Abstract
Coronary angiography is routinely used to assess the extent and severity of coronary artery disease and for decision-making during percutaneous coronary interventions (PCI). However, it is sometimes inadequate for deciding a strategy and defining optimal stenting outcomes. In this review, we present a comprehensive and practical approach to PCI using intravascular ultrasound or optical coherence tomography to optimize stent deployment and assess procedural complications after stent implantation.
Keywords: intravascular ultrasound, IVUS, optical coherence tomography, OCT, stent optimization, dissection, malapposition
INTRODUCTION
There have been a number of studies concluding that intravascular ultrasound (IVUS) assessment of minimum lumen area (MLA) enables the prediction of physiologically significant ischemia evaluated with fractional flow reserve (FFR).2–11 Moreover, meta-analyses have demonstrated that IVUS-guided percutaneous coronary interventions (PCI) for drug-eluting stent (DES) implantation reduces the risk of death, myocardial infarction (MI), stent thrombosis, and repeat revascularization.12–16 Optical coherence tomography (OCT) is a second intravascular imaging modality that uses the emission and reflection of light to evaluate coronary vessels. Providing 10 times higher resolution compared with IVUS,17,18 OCT facilitates the visualization of details including vessel anatomy and plaque morphology. In particular, its ability to determine plaque vulnerability (i.e., thin-cap fibrotic atheroma, high-attenuation plaque, and macrophage accumulation)19,20 is helpful in deciding a PCI strategy in high-risk patients. As with IVUS, the feasibility and safety of OCT-guided PCI have also been reported.21–23 Preprocedural OCT evaluation of MLA with a cut-off value of 1.6 to 1.9 mm2 is predictive of physiologically significant ischemia evaluated with FFR24,25 and also impacts decision-making with regard to PCI strategy and selection of stent size.21
After stent implantation, OCT can help optimize stent placement and detect procedure-related complications. This should be done according to the protocol adopted in the ILUMIEN III trial, which compared OCT-, IVUS-, and angiography-guided PCI.23 In ILUMIEN III, OCT-guided PCI was performed using a specific stent optimization strategy to establish stent length, diameter, and expansion according to reference segment external elastic lamina measurements. The primary end point was post-PCI minimal stent area measured by OCT, which is closely related to the risk of future stent failure. ILUMIEN III showed that all post-PCI parameters measured by OCT were comparable with those of IVUS and that both OCT and IVUS resulted in better post-PCI minimal stent area (MSA) compared to angiography. In effect, both IVUS and OCT can provide more precise planning and guidance for PCI.
STENT OPTIMIZATION
Stent Underexpansion
A number of IVUS studies demonstrate that the MSA is the strongest predictor of stent thrombosis and restenosis, 26,27 and a larger stent area has been associated with improved outcomes.13,14 Stent underexpansion, the most significant predictor of stent patency and clinical outcome, is diagnosed when a smaller in-stent lumen area is achieved compared with the reference lumen area. OCT can detect lumen contour and immediately provide luminal measurements in the lesions of interest. However, data regarding post-PCI OCT measurement (MSA, percentage area stenosis, etc.) to predict physiological ischemia or clinical outcomes are still limited pending results from the ongoing large-scale randomized ILUMIEN IV study.28
If stent underexpansion is detected, post-dilatation with an appropriately sized noncompliant balloon (based on reference lumen diameter) is recommended (Figure 1). On the other hand, post-dilatation in lesions with eccentric plaque, especially hard plaque, may risk coronary rupture. In such settings, physicians should consider the benefits and risks of further intervention to correct stent underexpansion. However, the most important priority is to avoid stent underexpansion altogether through adequate evaluation of underlying plaque morphology followed by appropriate lesion preparation.29 In this regard, intravascular imaging can help to decide PCI strategy, including lesion preparation, stent sizing, and stent optimization.
Figure 1.
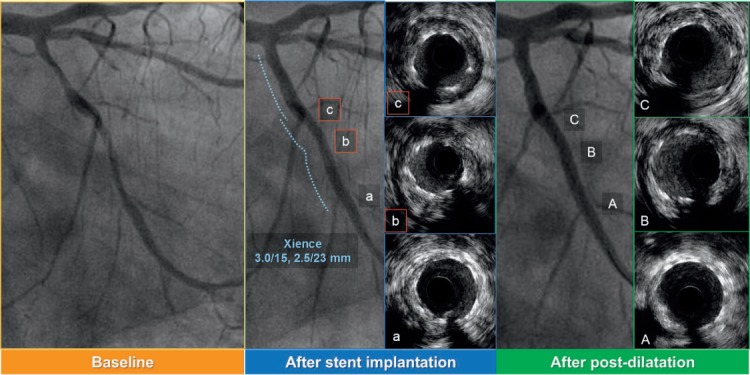
Intravascular ultrasound (IVUS)-guided post-dilatation for the stent underexpansion. (a–c) IVUS images after stent implantation (b and c show stent underexpansion). Stent area (SA) is 3.45 mm2 in panel b, 3.81 mm2 in panel c. (A–C) IVUS images after IVUS-guided post-dilatation with 2.5-mm noncompliant balloon. SA: 4.65 mm2 in panel B, 6.03 mm2 in panel C.
