Abstract
Due to advancing age and increasing comorbidities, the current population has a higher incidence of complex coronary artery disease, often without surgical options for revascularization. In this setting, hemodynamic support devices are an important adjunct in the interventionist's toolbox as they allow for a safer, more effective procedure. The following paper reviews the indications of various available mechanical support devices, highlights their clinical data and technical parameters, and offers a practical approach towards appropriate patient and device selection.
Keywords: mechanical circulatory support, high-risk, percutaneous coronary intervention, cardiogenic shock, stent, coronary artery disease
INTRODUCTION
Interventional cardiology has evolved tremendously in the last decade, with physicians gaining more experience and having an increasing number of tools at their disposal. As a result, complex percutaneous coronary interventions (PCI) are being performed on an older cohort of patients who have more comorbidities and more complex disease than in years past. Improvement and greater availability of mechanical circulatory support devices have made them a critical component of the interventionist's attempt at a successful outcome.
Mechanical circulatory support (MCS) devices have been used as an adjunct to PCI in two main clinical scenarios: cardiogenic shock complicating acute myocardial infarction (AMI) and technically complex high-risk PCI in the absence of overt shock.
CARDIOGENIC SHOCK
Cardiogenic shock is the combination of tissue hypoxia and decreased cardiac output in the setting of adequate intravascular volume. There are objective clinical and hemodynamic criteria that define cardiogenic shock. Clinical criteria include hypotension (a systolic blood pressure [SBP] of < 90 mm Hg for at least 30 minutes or the need for supportive measures to keep SBP ≥ 90) and evidence of end-organ hypoperfusion (urine output < 30 mL/h and a heart rate of ≥ 60 bpm with cool extremities); hemodynamic criteria include low cardiac index (< 2.2 mL/min/m2) and pulmonary capillary wedge pressure ≥ 15 mm Hg.1,2
Most cases of acute cardiogenic shock occur secondary to ventricular dysfunction after AMI with or without concomitant valvular dysfunction and vasodilatory abnormalities, although in rare instances mechanical complications of AMI can lead to sudden, acute cardiogenic shock in a previously stable patient.3,4 Cardiogenic shock complicating AMI has high rates of morbidity and mortality, with 30-day mortality approaching 50%. Even though interventional techniques and pharmacology have both improved dramatically in the last 3 decades, this has not translated to meaningful improvement in outcomes for cardiogenic shock; to date, only prompt revascularization has been shown to improve mortality in this setting.1 Over the last 20 years, newer percutaneous and surgical MCS devices have become available that aid in augmenting cardiac output and decreasing afterload and ventricular filling pressure, thereby supporting end-organ perfusion acting as a bridge to myocardial recovery or transplant and providing an excellent adjunct to PCI in these critical patients with cardiogenic shock.
HIGH-RISK PERCUTANEOUS CORONARY INTERVENTION
Coronary interventional techniques have progressed immensely since the inception of PCI 40 years ago. The availability of tools such as low-profile balloons, improved guidewires, coronary atherectomy devices, and superior stent designs that help improve deliverability have enabled physicians to perform coronary interventions in patients who would previously have been deemed untreatable. In addition, overall improvements in health care delivery have led to improved survival rates in patients with more advanced coronary artery disease to the point where revascularization can and should be performed. Furthermore, advancing age and increased comorbidities have put more patients at prohibitive risk for coronary artery bypass grafting, rendering high-risk PCI as the sole revascularization strategy for this population.5 Lastly, a large body of evidence now indicates that complete multivessel revascularization leads to significantly superior outcomes compared to partial/incomplete revascularization.6 These factors have led to the concept of CHIP: Complete revascularization for higher-risk indicated patients.
