cell engagers [89,90], modular extracellular sensor architecture (MESA) receptors, and various applications of CRISPR- Cas9 (other CD8+ T cell therapies reviewed in [75]). MESA receptors are tunable surface receptors that contain easily exchangeable ligand binding and transcription factor domains; when bound to the ligand, the receptor cleaves the transcription factor so that it may enter the nucleus and elicit a cellular response [91]. CRISPR-Cas9 is being used to alter CD8+ T cell functions in various ways; for example, it is used to introduce engineered TCRs or CARs into T cells [92].
NK cell cancer immunotherapies are only recently being considered. Data have shown that NK cells have many anti-tumor capabilities [27,93]. Currently, CAR-NK cells are being engineered with the same CD3ζ chain as CAR-T cells, with similar targets (CD19, CD20, or others) via retroviral-based transduction or plasmid electroporation transfection [94]. One of the advantages of developing CAR-NK cells over CAR-T cells is that NK cells actually inhibit graft vs. host disease (GvHD), and therefore may confer greater safety than T cells [94–101]. The Rezvani group showed that NK cells could be harvested from cord blood and developed into CAR-NK cells that can be readily available “off the shelf” rather than individually tailored [98]. In addition, NK cells are present in greater numbers in peripheral blood than T cells are, making them more readily available for harvest for therapy [99]. The main limitation of current CAR-NK strategies is that they are not designed for NK cells; rather, they simply borrow the concepts of CAR-T and only use NK cells as a surrogate of T cells. The true breakthrough in developing effective CAR-NK requires a good understanding of the NK cell recognition mechanism to design genuine NK-based CAR therapies. Antibody- based therapies are being engineered such that the Fc portion can more tightly bind to FcγRIIIA to induce a more robust ADCC activation targeted to tumor cells[25,102,103]. Finally, NK cells have been shown to be involved in the T cell checkpoint inhibitor responses [102,104,105], and the first NK cell checkpoint inhibitors [106–108] and CARs [109] are now in clinical trials.
Challenges in Cancer Immunotherapy
One of the major challenges of current immunotherapies is that neither checkpoint inhibitors nor CAR T-cells are tumor-specific; checkpoint inhibitors will have a full body response and can lead to autoimmune disease, and CARs have on-target but off-tumor effects. For example, anti-PD-1 and anti-CTLA-4 antibodies universally target all T cells expressing PD-1 and CTLA-4, causing off-target effects and may lead to the development of autoimmune diseases. The response rate of checkpoint inhibitors is also low at less than 20%, which, while this is significant compared to other cancer therapies, is far from ideal [2,110]. Many studies are looking for biomarkers to divide responders from non- responders, but there will still be a large cohort for whom these therapies do not work.
Non-tumor specific targeting is also a significant problem of CAR T cells. For example, CD19 CAR-T cells not only kill malignant B cells but also eliminate healthy B cells, which are essential in antibody generation [111,112]. In addition, CAR-T cell treatment faces the challenges of low T-cell proliferation [113,114], constant tumor mutation [115], and frequent tumor relapse [116,117]. Another major limitation of CAR-T cells is the ineffectiveness to solid tumors [4,118,119], although a few studies show promising outcomes [120–123]. Possible reasons could be the physical barrier and immunosuppressive microenvironment of solid tumors that prevent lymphocyte infiltration and survival. In addition, there are many associated toxicities, including neurological toxicity, cytokine release syndrome, anaphylaxis, and GvHD, which requires T cells to be HLA matched to the patient [4,124,125]. Many CAR-T clinical trials have been terminated due to patient death [4]. Groups are attempting to make a ‘universal’ CAR-T cell that erases the need for such transfer mechanisms, but these still are only targeting a few selected tumor antigens, which the cancer will soon evolve around [126]. Finally, the exact mechanism of action of CAR-T cells is not well understood; it is known that both CD4+ and CD8+ CAR-T cells are required for tumor suppression [126], but not why, and it is also known that CAR-T cells have much higher affinity for their targets and therefore become exhausted much more quickly than endogenous T cells. These mechanisms require more attention to remedy the resulting effects.
Specific challenges for NK cell therapy have yet to be identified, mostly due to the lack of research and application of NK cell based immunotherapies. One of the known challenges is the low transfection efficiency and the variable expansion process of NK cells compared to T cells [127].
Future Cancer Immunotherapy
From a science perspective, a comprehensive and systematic understanding of the complex immune system and tumor microenvironment must be acquired, as effective and precise therapies will rely on the identification of the exact mechanisms in each cancer type, as well as the unique characteristics of individual patients. While some mechanisms have been elucidated in CD8+ T cells, much work remains to be done in NK cells [22,93,128–131]. In our opinion, personalized cancer immunotherapy is the future, considering each tumor and each patient is different. However, personalized medicine is currently both incredibly expensive and time consuming. Future biotechnologies and visualization techniques will hopefully allow for quick, inexpensive, and comprehensive analysis of patient tumors to better screen for potential candidates, identify the targetable mutations, and generate personalized treatments; we need to know who will benefit from these treatments, know which treatments will be most effective for each patient, and be able to synthesize that personalized treatment accurately, efficiently, and inexpensively.
Clinically fast, effective, economic immunotherapies personalized to each cancer type should be created. Ideally, a theoretical solution could be to engineer a tunable cell to specifically combat tumor evolution. For example, NK cells could be engineered to express a specific ratio of surface receptors such that they specifically target a tumor. Alternatively, a CAR or TCR-engineered T cell could be created such that the antigen-recognition piece could be easily, quickly, and inexpensively swapped out to keep up with the evolving tumor as a personalized medicine technique. As technologies advance synchronously with our improving understanding, these feats may be monetarily feasible in the future. Another direction that should be taken is the combination of these therapies. While results are largely unimpressive for each therapy individually, it could be more effective to combine therapies in a manner reminiscent of the drug cocktails used to control HIV [132]. By incorporating aspects of both the innate and adaptive immune systems in a precisely target-specific manner, for example, combinatorial T cell and NK cell treatments could eradicate the cancer by beating the tumor evolution.
