Abstract
We initially tested the electrochemical activity of beta-carotene and lutein at unmodified glassy carbon electrodes. We found good sensitivity (1 nA/μM) at high, micromolar concentrations, but serum levels are at nanomolar concentrations. To enhance the electrochemical activity, we modified the sensor surface with β-cyclodextrin, which has a hydrophobic core. Our goal was that the beta-carotene will be attracted to the β-cyclodextrin core, increasing surface interaction and sensitivity. Instead we saw a decrease in electrochemical activity. Further investigation with a methylene blue mediator indicated two results. First, it is unlikely the beta-carotene strongly interacts with the β-cyclodextrin surface. And, second, the presence of a co-solvent or surfactant can greatly disrupt the surface β-cyclodextrin activity.
Background
Reactive oxygen species (ROS) has been linked to many diseases, including heart disease and epigenetic disorders.1–4 The body uses antioxidants to combat and remove ROS. Beta-carotene and lutein are common carotenoid pigments found in plants, and beta-carotene’s and lutein’s unique structures allow them to act as potent antioxidants. Beta-carotene and lutein are highly reactive with singlet oxygen and free radical oxygen species which will actively remove ROS.5 Initial studies have shown that beta-carotene and lutein may lower risk for a multitude of disease states including cancer, cardiovascular disease, and macular degeneration.6–8 Carotenoids can be monitored as a way of monitoring general ROS and patient health.
Serum antioxidant levels are presently detected through various analytical techniques, but each current method has limited capabilities. High performance liquid chromatography (HPLC) is currently the gold-standard to assess carotenoid levels. Yet, HPLC is typically operationally prohibitive to most hospitals and clinics due to its inability to handle high throughput volumes and high labor demands.9 Other non-invasive methods such as Raman spectroscopy have been developed to detect antioxidants. Raman spectroscopy based detection of carotenoid levels in skin or eye has been shown to be highly reproducible and correlate with serum levels of carotenoids.10–12 Lutein and zeaxanthin were detected due to double bond vibrations at a signal intensity of 1525−1 cm.13 However, Raman spectroscopic methods were unable to distinguish between specific types of carotenoids. Therefore, we attempted to develop an electrochemical method to measure specific carotenoids that could be eventually translated into a point-of-care assay.
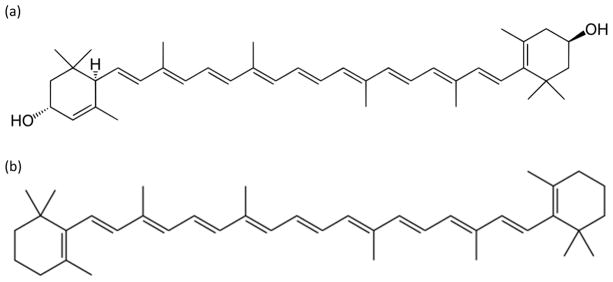
Previously published work has shown that beta-carotene and lutein are electrochemically active. Furthermore, good calibration (R2 = 0.999) is shown at high concentrations (0.5–76 uM) of lutein with a limit of detection of about 0.1 μM in tetrahydrafuran/ethanol solvent (1:9 volume ratio).5 The experimental setup utilized a bare glassy carbon working electrode, a platinum counter electrode, and an Ag/AgNO3 working electrode.5 It was expected that lutein and beta-carotene would display similar electrochemical properties as they are extremely similar in structure (Figure 1). The molecules differ only by the addition/subtraction of two hydroxyl groups and a rotation of the double bond in the 6-member ring terminals.
Figure 1.
Chemical structures of a) lutein and b) beta-carotene.
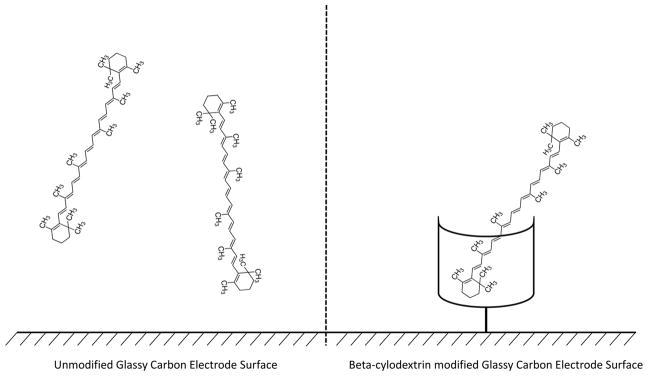
Previous bare electrode studies resulted only a 100 nM limit of detection, but our goal was to reduce the limit of detection to clinically relevant concentrations, 1–100 nM calibrated detection. In an attempt to increase sensitivity, a glassy carbon electrode was modified with β-cyclodextrin (Figure 2). We hypothesized that β-cyclodextrin’s large hydrophobic core would attract beta-carotene, a hydrophobic analyte. Molecular modeling studies indicated that beta-carotene was the most promising carotenoid to form complexes with β-cyclodextrin.14
Figure 2.