Stent Malapposition
Stent malapposition (SM) can be confirmed with IVUS when the stent struts float in the vessel lumen and are not apposed to the vessel wall (Figure 2). Stent malapposition with OCT is more accurate and is confirmed when the struts are not in contact with the vessel wall (Figure 3 and Online Video 1).30 Substantial malapposition delays stent endothelialization and can be a potential cause of stent thrombosis.31–33 Malapposition can occur in the presence of eccentric or excessively protruded plaque, severely calcified plaque, or with undersized stent implantation and is most frequently observed at stent edges.34,35
Figure 2.
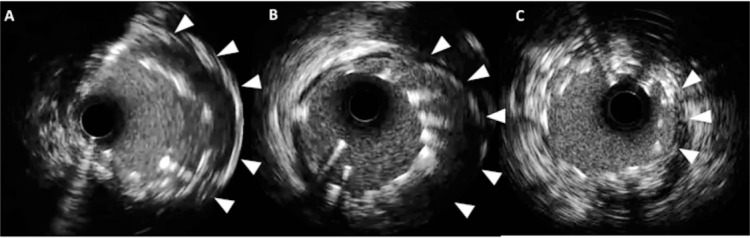
Three cases of stent malapposition with intravascular ultrasound. (A–C) Substantial malapposition (arrowheads) is identified between vessel wall and stent struts.
Figure 3 and Online Video 1.
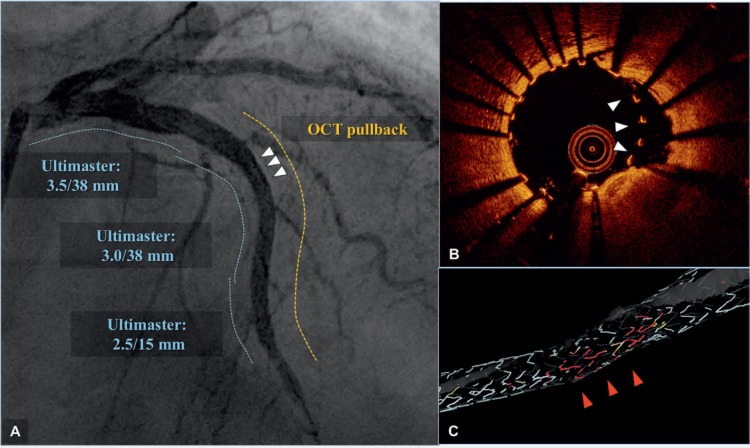
Automatic stent struts detection system (OPTISTM Stent Optimization Software) of optical coherence tomography (OCT). Angiographic results (A) were excellent after implantation of an Ultimaster (Terumo Corp.) drug-eluting stent for a diffusely diseased left anterior descending artery (dotted lines indicate DES-treated area before [blue] and after OCT pullback [yellow]). However, despite the excellent angiographic results, OCT (B) showed malapposed struts (white arrowheads in 3 A and B), which were automatically detected and highlighted with red in a 3-dimensional reconstruction image (C).
During follow-up, some cases of SM can resolve with reendothelialization. However, due to the multifactorial nature of these events (i.e., stent design, strut thickness, types of polymer, underlying plaque morphology), there is no consensus on the maximum distance between stent struts and vessel lumen that can be associated with endothelialization or adverse events.32,36 Therefore, the decision to perform further intervention on a SM is most likely left to operator discretion. Once SM is detected, the most frequent correction is post-dilatation with a noncompliant balloon. Selection of appropriate balloon size should, in most cases, be based on reference lumen diameters to avoid stent edge dissections. It is also important to take note of plaque morphology around the stent edge.
PROCEDURE-RELATED COMPLICATIONS
Stent Edge Dissection
Dissection or intramural hematoma are some of the important causes of peri-stent narrowing following implantation. Dissection can occur within the intima (intimal tear) or can reach the media (medial dissection) (Figure 4). As seen in Figure 5, an intramural hematoma can occur with medial dissection, which typically does not have a reentry site. In this case, an imaging study is indispensable to evaluate the location and length, depth (intimal or medial), circumferential and longitudinal extension, side branch involvement, and residual lumen area. Intramural hematoma generally is less likely to propagate since dissection at the proximal edge of the stent is retrograde, and the flap may be suppressed by antegrade flow. However, in cases of distal edge dissection, extreme care should be taken because antegrade dissection may turn into flap dissection and eventually lead to limited blood flow and coronary obstruction. The CLI-OPCI (Centro per la Lotta contro l'Infarto-Optimisation of Percutaneous Coronary Intervention) registry reported that dissections detected by OCT that were > 200 μm at the distal stent edge were independent predictors of adverse cardiac events (composite of all-cause death, MI, and target lesion revascularization, HR 2.54, 1.3–4.8).37 Conversely, some studies reported that edge dissections detected by OCT alone (i.e., undetectable with angiography) were not associated with restenosis and stent thrombosis at 1-year follow-up38; however, in these studies, edge dissection was defined only by the presence of luminal surface disruptions without cut-off values for width or length. Indeed, “minor” edge dissections, which are detectable with OCT alone and do not limit flow, can frequently heal and have no association with clinical events. Therefore, further prospective studies are needed to clarify cut-off values or features of OCT-detected edge dissections that may require additional intervention.
