Abstract
Background:
Obesity causes diastolic dysfunction, and is one of the leading causes of heart failure with preserved ejection fraction. Myocardial relaxation is determined by both active metabolic processes such as impaired energetic status and steatosis, as well as intrinsic myocardial remodelling. However, the relative contribution of each to diastolic dysfunction in obesity is currently unknown.
Methods:
Eighty adult subjects (48 male) with no cardiovascular risk factors across a wide range of body mass indices (18.4–53.0 kg m−2) underwent magnetic resonance imaging for abdominal visceral fat, left ventricular geometry (LV mass:volume ratio) and diastolic function (peak diastolic strain rate), and magnetic resonance spectroscopy for PCr/ATP and myocardial triglyceride content.
Results:
Increasing visceral obesity was related to diastolic dysfunction (peak diastolic strain rate, r=−0.46, P=0.001). Myocardial triglyceride content (β=−0.2, P=0.008), PCr/ATP (β=−0.22, P=0.04) and LV mass:volume ratio (β=−0.61, P=0.04) all independently predicted peak diastolic strain rate (model R2 0.36, P<0.001). Moderated multiple regression confirmed the full mediating roles of PCr/ATP, myocardial triglyceride content and LV mass:volume ratio in the relationship between visceral fat and peak diastolic strain rate. Of the negative effect of visceral fat on diastolic function, 40% was explained by increased myocardial triglycerides, 39% by reduced PCr/ATP and 21% by LV concentric remodelling.
Conclusions:
Myocardial energetics and steatosis are more important in determining LV diastolic function than concentric hypertrophy, accounting for more of the negative effect of obesity on diastolic function than LV geometric remodelling. Targeting these metabolic processes is an attractive strategy to treat diastolic dysfunction in obesity.
Introduction
It is now well established that obesity is intrinsically linked with the development of heart failure,1, 2 a condition with profound morbidity and mortality. The threatened global pandemic of obesity is likely to have a substantial impact on the incidence of heart failure. As the two conditions now frequently co-exist, the management of heart failure in the context of obesity is becoming a significant clinical concern.
Around 50% of patients presenting with new heart failure have normal systolic function, but rather pronounced diastolic impairment as the cause of their symptoms.3 This is important, as although linked with heart failure with reduced ejection fraction at a population level, most reports indicate that obesity itself is not usually associated with systolic dysfunction, at least when measured with standard clinical parameters such as ejection fraction.4, 5 Haemodynamic studies have shown that left ventricular end-diastolic pressure may be elevated at rest in obesity and frequently increases significantly with exercise.6, 7 Regardless of the technique used, non-invasive imaging studies have also consistently shown that, even without co-morbidities such as hypertension or diabetes, impairment of left ventricular diastolic function occurs in all classes of obesity,8, 9, 10, 11 and almost all obesity metrics correlate with progressive diastolic impairment.9, 12, 13 As a result, it is now clear that obesity contributes to diastolic dysfunction, and has been demonstrated to be one of the leading causes of heart failure with preserved ejection fraction (HFpEF).3, 14
Despite the disease prevalence, clinical trials in HFpEF have produced neutral results and treatments are largely directed towards associated conditions and symptoms rather than towards improving diastolic function. As a result, understanding the mechanisms by which obesity causes diastolic dysfunction is increasingly important and may yield additional therapeutic strategies to treat this major health burden. Myocardial relaxation involves both active processes such as calcium handling and myocardial energetics,15 and intrinsic physical properties of the LV (predominantly determined by chamber geometry and concentric hypertrophic remodelling16). In addition, myocardial steatosis and subsequent lipotoxicity are becoming increasingly recognised in human obesity and have been shown to be related to diastolic dysfunction.17
However, mitochondrial dysfunction, lipotoxicity and LV concentric remodelling are not completely distinct effects, and all three have been linked to an over-utilisation of fatty acids within the obese, insulin-resistant myocardium.18 Despite this, the progression of these pathways and the relative contribution of each to diastolic dysfunction in obesity are unclear, but are an important consideration when considering future targeted therapy.
Here, we have used state-of-the-art magnetic resonance imaging and multinuclear spectroscopy to investigate the relationship between, and relative contributions of, myocardial energetics, myocardial steatosis and concentric LV remodelling to diastolic dysfunction in a population with increasing adiposity.
Materials and methods
Ethics and study cohort
The study protocol was approved by the National Research Ethics Service (NRES ref 09/H0505/97, 10/H0604/95). Eighty adult subjects (32 female, 48 male) across a body mass index (BMI) range of 18.4–53.0 kg m−2 were recruited to the study from the Oxfordshire population. Written informed consent was obtained from all participants. Subjects were asked to fast overnight prior to the study day.
Inclusion criteria
Volunteers were excluded if they were prescribed any cardiac medication (including antihypertensive or lipid-lowering agents) or if they had any smoking history, diabetes mellitus (fasting glucose>7.0 mmol l−1), hyperlipidaemia (cholesterol>6.5 mmol l−1), hypertension (systolic>140 mm Hg; diastolic BP>90 mm Hg), obstructive sleep apnoea or an abnormal electrocardiogram. Patients with heart failure or valvular disease, or with any contraindication to MR scanning, were also excluded.
Anthropometric and biochemical measurements
Height and weight were measured using digital scales (Seca, Birmingham, UK) and used to calculate BMI. Fasting venous blood was drawn and sent for biochemical analysis of total cholesterol, serum triglyceride content, insulin and glucose. Blood analysis was performed by the Oxford University Hospitals clinical grade biochemistry laboratories. Blood pressure was measured as an average of three supine measures (DINAMAP-1846-SX, Critikon Corp).