(Left) Unmodified glassy carbon electrode interacting with beta-carotene. (Right) β-cylodextrin modified glassy carbon electrode interacting with beta-carotene.
Methods
Reagents and Equipment
Beta-carotene (97%), lutein (90%), and methylene blue were purchased from Acros organics. β-cyclodextrin was purchased from Sigma Aldrich. All other reagents were analytical grade and purchased from Fisher Scientific. Phosphate buffer saline solution (PBS) was produced using a standard protocol and made in-house to a pH of 7.3. 0.1 M tetrabutylammonium hexafluorophosphate (NBu4PF6) electrolyte was added for measurements in dimethyl sulfoxide (DMSO).
All electrochemical tests were run on a Gamry Reference 600 potentiostat using a a BASi C3 cell stand faraday cage. The electrode setup included a glassy carbon or platinum working electrode and a platinum auxiliary electrode. A Ag/AgNO3 reference electrode was used for DMSO electrolytes, and a Ag/AgCl reference electrode was used for aqueous electrolytes. Nitrogen was bubbled through the solution to create an anoxic environment. Stirring occurred in between electrochemical data points, but not during the
Electrochemical Settings
Cyclic voltammetry (CV) was typically conducted by sweeping from −0.6 to 0.6 V vs Ref at 100 mV/s. Six cycles were run for each concentration. The reported cyclic voltammograms were created by plotting voltage vs. current of only the second cycle. Additional data graphs can be requested by contacting the author.
Square wave voltammetry (SWV) was conducted by sweeping from −0.4 V to +0.55 V vs Ref at a frequency of 10 Hz and a pulse size of 25 mV. Only a single scan of SWV was run at each concentration. The reported square wave voltammogram is the forward current subtracted from the background current.
Serial Dilutions
For initial measurements conducted on unmodified electrodes, sample solutions were made at 2 mM carotenoid in 0.1 M NBu4PF6 in DMSO. When modified, an aqueous solution is needed for β-cyclodextrin hydrophobic entrapping forces; however, beta-carotene and lutein are not soluble in aqueous solvents. Therefore, a co-solvent was used for aqueous experiments. For carotenoid experiments, the sample solution was 0.5% Tween 20 in PBS. For cholesterol experiments, the sample solution was 10% ethanol in PBS. In all aqueous experiments, unless indicated, the base solution was pure PBS.
The sample solutions were added to the base solution in a serial mode. In other words, the concentration is constantly increasing, with experiments operating from low to high concentrations. A specific volume of the sample solution was added to achieve the diluted concentration in the base solution, typically ranging from 6 nM to 250 μM. Controls were conducted by adding an equivalent volume of the sample solution without analyte to the base solution.
Electrode Preparation
Prior to all unmodified electrode measurements, and before β-cyclodextrin attachment, the carbon and platinum electrodes were polished using 0.5 μm alumina. β-cyclodextrin was modified using a procedure adapted from previously published work.15 The electrode was placed in a 28.5 mM β-cyclodextrin, 1 mM ferrocene, and 0.6 M tetrabutylammonium methoxide in a 0.1 M NBu4PF6 DMSO solution with an applied voltage sweep of −0.5V to +0.8V vs Ag/AgNO3. β-cyclodextrin was covalently bound to the electrode surface through free-radical surface polymerization chemistry to create a covalent permanent attachment.15 For the methylene blue (MB) mediated experiments, the β-cyclodextrin modified electrode was soaked in 1 mM MB water solution prior to use.