Figure 4.
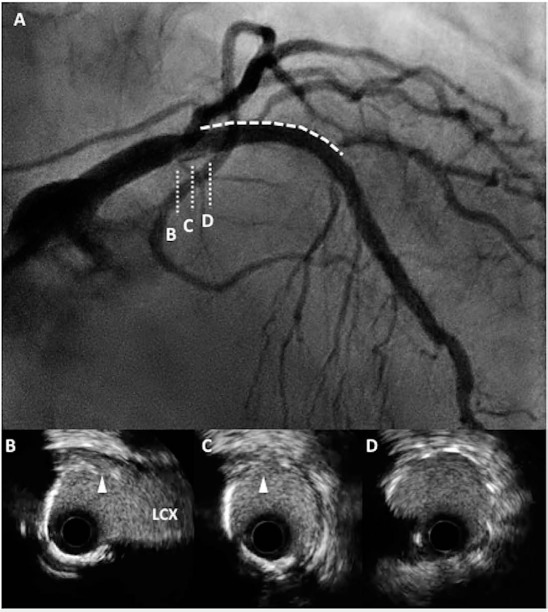
(A) Coronary angiography (CAG) demonstrates dissection from the proximal edge of the stent (dotted line and D) to the left main trunk (indicated as B, C). (B–D) Cross-sectional intravascular ultrasound images show medial dissection (arrowheads) close to left circumflex artery (LCX).
Figure 5.
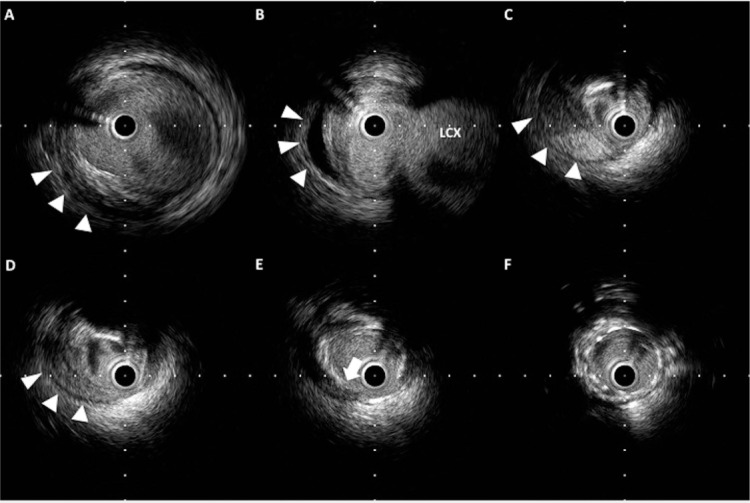
Retrograde dissection and intramural hematoma. (A–D) Hematoma is observed within the media (arrowheads) and has pushed the lumen inward. (E) The entry site of the hematoma (arrow) reaches the media. (F) Stent is implanted with good apposition.
Tissue Protrusion
Tissue protrusion (TP) within the stent strut can occasionally be identified after stent implantation. The incidence of TP can depend on the underlying plaque morphology (e.g., thin cap fibrotic atheroma and thrombus), clinical presentation, and procedure-related factors (e.g., type of stent, and post-dilatation). With coronary angiography, tissue protrusion is identified as a contrast defect within the stent and can sometimes appear as a stenosis. With IVUS, TP is revealed as a relatively high echogenic signal (Figure 6), whereas with OCT it appears as a smooth surface with high signal attenuation.39 Resolution with OCT is twice as high as with IVUS,22,32,39 enabling it to identify plaque protrusions with unprecedented precision; it also can detect plaques that are prone to rupture.39 One study using post-procedure OCT imaging to identify predictors for device-related clinical end points found a 92.9% incidence of smooth protrusion at 1-year follow-up.40 Despite its unprecedented resolution, the impact of OCT findings on outcomes/adverse events remains unclear.37,40
Figure 6.
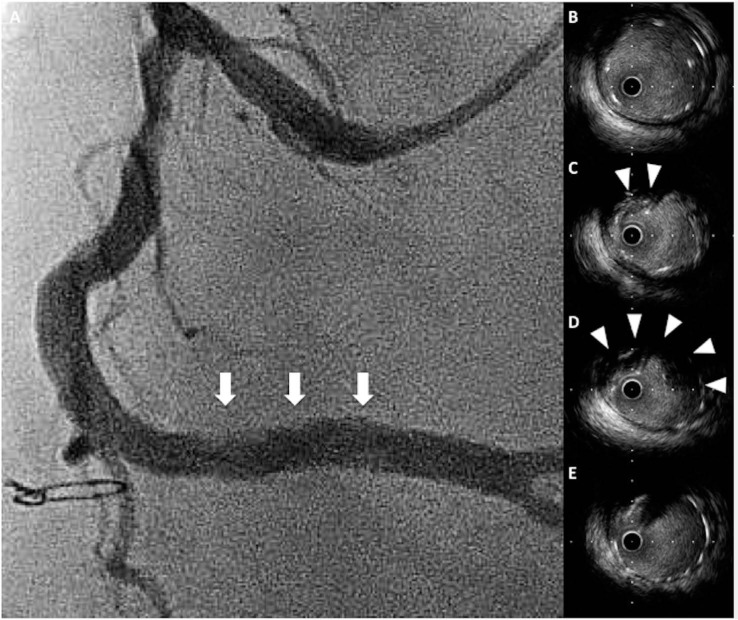
Tissue protrusion. (A) Coronary angiography demonstrates a hazy appearance within the stent (arrows). (C, D) Cross-sectional IVUS images show tissue protrusion into the stent struts (arrowheads). (B, E) Stent is well-expanded without tissue protrusion.