Adipose assessment
Total body fat content (kg) was assessed using bio-electrical impedance (Bodystat1500) in all patients. Dual X-ray absorptiometry (DXA, GE Lunar system, London, UK) assessment of total fat was also performed in 26 of the 80 patients across a wide range of fat mass (8.7–74.5 kg) for validation of the bio-electrical impedance measurement. This showed excellent correlation between the two measurements (r2=0.98, P<0.0001). To assess visceral fat mass, a water-suppressed turbo spin echo transverse 5 mm slice was performed at the fourth/fifth lumbar intervertebral disc and analysed by manual contouring of visceral fat volume.5
Data acquisition and analysis
Left ventricular imaging
Images to measure ventricular volumes and function were obtained using a steady state-free precession sequence with an echo time (TE) of 1.5 ms and a repetition time (TR) of 3.0 ms, as previously described, on a 1.5 T MR system (Siemens, Munich, Germany).5 Imaging was performed supine, prospectively gated and during end-expiratory breath-hold. All analysis was performed using cmr42 software (Circle Cardiovascular Imaging Inc, Calgary, Canada).16, 19 Global peak circumferential diastolic strain rates were determined by feature tracking analysis. Endocardial and epicardial LV contours were drawn on LV short axis and long axis images using a semi-automated process.
Proton magnetic resonance spectroscopy (1H MRS)
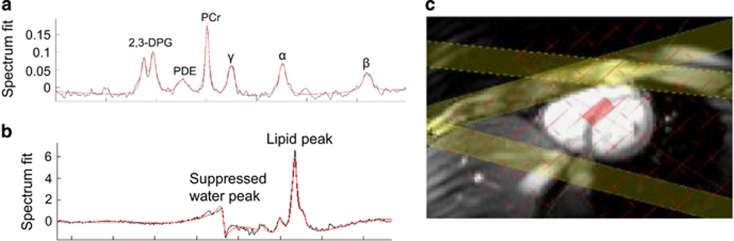
All 1H-MR spectroscopy, to determine both cardiac and hepatic lipid content, was performed on a 3.0 T MR system (Siemens) using a spectroscopic stimulated echo (STEAM) sequence, and water suppression using a T1- and B1-insensitive water suppression method for in vivo localised 1H NMR spectroscopy as previously described.20, 21 A cardiac voxel was selected in the mid septum as shown in Figure 1. The optimal water suppression pulse to determine the lipid signal at 1.3 ppm was calibrated with a pulse sequence. Five measurements were acquired per breath-hold. A 2 s TR allowed for relaxation of the lipid signal in between sequential pulses, which resulted in participants holding their breath for 12–14 s. Six breath-holds were taken—five with water suppression in order to acquire lipid spectra, and one with no water suppression to define the water signal.20, 22 Data were analysed using in-house tools (Matlab, AMARES algorithm in JMRUI). Lipid content is described as a percentage (signal amplitude of lipid/signal amplitude of water × 100).
Figure 1.
Representative myocardial spectra demonstrating 31P spectroscopy (a); 2,3-DPG, 2,3-diphosphoglycerate; PDE, phosphodiesterase; PCr, phosphocreatine; γ β α, gamma, beta and alpha phosphate groups of ATP), 1H spectroscopy (b) and the position of the basal septal voxel (c).
31Phosphorus magnetic resonance spectroscopy
All 31P-MRS was performed at 3 T (Siemens Tim Trio MR system, Germany) and were acquired fasted. Volunteers were positioned prone over a modified heart/liver Siemens coil.23 A 3D acquisition-weighted chemical shift imaging (CSI, TE=0.3 ms) sequence was used, together with an RF pulse centred between T and u resonances to reduce noise, minimise any artefact and deliver even excitation of spectra, as previously described.24 Voxel size was 16 × 8 × 8 mm, with field of view 240 × 240 × 200 mm. Nuclear Overhauser Enhancement was used to optimise the signal-to-noise ratio. Proton imaging was used to localise short-axis left ventricular slices. Saturation bands were placed over the skeletal muscle in the anterior chest wall, and the liver, in order to minimise potential signal contamination. The CSI grid was positioned to obtain one voxel containing mid-ventricular septal myocardium.24 Analysis was blinded to descriptive data, and voxels selected separately. Selected spectra were fitted using the automated processing algorithm AMARES within the jMRUI software package. The accuracy of the spectral fit was interrogated using the coefficient of variation in the PCr/ATP values based on the Cramer–Rao lower bounds. Spectra were assessed as being of satisfactory quality with a coefficient of variation under 20%.24
Statistical analysis
Statistics were analysed using SPSS 20 (SPSS Inc, Chicago, USA). Data were assessed with the Kolmogorov–Smirnov test in order to confirm normal distribution and are demonstrated as mean±standard deviation. Correlation, where relevant, was assessed with Pearson’s correlation analysis. To determine the contributors to diastolic strain rate, stepwise multiple linear regression models were used. These multivariate models included peak diastolic strain rate as the dependent variable, and those independent variables which were found to have a significant association with it in the linear regression analysis (PCr/ATP, myocardial triglyceride content (MTGC), LV mass:volume ratio, visceral fat). Collinearity was investigated with variance inflation factor (all variables <1.69). Residuals were normally distributed. To investigate in more detail the effect of myocardial energetic and myocardial triglyceride content on diastolic strain rate, mediator multiple regression was performed as per Preacher and Hayes,25 with 10 000 sample bootstrapping of indirect effects (dependent variable peak diastolic strain rate, independent variable visceral fat, moderator variables MCTG, PCr/ATP, LVMVR). A probability value of P<0.05 was considered significant.