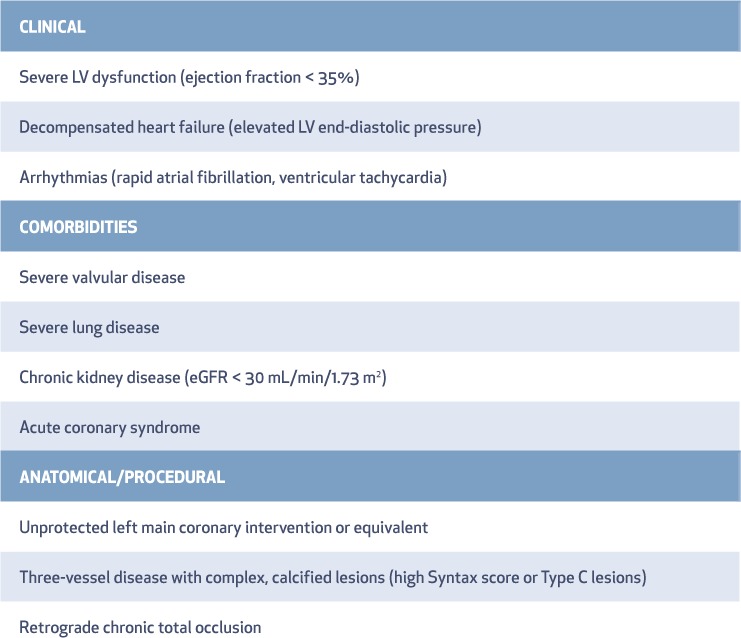
Several attributes may define a high-risk coronary intervention, although none are absolute (Table 1). These include patient characteristics such as left ventricular (LV) systolic function, end-organ dysfunction (baseline kidney disease or lung disease), clinical presentation with heart failure, and complex coronary anatomy. The goal of MCS in high-risk PCI is to provide sufficient forward cardiac output to maintain myocardial flow and end-organ perfusion and to unload the left ventricle during the procedure. This approach often requires multiple high-pressure balloon inflations and plaque modification procedures such as coronary atherectomy and intravascular imaging. These techniques are often time consuming, lead to prolonged periods of systemic and coronary hypoperfusion, and can ultimately result in myocardial depression and circulatory collapse7—although the latter is extremely uncommon due to improved equipment that minimizes ischemic time and quickly seals occlusive coronary dissections. Despite these concerns, use of an appropriate MCS device allows time to safely perform an optimal coronary intervention in brittle, critically ill patients with advanced coronary artery disease to enable superior short-and long-term outcomes. Therefore, an MCS device should be placed prior to initiating a high-risk coronary intervention. This support enables the operator to proceed confidently with high-risk PCI without the risk of inadvertent collapse and subsequent need for emergency implementation of hemodynamic support for bailout.
Table 1.
High-risk percutaneous coronary intervention. LV: left ventricular; eGFR: estimated glomerular filtration rate
PERCUTANEOUS MECHANICAL SUPPORT DEVICES
An ideal MCS device should provide adequate circulatory support that increases mean arterial pressure, thereby maintaining vital organ perfusion while simultaneously reducing myocardial oxygen demand through reductions in LV pressure (afterload) and volume (preload). Ultimately, the MCS device is meant to maximize cardiac power, which has been shown to be a strong independent predictor of outcomes in patients with cardiogenic shock and severe dysfunction.8 Several percutaneous MCS devices with variable impact on cardiovascular hemodynamics are available to the interventionist (Figure 1, Table 2).9 The following is a summary of approved devices and their published trial data.
Figure 1.
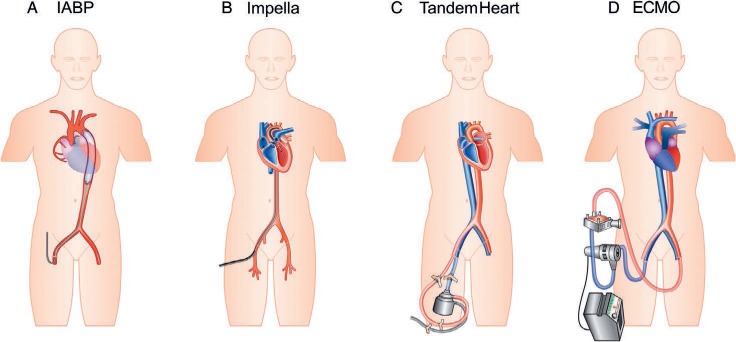
Pictorial representation of various mechanical circulatory support devices. IABP: intra-aortic balloon pump; ECMO: extracorporeal membrane oxygenation. Reprinted by permission of Oxford University Press.9
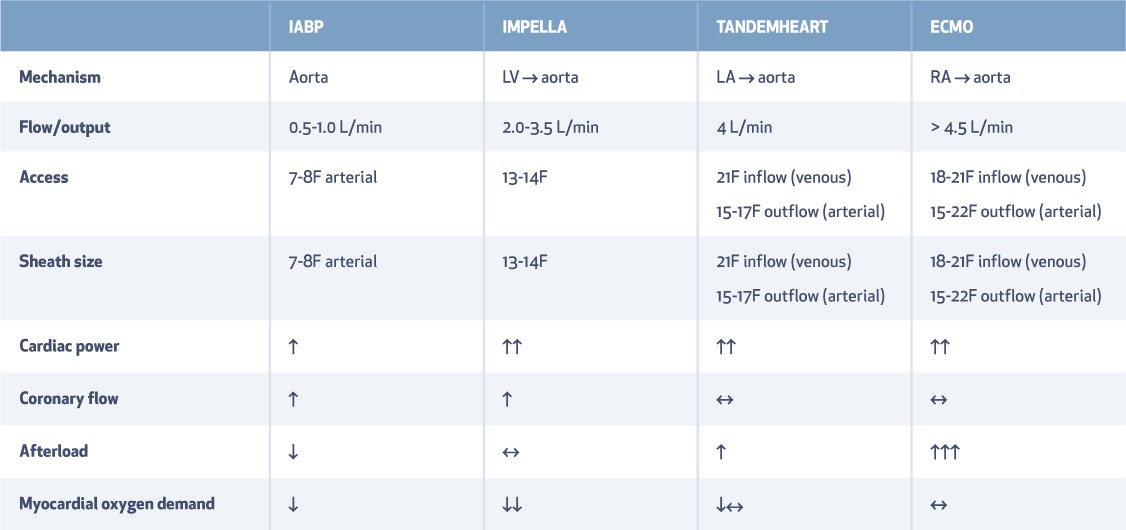
Table 2.