Tissue protrusion detected by IVUS is reported to be associated with poor short-term outcomes, including no-reflow phenomenon, periprocedural MI, and acute/subacute stent thrombosis.41 Serial OCT analyses showed that TP was detected in 28% of OCT cross-sections after stent implantation, but all of them resolved at the 8-month follow-up.32 According to available data, the majority of TPs detected by intravascular imaging can be considered benign; however, further investigations are needed to characterize TPs that require additional intervention. If further intervention is needed, larger-diameter and higher-pressure balloon inflation may push TPs toward the vessel wall, although there is a possibility of distal embolization that might lead to slow flow or no reflow phenomena. If post-dilatation is needed, a protection device should be considered to prevent distal embolization.
Plaque Shift
Mintz et al.42 reported that balloon angioplasty does not change plaque volume, and lumen enlargement is mainly due to combined plaque dissection, arterial expansion, and plaque redistribution. Although plaque volume reduction can be seen after stenting, especially in unstable lesions,43 longitudinal plaque redistribution is one of the important mechanisms of lumen enlargement and may result in longitudinal plaque shift.
Coronary Spasm
Coronary spasm represents one of the important causes of peristent narrowing after stent implantation. In cases of suspected spasm, an intracoronary injection of nitrate is the first step to determine whether the new stenosis might improve. If the nitrate injection does not resolve coronary narrowing at the edge of the stent, an imaging study can be useful in identifying the cause. Both IVUS and OCT can detect diffuse intimal and medial thickness and luminal narrowing at the spasm site.44,45 A pathology study suggested that coronary spasm is induced by medial thickening and folding of the internal elastic lamina (IEL).46 This folding occurs from shrinkage of coronary vessels due to decreased flow from severely stenotic arteries. A peri-medial high echoic band (PHB) detected by IVUS may indicate folding of the IEL and thus the potential to relieve the spasm without mechanical intervention. If the PHB can be seen distal to a chronic total occlusion after recanalization, vessel diameter will eventually enlarge.47
ADDITIONAL APPLICATIONS OF INTRAVASCULAR IMAGING
60-MHz High-Resolution IVUS
High-resolution IVUS has a higher frequency than the standard IVUS that is currently used (Figure 7), and three 60-MHz high-resolution systems are now available from ACIST Medical Systems, Boston Scientific, and Terumo. High-resolution IVUS technology can provide many potential advantages, including (1) evaluation after bioresorbable vascular scaffold implantation, (2) assessment of unstable plaque and plaque rupture, and (3) identification of intraplaque hemorrhage and macrophage accumulation. Further studies are warranted to identify the efficacy of high-resolution IVUS for assessing stent results.
Figure 7.
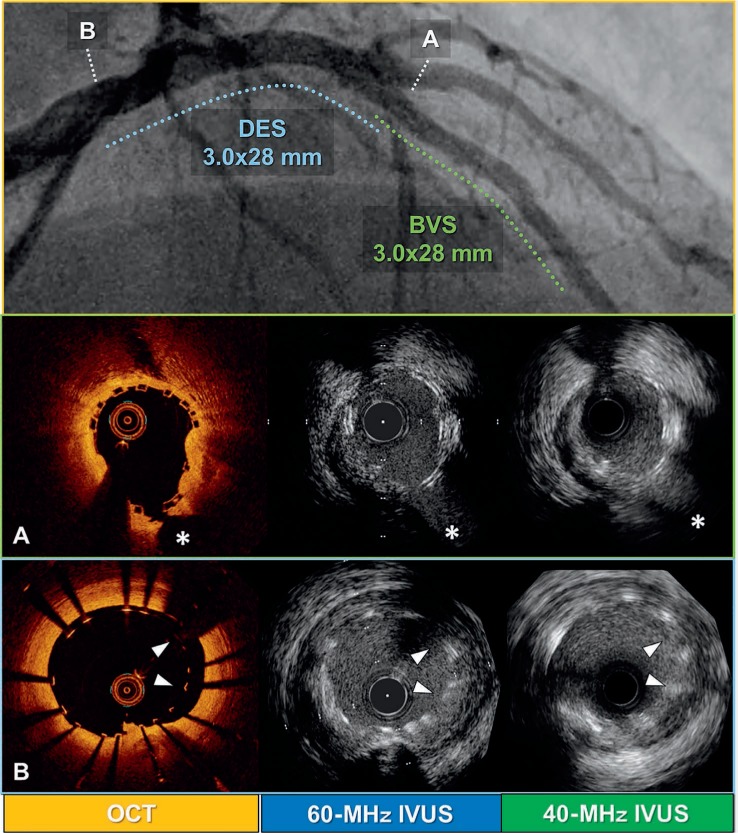
Comparison of different intravascular imaging modalities (OCT, 40-MHz IVUS, and 60-MHz IVUS) after left main trunk (LMT) to left anterior descending artery (LAD) percutaneous coronary intervention with drug-eluting stent (DES) and bioresorbable vascular scaffold (BVS). (A) Mid-LAD lesion treated with BVS (asterisks indicate septal branch). (B) LMT lesion treated with DES (arrowheads indicate malapposed struts). Top panel shows final coronary angiography after the procedure (dashed lines indicate lesions treated with BVS [green] and DES [blue]).