Conclusions
One central question in cancer immunology is why both CD8+ T cells in adaptive immunity and NK cells in innate immunity fail to recognize and attack the forming tumors. A theory for CD8+ T cells is that the CD4+ regulatory T cells have heightened activity in the hypoxic environment of the tumor, leading to increased suppression of the CD8+ cytolytic activity [133]. The discontinuity theory [134] states that while NK cells recognize the initial change in cell identity of tumor cells, the prolonged exposure (perseverance of the tumor) will cause desensitization and tolerance [135]. Additionally, it was recently shown that tumors might be converting NK effector cells into type 1 innate lymphoid cells [136]. Regardless, T- cell based immunotherapies such as checkpoint inhibitors have shown preliminary success in cancer treatments [68,74,78,82,90]. However, relapse from these therapies is generally the result of tumor down-regulation of MHCs, which could be combatted with a subsequent NK cell therapy. In addition, further understanding of memory development for both cell types may potentiate the ability to create a life-long single-dose treatment that could remain in cellular memory for an extended period of time. The development NK therapies could therefore have huge implications both as independent therapies and as a relapse treatment following T cell therapies. Before these therapies can be developed, however, it is imperative that we discover the precise molecular mechanisms of recognition, activation, specificity, effector function, and memory of these cells, as well as characterize the complex interactions among immune cells, in order to truly progress the field of cancer immunotherapy [51,137,138]. In conclusion, CD8+ T cells and NK cells share many similarities in overall functions and cytolytic activities, but also have many differences that could be utilized advantageously in the treatment of cancer.
Acknowledgments
This work was supported by NIH grants R00AI106941 and R21AI120010, NSF Career Award 1653782, and Young Investigator Award from the Cancer Research Foundation (To J.H.). J.R. is supported by NCI T32 CA009594 at the University of Chicago through the Committee on Cancer Biology. We would also like to acknowledge the advice and guidance from Dr. Barbara Kee (University of Chicago) for her NK cell expertise.
Footnotes
Conflicts of interest
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Recommended Reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 3.Gill S, June CH. Going viral: chimeric antigen receptor T-cell therapy for hematological malignancies. Immunol Rev. 2015;263:68–89. doi: 10.1111/imr.12243. [DOI] [PubMed] [Google Scholar]
- 4.Bonifant CL, Jackson HJ, Brentjens RJ, Curran KJ. Toxicity and management in CAR T-cell therapy. Mol Ther Oncolytics. 2016;3:16011. doi: 10.1038/mto.2016.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5•.Frigault MJ, Maus MV. Chimeric antigen receptor-modified T cells strike back. Int Immunol. 2016;28:355–363. doi: 10.1093/intimm/dxw018. Review describing current and past CAR-T cells, their efficacy, and room for improvement. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Resetca D, Neschadim A, Medin JA. Engineering Hematopoietic Cells for Cancer Immunotherapy: Strategies to Address Safety and Toxicity Concerns. J Immunother. 2016;39:249–259. doi: 10.1097/CJI.0000000000000134. [DOI] [PubMed] [Google Scholar]
- 7.Kärre K. Role of target histocompatibility antigens in regulation of natural killer activity: a reevaluation and a hypothesis. Mechanisms of Cytotoxicity by NK Cells. 1985:81–91. [Google Scholar]
- 8.Sun JC, Lanier LL. Natural killer cells remember: an evolutionary bridge between innate and adaptive immunity? European journal of immunology. 2009;39:2059–2064. doi: 10.1002/eji.200939435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lanier LL, Sun JC. Do the terms innate and adaptive immunity create artificial conceptual barriers? Nature reviews Immunology. 2009;9:302. doi: 10.1038/nri2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bezman NA, Kim CC, Sun JC, Min-Oo G, Hendricks DW, Kamimura Y, Best JA, Goldrath AW, Lanier LL Immunological Genome Project C. Molecular definition of the identity and activation of natural killer cells. Nat Immunol. 2012;13:1000–1009. doi: 10.1038/ni.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jorgensen JL, Reay PA, Ehrich EW, Davis MM. Molecular components of T-cell recognition. Annu Rev Immunol. 1992;10:835–873. doi: 10.1146/annurev.iy.10.040192.004155. [DOI] [PubMed] [Google Scholar]
- 12.Li Y, Yin Y, Mariuzza RA. Structural and biophysical insights into the role of CD4 and CD8 in T cell activation. Front Immunol. 2013;4:206. doi: 10.3389/fimmu.2013.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis MM, Bjorkman PJ. T-cell antigen receptor genes and T-cell recognition. Nature. 1988;334:395–402. doi: 10.1038/334395a0. [DOI] [PubMed] [Google Scholar]
- 14.Malissen B, Gregoire C, Malissen M, Roncagalli R. Integrative biology of T cell activation. Nat Immunol. 2014;15:790–797. doi: 10.1038/ni.2959. [DOI] [PubMed] [Google Scholar]
- 15.Lillemeier BF, Mortelmaier MA, Forstner MB, Huppa JB, Groves JT, Davis MM. TCR and Lat are expressed on separate protein islands on T cell membranes and concatenate during activation. Nat Immunol. 2010;11:90–96. doi: 10.1038/ni.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13:227–242. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pegram HJ, Andrews DM, Smyth MJ, Darcy PK, Kershaw MH. Activating and inhibitory receptors of natural killer cells. Immunol Cell Biol. 2011;89:216–224. doi: 10.1038/icb.2010.78. [DOI] [PubMed] [Google Scholar]