Types of mechanical circulatory support: access and hemodynamic impact. LV: left ventricle; LA: left atrium; RA: right atrium; IABP: intra-aortic balloon pump; ECMO: extracorporeal membrane oxygenation
INTRA-AORTIC BALLOON PUMP
For 40 years, the intra-aortic balloon pump (IABP) has been the most widely used mechanical assist device in hemodynamically unstable patients with AMI. Mounted on a catheter and inserted through the femoral or axillary artery, the IABP displaces blood volume in the descending aorta by inflating during diastole and creating a potential space during systole that facilitates cardiac filling of the aorta. The IABP console delivers a specific volume of gas (usually helium) into the balloon with rapid entry and retrieval. The primary impact of the IABP is to reduce myocardial work and oxygen demand by decreasing the duration of the isometric phase of LV contraction. The cardiovascular changes reported with IABP can result in decreased end-diastolic aortic and systolic blood pressures of up to 30% and 10%, respectively, indicating LV systolic unloading and afterload reduction and resulting in a decrease in LV diastolic volume.10 However, the IABP provides only a modest increase in cardiac index and requires a certain level of basal ventricular function due to the lack of active cardiac support (Table 2).9 In contrast to the continuous-flow devices described elsewhere in this review, the IABP requires a stable electrical rhythm or pressure tracing for optimal timing and function.
The use of IABP in cardiogenic shock and AMI has been studied extensively. A possible benefit of IABP use was initially reported in patients receiving thrombolytic therapy for ST-segment AMI.11,12 However, the treatment strategy for ST-segment AMI has evolved over the past decades to default use of primary PCI. The use of IABP in this setting was tested in the IABP Shock II trial that included patients with AMI and cardiogenic shock. This randomized trial showed no improvement in survival with IABP either during hospitalization or at 1-year follow-up. In the realm of high-risk PCI, neither randomized clinical trials nor registry data have demonstrated benefit for routine periprocedural use of IABP.13–16
IMPELLA
The Impella (ABIOMED) is a continuous nonpulsatile microaxial pump mounted on a 9F catheter that aspirates blood from the LV cavity and expels it to the ascending aorta. The efficient extraction of blood from the LV immediately reduces myocardial wall stress, pulmonary capillary wedge pressure, and myocardial oxygen consumption. The Impella comes in various sizes that reflect relative stepwise increases in cardiac output from 2.5 to 5 L/min, with a concomitant increase in sheath sizes up to the largest, Impella 5.0 (21F sheath), which usually requires surgical placement of an access conduit. The Impella 2.5 device was first tested and compared against conventional IABP therapy in a small randomized trial that included patients with cardiogenic shock.17 Although it did not show a significant decrease in mortality at 30 days, this trial demonstrated superior hemodynamic improvement (cardiac output and mean arterial pressure) with the Impella device in the short term compared with the IABP. A more recent randomized study comparing the larger Impella CP to IABP again showed similar findings, with no differences in mortality at 30 days and 6 months.18
The Impella device was subsequently tested in an elective high-risk PCI population against preprocedural IABP in the PROTECT-II study.19 This trial randomized approximately 500 patients with depressed ventricular function and multivessel coronary artery disease to IABP or Impella 2.5 at the time of PCI. The trial was stopped early after an interim analysis showed that it would not reach its primary end point of 30-day major adverse cardiac events. However, a subsequent post-hoc analysis revealed a lower rate of composite major adverse cardiac events with Impella compared to IABP at 90 days (40.8% vs 51.4%; P = .029). Real-world results using adjunctive Impella technology in patients undergoing high-risk PCI were also reported in the observational multicenter USpella registry.20,21 Data from this registry substantiated the feasibility, safety, and hemodynamic usefulness of the Impella device for high-risk PCI with favorable short- and mid-term angiographic, procedural, and clinical outcomes. The FDA has approved Impella devices for use in cardiogenic shock after AMI or open-heart surgery (Impella 2.5 and Impella CP for < 4 days and Impella 5.0 for < 6 days) and for use in urgent and elective high-risk PCI (Impella 2.5 and Impella CP ≤ 6 hours).