Results
Anthropometric data
For this analysis, the cohort was divided into quartiles of visceral fat. Owing to the stringent inclusion criteria, all quartiles were well-matched for age, blood pressure and serum cholesterol (Table 1). Although fasting glucose levels were well within the normal range for all quartiles, fasting insulin and HOMA levels were higher in the highest visceral fat quartile in line with a degree of insulin resistance with increases in visceral fat (Table 1).
Table 1. Anthropometric data for the study group separated into quartiles.
|
Visceral fat quartile |
||||
|---|---|---|---|---|
| I n=20 | II n=20 | III n=20 | IV n=20 | |
| Age (years) | 34.3±11.0 | 43.0±13.0 | 43.3±15.0 | 45±12.0 |
| BMI (kg m−2) | 22.8±3.2§ | 24.7±3.3§ | 26.8±4.4§ | 36.6±7.4*†‡ |
| Visceral fat (cm2) | 28±9 | 59±7*‡§ | 94±16*†§ | 182±45*†‡ |
| Total fat mass (kg) | 16±7 | 20±6§ | 21±8*§ | 42±17*†‡ |
| Systolic blood pressure (mm Hg) | 118±9 | 123±12 | 134±14 | 129±18 |
| Diastolic blood pressure (mm Hg) | 73±8 | 75±8 | 79±8 | 81±8 |
| Fasting glucose (mmol l−1) | 4.7±0.5‡§ | 4.9±0.6§ | 5.2±0.6 | 5.4±0.6 |
| Fasting cholesterol (mmol l−1) | 4.5±0.8 | 4.6±1.4 | 4.6±1.1 | 5.0±1.2 |
| Insulin (mmol l−1) | 8.6±5.0 | 9.7±6.8§ | 8.9±8.0 | 11.8±6.6 |
| HOMA-IR | 1.8±1.0 | 2.3±1.7§ | 2.1±1.9 | 2.7±1.5 |
Abbreviation: BMI, body mass index.
*P<0.05 vs Quartile I.
†P<0.05 vs Quartile II.
‡P<0.05 vs Quartile III.
§P<0.05 vs Quartile IV.
Left ventricular structure
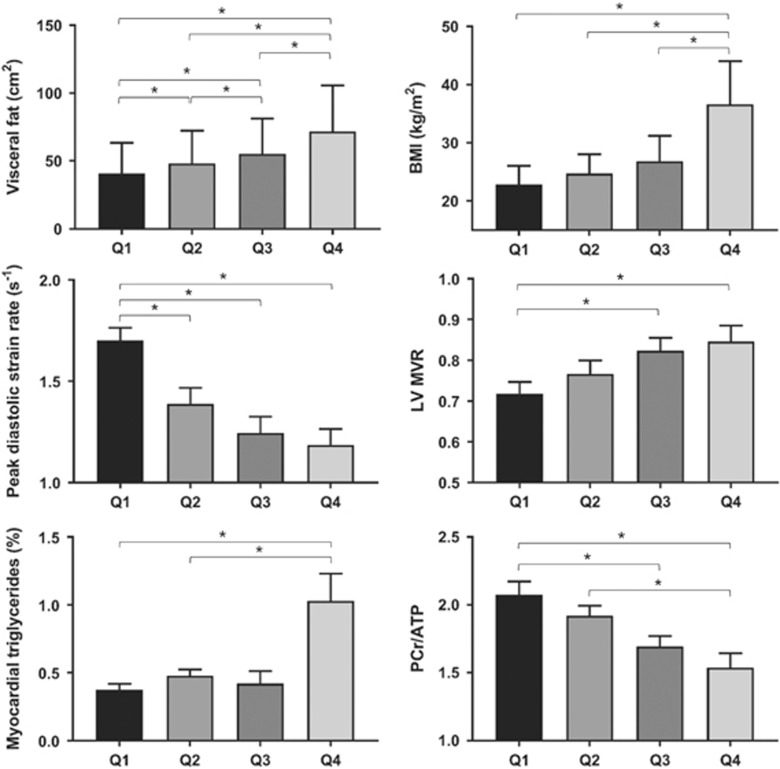
As expected, increasing BMI, total fat mass and visceral fat were associated with increasing LV mass (BMI, r=0.30, P=0.008; fat mass, r=0.22, P=0.055; visceral fat, r=0.39; P<0.001) and with concentric LV remodelling (BMI, r=0.25, P=0.03; total fat mass, r=0.22, P=0.053; visceral fat r=0.39, P<0.001).
Left ventricular diastolic function
Diastolic function was impaired in obesity, with BMI (r=−0.37, P<0.001), total body fat (r=−0.43, P=0.001) and visceral fat (r=−0.46, P=0.001) all being negatively correlated with diastolic strain rate. As expected, both increasing LV concentric remodelling (r=−0.28, P=0.01) and insulin resistance (HOMA, r=−0.26, P=0.034) were also correlated with diastolic function. In addition, myocardial PCr/ATP ratio (r=0.38, P=0.001) and MTGC (r=−0.42, P<0.001) were related to diastolic dysfunction (Table 2, Figure 2). Stepwise multivariable regression of these variables showed that both MTGC (β=−0.2, P=0.042) and PCr/ATP (β=−0.23, P=0.046) were independent predictors of diastolic function (overall R2 of the model 0.29, P=0.001, Table 3).