- 18.Romee R, Schneider SE, Leong JW, Chase JM, Keppel CR, Sullivan RP, Cooper MA, Fehniger TA. Cytokine activation induces human memory-like NK cells. Blood. 2012;120:4751–4760. doi: 10.1182/blood-2012-04-419283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19•.Brodin P, Karre K, Hoglund P. NK cell education: not an on-off switch but a tunable rheostat. Trends Immunol. 2009;30:143–149. doi: 10.1016/j.it.2009.01.006. First paper to propose the idea that NK cells are not simply activated or inactivated, but rather there can be an array of responses according to the receptors that are engaged. [DOI] [PubMed] [Google Scholar]
- 20.Brodin P, Lakshmikanth T, Johansson S, Karre K, Hoglund P. The strength of inhibitory input during education quantitatively tunes the functional responsiveness of individual natural killer cells. Blood. 2009;113:2434–2441. doi: 10.1182/blood-2008-05-156836. [DOI] [PubMed] [Google Scholar]
- 21.Hoglund P, Brodin P. Current perspectives of natural killer cell education by MHC class I molecules. Nat Rev Immunol. 2010;10:724–734. doi: 10.1038/nri2835. [DOI] [PubMed] [Google Scholar]
- 22.Moretta L, Montaldo E, Vacca P, Del Zotto G, Moretta F, Merli P, Locatelli F, Mingari MC. Human natural killer cells: origin, receptors, function, and clinical applications. Int Arch Allergy Immunol. 2014;164:253–264. doi: 10.1159/000365632. [DOI] [PubMed] [Google Scholar]
- 23.Kärre K. On the Immunobiology of Natural Killer Cells. Karolinska Institute. 1981 [Google Scholar]
- 24.Kärre K. Natural killer cell recognition of missing self. Nature immunology. 2008;9:477–480. doi: 10.1038/ni0508-477. [DOI] [PubMed] [Google Scholar]
- 25.Wang W, Erbe AK, Hank JA, Morris ZS, Sondel PM. NK Cell-Mediated Antibody-Dependent Cellular Cytotoxicity in Cancer Immunotherapy. Front Immunol. 2015;6:368. doi: 10.3389/fimmu.2015.00368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takaki R, Hayakawa Y, Nelson A, Sivakumar PV, Hughes S, Smyth MJ, Lanier LL. IL-21 enhances tumor rejection through a NKG2D-dependent mechanism. Journal of Immunology. 2005;175:2167–2173. doi: 10.4049/jimmunol.175.4.2167. [DOI] [PubMed] [Google Scholar]
- 27.Wu J, Lanier LL. Natural killer cells and cancer. Adv Cancer Res. 2003;90:127–156. doi: 10.1016/s0065-230x(03)90004-2. [DOI] [PubMed] [Google Scholar]
- 28.Hesslein DG, Palacios EH, Sun JC, Beilke JN, Watson SR, Weiss A, Lanier LL. Differential requirements for CD45 in NK-cell function reveal distinct roles for Syk-family kinases. Blood. 2011;117:3087–3095. doi: 10.1182/blood-2010-06-292219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marcus A, Raulet DH. Evidence for natural killer cell memory. Curr Biol. 2013;23:R817–820. doi: 10.1016/j.cub.2013.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keppel MP, Yang L, Cooper MA. Murine NK cell intrinsic cytokine-induced memory-like responses are maintained following homeostatic proliferation. J Immunol. 2013;190:4754–4762. doi: 10.4049/jimmunol.1201742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang J, Zarnitsyna VI, Liu B, Edwards LJ, Jiang N, Evavold BD, Zhu C. The kinetics of two-dimensional TCR and pMHC interactions determine T-cell responsiveness. Nature. 2010;464:932–936. doi: 10.1038/nature08944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kersh GJ, Kersh EN, Fremont DH, Allen PM. High- and low-potency ligands with similar affinities for the TCR: the importance of kinetics in TCR signaling. Immunity. 1998;9:817–826. doi: 10.1016/s1074-7613(00)80647-0. [DOI] [PubMed] [Google Scholar]
- 33.Alam SM, Travers PJ, Wung JL, Nasholds W, Redpath S, Jameson SC, Gascoigne NR. T-cell-receptor affinity and thymocyte positive selection. Nature. 1996;381:616–620. doi: 10.1038/381616a0. [DOI] [PubMed] [Google Scholar]
- 34.Irvine DJ, Purbhoo MA, Krogsgaard M, Davis MM. Direct observation of ligand recognition by T cells. Nature. 2002;419:845–849. doi: 10.1038/nature01076. [DOI] [PubMed] [Google Scholar]
- 35.Purbhoo MA, Irvine DJ, Huppa JB, Davis MM. T cell killing does not require the formation of a stable mature immunological synapse. Nat Immunol. 2004;5:524–530. doi: 10.1038/ni1058. [DOI] [PubMed] [Google Scholar]
- 36••.Huang J, Brameshuber M, Zeng X, Xie J, Li QJ, Chien YH, Valitutti S, Davis MM. A single peptide-major histocompatibility complex ligand triggers digital cytokine secretion in CD4(+) T cells. Immunity. 2013;39:846–857. doi: 10.1016/j.immuni.2013.08.036. First paper to describe that multiple pMHC are not necessary to induce a full T cell response. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chakraborty AK, Weiss A. Insights into the initiation of TCR signaling. Nat Immunol. 2014;15:798–807. doi: 10.1038/ni.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van der Merwe PA, Dushek O. Mechanisms for T cell receptor triggering. Nat Rev Immunol. 2010;11:47–55. doi: 10.1038/nri2887. [DOI] [PubMed] [Google Scholar]
- 39.Li YC, Chen BM, Wu PC, Cheng TL, Kao LS, Tao MH, Lieber A, Roffler SR. Cutting Edge: mechanical forces acting on T cells immobilized via the TCR complex can trigger TCR signaling. J Immunol. 2010;184:5959–5963. doi: 10.4049/jimmunol.0900775. [DOI] [PubMed] [Google Scholar]
- 40.Gil D, Schamel WW, Montoya M, Sanchez-Madrid F, Alarcon B. Recruitment of Nck by CD3 epsilon reveals a ligand-induced conformational change essential for T cell receptor signaling and synapse formation. Cell. 2002;109:901–912. doi: 10.1016/s0092-8674(02)00799-7. [DOI] [PubMed] [Google Scholar]
- 41.Lanzavecchia A, Iezzi G, Viola A. From TCR engagement to T cell activation: a kinetic view of T cell behavior. Cell. 1999;96:1–4. doi: 10.1016/s0092-8674(00)80952-6. [DOI] [PubMed] [Google Scholar]