Technical Considerations
The Impella device requires large-bore arterial cannulation (13F for Impella 2.5 and 14F for Impella CP) and therefore requires assessment for adequate iliac and femoral artery diameters. A fluid bolus of 250 to 500 mL of normal saline is usually given to sustain ventricular filling before initiating Impella support. For most patients, the device is removed at the end of the coronary intervention after high-risk PCI. However, if retained following the intervention (i.e., in those with cardiogenic shock), the device poses some unique challenges. Adequate positioning in the LV cavity is crucial for optimum functioning, and device migration can lead to low flow, entanglement in subvalvular mitral valve apparatus, ventricular arrhythmias, and hemolysis (Tables 2, 3). In such cases, the device can usually be repositioned at bedside with transthoracic echocardiographic guidance without the need for fluoroscopy.
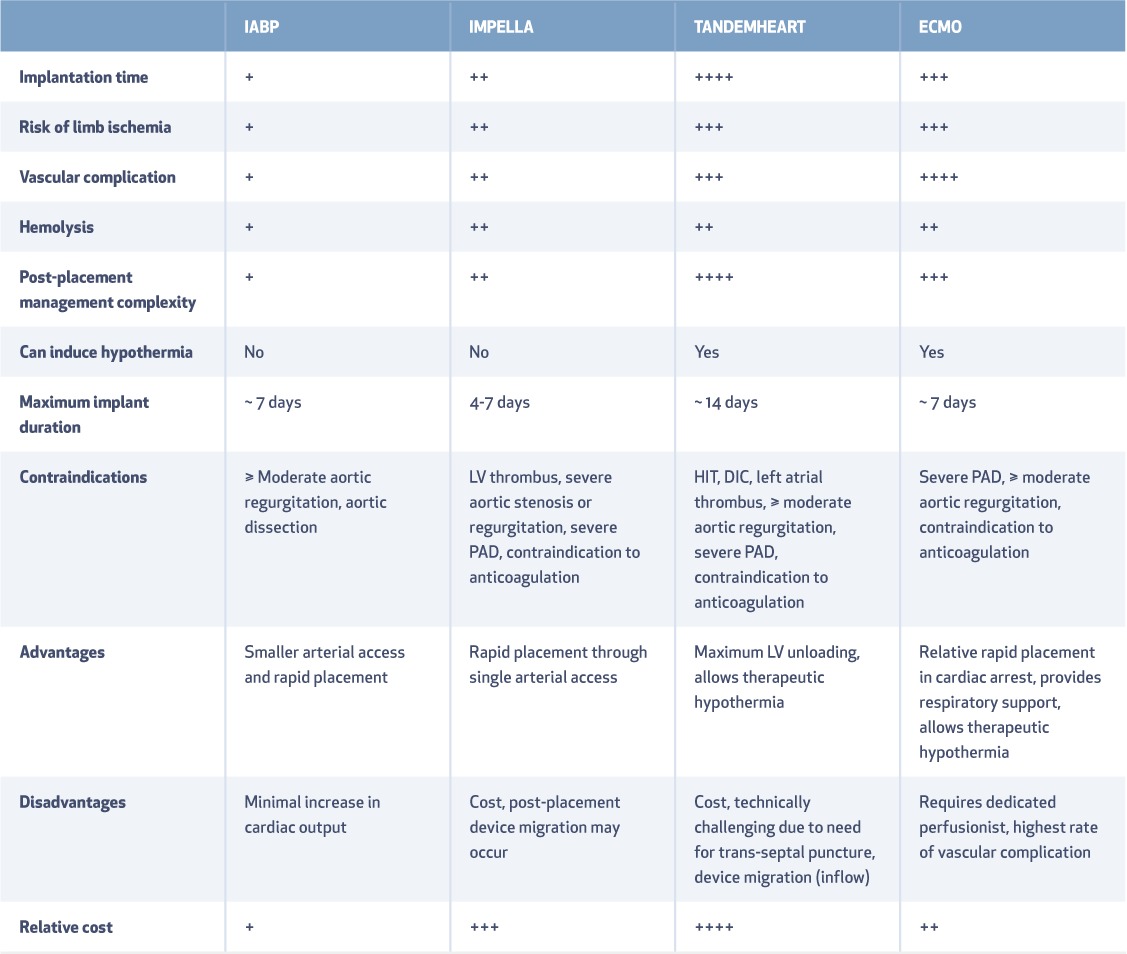
Table 3.
Technical considerations with mechanical circulatory support. IABP: intra-aortic balloon pump; ECMO: extracorporeal membrane oxygenation; HIT: heparin-induced thrombocytopenia; DIC: disseminated intravascular coagulation; PAD: peripheral arterial disease
TANDEMHEART
The TandemHeart LV assist device (CardiacAssist, Inc.) is a continuous-flow centrifugal pump wherein oxygenated blood is withdrawn from the left atrium through a 21F inflow cannula, propelled by a magnetically driven six-bladed impeller, and delivered to the femoral artery via a 17F or 19F arterial cannula.22 The device delivers up to 4 L/min of flow and is approved for use in cardiogenic shock for short-term support ranging from a few hours to 14 days.