Table 2. Left ventricular data for the study group separated into quartiles.
|
Visceral fat quartile |
||||
|---|---|---|---|---|
| I n=20 | II n=20 | III n=20 | IV n=20 | |
| End-diastolic volume (ml) | 147±33 | 150±29 | 151±25 | 164±25 |
| Stroke volume (ml) | 100±15 | 103±19 | 107±16 | 116±19 |
| Ejection fraction (%) | 69±6 | 69±5 | 71±4 | 70±6 |
| Mass (g) | 104±33 | 113±32 | 125±26 | 137±33 |
| Mass:volume ratio | 0.70±0.09 | 0.75±0.14 | 0.83±0.12* | 0.83±0.17* |
| Peak systolic strain (εcc) | −19.7±1.7 | −19.2±2.2 | −18.6±1.7 | −17.7±2.2*† |
| Peak diastolic strain rate (s−1/s) | 1.74±0.26 | 1.44±0.34* | 1.42±0.29* | 1.21±0.32* |
| PCr/ATP | 2.06±0.45 | 1.98±0.31 | 1.69±0.32* | 1.57±0.38*† |
| Triglyceride content (% water signal) | 0.39±0.20 | 0.41±0.19 | 0.51±0.43 | 0.84±0.72*†‡§ |
*P<0.05 vs Quartile I.
†P<0.05 vs Quartile II.
‡P<0.05 vs Quartile III.
§P<0.05 vs Quartile IV.
Figure 2.
The effect of increasing visceral fat on diastolic strain rate, LV concentric remodelling, myocardial triglyceride content, and myocardial PCr/ATP ratio (* indicates P<0.05, **P<0.01, ***P<0.001).
Table 3. Regression analysis for peak diastolic strain rate.
| Peak diastolic strain rate |
Bivariate |
Multivariate |
||
|---|---|---|---|---|
| r | P | β | P | |
| MTGC (% water) | −0.42 | <0.001 | −0.20 | 0.038 |
| PCr/ATP ratio | 0.38 | 0.001 | 0.23 | 0.038 |
| LV mass:volume ratio | −0.28 | 0.012 | −0.48 | 0.12 |
| Visceral fat mass (cm2) | −0.44 | <0.001 | −0.01 | 0.34 |
Abbreviations: LV, left ventricular; MTGC, myocardial triglyceride content.
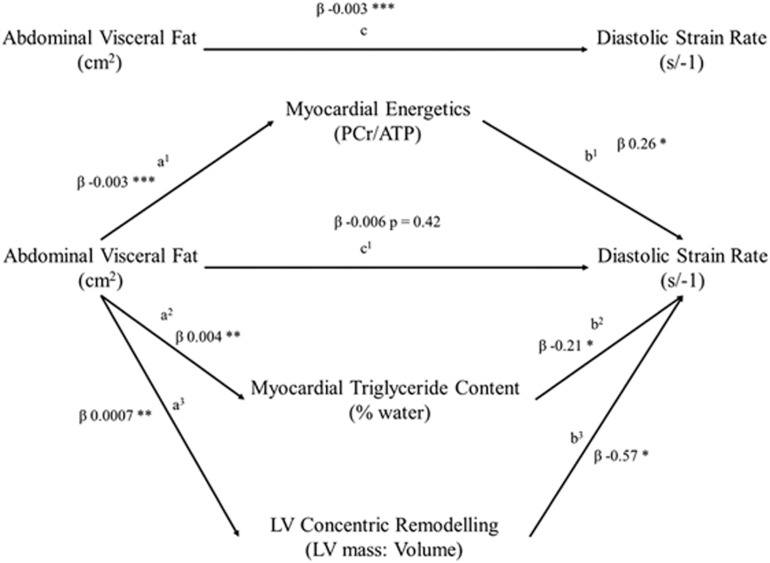
To further explore the relationship between obesity, diastolic function, MTGC and PCr/ATP, moderated multiple regression was performed (dependent variable peak diastolic filling rate, independent variable visceral fat, moderators PCr/ATP, MTGC and LVMVR, Table 4). In this visceral fat was positively related to PDSR (c pathway; β=−0.003, P=<0.001), related negatively to PCr/ATP (a1 pathway; β=−0.003, P=<0.01), and positively to both MTGC (a2 pathway; β=0.004, P=<0.01) and LVMVR (a3 pathway; β=0.0007, P=<0.01). In addition, the mediators PCr/ATP (b1 pathway β=0.26, P=0.032), MTGC (b2 pathway; β=−0.21, P=0.016) and LVMR (b3 pathway β=−0.57, P=0.047) were all related to PDSR. As both a and b pathways were found to be significant, mediation analysis was performed using 10 000 bootstrap resamples to calculate a bias-corrected 95% confidence interval of the indirect effect (Figure 3). This confirmed the mediating roles of PCr/ATP (β=−0.008, CI=−0.0015 to −0.003), MTGC (β=−0.008, CI=−0.002 to −0.0002) and LVMVR (β=−0.004, CI=−0.001 to −0.0002) in the relationship between visceral fat and PDSR. As the direct effect of body fat became non-significant (β=−0.006, P=0.427), this suggests full mediation with all the effects of fat mass on diastolic function being mediated via PCr/ATP, MTGC and LVMVR in this model. When considering the negative effect of visceral fat on diastolic function, 40% was explained by increased MTGC, 39% by reduced PCr/ATP and 21% by concentric remodelling.
Table 4. Moderated regression analysis for peak diastolic strain rate.
| Coefficient | s.e. | t | p | |
|---|---|---|---|---|
| Visceral fat to PCr/ATP effect (a1 pathway) | −0.0029 | 0.0007 | −4.12 | 0.0001 |
| Visceral fat to MTGC effect (a2 pathway) | 0.0037 | 0.0008 | 4.53 | <0.0001 |
| Visceral fat to LVMVR effect (a3 pathway) | 0.0007 | 0.0002 | 2.88 | 0.005 |
| Direct effect of PCr/ATP on PDSR (b1 pathway) | 0.26 | 0.1 | 2.64 | 0.01 |
| Direct effect of MTGC on PDSR (b2 pathway) | −0.21 | 0.09 | −2.44 | 0.017 |
| Direct effect of LVMVR on PDSR (b3 pathway) | −0.57 | 0.28 | −2.02 | 0.047 |
| Total effect of visceral fat on PDSR (c pathway) | −0.003 | 0.0006 | −4.02 | 0.0001 |
| Direct effect of visceral fat on PDSR (c1 pathway) | −0.006 | 0.0008 | −0.79 | 0.42 |
| Model summary | R2=0.35, F=8.9, P<0.0001 | |||
Abbreviation: MTGC, myocardial triglyceride content.
