Abstract
Mammalian Trp proteins are candidates for plasma membrane calcium channels regulated by receptor activation or by intracellular calcium store depletion [capacitative calcium entry (CCE)]. One extensively investigated member of the Trp family, the human Trp3 (hTrp3), behaves as a receptor-activated, calcium-permeable, nonselective cation channel when expressed in cell lines and does not appear to be activated by store depletion. Nonetheless, there is good evidence that Trp3 can be regulated by interacting with inositol trisphosphate receptors (IP3Rs), reminiscent of the conformational coupling mode of CCE. To investigate the role of Trp3 in CCE, and its regulation by IP3R, we transiently expressed hTrp3 in the wild-type DT40 chicken B lymphocyte cell line and its variant lacking IP3R. Expression of hTrp3 in either wild-type or IP3R-knockout cells did not increase basal membrane permeability, but resulted in a substantially greater divalent cation entry after thapsigargin-induced store depletion. This hTrp3-dependent divalent cation entry was significantly greater in the wild type than in IP3R-knockout cells. Thus, it appears that in this cell line, hTrp3 forms channels that are store-operated by both IP3R-dependent and IP3R-independent mechanisms. Trp3, or one of its structural relatives, is a candidate for the store-operated, nonselective cation channels observed in smooth muscle cells and other cell types.
Agonist-dependent regulation of intracellular Ca2+ levels in most cell types involves modulation of Ca2+ release from intracellular stores, stimulation of Ca2+ entry from the outside through Ca2+ channels in the plasma membrane, or both (1–3). As a result of this regulation, cytosolic Ca2+ levels control diverse cellular processes such as muscle contraction, secretion, cellular proliferation and differentiation, and apoptosis. In many cell types, the major Ca2+-signaling pathway is initiated by release of intracellular Ca2+ as a result of activation of the phospholipase C (PLC)–inositol trisphosphate (IP3) pathway (4). This release of intracellular Ca2+ also triggers an influx of Ca2+ across the plasma membrane, a process known as capacitative Ca2+ entry (CCE). The mechanism underlying the activation of the calcium-permeable channels is not known with certainty, but the initiating signal somehow is derived from depletion of endoplasmic reticulum luminal Ca2+ content (1, 5, 6). Two leading hypotheses have been proposed to account for this signaling mechanism, and these are perhaps not mutually exclusive. One, the conformational coupling model (7), involves direct communication between IP3 receptors (IP3Rs) in the intracellular stores and plasma membrane Ca2+ channels (8). Alternatively, communication between intracellular stores and plasma membrane channels may occur as a result of the release from the stores of a diffusible signal, the so-called calcium influx factor (9, 10). In either case, Ca2+ is presumed to enter cells through store-operated Ca2+ channels (SOC), which apparently vary in their ion selectivity in different cell types.
The most Ca2+-selective channels appear to be those mediating the well-characterized Ca2+ release-activated Ca2+ current [calcium release-activated calcium current (Icrac)], found predominantly in hematopoietic cells (11). In other cell types, currents have been observed that have moderate divalent cation selectivity (12, 13), and there are a number of examples of store-operated, nonselective cation currents (14–18).
Studies on the molecular nature of SOCs have focused primarily on mammalian homologues of the Drosophila Trp channel (19). Candidates for the highly Ca2+-selective Icrac channels include the mammalian Trp homolog, Trp4 (20), or a more distantly related member of the Trp superfamily, CaT1 (21). However, as yet, there are no clear candidates for other, less calcium-selective, store-operated channels.
The first mammalian Trps to be cloned were those most structurally similar to the Drosophila photoreceptor Trp channel (22–24). These canonical Trps (with a conserved, C-terminal, 6-aa signature sequence: EWKFAR) now form part of a larger Trp superfamily (25). The members of the canonical Trp subfamily, designated Trp1 through Trp7 (or TRPC1 through TRPC7) are also the ones most extensively investigated, largely by heterologous expression in cell lines. When expressed in cell lines, these proteins most often form nonselective cation channels (26–28), although not exclusively (e.g., Trp4, see above). Thus, the canonical Trp subfamily could be candidates for store-operated Ca2+ entry in those specific instances in which store-operated entry involves nonselective cation channels. One of the most extensively characterized members of this group is the human isoform of Trp3 (hTrp3), originally cloned by Zhu et al. (24). Although initial studies indicated that expression of Trp3 increased CCE (24, 29), it was shown subsequently that this likely reflects a constitutive rather than regulated mode of Ca2+ entry (30). Thus, Trp3 appears to behave as a receptor-activated channel that cannot be activated by store depletion (26, 30–33). Several studies also have suggested that Trp3 activation involves interaction with underlying IP3Rs or, in some instances, ryanodine receptors (34–36). There is also evidence that CCE may involve such an interaction (37, 38), in support of the proposed conformational coupling model for the regulation of SOCs (7, 8). Thus, despite the fact that expressed Trp3 apparently is not regulated by store depletion, its interaction with IP3Rs has been investigated as a model for understanding the conformational coupling mechanism. On the other hand, Trp3 and its close structural relative, Trp6, also are activated by diacylglycerols (31), and it has been suggested that this may reflect their mode of activation by PLC-linked receptors, rather than the IP3R (39). Finally, despite evidence in some cell lines suggesting that the IP3R is involved in activation of endogenous CCE channels, there is also considerable evidence against this idea (40, 41), at least in certain cell types. Thus, a major problem with a proposed function of Trp3 as a store-operated channel is the inability to demonstrate its regulation by store depletion. There is also controversy as to whether the mechanism of regulation of Trp3 and of endogenous CCE channels involves conformational coupling with the IP3R.