Results and Discussion
Electrochemical Evaluation from an Unmodified Electrode with Cyclic Voltammetry
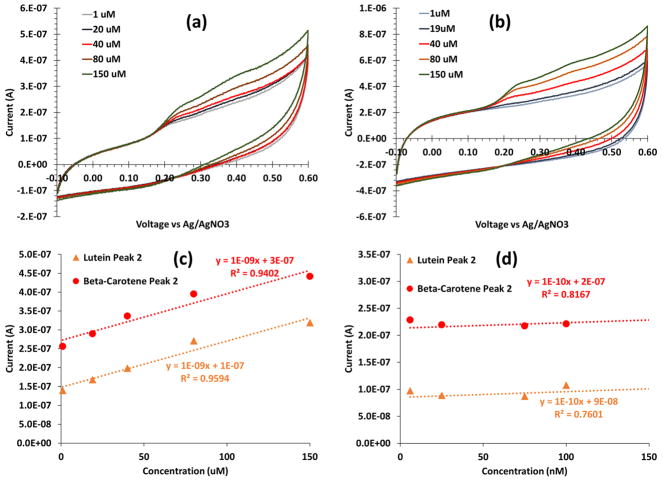
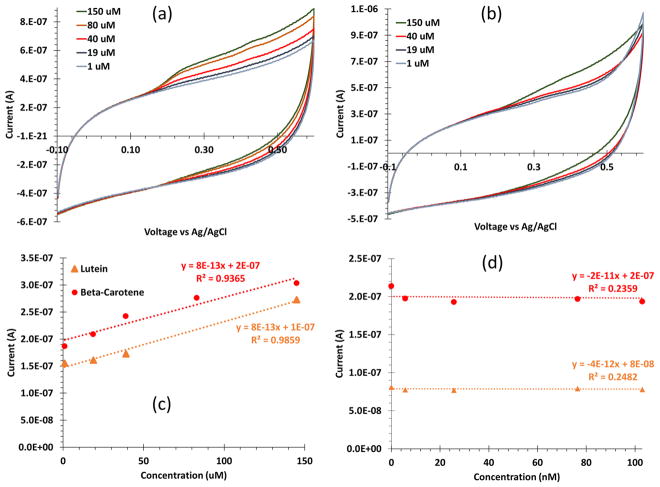
Using a glassy carbon electrode and cyclic voltammetry, a serial dilution including a baseline (i.e. 0 M or no addition of the sample solution), low concentrations (25 nM to 900 nM), and high concentrations (1 μM to 250 μM) of beta-carotene yielded two clear oxidation peaks at 256 mV and 420 mV (Figure 3a). The serial dilution at the same concentrations and cyclic voltammetry settings for lutein yielded two clear oxidation peaks at 338 mV and 412 mV (Figure 3b).
Figure 3.
Cyclic voltammograms of (a) beta-carotene and (b) lutein using an unmodified glassy carbon electrode with five concentrations ranging 1 to 150 μM. Beta-carotene had two oxidation peaks at 256 mV and 420 mV, and lutein had two oxidation peaks 338 mV and 417 mV. (c) A serial dilution calibration curve for the second peak of lutein (orange triangles) and beta-carotene (red circles) shows a good calibration from 1–150 μM, both with sensitivity of 1 nA/μM. (d) A weak calibration is observed at low concentrations, 5–150 nM, indicated by the R2 values.
Both antioxidants showed very similar electrochemical activity. The cyclic voltammograms revealed good and consistent calibration (R2 > 0.99) at high concentrations (1–150 μM) with a 1 nA/μM sensitivity (Figure 3c); however, a poor calibration was observed for low concentrations (5–900 nM) (Figure 3d) The poor, low concentration calibration indicates that the basic unmodified sensor will not accurately produce needed sensitivity for serum relevant concentrations.
Serum levels, and therefore desired observable changes, of beta-carotene and lutein are on the nanomolar scale.16 The sensitivity produced by the bare electrode method was not within range to accurately detect low levels of antioxidants in serum on the nanomolar scale (Figure 3d). We explored β-cyclodextrin modifications to improve sensitivity of carotenoids, specifically for beta-carotene and lutein.