Bifurcation Stenting
Bifurcation lesions are some of the most challenging lesion subsets for PCI due to the higher incidence of restenosis and stent thrombosis.48,49 For most bifurcation lesions, the provisional single-stent approach is the gold standard in terms of short-term efficacy and safety and lower long-term mortality.50
However, some complex lesions may require a two-stent strategy. In these cases, OCT can immediately provide online 3-dimensional (3D) reconstruction images to help the physician visualize and understand complex bifurcation anatomy and determine the most effective PCI strategy.51 Moreover, automatic stent struts detection systems52 can help operators evaluate wire re-crossing points of the main branch stent struts towards the side branch, which may optimize the kissing balloon inflation technique and lead to more favorable results.53
LESIONS TREATED WITH BIORESORBABLE VASCULAR SCAFFOLDS
For the best possible results after bioresorbable vascular scaffold (BVS) implantation, the recommended technique is the PSP method: Prepare the lesion using adequate predilatation, Size the vessel/scaffold correctly, and Post-dilate the scaffold to avoid underexpansion.54 In this regard, intravascular imaging evaluation with IVUS or OCT should be mandatory. Improper implantation of current-generation BVSs have been associated with unfavorable findings such as malapposition, underexpansion, and incorrect sizing (e.g., large BVS implanted in a small vessel) that are associated with a higher risk of scaffold thrombosis.55 The differential impact between IVUS and OCT guidance on acute procedural results and follow-up clinical outcomes remains unclear; however, with this specific device, OCT plays a crucial role in order to precisely evaluate these results after implantation.
CONCLUSIONS
Intravascular imaging after stent implantation is very helpful in optimizing stent results and contributes to favorable clinical outcomes. In clinical settings, physicians should consider the different features of IVUS versus OCT and decide the best possible strategy based on this assessment. New technologies such as high-resolution IVUS and OCT 3D reconstruction will hopefully provide further clinical implications of intravascular image-guided PCI.
KEY POINTS
Intravascular imaging can provide accurate assessment of stent deployment and procedural complications.
Stent optimization includes the assessment of stent expansion and malapposition.
Intravascular imaging is useful in evaluating complications after stent implantation, including stent edge dissection, tissue protrusion, plaque shift, and coronary spasm.
Additional applications of intravascular imaging including 60-MHz high-resolution IVUS, assessment of bifurcation stenting, and bioresorbable vascular scaffold-treated lesions help optimize both procedural and long-term clinical outcomes.
Supplementary Material
Footnotes
Drs. Hachinohe and Mitomo contributed equally and are joint first authors.
Conflict of Interest Disclosure: Dr. Latib is a formal advisor for 4-Tech, Valtech Cardio, Mitralign, Medtronic, Edwards, and Abbott Vascular.