In studies comparing the TandemHeart device with IABP in patients with cardiogenic shock,23,24 the TandemHeart consistently demonstrated superiority in hemodynamic parameters (e.g., cardiac output, mean arterial pressure) although it showed no significant survival benefit. This device has also been used with good short-term success as an adjunctive tool for high-risk PCI.25
Technical Considerations
Although it provides significant support, the TandemHeart device has several limitations that curtail its use. One limitation is the requirement of transseptal puncture for placement of a 21F inflow cannula. Transseptal puncture is associated with longer initiation times and higher complication rates when performed by inexperienced providers, and it often requires additional imaging modalities—such as intracardiac or transesophageal echocardiogram—for adequate placement (Tables 2, 3). Other drawbacks include an increased risk of hemolysis, limb ischemia, device migration, and infection with longer support times.
EXTRACORPOREAL MEMBRANE OXYGENATION
Extracorporeal membrane oxygenation (ECMO) is a modified percutaneous heart-lung bypass machine that consists of a centrifugal pump, heat exchanger, and membrane oxygenator.26 This device drains venous blood from the right atrium via an 18F to 21F inflow cannula into the external centrifugal pump, where it is sent to the oxygenator. Gaseous exchange occurs through a semipermeable membrane as blood flows through the oxygenator, and the oxygenated blood is then returned to the descending aorta via a 14F to 19F outflow cannula placed in the femoral artery. Referred to as venoarterial ECMO, this system can provide cardiac flow from between 3 and 7 L/min and can potentially be maintained for weeks. Venoarterial (VA) ECMO has largely been studied in patients who have profound cardiogenic shock with respiratory failure and cardiac arrest.27,28 Small studies evaluating patients with post-STEMI cardiogenic shock have indicated higher rates of survival to hospital discharge in patients receiving ECMO-assisted PCI compared with primary PCI alone.29
ECMO holds several advantages over other MCS devices. It can be inserted quickly at bedside and provides complete cardiopulmonary support, making it especially useful in patients who are undergoing cardiopulmonary resuscitation at the time PCI is initiated. It also provides right ventricular and/or biventricular support and has the potential to support patients with concomitant pulmonary disease due to its ability to oxygenate venous blood (Tables 2, 3). Finally, since it can be maintained for longer durations, ECMO can be used as a bridge to permanent LV assist device placement or transplant in patients with persistent cardiogenic shock.
Technical Considerations
While ECMO has several benefits over the other MCS devices, it also has limitations. For example, the risk for limb ischemia is high due to large-bore arterial access; as a result, this often requires placement of an antegrade sheath in the ipsilateral femoral artery to allow for adequate distal limb perfusion. Another major disadvantage of VA ECMO is its inability to provide adequate ventricular unloading. This can lead to increased afterload, LV distention, increased myocardial oxygen demand, and an ultimate decline in myocardial perfusion. The distension can be countered with inotropes or high-dose diuretics but often requires additional support via IABP or Impella to vent the left ventricle. Due to the complexities involved with this device, its use is only recommended in experienced centers with dedicated ECMO teams that include a perfusionist.
PRACTICAL APPROACH AND DEVICE SELECTION
Cardiogenic shock is a clinical continuum that varies from pre-shock/early shock (low cardiac index with SBP > 90 mm Hg) to severe shock (hypotension, end-organ dysfunction, and need for multiple vasopressors). For adequate device selection, the clinician must rapidly assess the severity of shock to determine the appropriate degree of cardiac support needed for PCI. This may require a right heart catheterization or assessment of LV end-diastolic pressure to determine cardiac output in borderline cases. Intricate preprocedural planning is paramount, as device selection should be made prior to intitiating the procedure rather than on an ad hoc basis. A team approach that involves an advanced heart failure specialist, cardiothoracic surgeon, and interventional cardiologist can be beneficial, although it may not be feasible in all cardiogenic shock patients who require immediate intervention.
The timing of device placement is critical for maximum benefit and successful outcomes. Prolonged cardiogenic shock results in end-organ (renal, liver, and cerebral) dysfunction, which can rapidly decline to a “hemo-metabolic” state that may not subsequently respond to improvement in cardiac output alone. Once prolonged periods of shock and multiorgan dysfunction have occurred, MCS devices have limited utility and impact.
To address this, the concept of “door to support”—placement of MCS prior to percutaneous intervention—in patients with cardiogenic shock and AMI has been proposed and, in small studies, has shown to improve outcomes.30,31 Given our experience with these devices, we recommend rapid placement of an appropriate MCS device before coronary intervention in these patients. For high-risk PCI, most cases should be elective, and MCS should be instituted by default in the catheterization laboratory prior to initiating coronary intervention.