Figure 3.
Moderated regression modelling of the effect of increasing visceral fat on diastolic strain rate, with moderators of LV concentric remodelling, myocardial triglyceride content and myocardial PCr/ATP (* indicates P<0.05, **P<0.01, ***P<0.001).
Myocardial energetics
Increasing obesity, whether measured by BMI (r=–0.48, P<0.001), fat mass (r=−0.48, P<0.001) or visceral fat (r=−0.50, P<0.001), was associated with a decreasing PCr/ATP ratio. Within this cohort, myocardial energetics were also related to fasting glucose concentration (r=−0.37, P=0.001) and there was a trend towards relation with fasting triglyceride levels (r=−0.23, P=0.053).
Myocardial triglyceride content (steatosis)
Increasing obesity, whether measured by BMI (r=0.42, P<0.001), fat mass (r=0.47, P<0.001) or visceral fat (r=0.46, P<0.001), was associated with increasing MTGC. However, unlike myocardial energetics which showed a progressive and equally spread decrease across the increasing visceral fat quartiles, the largest increase in MTGC occurred between quartiles III and IV, where a 79% increase occurred (from 0.48±36 to 0.86±0.67%, P=0.048, Figure 2). In addition, while across quartiles I-to-III there was no significant increase in MTGC with increased visceral fat (P=0.238), between quartiles III and IV there was a significant MTGC increase per cm2 visceral fat increase (↑MTGC 0.002% per cm2 increase in visceral fat, P=0.0124). MTGC was also correlated with left ventricular hypertrophy (LV mass; r=0.28, P=0.0121) and with concentric LV remodelling (LVMVR r=0.24, P=0.033). Increasing fasting glucose (r=0.25, P=0.032), insulin levels (r=0.33, P=0.004) and insulin resistance (HOMA, r=0.34, P=0.003) were all correlated with increasing MTGC. Importantly, MTGC and PCr/ATP were not correlated in this study (r=−0.17, P=0.138).
Discussion
The face of heart failure has changed over recent years, with now half of new diagnoses involving preserved, as opposed to reduced, ejection fraction. Despite this, clinical trials in HFpEF have been disappointing, and current treatments are directed to targeting the co-morbidities and symptoms, rather than the intrinsic changes in diastolic function that characterise the condition. With its prevalence rapidly increasing on a global scale, obesity is one of the main contributors to HFpEF. However, the relative contributions of the pathophysiological mechanisms by which it causes diastolic dysfunction, including impaired myocardial energetics, lipotoxicity and concentric LV hypertrophic remodelling, remain unclear. Here we have isolated the effects of obesity on diastolic function by excluding participants with other cardiovascular risk factors, such as diabetes and hypertension, that are themselves implicated in diastolic dysfunction. As a result, we have shown that, in this model, impaired myocardial energetics, myocardial steatosis and concentric LV hypertrophic remodelling are independent predictors of diastolic impairment. This is the first study to suggest that metabolic processes, such as energetics and lipotoxicity, may have a greater contribution to impaired myocardial relaxation than changes in LV geometry and concentric LV remodelling.
The impact of myocardial energetics on diastolic function in obesity
In this study we confirm that visceral obesity is not only related to impaired myocardial energetics (by 24% in the highest visceral fat quartile), but also that this fall in energetics is associated with diastolic dysfunction. Overall, 39% of the negative effect of visceral fat on diastolic dysfunction is mediated through reduced myocardial energetics.
The most likely mechanism for the reduced PCr/ATP seen at rest here with increasing visceral obesity is a diminished total creatine store, which is proportional to the reduction in PCr, and typically seen in cardiac hypertrophy.26 If [ATP] remains constant, it follows from the creatine kinase equilibrium expression that PCr depletion implies increased steady-state [ADP] and reduced energy generated by hydrolysis of ATP’s gamma-phosphoryl moiety. Increased metabolism of free fatty acids in obesity alters the mitochondrial redox state and reduces mitochondrial efficiency—as a result the overall ATP yield normalised to oxygen consumption is likely to be lower.18, 27, 28, 29 Indeed, it has been shown that if the isolated pig heart is perfused with fatty acids, the same workload requires additional oxygen by more than 25% in comparison with glucose.30 This inefficiency has also been shown in obese humans using PET.31 The diastolic phase of the cardiac cycle is more sensitive to energetic depletion, due to the higher energy demands of active calcium uptake via SERCA, relative to myosin ATPase. It is therefore logical that compromises in myocardial energetics are linked to diastolic dysfunction.
Adipose tissue expansion and myocardial lipotoxicity
The most physiological depot in which to store excess lipids is the white subcutaneous adipose tissue. However, once its capacity is replete, excess fat can ‘overspill’ into less physiologically appropriate, non-adipose tissues.32 The deposition of fat into non-adipose depots occurs in both the apparently opposite pathological states of lipodystrophy33 and obesity,17 where storage capacity is exceeded either due to a congenital absence of white adipose tissue, or via an excess supply of lipid, respectively. When the excess fat is directed to the heart, the initial response is to store the surplus in the form of triacylglycerol. However, the buffering capability in the heart is limited, and thereafter lipids enter alternative pathways, resulting in creation of toxic reactive lipid species (such as ceramides and diacyl-glycerol).34
We have shown here that a nearly twofold greater increase in MTGC occurs between visceral fat quartiles III and IV when compared to the increment between quartiles I and III. This is in line with the hypothesis that there is an initial protective buffering effect of visceral fat, which, when saturated, results in much greater cardiac TG deposition.