REFERENCES
- 1. Windecker S, Kolh P, Alfonso F, . et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2014. October 1; 35 37: 2541– 619. [DOI] [PubMed] [Google Scholar]
- 2. Tobis J, Azarbal B, Slavin L.. Assessment of intermediate severity coronary lesions in the catheterization laboratory. J Am Coll Cardiol. 2007. February 27; 49 8: 839– 48. [DOI] [PubMed] [Google Scholar]
- 3. Nam CW, Yoon HJ, Cho YK, . et al. Outcomes of percutaneous coronary intervention in intermediate coronary artery disease: fractional flow reserve-guided versus intravascular ultrasound-guided. JACC Cardiovasc Interv. 2010. August; 3 8: 812– 7. [DOI] [PubMed] [Google Scholar]
- 4. Ben-Dor I, Torguson R, Gaglia MA Jr, . et al. Correlation between fractional flow reserve and intravascular ultrasound lumen area in intermediate coronary artery stenosis. EuroIntervention. 2011. June; 7 2: 225– 33. [DOI] [PubMed] [Google Scholar]
- 5. Kang SJ, Lee JY, Ahn JM, . et al. Intravascular ultrasound-derived predictors for fractional flow reserve in intermediate left main disease. JACC Cardiovasc Interv. 2011. November; 4 11: 1168– 74. [DOI] [PubMed] [Google Scholar]
- 6. Koo BK, Yang HM, Doh JH, . et al. Optimal intravascular ultrasound criteria and their accuracy for defining the functional significance of intermediate coronary stenoses of different locations. JACC Cardiovasc Interv. 2011. July; 4 7: 803– 11. [DOI] [PubMed] [Google Scholar]
- 7. Puri R, Kapadia SR, Nicholls SJ, Harvey JE, Kataoka Y, Tuzcu EM.. Optimizing outcomes during left main percutaneous coronary intervention with intravascular ultrasound and fractional flow reserve: the current state of evidence. JACC Cardiovasc Interv 2012; 5 7: 697– 707. [DOI] [PubMed] [Google Scholar]
- 8. de la Torre Hernandez JM, Lopez-Palop R, Garcia Camarero T, . et al. Clinical outcomes after intravascular ultrasound and fractional flow reserve assessment of intermediate coronary lesions. Propensity score matching of large cohorts from two institutions with a differential approach. EuroIntervention. 2013. November; 9 7: 824– 30. [DOI] [PubMed] [Google Scholar]
- 9. Park SJ, Ahn JM, Kang SJ, . et al. Intravascular ultrasound-derived minimal lumen area criteria for functionally significant left main coronary artery stenosis. JACC Cardiovasc Interv. 2014. August; 7 8: 868– 74. [DOI] [PubMed] [Google Scholar]
- 10. D'Ascenzo F, Barbero U, Cerrato E, . et al. Accuracy of intravascular ultrasound and optical coherence tomography in identifying functionally significant coronary stenosis according to vessel diameter: A meta-analysis of 2,581 patients and 2,807 lesions. Am Heart J. 2015. May; 169 5: 663– 73. [DOI] [PubMed] [Google Scholar]
- 11. Jin XJ, Tahk SJ, Yang HM, . et al. The relationship between intravascular ultrasound-derived percent total atheroma volume and fractional flow reserve in the intermediate stenosis of proximal or middle left anterior descending coronary artery. Int J Cardiol. 2015. April 15; 185: 56– 61. [DOI] [PubMed] [Google Scholar]
- 12. Klersy C, Ferlini M, Raisaro A, . et al. Use of IVUS guided coronary stenting with drug eluting stent: a systematic review and meta-analysis of randomized controlled clinical trials and high quality observational studies. Int J Cardiol. 2013. December 5; 170 1: 54– 63. [DOI] [PubMed] [Google Scholar]
- 13. Jang JS, Song YJ, Kang W, . et al. Intravascular ultrasound-guided implantation of drug-eluting stents to improve outcome: a meta-analysis. JACC Cardiovasc Interv. 2014. March; 7 3: 233– 43. [DOI] [PubMed] [Google Scholar]
- 14. Ahn JM, Kang SJ, Yoon SH, . et al. Meta-analysis of outcomes after intravascular ultrasound-guided versus angiography-guided drug-eluting stent implantation in 26,503 patients enrolled in three randomized trials and 14 observational studies. Am J Cardiol. 2014. April 15; 113 8: 1338– 47. [DOI] [PubMed] [Google Scholar]
- 15. Elgendy IY, Mahmoud AN, Elgendy AY, Bavry AA.. Outcomes With Intravascular Ultrasound-Guided Stent Implantation: A Meta-Analysis of Randomized Trials in the Era of Drug-Eluting Stents. Circ Cardiovasc Interv. 2016. April; 9 4: e003700. [DOI] [PubMed] [Google Scholar]
- 16. Zhang Y, Farooq V, Garcia-Garcia HM, . et al. Comparison of intravascular ultrasound versus angiography-guided drug-eluting stent implantation: a meta-analysis of one randomised trial and ten observational studies involving 19,619 patients. EuroIntervention. 2012. November 22; 8 7: 855– 65. [DOI] [PubMed] [Google Scholar]
- 17. Bezerra HG, Costa MA, Guagliumi G, Rollins AM, Simon DI.. Intracoronary optical coherence tomography: a comprehensive review clinical and research applications. JACC Cardiovasc Interv. 2009. November; 2 11: 1035– 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Costopoulos C, Brown AJ, Teng Z, . et al. Intravascular ultrasound and optical coherence tomography imaging of coronary atherosclerosis. Int J Cardiovasc Imaging. 2016. January; 32 1: 189– 200. [DOI] [PubMed] [Google Scholar]