- 42.Giannone G, Sheetz MP. Substrate rigidity and force define form through tyrosine phosphatase and kinase pathways. Trends in cell biology. 2006;16:213–223. doi: 10.1016/j.tcb.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 43.Kim ST, Takeuchi K, Sun Z-YJ, Touma M, Castro CE, Fahmy A, Lang MJ, Wagner G, Reinherz EL. The αβ T cell receptor is an anisotropic mechanosensor. Journal of Biological Chemistry. 2009;284:31028–31037. doi: 10.1074/jbc.M109.052712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dustin ML. Cell adhesion molecules and actin cytoskeleton at immune synapses and kinapses. Curr Opin Cell Biol. 2007;19:529–533. doi: 10.1016/j.ceb.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu C, Gagnon E, Call ME, Schnell JR, Schwieters CD, Carman CV, Chou JJ, Wucherpfennig KW. Regulation of T cell receptor activation by dynamic membrane binding of the CD3epsilon cytoplasmic tyrosine-based motif. Cell. 2008;135:702–713. doi: 10.1016/j.cell.2008.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuhns MS, Davis MM, Garcia KC. Deconstructing the form and function of the TCR/CD3 complex. Immunity. 2006;24:133–139. doi: 10.1016/j.immuni.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 47.Valitutti S, Muller S, Cella M, Padovan E, Lanzavecchia A. Serial triggering of many T-cell receptors by a few peptide-MHC complexes. Nature. 1995;375:148–151. doi: 10.1038/375148a0. [DOI] [PubMed] [Google Scholar]
- 48.Xie J, Huppa JB, Newell EW, Huang J, Ebert PJ, Li QJ, Davis MM. Photocrosslinkable pMHC monomers stain T cells specifically and cause ligand-bound TCRs to be ‘preferentially’ transported to the cSMAC. Nat Immunol. 2012;13:674–680. doi: 10.1038/ni.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Valitutti S. The Serial Engagement Model 17 Years After: From TCR Triggering to Immunotherapy. Front Immunol. 2012;3:272. doi: 10.3389/fimmu.2012.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.San Jose E, Borroto A, Niedergang F, Alcover A, Alarcon B. Triggering the TCR complex causes the downregulation of nonengaged receptors by a signal transduction-dependent mechanism. Immunity. 2000;12:161–170. doi: 10.1016/s1074-7613(00)80169-7. [DOI] [PubMed] [Google Scholar]
- 51•.Crouse J, Xu HC, Lang PA, Oxenius A. NK cells regulating T cell responses: mechanisms and outcome. Trends Immunol. 2015;36:49–58. doi: 10.1016/j.it.2014.11.001. First paper to report evidence for an innate immune cell regulating an adaptive immune cell. [DOI] [PubMed] [Google Scholar]
- 52.Dutton RW, Bradley LM, Swain SL. T cell memory. Annu Rev Immunol. 1998;16:201–223. doi: 10.1146/annurev.immunol.16.1.201. [DOI] [PubMed] [Google Scholar]
- 53.Blattman JN, Antia R, Sourdive DJ, Wang X, Kaech SM, Murali-Krishna K, Altman JD, Ahmed R. Estimating the precursor frequency of naive antigen-specific CD8 T cells. J Exp Med. 2002;195:657–664. doi: 10.1084/jem.20001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kaech SM, Wherry EJ. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity. 2007;27:393–405. doi: 10.1016/j.immuni.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gerritsen B, Pandit A. The memory of a killer T cell: models of CD8(+) T cell differentiation. Immunol Cell Biol. 2016;94:236–241. doi: 10.1038/icb.2015.118. [DOI] [PubMed] [Google Scholar]
- 56.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 57.Mahnke YD, Brodie TM, Sallusto F, Roederer M, Lugli E. The who’s who of T-cell differentiation: human memory T-cell subsets. Eur J Immunol. 2013;43:2797–2809. doi: 10.1002/eji.201343751. [DOI] [PubMed] [Google Scholar]
- 58.Lauvau G, Soudja SMH. Springer, editor. Crossroads Between Innate and Adaptive Immunity V. 2015. Mechanisms of Memory T Cell Activation and Effective Immunity; pp. 73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457:557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Paust S, von Andrian UH. Natural killer cell memory. Nat Immunol. 2011;12:500–508. doi: 10.1038/ni.2032. [DOI] [PubMed] [Google Scholar]
- 61.O’Sullivan TE, Sun JC, Lanier LL. Natural killer cell memory. Immunity. 2015;43:634–645. doi: 10.1016/j.immuni.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cerwenka A, Lanier LL. Natural killer cell memory in infection, inflammation and cancer. Nat Rev Immunol. 2016;16:112–123. doi: 10.1038/nri.2015.9. [DOI] [PubMed] [Google Scholar]
- 63.Sun JC, Lopez-Verges S, Kim CC, DeRisi JL, Lanier LL. NK cells and immune “memory”. J Immunol. 2011;186:1891–1897. doi: 10.4049/jimmunol.1003035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sun JC, Lanier LL. Versatility in NK cell memory. Immunol Cell Biol. 2011;89:327–329. doi: 10.1038/icb.2010.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Biron CA, Tarrio ML. Immunoregulatory cytokine networks: 60 years of learning from murine cytomegalovirus. Med Microbiol Immunol. 2015;204:345–354. doi: 10.1007/s00430-015-0412-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sgambato A, Casaluce F, Sacco PC, Palazzolo G, Maione P, Rossi A, Ciardiello F, Gridelli C. Anti PD-1 and PDL-1 Immunotherapy in the Treatment of Advanced Non-Small Cell Lung Cancer (NSCLC): A Review on Toxicity Profile and its Management. Curr Drug Saf. 2016;11:62–68. doi: 10.2174/1574886311207040289. [DOI] [PubMed] [Google Scholar]