Concentric left ventricular hypertrophy and diastolic function
Cardiac output and circulating volume are both increased in obesity, which over time leads to cardiovascular volume overload. Contrary to earlier studies which described an association with concentric LV remodelling,35, 36 it is now clear that obesity leads to a more eccentric pattern of remodelling, with increases in both cavity size and wall thickness. This increase in wall thickness changes the intrinsic mechanical properties of the myocardium, acting as one of the main passive mechanisms of diastolic impairment. Here we have shown that concentric LV remodelling indeed independently predicts diastolic function—however, it only accounts for around 20% of the effect of visceral fat on diastolic function, around half of the effect of MTGC and energetics.
The severity of concentric LV remodelling seen in obesity5, 37 may reflect altered adipokine environment with hyperleptinaemia and hyperinsulinaemia both being linked to increased cardiomyocyte size and left ventricular hypertrophy in human obesity.38, 39 The myocardium itself expresses isoforms of the leptin receptor,40 and even in isolation, activation of these induces hypertrophy in cardiomyocyte culture.41, 42, 43, 44 While leptin leads to hypertrophy via pathways involving JAK/STAT, MAPK, protein kinase C and Rho/ROCK dependent kinases,45, 46, 47 hyperinsulinaemia also has a role, acting via the plentiful myocardial insulin-like growth factor 1 receptors.48 In addition, as described above, the presence of myocardial steatosis is also linked to concentric LV hypertrophy and remodelling, thus linking altered myocardial substrate utilisation, via overuse of fatty acid, again to diastolic dysfunction via concentric LV remodelling.
Potential treatments for diastolic dysfunction in obesity
This study reaffirms the importance of myocardial metabolism for diastolic function in obesity, perhaps contributing more than the intrinsic changes in myocardial stiffness brought about by LV concentric hypertrophy. As lipid metabolism seems to underpin both the metabolic and hypertrophic mechanisms seen here, this may be a common pathway to target as therapy, and animal models have shown that targeting the lipid metabolism pathway has the potential to be beneficial. Treatment of MHC-ACS mouse with a hepatic adenovirus encoding leptin improved contractility, presumably via increased myocardial fatty acid oxidation rates and reducing lipotoxicity.49 In addition, treatment of the obese Zucker Diabetic Fatty rats with troglitazone reduces cardiac triglyceride, and prevents both apoptosis and loss of function.50 Whether one specific therapeutic target can address diastolic dysfunction in human obesity remains unclear, but as reduced myocardial energetics, myocardial steatosis and concentric remodelling have all been related to overuse of lipids, this is a potential metabolic hub to address diastolic dysfunction. Ideally this would be achieved through successful weight loss interventions, which improve energetics,51 steatosis52 and LV remodelling,10 but the poor sustainability of non-surgical weight loss interventions makes a pharmacological approach potentially a more realistic prospect.
Conclusion
Although obesity is a prime contributor to diastolic dysfunction, the mechanisms that underlie this are not well understood. Here we have shown that visceral obesity is not only related to diastolic dysfunction, but also that all of the negative effects of visceral fat on diastolic function can be accounted for by concentric LV remodelling, elevated myocardial triglyceride levels and impaired energetics. We have also shown for the first time that metabolic mechanisms may be more important than structural factors in mediating the negative effects of obesity on diastolic function. A therapeutic approach that alters myocardial substrate selection may target both the cardiac metabolic and structural effects of obesity, and is likely to be effective in treating cardiac dysfunction in obesity.
Acknowledgments
We acknowledge Professor Bernard Fingleton (CSTAT) for his assistance and guidance with the statistical methods and analysis in this manuscript. The study was supported by a grant from the British Heart Foundation FS/08/074/26233. SN and OJR acknowledge support from the Oxford BHF Centre of Research Excellence and the Oxford NIHR Biomedical Research Centre.
Footnotes
The authors declare no conflict of interest.