- 19. Tearney GJ, Yabushita H, Houser SL, . et al. Quantification of macrophage content in atherosclerotic plaques by optical coherence tomography. Circulation. 2003. January 7; 107 1: 113– 9. [DOI] [PubMed] [Google Scholar]
- 20. Otsuka F, Joner M, Prati F, Virmani R, Narula J.. Clinical classification of plaque morphology in coronary disease. Nat Rev Cardiol. 2014. July; 11 7: 379– 89. [DOI] [PubMed] [Google Scholar]
- 21. Wijns W, Shite J, Jones MR, . et al. Optical coherence tomography imaging during percutaneous coronary intervention impacts physician decision-making: ILUMIEN I study. Eur Heart J. 2015. December 14; 36 47: 3346– 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Maehara A, Ben-Yehuda O, Ali Z, . et al. Comparison of Stent Expansion Guided by Optical Coherence Tomography Versus Intravascular Ultrasound: The ILUMIEN II Study (Observational Study of Optical Coherence Tomography [OCT] in Patients Undergoing Fractional Flow Reserve [FFR] and Percutaneous Coronary Intervention). JACC Cardiovasc Interv. 2015. November; 8 13: 1704– 14. [DOI] [PubMed] [Google Scholar]
- 23. Ali ZA1, Maehara A, Généreux P, . et al. Optical coherence tomography compared with intravascular ultrasound and with angiography to guide coronary stent implantation (ILUMIEN III: OPTIMIZE PCI): a randomised controlled trial. Lancet. 2016. November 26; 388 10060: 2618– 28. [DOI] [PubMed] [Google Scholar]
- 24. Shiono Y, Kitabata H, Kubo T, . et al. Optical coherence tomography-derived anatomical criteria for functionally significant coronary stenosis assessed by fractional flow reserve. Circ J. 2012; 76 9: 2218– 25. [DOI] [PubMed] [Google Scholar]
- 25. Zafar H, Ullah I, Dinneen K, . et al. Evaluation of hemodynamically severe coronary stenosis as determined by fractional flow reserve with frequency domain optical coherence tomography measured anatomical parameters. J Cardiol. 2014. July; 64 1: 19– 24. [DOI] [PubMed] [Google Scholar]
- 26. Doi H, Maehara A, Mintz GS, . et al. Impact of post-intervention minimal stent area on 9-month follow-up patency of paclitaxeleluting stents: an integrated intravascular ultrasound analysis from the TAXUS IV, V, and VI and TAXUS ATLAS Workhorse, Long Lesion, and Direct Stent Trials. JACC Cardiovasc Interv. 2009. December; 2 12: 1269– 75. [DOI] [PubMed] [Google Scholar]
- 27. Fujii K, Carlier SG, Mintz GS, . et al. Stent underexpansion and residual reference segment stenosis are related to stent thrombosis after sirolimus-eluting stent implantation: an intravascular ultrasound study. J Am Coll Cardiol. 2005. April 5; 45 7: 995– 8. [DOI] [PubMed] [Google Scholar]
- 28. Reith S, Battermann S, Hellmich M, Marx N, Burgmaier M.. Correlation between OCT-derived intrastent dimensions and fractional flow reserve measurements after coronary stent implantation and impact on clinical outcome. J Invasive Cardiol. 2015. May; 27 5: 222– 8. [PubMed] [Google Scholar]
- 29. de Ribamar Costa J Jr, Mintz GS, Carlier SG, . et al. Nonrandomized comparison of coronary stenting under intravascular ultrasound guidance of direct stenting without predilation versus conventional predilation with a semi-compliant balloon versus predilation with a new scoring balloon. Am J Cardiol. 2007. September 1; 100 5: 812– 7. [DOI] [PubMed] [Google Scholar]
- 30. Tearney GJ, Regar E, Akasaka T, . et al. Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: a report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J Am Coll Cardiol. 2012. March 20; 59 12: 1058– 72. [DOI] [PubMed] [Google Scholar]
- 31. Nakazawa G, Finn AV, Vorpahl M, Ladich ER, Kolodgie FD, Virmani R.. Coronary responses and differential mechanisms of late stent thrombosis attributed to first-generation sirolimus- and paclitaxel-eluting stents. J Am Coll Cardiol. 2011. January 25; 57 4: 390– 8. [DOI] [PubMed] [Google Scholar]
- 32. Kawamori H, Shite J, Shinke T, . et al. Natural consequence of post-intervention stent malapposition, thrombus, tissue prolapse, and dissection assessed by optical coherence tomography at mid-term follow-up. Eur Heart J Cardiovasc Imaging. 2013. September; 14 9: 865– 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Taniwaki M, Radu MD, Zaugg S, . et al. Mechanisms of Very Late Drug-Eluting Stent Thrombosis Assessed by Optical Coherence Tomography. Circulation. 2016. February 16; 133 7: 650– 60. [DOI] [PubMed] [Google Scholar]
- 34. Attizzani GF, Capodanno D, Ohno Y, Tamburino C.. Mechanisms, pathophysiology, and clinical aspects of incomplete stent apposition. J Am Coll Cardiol. 2014. April 15; 63 14: 1355– 67. [DOI] [PubMed] [Google Scholar]
- 35. Im E, Kim BK, Ko YG, . et al. Incidences, predictors, and clinical outcomes of acute and late stent malapposition detected by optical coherence tomography after drug-eluting stent implantation. Circ Cardiovasc Interv. 2014. February; 7 1: 88– 96. [DOI] [PubMed] [Google Scholar]