- 67.Li K, Cheng X, Tilevik A, Davis SJ, Zhu C. In situ and in silico kinetic analyses of programmed cell death-1 (PD-1) receptor, programmed cell death ligands, and B7–1 protein interaction network. J Biol Chem. 2017;292:6799–6809. doi: 10.1074/jbc.M116.763888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mahoney KM, Freeman GJ, McDermott DF. The Next Immune-Checkpoint Inhibitors: PD-1/PD-L1 Blockade in Melanoma. Clin Ther. 2015;37:764–782. doi: 10.1016/j.clinthera.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gros A, Robbins PF, Yao X, Li YF, Turcotte S, Tran E, Wunderlich JR, Mixon A, Farid S, Dudley ME, et al. PD-1 identifies the patient-specific CD8(+) tumor-reactive repertoire infiltrating human tumors. J Clin Invest. 2014;124:2246–2259. doi: 10.1172/JCI73639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fedorov VD, Themeli M, Sadelain M. PD-1- and CTLA-4-based inhibitory chimeric antigen receptors (iCARs) divert off-target immunotherapy responses. Sci Transl Med. 2013;5:215ra172. doi: 10.1126/scitranslmed.3006597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ghiotto M, Gauthier L, Serriari N, Pastor S, Truneh A, Nunes JA, Olive D. PD-L1 and PD- L2 differ in their molecular mechanisms of interaction with PD-1. Int Immunol. 2010;22:651–660. doi: 10.1093/intimm/dxq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Grigg C, Rizvi NA. PD-L1 biomarker testing for non-small cell lung cancer: truth or fiction? J Immunother Cancer. 2016;4:48. doi: 10.1186/s40425-016-0153-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73•.Kyi C, Postow MA. Immune checkpoint inhibitor combinations in solid tumors: opportunities and challenges. Immunotherapy. 2016;8:821–837. doi: 10.2217/imt-2016-0002. Review describing the current inability for checkpoint inhibitor therapies to reach solid tumors, despite their success in leukemias. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Johnson DB, Rioth MJ, Horn L. Immune checkpoint inhibitors in NSCLC. Curr Treat Options Oncol. 2014;15:658–669. doi: 10.1007/s11864-014-0305-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Michot JM, Bigenwald C, Champiat S, Collins M, Carbonnel F, Postel-Vinay S, Berdelou A, Varga A, Bahleda R, Hollebecque A, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. 2016;54:139–148. doi: 10.1016/j.ejca.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 76.Alegre ML, Frauwirth KA, Thompson CB. T-cell regulation by CD28 and CTLA-4. Nat Rev Immunol. 2001;1:220–228. doi: 10.1038/35105024. [DOI] [PubMed] [Google Scholar]
- 77.Lee S, Margolin K. Cytokines in cancer immunotherapy. Cancers (Basel) 2011;3:3856–3893. doi: 10.3390/cancers3043856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pilipow K, Roberto A, Roederer M, Waldmann TA, Mavilio D, Lugli E. IL15 and T-cell Stemness in T-cell-Based Cancer Immunotherapy. Cancer Res. 2015;75:5187–5193. doi: 10.1158/0008-5472.CAN-15-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Reiser J, Banerjee A. Effector, Memory, and Dysfunctional CD8(+) T Cell Fates in the Antitumor Immune Response. J Immunol Res. 2016;2016:8941260. doi: 10.1155/2016/8941260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, Rosenberg SA. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537–1544. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gros A, Parkhurst MR, Tran E, Pasetto A, Robbins PF, Ilyas S, Prickett TD, Gartner JJ, Crystal JS, Roberts IM, et al. Prospective identification of neoantigen-specific lymphocytes in the peripheral blood of melanoma patients. Nat Med. 2016;22:433–438. doi: 10.1038/nm.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Moon EK, Langer CJ, Albelda SM. The Era of Checkpoint Blockade in Lung Cancer: Taking the Brakes Off the Immune System. Ann Am Thorac Soc. 2017 doi: 10.1513/AnnalsATS.201702-152FR. [DOI] [PubMed] [Google Scholar]
- 83.Djenidi F, Adam J, Goubar A, Durgeau A, Meurice G, de Montpreville V, Validire P, Besse B, Mami-Chouaib F. CD8+CD103+ tumor-infiltrating lymphocytes are tumor-specific tissue-resident memory T cells and a prognostic factor for survival in lung cancer patients. J Immunol. 2015;194:3475–3486. doi: 10.4049/jimmunol.1402711. [DOI] [PubMed] [Google Scholar]
- 84.Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, Robbins PF, Huang J, Citrin DE, Leitman SF, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26:5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 86.Kmiecik J, Poli A, Brons NHC, Waha A, Eide GE, Enger PO, Zimmer J, Chekenya M. Elevated CD3(+) and CD8(+) tumor-infiltrating immune cells correlate with prolonged survival in glioblastoma patients despite integrated immunosuppressive mechanisms in the tumor microenvironment and at the systemic level. Journal of Neuroimmunology. 2013;264:71–83. doi: 10.1016/j.jneuroim.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 87.Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AH, Ellis IO, Green AR. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol. 2011;29:1949–1955. doi: 10.1200/JCO.2010.30.5037. [DOI] [PubMed] [Google Scholar]
- 88.Rapoport AP, Stadtmauer EA, Binder-Scholl GK, Goloubeva O, Vogl DT, Lacey SF, Badros AZ, Garfall A, Weiss B, Finklestein J, et al. NY-ESO-1-specific TCR-engineered T cells mediate sustained antigen-specific antitumor effects in myeloma. Nat Med. 2015;21:914–921. doi: 10.1038/nm.3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Suryadevara CM, Gedeon PC, Sanchez-Perez L, Verla T, Alvarez-Breckenridge C, Choi BD, Fecci PE, Sampson JH. Are BiTEs the “missing link” in cancer therapy? Oncoimmunology. 2015;4:e1008339. doi: 10.1080/2162402X.2015.1008339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Klinger M, Benjamin J, Kischel R, Stienen S, Zugmaier G. Harnessing T cells to fight cancer with BiTE (R) antibody constructs -past developments and future directions. Immunological Reviews. 2016;270:193–208. doi: 10.1111/imr.12393. [DOI] [PubMed] [Google Scholar]