References
- Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG et al. Obesity and the risk of heart failure. N Engl J Med 2002; 347: 305–313. [DOI] [PubMed] [Google Scholar]
- Alpert MA, Terry BE, Mulekar M, Cohen MV, Massey CV, Fan TM et al. Cardiac morphology and left ventricular function in normotensive morbidly obese patients with and without congestive heart failure, and effect of weight loss. Am J Cardiol 1997; 80: 736–740. [DOI] [PubMed] [Google Scholar]
- Owan TE, Redfield MM. Epidemiology of diastolic heart failure. Prog Cardiovasc Dis 2005; 47: 320–332. [DOI] [PubMed] [Google Scholar]
- Powell BD, Redfield MM, Bybee KA, Freeman WK, Rihal CS. Association of obesity with left ventricular remodeling and diastolic dysfunction in patients without coronary artery disease. Am J Cardiol 2006; 98: 116–120. [DOI] [PubMed] [Google Scholar]
- Rider OJ, Francis JM, Ali MK, Byrne J, Clarke K, Neubauer S et al. Determinants of left ventricular mass in obesity; a cardiovascular magnetic resonance study. J Cardiovasc Magn Reson 2009; 11: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander JK. Obesity and cardiac performance. Am J Cardiol 1964; 14: 860–865. [DOI] [PubMed] [Google Scholar]
- Kaltman AJ, Goldring RM. Role of circulatory congestion in the cardiorespiratory failure of obesity. Am J Med 1976; 60: 645–653. [DOI] [PubMed] [Google Scholar]
- Otto ME, Belohlavek M, Khandheria B, Gilman G, Svatikova A, Somers V. Comparison of right and left ventricular function in obese and nonobese men. Am J Cardiol 2004; 93: 1569–1572. [DOI] [PubMed] [Google Scholar]
- Peterson LR, Waggoner AD, Schechtman KB, Meyer T, Gropler RJ, Barzilai B et al. Alterations in left ventricular structure and function in young healthy obese women: assessment by echocardiography and tissue Doppler imaging. J Am Coll Cardiol 2004; 43: 1399–1404. [DOI] [PubMed] [Google Scholar]
- Rider OJ, Francis JM, Ali MK, Petersen SE, Robinson M, Robson MD et al. Beneficial cardiovascular effects of bariatric surgical and dietary weight loss in obesity. J Am Coll Cardiol 2009; 54: 718–726. [DOI] [PubMed] [Google Scholar]
- Rider OJ, Francis JM, Ali MK, Holloway C, Pegg T, Robson MD et al. Effects of catecholamine stress on diastolic function and myocardial energetics in obesity. Circulation 2012; 125: 1511–1519. [DOI] [PubMed] [Google Scholar]
- Pascual M, Pascual DA, Soria F, Vicente T, Hernandez AM, Tebar FJ et al. Effects of isolated obesity on systolic and diastolic left ventricular function. Heart 2003; 89: 1152–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kossaify A, Nicolas N. Impact of overweight and obesity on left ventricular diastolic function and value of tissue Doppler echocardiography. Clin Med Insights Cardiol 2013; 7: 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton CB, Pettinger M, Rossouw J, Martin LW, Foraker R, Quddus A et al. Risk factors for incident hospitalized heart failure with preserved versus reduced ejection fraction in a multiracial cohort of postmenopausal women. Circ Heart Fail 2016; 9: pii: e002883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rider OJ, Francis JM, Tyler D, Byrne J, Clarke K, Neubauer S. Effects of weight loss on myocardial energetics and diastolic function in obesity. Int J Cardiovasc Imaging 2013; 29: 1043–1050. [DOI] [PubMed] [Google Scholar]
- Rider OJ, Lewandowski A, Nethononda R, Petersen SE, Francis JM, Pitcher A et al. Gender-specific differences in left ventricular remodelling in obesity: insights from cardiovascular magnetic resonance imaging. Eur Heart J 2013; 34: 292–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee R, Rial B, Holloway CJ, Lewandowski AJ, Robson MD, Osuchukwu C et al. Evidence of a direct effect of myocardial steatosis on LV hypertrophy and diastolic dysfunction in adult and adolescent obesity. JACC Cardiovasc Imaging 2015; 8: 1468–1470. [DOI] [PubMed] [Google Scholar]
- Rider OJ, Cox P, Tyler D, Clarke K, Neubauer S. Myocardial substrate metabolism in obesity. Int J Obes (Lond) 2013; 37: 972–979. [DOI] [PubMed] [Google Scholar]
- Zeidan Z, Erbel R, Barkhausen J, Hunold P, Bartel T, Buck T. Analysis of global systolic and diastolic left ventricular performance using volume-time curves by real-time three-dimensional echocardiography. J Am Soc Echocardiogr 2003; 16: 29–37. [DOI] [PubMed] [Google Scholar]
- Rider OJ, Banerjee R, Rayner JJ, Shah R, Murthy VL, Robson MD et al. Investigating a liver fat: arterial stiffening pathway in adult and childhood obesity. Arterioscler Thromb Vasc Biol 2016; 36: 198–203. [DOI] [PubMed] [Google Scholar]
- Rial B, Robson MD, Neubauer S, Schneider JE. Rapid quantification of myocardial lipid content in humans using single breath-hold 1H MRS at 3 Tesla. Magn Reson Med 2011; 66: 619–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamopoulos C, Meyer P, Desai RV, Karatzidou K, Ovalle F, White M et al. Absence of obesity paradox in patients with chronic heart failure and diabetes mellitus: a propensity-matched study. Eur J Heart Fail 2011; 13: 200–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levelt E, Rodgers CT, Clarke WT, Mahmod M, Ariga R, Francis JM et al. Cardiac energetics, oxygenation, and perfusion during increased workload in patients with type 2 diabetes mellitus. Eur Heart J 2015; 65: 44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway CJ, Dass S, Suttie JJ, Rider OJ, Cox P, Cochlin LE et al. Exercise training in dilated cardiomyopathy improves rest and stress cardiac function without changes in cardiac high energy phosphate metabolism. Heart 2012; 98: 1083–1090. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods 2008; 40: 879–891. [DOI] [PubMed] [Google Scholar]
- Neubauer S. The failing heart—an engine out of fuel. N Engl J Med 2007; 356: 1140–1151. [DOI] [PubMed] [Google Scholar]