Before selecting MCS, one must consider contraindications and nuances involved with each device as well as individual and institutional experience. In some cases of pre-shock/early shock, it may be reasonable to start with IABP and then reassess hemodynamic response. For severe cardiogenic shock, a device with a greater impact on cardiac power should be initiated, such as the Impella, VA ECMO, or TandemHeart. Specific considerations should be kept in mind; for example, the ECMO device allows for biventricular support and can be used in subjects with concomitant pulmonary dysfunction, unlike the other described devices, and an Impella device may be the easiest and quickest to initiate due to single arterial access and a simple set-up. In the setting of cardiogenic shock secondary to acute right ventricular systolic dysfunction, the main options for hemodynamic support include Tandem-Heart ProTek Duo (CardiacAssist, Inc.), Impella RP (Abiomed), and VA ECMO.32 When right ventricular function is marginal, further deterioration may occur after initiating left-sided support due to “flooding” of the right ventricle; this should be anticipated and may require initiation of right-side support. In patients with biventricular failure, percutaneous options include VA ECMO or a combination of percutaneous right ventricular assist devices such as Impella RP with an Impella CP or 5.0 or TandemHeart.
Pelvic angiography is also essential to assess peripheral arterial size and the presence of atherosclerosis and/or significant tortuosity that may complicate device placement. While the femoral artery is the default access site, the axillary or subclavian artery can be used in cases of severe femoral arterial disease.33 Obtaining arterial vascular access should be done with great precision using micropuncture techniques and/or ultrasound guidance for appropriate anterior wall cannulation above the femoral artery bifurcation. For large-bore devices (Impella, TandemHeart, or VA ECMO), preclosure of arterial access with one or two Perclose Proglide devices (Abbott Vascular, Inc.) should be strongly considered before upsizing the arteriotomy to > 8F. Additionally, a stiff 0.035-in wire such as the Amplatz Super Stiff Guidewire (Boston Scientific Corp.) or Lunderquist Extra-Stiff Wire (Cook Medical, LLC) may be inserted through a diagnostic catheter and used as a rail to overcome arterial tortuosity and ensure atraumatic delivery of a large-bore arterial cannula.32
POSTPROCEDURAL CARE AND DEVICE WEAN
Immaculate postprocedural care is essential to ensure safe and effective use of MCS. Training of the nursing staff and house staff physicians/intensivists is paramount to minimize complications and troubleshoot device-related issues. All MCS devices require systemic anticoagulation, and appropriate protocols should be established and strictly instituted for each device.
Once shock has resolved, or at the end of an elective high-risk PCI, early weaning or de-escalation of the MCS device should be immediately considered to minimize device-related complications from protracted dwell time. The device can be rapidly weaned in the catheterization laboratory in cases of high-risk PCI and removed prior to transferring to a nursing floor, whereas device weaning at the bedside should be slower (over hours). End-organ perfusion and cardiac output should be assessed during device wean and at a minimum level of support prior to discontinuing the device to avoid emergent repeat MCS. Additionally, the interventional physician using such devices must become facile with large-bore access closure techniques. While manual pressure can be successful in most patients, this should not be routinely used as default strategy especially for large-bore (> 10F) arterial access. In select cases or in cases of significantly diseased peripheral vasculature, surgical closure with cut-down may be considered as an alternate strategy.
CONCLUSIONS
Cardiogenic shock complicating AMI and complex high-risk coronary intervention are associated with a high incidence of morbidity and mortality. Percutaneous MCS devices have evolved dramatically in the last decade and have the potential to improve outcomes in these critical patients. With widespread availability of these devices, interventional physicians need to understand specific indications, contraindications, device placement, and postplacement monitoring techniques. A multidisciplinary team approach that involves adequately trained catheterization laboratory and nursing staff in addition to an interventional cardiologist, advanced heart failure physician, and/or cardiothoracic surgeon remains the cornerstone for a successful MCS program.
KEY POINTS
Patients with anatomically complex multivessel disease and acute myocardial infarction with cardiogenic shock represent a high-risk cohort.
Mechanical circulatory support devices act as an adjunct to percutaneous coronary intervention, enabling safe and successful coronary revascularization.
Mechanical circulatory support device selection should be carefully individualized with continued management using a specialized, multidisciplinary team approach.
Footnotes
Conflict of Interest Disclosure: The authors have completed and submitted the Methodist DeBakey Cardiovascular Journal Conflict of Interest Statement and none were reported.