- 91•.Daringer NM, Dudek RM, Schwarz KA, Leonard JN. Modular extracellular sensor architecture for engineering mammalian cell-based devices. ACS Synth Biol. 2014;3:892–902. doi: 10.1021/sb400128g. Interesting and unique example of an easily tunable engineered receptor to target cancer cells; if used correctly, this method could be utilized combat tumor evolution with personalized medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sanchez-Rivera FJ, Jacks T. Applications of the CRISPR-Cas9 system in cancer biology. Nat Rev Cancer. 2015;15:387–395. doi: 10.1038/nrc3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93•.Cantoni C, Huergo-Zapico L, Parodi M, Pedrazzi M, Mingari MC, Moretta A, Sparatore B, Gonzalez S, Olive D, Bottino C, et al. NK Cells, Tumor Cell Transition, and Tumor Progression in Solid Malignancies: New Hints for NK-Based Immunotherapy? J Immunol Res. 2016;2016:4684268. doi: 10.1155/2016/4684268. Review describing in detail the potential for NK cells to be used in cancer immunotherapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Glienke W, Esser R, Priesner C, Suerth JD, Schambach A, Wels WS, Grez M, Kloess S, Arseniev L, Koehl U. Advantages and applications of CAR-expressing natural killer cells. Front Pharmacol. 2015;6:21. doi: 10.3389/fphar.2015.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shlomchik WD, Couzens MS, Tang CB, McNiff J, Robert ME, Liu J, Shlomchik MJ, Emerson SG. Prevention of graft versus host disease by inactivation of host antigen-presenting cells. Science. 1999;285:412–415. doi: 10.1126/science.285.5426.412. [DOI] [PubMed] [Google Scholar]
- 96.Olson JA, Leveson-Gower DB, Gill S, Baker J, Beilhack A, Negrin RS. NK cells mediate reduction of GVHD by inhibiting activated, alloreactive T cells while retaining GVT effects. Blood. 2010;115:4293–4301. doi: 10.1182/blood-2009-05-222190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, Posati S, Rogaia D, Frassoni F, Aversa F, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 98••.Liu E, Tong Y, Dotti G, Shaim H, Savoldo B, Mukherjee M, Orange J, Wan X, Lu X, Reynolds A. Cord blood NK cells engineered to express IL-15 and a CD19-targeted CAR show long-term persistence and potent anti-tumor activity. Leukemia. 2017 doi: 10.1038/leu.2017.226. First paper to show the ability of CAR-NK cells to be engineered from cord blood and safely administered without HLA-matching. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hu Y, Tian ZG, Zhang C. Chimeric antigen receptor (CAR)-transduced natural killer cells in tumor immunotherapy. Acta Pharmacol Sin. 2017 doi: 10.1038/aps.2017.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nowakowska P, Romanski A, Miller N, Odendahl M, Bonig H, Zhang C, Seifried E, Wels WS, Tonn T. Clinical grade manufacturing of genetically modified, CAR-expressing NK-92 cells for the treatment of ErbB2-positive malignancies. Cancer Immunol Immunother. 2017:1–14. doi: 10.1007/s00262-017-2055-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Equipping NK. Cells with CARs. Cancer Discov. 2017 doi: 10.1158/2159-8290.CD-NB2017-124. [DOI] [PubMed] [Google Scholar]
- 102.Trivedi S, Srivastava RM, Concha-Benavente F, Ferrone S, Garcia-Bates TM, Li J, Ferris RL. Anti-EGFR Targeted Monoclonal Antibody Isotype Influences Antitumor Cellular Immunity in Head and Neck Cancer Patients. Clin Cancer Res. 2016;22:5229–5237. doi: 10.1158/1078-0432.CCR-15-2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schlaeth M, Berger S, Derer S, Klausz K, Lohse S, Dechant M, Lazar GA, Schneider-Merck T, Peipp M, Valerius T. Fc-engineered EGF-R antibodies mediate improved antibody-dependent cellular cytotoxicity (ADCC) against KRAS-mutated tumor cells. Cancer science. 2010;101:1080–1088. doi: 10.1111/j.1349-7006.2010.01505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Varchetta S, Gibelli N, Oliviero B, Nardini E, Gennari R, Gatti G, Silva LS, Villani L, Tagliabue E, Menard S, et al. Elements related to heterogeneity of antibody-dependent cell cytotoxicity in patients under trastuzumab therapy for primary operable breast cancer overexpressing Her2. Cancer Res. 2007;67:11991–11999. doi: 10.1158/0008-5472.CAN-07-2068. [DOI] [PubMed] [Google Scholar]
- 105•.Awasthi A, Ayello J, Van de Ven C, Elmacken M, Sabulski A, Barth MJ, Czuczman MS, Islam H, Klein C, Cairo MS. Obinutuzumab (GA101) compared to rituximab significantly enhances cell death and antibody-dependent cytotoxicity and improves overall survival against CD20+ rituximab-sensitive/-resistant Burkitt lymphoma (BL) and precursor B-acute lymphoblastic leukaemia (pre- B-ALL): potential targeted therapy in patients with poor risk CD20+ BL and pre-B-ALL. British journal of haematology. 2015;171:763–775. doi: 10.1111/bjh.13764. First completed report of a clinical trial involving manipulation of NK cells. [DOI] [PubMed] [Google Scholar]
- 106.Muntasell A, Ochoa MC, Cordeiro L, Berraondo P, Lopez-Diaz de Cerio A, Cabo M, Lopez-Botet M, Melero I. Targeting NK-cell checkpoints for cancer immunotherapy. Curr Opin Immunol. 2017;45:73–81. doi: 10.1016/j.coi.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 107•.Carlsten M, Korde N, Kotecha R, Reger R, Bor S, Kazandjian D, Landgren O, Childs RW. Checkpoint Inhibition of KIR2D with the Monoclonal Antibody IPH2101 Induces Contraction and Hyporesponsiveness of NK Cells in Patients with Myeloma. Clin Cancer Res. 2016;22:5211–5222. doi: 10.1158/1078-0432.CCR-16-1108. Clinical trial results from one of the first NK cell chcekpoint inhibition therapies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vey N, Bourhis JH, Boissel N, Bordessoule D, Prebet T, Charbonnier A, Etienne A, Andre P, Romagne F, Benson D, et al. A phase 1 trial of the anti-inhibitory KIR mAb IPH2101 for AML in complete remission. Blood. 2012;120:4317–4323. doi: 10.1182/blood-2012-06-437558. [DOI] [PubMed] [Google Scholar]