Cyclodextrin Modification
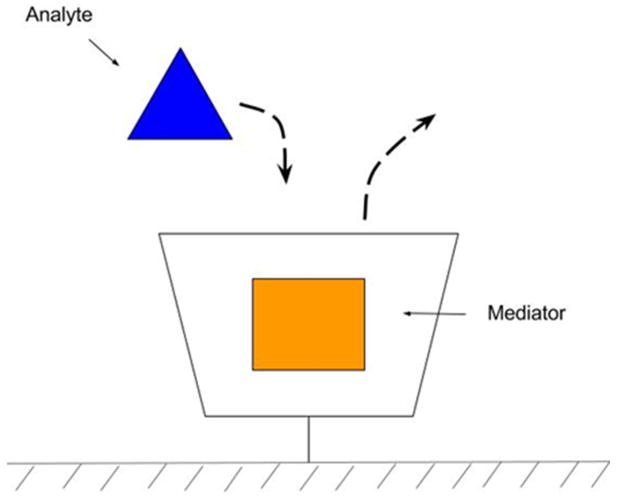
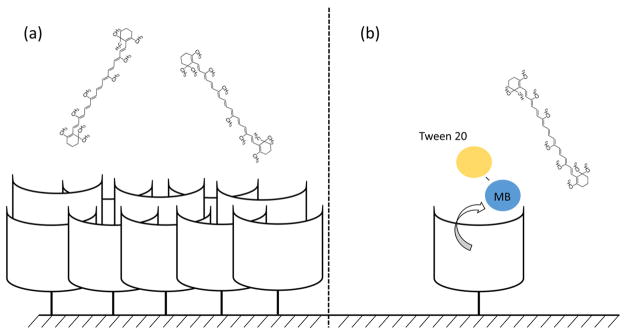
Cyclodextrin surface modifications can work one of two ways. First, β-cyclodextrin surfaces can attract hydrophobic molecules to the electrode and encapsulate them in the hydrophobic core increasing their surface interaction (Figure 2). The attraction allows greater contact between the analyte and the electrode surface which has been shown to increase sensitivity. Second, a mediator molecule, such as methylene blue (MB), can be added to the β-cyclodextrin. A hydrophobic analyte will displace the MB, allowing the detection of electrochemically inactive (or low activity) molecules (Figure 4). Using a mediator increases the detection possibilities for analytes that are normally undetectable or have weak detection via traditional methods. In one study, the displacement of MB was shown to accurately detect cholesterol, where cholesterol displaces MB causing a redox reaction.17
Figure 4.
β-cyclodextrin is grafted to the surface. A MB mediator is entrapped inside the β-cyclodextrin. When an analyte is introduced to the system, the analyte will displace the mediator. This mediator can then be measured electrochemically.
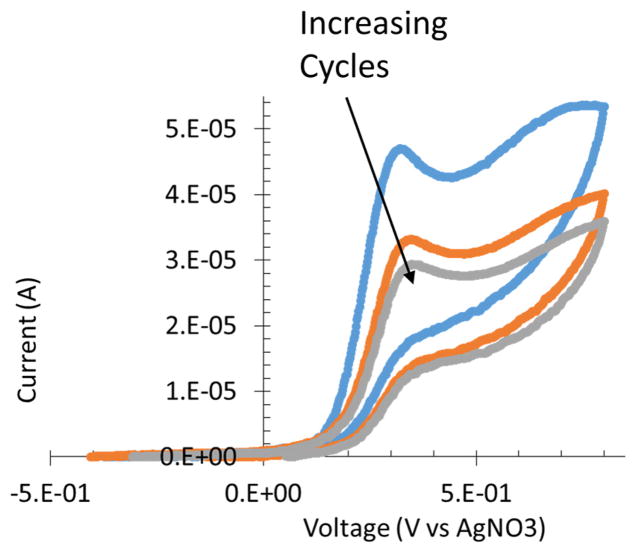
Cyclic voltammograms of β-cyclodextrin attachment to a bare glassy carbon electrode shows a change in current at differing cycles in the +0.3 – +0.7 V vs Ag/AgNO3 (Figure 5). Changes in current decreased as the cycles progressed. The reduced charge transferred indicates the level of surface attachment.
Figure 5.
A scan from −400 to +700 mV vs. Ag/AgNO3 in the β-cyclodextrin ferrocene electrochemical grafting solution. A decrease in observed charge from +300 to +700 mV as cycles increases indicates attachment.
A serial dilution at high (uM) and low (nM) concentrations of beta-carotene yielded cyclic voltammograms with two clear oxidation peaks. The oxidation peaks were seen at 250 mV and 420 mV (Figure 6a). The serial dilution for lutein yielded cyclic voltammograms with one broad oxidation peak at 412 mV (Figure 6b). The cyclic voltammograms indicate a reduction of electrochemical activity.