REFERENCES
- 1. Hochman JS, Sleeper LA, Webb JG, . et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock. N Engl J Med. 1999. August 26; 341 9: 625– 34. [DOI] [PubMed] [Google Scholar]
- 2. van Diepen S, Katz JN, Albert NM, . et al. Contemporary Management of Cardiogenic Shock: A Scientific Statement From the American Heart Association. Circulation. 2017. October 17; 136 16: e232– e268. [DOI] [PubMed] [Google Scholar]
- 3. Nguyen HL, Yarzebski J, Lessard D, Gore JM, McManus DD, Goldberg RJ.. Ten-Year (2001–2011) Trends in the Incidence Rates and Short-Term Outcomes of Early Versus Late Onset Cardiogenic Shock After Hospitalization for Acute Myocardial Infarction. J Am Heart Assoc. 2017. June 7; 6 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goldberg RJ, Gore JM, Alpert JS, . et al. Cardiogenic shock after acute myocardial infarction. Incidence and mortality from a community-wide perspective, 1975 to 1988. N Engl J Med. 1991. October 17; 325 16: 1117– 22. [DOI] [PubMed] [Google Scholar]
- 5. Waldo SW, Secemsky EA, O'Brien C, . et al. Surgical ineligibility and mortality among patients with unprotected left main or multivessel coronary artery disease undergoing percutaneous coronary intervention. Circulation. 2014. December 23; 130 25: 2295– 301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gössl M, Faxon DP, Bell MR, Holmes DR, Gersh BJ.. Complete versus incomplete revascularization with coronary artery bypass graft or percutaneous intervention in stable coronary artery disease. Circ Cardiovasc Interv. 2012. August 1; 5 4: 597– 604. [DOI] [PubMed] [Google Scholar]
- 7. Nellis SH, Liedtke AJ, Whitesell L.. Small coronary vessel pressure and diameter in an intact beating rabbit heart using fixed-position and free-motion techniques. Circ Res. 1981. August; 49 2: 342– 53. [DOI] [PubMed] [Google Scholar]
- 8. Fincke R, Hochman JS, Lowe AM, . et al .; SHOCK Investigators Cardiac power is the strongest hemodynamic correlate of mortality in cardiogenic shock: a report from the SHOCK trial registry. J Am Coll Cardiol. 2004. July 21; 44 2: 340– 8. [DOI] [PubMed] [Google Scholar]
- 9. Thiele H, Smalling RW, Schuler GC.. Percutaneous left ventricular assist devices in acute myocardial infarction complicated by cardiogenic shock. Eur Heart J. 2007. September; 28 17: 2057– 63. [DOI] [PubMed] [Google Scholar]
- 10. Parissis H, Graham V, Lampridis S, Lau M, Hooks G, Mhandu PC.. IABP: history-evolution-pathophysiology-indications: what we need to know. J Cardiothorac Surg. 2016. August 4; 11 1: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barron HV, Every NR, Parsons LS, . et al .; Investigators in the National Registry of Myocardial Infarction 2 The use of intraaortic balloon counterpulsation in patients with cardiogenic shock complicating acute myocardial infarction: data from the National Registry of Myocardial Infarction 2. Am Heart J. 2001. June; 141 6: 933– 9. [DOI] [PubMed] [Google Scholar]
- 12. Ohman EM, Nanas J, Stomel RJ, . et al. Thrombolysis and counterpulsation to improve survival in myocardial infarction complicated by hypotension and suspected cardiogenic shock or heart failure: results of the TACTICS Trial. J Thromb Thrombolysis. 2005. February; 19 1: 33– 9. [DOI] [PubMed] [Google Scholar]
- 13. Perera D, Stables R, Thomas M, . et al. Elective intra-aortic balloon counterpulsation during high-risk percutaneous coronary intervention: a randomized controlled trial. JAMA. 2010. August 25; 304 8: 867– 74 [DOI] [PubMed] [Google Scholar]
- 14. Patel MR, Smalling RW, Thiele H, . et al. Intra-aortic balloon counterpulsation and infarct size in patients with acute anterior myocardial infarction without shock: the CRISP AMI randomized trial. JAMA. 2011. September 28; 306 12: 1329– 37. [DOI] [PubMed] [Google Scholar]
- 15. Curtis JP, Rathore SS, Wang Y, Chen J, Nallamothu BK, Krumholz HM.. Use and effectiveness of intra-aortic balloon pumps among patients undergoing high risk percutaneous coronary intervention: insights from the National Cardiovascular Data Registry. Circ Cardiovasc Qual Outcomes. 2012. January; 5 1: 21– 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Briguori C, Airoldi F, Chieffo A, . et al. Elective versus provisional intraaortic balloon pumping in unprotected left main stenting. Am Heart J. 2006. September; 152 3: 565– 72. [DOI] [PubMed] [Google Scholar]
- 17. Seyfarth M, Sibbing D, Bauer I, . et al. A randomized clinical trial to evaluate the safety and efficacy of a percutaneous left ventricular assist device versus intra-aortic balloon pumping for treatment of cardiogenic shock caused by myocardial infarction. J Am Coll Cardiol. 2008. November 4; 52 19: 1584– 8. [DOI] [PubMed] [Google Scholar]