- 109•.Rezvani K, Rouce R, Liu E, Shpall E. Engineering natural killer cells for cancer immunotherapy. Molecular Therapy. 2017 doi: 10.1016/j.ymthe.2017.06.012. Review of the current CAR-NK cells in clinical trials. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Restifo NP, Smyth MJ, Snyder A. Acquired resistance to immunotherapy and future challenges. Nat Rev Cancer. 2016;16:121–126. doi: 10.1038/nrc.2016.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kochenderfer JN, Dudley ME, Feldman SA, Wilson WH, Spaner DE, Maric I, Stetler-Stevenson M, Phan GQ, Hughes MS, Sherry RM, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119:2709–2720. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kochenderfer JN, Wilson WH, Janik JE, Dudley ME, Stetler-Stevenson M, Feldman SA, Maric I, Raffeld M, Nathan DA, Lanier BJ, et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 2010;116:4099–4102. doi: 10.1182/blood-2010-04-281931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Neese RA, Misell LM, Turner S, Chu A, Kim J, Cesar D, Hoh R, Antelo F, Strawford A, McCune JM, et al. Measurement in vivo of proliferation rates of slow turnover cells by 2H2O labeling of the deoxyribose moiety of DNA. Proc Natl Acad Sci U S A. 2002;99:15345–15350. doi: 10.1073/pnas.232551499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tanchot C, Lemonnier FA, Perarnau B, Freitas AA, Rocha B. Differential requirements for survival and proliferation of CD8 naive or memory T cells. Science. 1997;276:2057–2062. doi: 10.1126/science.276.5321.2057. [DOI] [PubMed] [Google Scholar]
- 115.Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA, Jr, Kinzler KW. Cancer genome landscapes. Science. 2013;339:1546–1558. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Garrido F, Aptsiauri N, Doorduijn EM, Garcia Lora AM, van Hall T. The urgent need to recover MHC class I in cancers for effective immunotherapy. Curr Opin Immunol. 2016;39:44–51. doi: 10.1016/j.coi.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ruella M, Maus MV. Catch me if you can: Leukemia Escape after CD19-Directed T Cell Immunotherapies. Computational and Structural Biotechnology Journal. 2016;14:357–362. doi: 10.1016/j.csbj.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kakarla S, Gottschalk S. CAR T cells for solid tumors: armed and ready to go? Cancer J. 2014;20:151–155. doi: 10.1097/PPO.0000000000000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Newick K, Moon E, Albelda SM. Chimeric antigen receptor T-cell therapy for solid tumors. Mol Ther Oncolytics. 2016;3:16006. doi: 10.1038/mto.2016.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Feng K, Guo Y, Dai H, Wang Y, Li X, Jia H, Han W. Chimeric antigen receptor-modified T cells for the immunotherapy of patients with EGFR-expressing advanced relapsed/refractory non-small cell lung cancer. Sci China Life Sci. 2016;59:468–479. doi: 10.1007/s11427-016-5023-8. [DOI] [PubMed] [Google Scholar]
- 121.Adusumilli PS, Cherkassky L, Villena-Vargas J, Colovos C, Servais E, Plotkin J, Jones DR, Sadelain M. Regional delivery of mesothelin-targeted CAR T cell therapy generates potent and long-lasting CD4-dependent tumor immunity. Sci Transl Med. 2014;6:261ra151. doi: 10.1126/scitranslmed.3010162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Posey AD, Jr, Schwab RD, Boesteanu AC, Steentoft C, Mandel U, Engels B, Stone JD, Madsen TD, Schreiber K, Haines KM, et al. Engineered CAR T Cells Targeting the Cancer-Associated Tn-Glycoform of the Membrane Mucin MUC1 Control Adenocarcinoma. Immunity. 2016;44:1444–1454. doi: 10.1016/j.immuni.2016.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Katz SC, Burga RA, McCormack E, Wang LJ, Mooring W, Point GR, Khare PD, Thorn M, Ma Q, Stainken BF, et al. Phase I Hepatic Immunotherapy for Metastases Study of Intra-Arterial Chimeric Antigen Receptor-Modified T-cell Therapy for CEA+ Liver Metastases. Clin Cancer Res. 2015;21:3149–3159. doi: 10.1158/1078-0432.CCR-14-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kalaitsidou M, Kueberuwa G, Schutt A, Gilham DE. CAR T-cell therapy: toxicity and the relevance of preclinical models. Immunotherapy. 2015;7:487–497. doi: 10.2217/imt.14.123. [DOI] [PubMed] [Google Scholar]
- 125.Zheng H, Matte-Martone C, Jain D, McNiff J, Shlomchik WD. Central memory CD8+ T cells induce graft-versus-host disease and mediate graft-versus-leukemia. J Immunol. 2009;182:5938–5948. doi: 10.4049/jimmunol.0802212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126••.Ren J, Liu X, Fang C, Jiang S, June CH, Zhao Y. Multiplex Genome Editing to Generate Universal CAR T Cells Resistant to PD1 Inhibition. Clin Cancer Res. 2017;23:2255–2266. doi: 10.1158/1078-0432.CCR-16-1300. First paper to propose a “universal” CAR-T cell created with CRISPR/Cas9 technology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Romanski A, Uherek C, Bug G, Seifried E, Klingemann H, Wels WS, Ottmann OG, Tonn T. CD19-CAR engineered NK-92 cells are sufficient to overcome NK cell resistance in B-cell malignancies. J Cell Mol Med. 2016;20:1287–1294. doi: 10.1111/jcmm.12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zamora AE, Grossenbacher SK, Aguilar EG, Murphy WJ. Models to Study NK Cell Biology and Possible Clinical Application. Curr Protoc Immunol. 2015;110:14 37 11–14. doi: 10.1002/0471142735.im1437s110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Levi I, Amsalem H, Nissan A, Darash-Yahana M, Peretz T, Mandelboim O, Rachmilewitz J. Characterization of tumor infiltrating natural killer cell subset. Oncotarget. 2015;6:13835–13843. doi: 10.18632/oncotarget.3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Deng WW, Gowen BG, Zhang L, Wang L, Lau S, Iannello A, Xu JF, Rovis TL, Xiong N, Raulet DH. A shed NKG2D ligand that promotes natural killer cell activation and tumor rejection. Science. 2015;348:136–139. doi: 10.1126/science.1258867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Campbell AR, Duggan MC, Suarez-Kelly LP, Bhave N, Opheim KS, McMichael E, Trikha P, Parihar R, Luedke E, Lewis A, et al. MICA-expressing monocytes enhance natural killer cell Fc receptor-mediated antitumor functions. Cancer Immunol Res. 2017 doi: 10.1158/2326-6066.CIR-16-0005. canimm. 0005.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cohen J. HIV/AIDS Prevention cocktails: combining tools to Stop HIV’s spread. Science. 2005;309:1002–1005. doi: 10.1126/science.309.5737.1002. [DOI] [PubMed] [Google Scholar]