Figure 6.
Cyclic voltammograms of (a) beta-carotene and (b) lutein using an β-cyclodextrin modified electrode with five concentrations 1 to 150 μM. Beta-carotene had two oxidation peaks at 250 mV and 420 mV, and lutein had only one oxidation peak at 412 mV. (c) A serial dilution calibration curve of lutein (orange triangles) and beta-carotene (red circles) shows weaker sensitivity from 1–150 μM, compared to unmodified electrodes. (d) Also, a weak calibration is observed at low concentrations, 5–100 nM.
When compared to the unmodified electrodes, the β-cyclodextrin-modified electrodes showed a reduction in sensitivity (Figure 6c and 6d). At high concentrations (5–150 μM), a linear calibration of beta-carotene and lutein showed a good fit, but a large decrease in sensitivity, now 10−4 nA/μM (Figure 6c). Still, at low concentrations (5–150 nM), a weak calibration curve is seen (Figure 6d).
We can draw different conclusions from this data. First, we know the β-cyclodextrin modification is successful due to a reduction of charge transfer during the attachment procedure and a change in electrical behavior after modification. However, because of the free-radical nature of the attachment, we could be getting a multi-layered aggregate on the surface instead of a monolayer (Figure 8a).14 The lack of sensitivity could be because the β-cyclodextrin film thickness was too large for an interaction with the carotenoid analytes. Instead of enhancing the surface interaction, the β-cyclodextrin surface may be too thick hindering the ability for the carotenoid to reach the surface applied potential. Alternatively, the beta-carotene may not interact with the β-cyclodextrin contrary to published theorized work.18 To determine which theory is correct, we introduce an electrochemically active mediator, MB, to bind with the β-cyclodextrin-modified surface. If the beta-carotene is not interacting with the β-cyclodextrin, the MB should create no signal. However, if a signal is observed, the beta-carotene is to sterically large to reach the sensor surface.
Figure 8.
We propose two theories with the problems observed in our experiments. (a) Multiple layers of β-cyclodextrin are bound to the surface because of the free-radical surface attachment. The analyte surface can become impeded due to the thickness. (b) The Tween 20 surfactant solubilizes and removes the MB from the β-cyclodextrin independent of the carotenoid interaction.
Cyclodextrin Modified Surface with a Mediator
Previously published work has shown that MB can act as a redox mediator for the detection of cholesterol on a β-cyclodextrin modified graphene sheet.17 β-cyclodextrin-modified graphene can be loaded with MB through a simple soaking procedure. MB elutes from the β-cyclodextrin core as it is exposed to and replaced by a more hydrophobic analyte (Figure 2). Once the analyte replaces the MB from the β-cyclodextrin core, the MB will create an electrochemical signal and should be directly proportional to the amount of analyte added, resulting in a calibration curve.
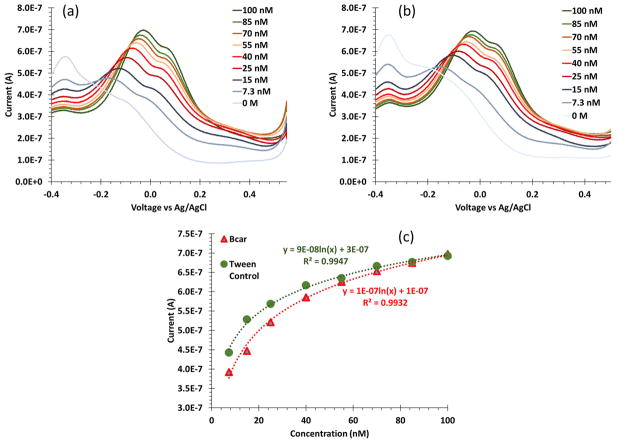
The MB mediated experiments used SWV centered around the oxidation of MB to measure any release from the β-cyclodextrin. Initial serial dilutions of beta-carotene with an MB loaded β-cyclodextrin surface were conducted. The calibration was logarithmic, and we achieved consistent detectable levels of beta-carotene on the low nano-molar scale (Figure 7a). Note, the peaks detected, −33mV and +72 mV, are the oxidation response of MB. To confirm the detection of nanomolar concentration of beta carotene a background dilution of 0.5% Tween 20 in PBS sample solution with equivalent volumes yielded nearly identical results (Figure 7b). Therefore, instead of eluting from the pocket when exposed to beta-carotene, the MB eluted from β-cyclodextrin due solubilizing from interacting with the Tween 20 surfactant. These data indicate that the surface is not too thick to hinder electrical transfer with analytes, and beta-carotene is not interacting with the β-cyclodextrin surface.