- Veech RL. The determination of the redox states and phosphorylation potential in living tissues and their relationship to metabolic control of disease phenotypes. Biochem Mol Biol Educ 2006; 34: 168–179. [DOI] [PubMed] [Google Scholar]
- Veech RL, Chance B, Kashiwaya Y, Lardy HA, Cahill GR. Ketone bodies, potential therapeutic uses. IUBMB Life 2001; 51: 241–247. [DOI] [PubMed] [Google Scholar]
- Hue L, Taegtmeyer H. The Randle cycle revisited: a new head for an old hat. Am J Physiol-Endocrinol Metab 2009; 297: E578–E591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korvald C, Elvenes OP, Myrmel T. Myocardial substrate metabolism influences left ventricular energetics in vivo. Am J Physiol-Heart Circulat Physiol 2000; 278: H1345–H1351. [DOI] [PubMed] [Google Scholar]
- Peterson LR, Herrero P, Schechtman KB, Racette SB, Waggoner AD, Kisrieva-Ware Z et al. Effect of obesity and insulin resistance on myocardial substrate metabolism and efficiency in young women. Circulation 2004; 109: 2191–2196. [DOI] [PubMed] [Google Scholar]
- Virtue S, Vidal-Puig A. Adipose tissue expandability, lipotoxicity and the metabolic syndrome—an allostatic perspective. Biochim Biophys Acta 2010; 1801: 338–349. [DOI] [PubMed] [Google Scholar]
- Nelson MD, Victor RG, Szczepaniak EW, Simha V, Garg A, Szczepaniak LS. Cardiac steatosis and left ventricular hypertrophy in patients with generalized lipodystrophy as determined by magnetic resonance spectroscopy and imaging. Am J Cardiol 2013; 112: 1019–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze PC, Drosatos K, Goldberg IJ. Lipid use and misuse by the heart. Circ Res 2016; 118: 1736–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpert MA. Obesity cardiomyopathy: pathophysiology and evolution of the clinical syndrome. Am J Med Sci 2001; 321: 225–236. [DOI] [PubMed] [Google Scholar]
- Alexander JK. Obesity and the heart. Heart Dis Stroke 1993; 2: 317–321. [PubMed] [Google Scholar]
- Turkbey EB, McClelland RL, Kronmal RA, Burke GL, Bild DE, Tracy RP et al. The impact of obesity on the left ventricle: the Multi-Ethnic Study of Atherosclerosis (MESA). JACC Cardiovasc Imaging 2010; 3: 266–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perego L, Pizzocri P, Corradi D, Maisano F, Paganelli M, Fiorina P et al. Circulating leptin correlates with left ventricular mass in morbid (grade III) obesity before and after weight loss induced by bariatric surgery: a potential role for leptin in mediating human left ventricular hypertrophy. J Clin Endocrinol Metab 2005; 90: 4087–4093. [DOI] [PubMed] [Google Scholar]
- Rider OJ, Petersen SE, Francis JM, Ali MK, Hudsmith LE, Robinson MR et al. Ventricular hypertrophy and cavity dilatation in relation to body mass index in females with uncomplicated obesity. Heart 2011; 97: 203–208. [DOI] [PubMed] [Google Scholar]
- Purdham DM, Zou MX, Rajapurohitam V, Karmazyn M. Rat heart is a site of leptin production and action. Am J Physiol Heart Circ Physiol 2004; 287: H2877–H2884. [DOI] [PubMed] [Google Scholar]
- Madani S, De Girolamo S, Munoz DM, Li RK, Sweeney G. Direct effects of leptin on size and extracellular matrix components of human pediatric ventricular myocytes. Cardiovasc Res 2006; 69: 716–725. [DOI] [PubMed] [Google Scholar]
- Rajapurohitam V, Gan XT, Kirshenbaum LA, Karmazyn M. The obesity-associated peptide leptin induces hypertrophy in neonatal rat ventricular myocytes. Circ Res 2003; 93: 277–279. [DOI] [PubMed] [Google Scholar]
- Tajmir P, Ceddia RB, Li RK, Coe IR, Sweeney G. Leptin increases cardiomyocyte hyperplasia via extracellular signal-regulated kinase- and phosphatidylinositol 3-kinase-dependent signaling pathways. Endocrinology 2004; 145: 1550–1555. [DOI] [PubMed] [Google Scholar]
- Abe Y, Ono K, Kawamura T, Wada H, Kita T, Shimatsu A et al. Leptin induces elongation of cardiac myocyte and causes eccentric left ventricular dilatation with compensation. Am J Physiol Heart Circ Physiol 2007; 292: H2387–H2396. [DOI] [PubMed] [Google Scholar]
- Zeidan A, Javadov S, Karmazyn M. Essential role of Rho/ROCK-dependent processes and actin dynamics in mediating leptin-induced hypertrophy in rat neonatal ventricular myocytes. Cardiovasc Res 2006; 72: 101–111. [DOI] [PubMed] [Google Scholar]
- Banks AS, Davis SM, Bates SH, Myers MG Jr. Activation of downstream signals by the long form of the leptin receptor. J Biol Chem 2000; 275: 14563–14572. [DOI] [PubMed] [Google Scholar]
- Ghilardi N, Skoda RC. The leptin receptor activates janus kinase 2 and signals for proliferation in a factor-dependent cell line. Mol Endocrinol 1997; 11: 393–399. [DOI] [PubMed] [Google Scholar]
- Cittadini A, Stromer H, Katz SE, Clark R, Moses AC, Morgan JP et al. Differential cardiac effects of growth hormone and insulin-like growth factor-1 in the rat. A combined in vivo and in vitro evaluation. Circulation 1996; 93: 800–809. [DOI] [PubMed] [Google Scholar]
- Lee Y, Naseem RH, Duplomb L, Park BH, Garry DJ, Richardson JA et al. Hyperleptinemia prevents lipotoxic cardiomyopathy in acyl CoA synthase transgenic mice. Proc Natl Acad Sci USA 2004; 101: 13624–13629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou YT, Grayburn P, Karim A, Shimabukuro M, Higa M, Baetens D et al. Lipotoxic heart disease in obese rats: implications for human obesity. Proc Natl Acad Sci USA 2000; 97: 1784–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rider OJ, Francis JM, Tyler D, Byrne J, Clarke K, Neubauer S. Effects of weight loss on myocardial energetics and diastolic function in obesity. Int J Cardiovasc Imaging 2012; 29: 1043–1050. [DOI] [PubMed] [Google Scholar]
- Utz W, Engeli S, Haufe S, Kast P, Bohnke J, Haas V et al. Moderate dietary weight loss reduces myocardial steatosis in obese and overweight women. Int J Cardiol 2012; 167: 905–909. [DOI] [PubMed] [Google Scholar]