- 133.Angelin A, Gil-de-Gomez L, Dahiya S, Jiao J, Guo L, Levine MH, Wang Z, Quinn WJ, 3rd, Kopinski PK, Wang L, et al. Foxp3 Reprograms T Cell Metabolism to Function in Low-Glucose, High-Lactate Environments. Cell Metab. 2017;25:1282–1293. e1287. doi: 10.1016/j.cmet.2016.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pradeu T, Jaeger S, Vivier E. The speed of change: towards a discontinuity theory of immunity? Nat Rev Immunol. 2013;13:764–769. doi: 10.1038/nri3521. [DOI] [PubMed] [Google Scholar]
- 135.Vitale M, Cantoni C, Pietra G, Mingari MC, Moretta L. Effect of tumor cells and tumor microenvironment on NK-cell function. Eur J Immunol. 2014;44:1582–1592. doi: 10.1002/eji.201344272. [DOI] [PubMed] [Google Scholar]
- 136••.Gao YL, Souza-Fonseca-Guimaraes F, Bald T, Ng SS, Young A, Ngiow SF, Rautela J, Straube J, Waddell N, Blake SJ, et al. Tumor immunoevasion by the conversion of effector NK cells into type 1 innate lymphoid cells. Nature Immunology. 2017;18:1004. doi: 10.1038/ni.3800. First paper suggesting that the tumor microenvironment may be causing NK cells to convert into progenitor cells that do not possess effector capabilites. [DOI] [PubMed] [Google Scholar]
- 137•.Svensson MC, Warfvinge CF, Fristedt R, Hednet C, Borg D, Eberhard J, Micke P, Nodin B, Leandersson K, Jirström K. The integrative clinical impact of tumor-infiltrating T lymphocytes and NK cells in relation to B lymphocyte and plasma cell density in esophageal and gastric adenocarcinoma. Oncotarget. 2017 doi: 10.18632/oncotarget.19437. Study showing a correlation between T cells, NK cells, and cancer prognosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Henrickson SE, Mempel TR, Mazo IB, Liu B, Artyomov MN, Zheng H, Peixoto A, Flynn MP, Senman B, Junt T, et al. T cell sensing of antigen dose governs interactive behavior with dendritic cells and sets a threshold for T cell activation. Nat Immunol. 2008;9:282–291. doi: 10.1038/ni1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kaufman HL, Russell J, Hamid O, Bhatia S, Terheyden P, D’Angelo SP, Shih KC, Lebbé C, Linette GP, Milella M. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. The Lancet Oncology. 2016;17:1374–1385. doi: 10.1016/S1470-2045(16)30364-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, Park K, Smith D, Artal-Cortes A, Lewanski C. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. The Lancet. 2016;387:1837–1846. doi: 10.1016/S0140-6736(16)00587-0. [DOI] [PubMed] [Google Scholar]
- 141.Swaika A, Hammond WA, Joseph RW. Current state of anti-PD-L1 and anti-PD-1 agents in cancer therapy. Molecular immunology. 2015;67:4–17. doi: 10.1016/j.molimm.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 142.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. New England journal of medicine. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wu J. IL-15 agonists: the cancer cure cytokine. Journal of molecular and genetic medicine: an international journal of biomedical research. 2013;7:85. doi: 10.4172/1747-0862.1000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mueller KT, Maude SL, Porter DL, Frey N, Wood P, Han X, Waldron E, Chakraborty A, Awasthi R, Levine BL. Cellular kinetics of CTL019 in relapsed/refractory B-cell acute lymphoblastic leukemia and chronic lymphocytic leukemia. Blood. 2017 doi: 10.1182/blood-2017-06-786129. blood-2017-2006-786129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Topp MS, Gökbuget N, Stein AS, Zugmaier G, O’Brien S, Bargou RC, Dombret H, Fielding AK, Heffner L, Larson RA. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. The Lancet Oncology. 2015;16:57–66. doi: 10.1016/S1470-2045(14)71170-2. [DOI] [PubMed] [Google Scholar]
- 146.Hipp S, Tai Y, Blanset D, Deegen P, Wahl J, Thomas O, Rattel B, Adam P, Anderson K, Friedrich M. A novel BCMA/CD3 bispecific T-cell engager for the treatment of multiple myeloma induces selective lysis in vitro and in vivo. Leukemia. 2016 doi: 10.1038/leu.2016.388. [DOI] [PubMed] [Google Scholar]
- 147.Huehls AM, Coupet TA, Sentman CL. Bispecific T cell engagers for cancer immunotherapy. Immunology and cell biology. 2015;93:290. doi: 10.1038/icb.2014.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.McWilliams EM, Mele JM, Cheney C, Timmerman EA, Fiazuddin F, Strattan EJ, Mo X, Byrd JC, Muthusamy N, Awan FT. Therapeutic CD94/NKG2A blockade improves natural killer cell dysfunction in chronic lymphocytic leukemia. Oncoimmunology. 2016;5:e1226720. doi: 10.1080/2162402X.2016.1226720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Alici E. IPH-2101, a fully human anti-NK-cell inhibitory receptor mAb for the potential treatment of hematological cancers. Current opinion in molecular therapeutics. 2010;12:724–733. [PubMed] [Google Scholar]
- 150.Yonezawa A, Dutt S, Chester C, Kim J, Kohrt HE. Boosting cancer immunotherapy with anti-CD137 antibody therapy. Clinical Cancer Research. 2015;21:3113–3120. doi: 10.1158/1078-0432.CCR-15-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151•.Guillerey C, Huntington ND, Smyth MJ. Targeting natural killer cells in cancer immunotherapy. Nature immunology. 2016;17:1025–1036. doi: 10.1038/ni.3518. Review on current NK cell cancer therapeutics and potential. [DOI] [PubMed] [Google Scholar]