Figure 7.
Square wave voltammograms of (a) beta-carotene and (b) equivalent volume of Tween 20 using a β-cyclodextrin modified electrode loaded with MB at low, nanomolar concentrations. The two SWV look similar because both are measuring the elution of MB. (c) The calibration curves of the beta-carotene and blank Tween 20 control indicate that the measurement is primarily Tween 20 solubilizing the MB.
Additional Need for Controls
Based on the MB data, we have arrived at two interesting observations. First, it appears that the beta-carotene does not interact strongly with β-cyclodextrin, against previous modeling data. Second, it appears that the Tween 20 surfactant can have a greater influence on the electrochemical behavior compared to the analyte of interest. Within this section, we will further expand on the later, specifically, the influence of surfactant and co-solvents on the analyte with β-cyclodextrin.
We decided to test cholesterol using a 10% ethanol co-solvent in water sample solution, which was based on previous work; however, there are some differences with the previous published work and our experiments.17 In this work, we directly modified β-cyclodextrin to the sensor surface through an electrochemical free-radical grafting technique which can cause multiple layers of β-cyclodextrin (Figure 8a).
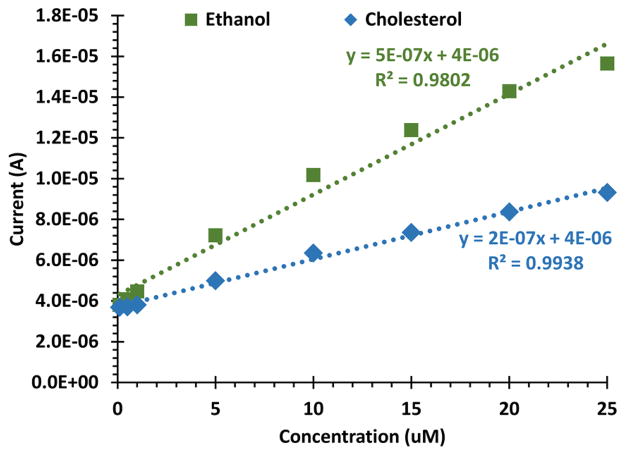
Serial dilutions of cholesterol using MB loaded β-cyclodextrin surfaces showed that the calibration curve was more dependent on the ethanol co-solvent content compared to the analyte. A calibration curve was generated upon addition of cholesterol and ethanol co-solvent to the base buffered solution (Figure 9, purple diamonds). This calibration curve was similar, yet reduced sensitivity, compared to a control, where the sample solution only contained ethanol co-solvent without analyte (Figure 9). In other words, 10% ethanol in PBS was added in equivalent volumes to the base solution which produced greater electrochemical activity compared to a sample solution with cholesterol.
Figure 9.
A serial dilution of cholesterol dissolved in a 10% ethanol PBS solution added to the base PBS buffered solution (blue diamonds). We found that additions of a control, a sample solution without cholesterol analyte, had a larger impact on the current measurement, measured by MB released (green squares).
Conclusions
Initial measurements of beta-carotene and lutein from unmodified electrodes achieved good measurements at high concentrations. To improve sensitivity, β-cyclodextrin modifications were attempted. Instead of measuring beta-carotene’s interaction with β-cyclodextrin, we found that the co-solvent or surfactant had a greater effect on the electrochemical measurements. Based on the data presented, it is unlikely beta-carotene interacts with β-cyclodextrin to produce a positive influence the electrochemical measurement.
Ethanol and Tween 20 are potent co-solvent and surfactant respectively, and the addition of a co-solvent or surfactant caused methylene blue to elute from the β-cyclodextrin independent of the analyte. We conclude that, whenever a co-solvent or surfactant is added to a sample solution without being present in the base solution, the co-solvent or surfactant has the ability to significantly disrupt the β-cyclodextrin activity. This might appear obvious, but care in these experiments may be overlooked. In the case of using β-cyclodextrin, including using non-electrochemically active co-solvents or surfactants, the hydrophobic/hydrophilic interactive behavior of β-cyclodextrin can become disrupted. Placing the co-solvent and surfactant in both the sample and base solutions could improve accuracy of the sensor. However, because of the disruption that non-polar solvents or surfactants can have to the β-cyclodextrin activity, it is best to avoid them entirely.