- 18. Ouweneel DM, Eriksen E, Sjauw KD, . et al. Percutaneous mechanical circulatory support versus intra-aortic balloon pump in cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol. 2017. January 24; 69 3: 278– 87. [DOI] [PubMed] [Google Scholar]
- 19. O'Neill WW, Kleiman NS, Moses J, . et al. A prospective, randomized clinical trial of hemodynamic support with Impella 2.5 versus intra-aortic balloon pump in patients undergoing high-risk percutaneous coronary intervention: the PROTECT II study. Circulation. 2012. October 2; 126 14: 1717– 27. [DOI] [PubMed] [Google Scholar]
- 20. Schreiber T, Wah Htun W, Blank N, . et al. Real-world supported unprotected left main percutaneous coronary intervention with impella device; data from the USpella registry. Catheter Cardiovasc Interv. 2017. October 1; 90 4: 576– 581. [DOI] [PubMed] [Google Scholar]
- 21. Maini B, Naidu SS, Mulukutla S, . et al. Real-world use of the Impella 2.5 circulatory support system in complex high-risk percutaneous coronary intervention: the USpella Registry. Catheter Cardiovasc Interv. 2012. November 1; 80 5: 717– 25. [DOI] [PubMed] [Google Scholar]
- 22. Thiele H, Lauer B, Hambrecht R, Boudriot E, Cohen HA, Schuler G.. Reversal of cardiogenic shock by percutaneous left atrial-to-femoral arterial bypass assistance. Circulation. 2001. December 11; 104 24: 2917– 22. [DOI] [PubMed] [Google Scholar]
- 23. Burkhoff D, Cohen H, Brunckhorst C, O'Neill WW; TandemHeart Investigators Group. . A randomized multicenter clinical study to evaluate the safety and efficacy of the TandemHeart percutaneous ventricular assist device versus conventional therapy with intraaortic balloon pumping for treatment of cardiogenic shock. Am Heart J. 2006. September; 152 3: 469.e1– 8. [DOI] [PubMed] [Google Scholar]
- 24. Thiele H, Sick P, Boudriot E, . et al. Randomized comparison of intra-aortic balloon support with a percutaneous left ventricular assist device in patients with revascularized acute myocardial infarction complicated by cardiogenic shock. Eur Heart J. 2005. July; 26 13: 1276– 83. [DOI] [PubMed] [Google Scholar]
- 25. Alli OO, Singh IM, Holmes DR Jr, Pulido JN, Park SJ, Rihal CS.. Percutaneous left ventricular assist device with TandemHeart for high-risk percutaneous coronary intervention: the Mayo Clinic experience. Catheter Cardiovasc Interv. 2012. November 1; 80 5: 728– 34. [DOI] [PubMed] [Google Scholar]
- 26. MacLaren G, Combes A, Bartlett RH.. Contemporary extracorporeal membrane oxygenation for adult respiratory failure: life support in the new era. Intensive Care Med. 2012. February; 38 2: 210– 20. [DOI] [PubMed] [Google Scholar]
- 27. Nichol G, Karmy-Jones R, Salerno C, Cantore L, Becker L.. Systematic review of percutaneous cardiopulmonary bypass for cardiac arrest or cardiogenic shock states. Resuscitation. 2006. September; 70 3: 381– 94. [DOI] [PubMed] [Google Scholar]
- 28. Takayama H, Truby L, Koekort M, . et al. Clinical outcome of mechanical circulatory support for refractory cardiogenic shock in the current era. J Heart Lung Transplant. 2013. January; 32 1: 106– 11. [DOI] [PubMed] [Google Scholar]
- 29. Sheu JJ, Tsai TH, Lee FY, . et al. Early extracorporeal membrane oxygenator-assisted primary percutaneous coronary intervention improved 30-day clinical outcomes in patients with ST-segment elevation myocardial infarction complicated with profound cardiogenic shock. Crit Care Med. 2010. September; 38 9: 1810– 7. [DOI] [PubMed] [Google Scholar]
- 30. Basir MB, Schreiber TL, Grines CL, . et al. Effect of Early Initiation of Mechanical Circulatory Support on Survival in Cardiogenic Shock. Am J Cardiol. 2017. March 15; 119 6: 845– 51. [DOI] [PubMed] [Google Scholar]
- 31. O'Neill WW, Schreiber T, Wohns DH, . et al. The current use of Impella 2.5 in acute myocardial infarction complicated by cardiogenic shock: results from the USpella Registry. J J Interv Cardiol. 2014. February; 27 1: 1– 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Atkinson TM, Ohman EM, O'Neill WW, Rab T, Cigarroa JE; Interventional Scientific Council of the American College of Cardiology. . A Practical Approach to Mechanical Circulatory Support in Patients Undergoing Percutaneous Coronary Intervention: An Interventional Perspective. JACC Cardiovasc Interv. 2016. May 9; 9 9: 871– 83. [DOI] [PubMed] [Google Scholar]
- 33. Mathur M, Hira RS, Smith BM, Lombardi WL, McCabe JM.. Fully Percutaneous Technique for Transaxillary Implantation of the Impella CP. JACC Cardiovasc Interv. 2016. June 13; 9 11: 1196– 8. [DOI] [PubMed] [Google Scholar]