- 36. Inoue T, Shinke T, Otake H, . et al. Impact of strut-vessel distance and underlying plaque type on the resolution of acute strut malapposition: serial optimal coherence tomography analysis after everolimus-eluting stent implantation. Int J Cardiovasc Imaging. 2014. June; 30 5: 857– 65. [DOI] [PubMed] [Google Scholar]
- 37. Prati F, Romagnoli E, Burzotta F, . et al. Clinical Impact of OCT Findings During PCI: The CLI-OPCI II Study. JACC Cardiovasc Imaging. 2015. November; 8 11: 1297– 305. [DOI] [PubMed] [Google Scholar]
- 38. Radu MD, Räber L, Heo J, . et al. Natural history of optical coherence tomography-detected non-flow-limiting edge dissections following drug-eluting stent implantation. EuroIntervention. 2014. January 22; 9 9: 1085– 94. [DOI] [PubMed] [Google Scholar]
- 39. Roleder T, Jąkała J, Kałuża GL, . et al. The basics of intravascular optical coherence tomography. Postepy Kardiol Interwencyjnej. 2015; 11 2: 74– 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Soeda T, Uemura S, Park SJ, . et al. Incidence and Clinical Significance of Poststent Optical Coherence Tomography Findings: One-Year Follow-Up Study From a Multicenter Registry. Circulation. 2015. September 15; 132 11: 1020– 9. [DOI] [PubMed] [Google Scholar]
- 41. Hong YJ, Jeong MH, Choi YH, . et al. Impact of tissue prolapse after stent implantation on short- and long-term clinical outcomes in patients with acute myocardial infarction: an intravascular ultrasound analysis. Int J Cardiol. 2013. July 1; 166 3: 646– 51. [DOI] [PubMed] [Google Scholar]
- 42. Mintz GS, Pichard AD, Kent KM, Satler LF, Popma JJ, Leon MB.. Axial plaque redistribution as a mechanism of percutaneous transluminal coronary angioplasty. Am J Cardiol. 1996. February 15; 77 5: 427– 30. [DOI] [PubMed] [Google Scholar]
- 43. Prati F, Pawlowski T, Gil R, . et al. Stenting of culprit lesions in unstable angina leads to a marked reduction in plaque burden: a major role of plaque embolization? A serial intravascular ultrasound study. Circulation. 2003. May 13; 107 18: 2320– 5. [DOI] [PubMed] [Google Scholar]
- 44. Ota H, Kawase Y, Kondo H, . et al. A case report of acute myocardial infarction induced by coronary spasm. Intravascular findings. Int Heart J. 2013; 54 4: 237– 9. [DOI] [PubMed] [Google Scholar]
- 45. Tanaka A, Shimada K, Tearney GJ, . et al. Conformational change in coronary artery structure assessed by optical coherence tomography in patients with vasospastic angina. J Am Coll Cardiol. 2011. October 4; 58 15: 1608– 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Uchida Y, Uchida Y, Matsuyama A, . et al. Functional medial thickening and folding of the internal elastic lamina in coronary spasm. Am J Physiol Heart Circ Physiol. 2011. February; 300 2: H423– 30. [DOI] [PubMed] [Google Scholar]
- 47. Neishi Y, Okura H, Kume T, Fukuhara K, Yamada R, Yoshida K.. Prediction of chronic vessel enlargement by a novel intravascular ultrasound finding. Circ J. 2015; 79 3: 607– 12. [DOI] [PubMed] [Google Scholar]
- 48. Colombo A, Moses JW, Morice MC, . et al. Randomized study to evaluate sirolimus-eluting stents implanted at coronary bifurcation lesions. Circulation. 2004. March 16; 109 10: 1244– 9. [DOI] [PubMed] [Google Scholar]
- 49. Iakovou I, Schmidt T, Bonizzoni E, . et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA. 2005. May 4; 293 17: 2126– 30. [DOI] [PubMed] [Google Scholar]
- 50. Behan MW, Holm NR, de Belder AJ, . et al. Coronary bifurcation lesions treated with simple or complex stenting: 5-year survival from patient-level pooled analysis of the Nordic Bifurcation Study and the British Bifurcation Coronary Study. Eur Heart J. 2016. June 21; 37 24: 1923– 8. [DOI] [PubMed] [Google Scholar]
- 51. Onuma Y, Okamura T, Muramatsu T, Uemura S, Serruys PW.. New implication of three-dimensional optical coherence tomography in optimising bifurcation PCI. EuroIntervention. 2015; 11 Suppl V: V71– 4. [DOI] [PubMed] [Google Scholar]
- 52. Wang A, Eggermont J, Dekker N, . et al. Automatic stent strut detection in intravascular optical coherence tomographic pullback runs. Int J Cardiovasc Imaging. 2013. January; 29 1: 29– 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Murasato Y, Iwasaki K, Yamamoto T, . et al. Optimal kissing balloon inflation after single-stent deployment in a coronary bifurcation model. EuroIntervention. 2014. December; 10 8: 934– 41. [DOI] [PubMed] [Google Scholar]
- 54. Puricel S, Cuculi F, Weissner M, . et al. Bioresorbable Coronary Scaffold Thrombosis: Multicenter Comprehensive Analysis of Clinical Presentation, Mechanisms, and Predictors. J Am Coll Cardiol. 2016. March 1; 67 8: 921– 31. [DOI] [PubMed] [Google Scholar]
- 55. Sotomi Y, Suwannasom P, Serruys PW, Onuma Y.. Possible mechanical causes of scaffold thrombosis: insights from case reports with intracoronary imaging. EuroIntervention. 2017. February 20; 12 14: 1747– 56. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