In conclusions, in measuring the change of β-cyclodextrin binding characteristics, it becomes unclear if the measurement effect is due to the analyte or the disruption of β-cyclodextrin activity. These data indicate that it is best to avoid, when possible, co-solvents and surfactants that can disrupt β-cyclodextrin’s hydrophobic/hydrophilic behavior in future experiments. Future tests might include investigating if the co-solvent and surfactant still effects the elution of methylene blue if it is present in both the sample and base solution.
Acknowledgments
The authors would like to thank NSF EAGER (CBET 1638896), NIH COBRE (P20GM113131), the Hamel Center for Undergraduate Research, and College of Engineering and Physical Sciences at the University of New Hampshire for funding and support for this work. Also, the authors would like to thank Nicholas Sweeny-Cook for assisting on developing the SOP for β-cyclodextrin attachment to our sensor surfaces.
References
- 1.Lim SO, et al. Gastroenterology. 2008;135:2128–2140.e8. doi: 10.1053/j.gastro.2008.07.027. [DOI] [PubMed] [Google Scholar]
- 2.Halliwell B. Am J Med. 1991;91:S14–S22. [Google Scholar]
- 3.Ziech D, Franco R, Pappa A, Panayiotidis MI. Mutat Res Mol Mech Mutagen. 2011;711:167–173. doi: 10.1016/j.mrfmmm.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 4.Taniyama Y, Griendling KK. Hypertension. 2003;42:1075–1081. doi: 10.1161/01.HYP.0000100443.09293.4F. [DOI] [PubMed] [Google Scholar]
- 5.Yue Y, Liang Q, Liao Y, Guo Y, Shao S. J Electroanal Chem. 2012;682:90–94. [Google Scholar]
- 6.Mayne ST, Taylor S. FASEB J. 1996;10:690–701. [PubMed] [Google Scholar]
- 7.Siegel RL, Miller KD, Jemal A. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 8.Rissanen TH, et al. Br J Nutr. 2001;85:749–754. doi: 10.1079/bjn2001357. [DOI] [PubMed] [Google Scholar]
- 9.Vogeser M, Seger C. Clin Biochem. 2008;41:649–662. doi: 10.1016/j.clinbiochem.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 10.Hata TR, et al. J Invest Dermatol. 2000;115:441–448. doi: 10.1046/j.1523-1747.2000.00060.x. [DOI] [PubMed] [Google Scholar]
- 11.Stahl W, et al. J Nutr. 1998;128:903–907. doi: 10.1093/jn/128.5.903. [DOI] [PubMed] [Google Scholar]
- 12.Stahl W, Sies H. Mol Aspects Med. 2003;24:345–351. doi: 10.1016/s0098-2997(03)00030-x. [DOI] [PubMed] [Google Scholar]
- 13.Sharifzadeh M, Zhao DY, Bernstein PS, Gellermann W. J Opt Soc Am A Opt Image Sci Vis. 2008;25:947–957. doi: 10.1364/josaa.25.000947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bikádi Z, Kurdi R, Balogh S, Szemán J, Hazai E. Chem Biodivers. 2006;3:1266–1278. doi: 10.1002/cbdv.200690129. [DOI] [PubMed] [Google Scholar]
- 15.Guzmán-Hernández DS, et al. Electrochim Acta. 2014;140:535–540. [Google Scholar]
- 16.Street DA, Comstock GW, Salkeld RM, Schuep W, Klag MJ. Circulation. 1994;90:1154–1161. doi: 10.1161/01.cir.90.3.1154. [DOI] [PubMed] [Google Scholar]
- 17.Agnihotri N, Chowdhury AD, De A. Biosens Bioelectron. 2015;63:212–217. doi: 10.1016/j.bios.2014.07.037. [DOI] [PubMed] [Google Scholar]
- 18.Háda M, Nagy V, Deli J, Agócs J. Molecules. 2012;17:5003–5012. doi: 10.3390/molecules17055003. [DOI] [PMC free article] [PubMed] [Google Scholar]