DT40 is an avian leukosis virus-induced chicken pre-B cell line that expresses an αIgM isotype B cell receptor in the plasma membrane (40, 42, 43). B cell receptor stimulation can be accomplished by anti-IgM-induced cross-linking of the receptor that leads to activation of nonreceptor tyrosine kinases, PLCγ activation, and IP3-induced release of Ca2+ from thapsigargin-sensitive endogenous stores. This release then triggers Ca2+ entry from the outside through the CCE pathway. A variant of these cells has been generated (40) in which the genes coding for the three IP3R subtypes normally expressed in the wild-type cells have been disrupted by homologous recombination. Such knockout cells (IP3R-KO) are able to respond to PLC-coupled receptors with the generation of IP3, but they do not produce PLC-linked cytosolic Ca2+ signals. However, normal CCE upon thapsigargin-induced store depletion occurs (40, 41).
As a hematopoietic cell line, DT40 would be expected to express only the highly calcium-selective mode of CCE (Icrac). Thus, we considered that the DT40 wild-type and IP3R-KO cell lines may provide an ideal environment to investigate the regulation of transiently expressed hTrp3 nonselective cation channels, especially regarding the role of IP3Rs. Two rather surprising results were obtained. In contrast to findings in other cell lines, hTrp3 was activated robustly by the depletion of intracellular Ca2+ stores. Additionally, this activation occurred in both the wild-type and IP3R-KO cell lines. This represents a clear example of store-dependent regulation of Trp3 channels and also an example of the regulation of these channels by an IP3R-independent mechanism. Thus, Trp3 appears to be a good candidate for store-operated, nonselective cation channels.
Materials and Methods
Cell Culture, Transfection, and Measurement of Intracellular Calcium.
The immortalized chicken B lymphocyte cell line, DT40 [The Institute of Physical and Chemical Research (RIKEN) Cell Bank no. RCB1464], and the mutant variant in which the genes for all three IP3R types were disrupted (RIKEN Cell Bank no. RCB1467) were obtained through the courtesy of Tomohiro Kurosaki (Kansai Medical University, Kansai, Japan). They were cultured essentially as described in ref. 40. For experiments, both cell types were allowed to attach to glass coverslips for 20 min at 40°C before Fura-2 loading. DT40 cells attached to glass coverslips were mounted in a Teflon chamber and incubated with 2 μM Fura-2/AM (Molecular Probes) for 30 min at room temperature. The cells then were washed and bathed in a Hepes-buffered physiological saline solution (HBSS composition: 140 mM NaCl/4.7 mM KCl/1 mM MgCl2/1.5 mM CaCl2/10 mM glucose/10 mM Hepes, pH 7.4) at room temperature at least 15 min before Ca2+ measurements were performed. Experiments were initiated in a nominally Ca2+-free medium, which was identical in composition except for the omission of added CaCl2.
DT40 cells were transiently transfected by electroporation using a Gene Pulser apparatus (Bio-Rad) with either the human isoform of Trp3 (hTrp3 into pcDNA3 vector, provided by Lutz Birnbaumer, University of California, Los Angeles) or its vector (pcDNA3, mock-transfected cells), along with either peGFP-C3 or DsRed-Mito vectors (CLONTECH) as markers for transfection. Cells were assayed 18–30 h posttransfection. Fluorescence measurements were performed under the conditions indicated with single enhanced green fluorescent protein (eGFP)-positive or DsRed-Mito-positive cells, which were selected by their green or red fluorescence when excited at 488 nm and 558 nm, respectively, and emission wavelengths observed at 520 nm and 610 nm, respectively. Fura-2 was not excited at 488 nm. The fluorescence of Fura-2-loaded DT40 cells was monitored with a photomultiplier-based system, mounted on a Nikon Diaphot 300 inverted microscope equipped with a Nikon ×40 (1.3 numerical aperture) Neofluor objective. The fluorescence light source was provided by a Deltascan D101 (Photon Technology International, Princeton), equipped with a light path chopper and dual excitation monochromators, which enabled rapid interchange between two excitation wavelengths (340 and 380 nm), the emission fluorescence being selected at 510 nm through a barrier filter and detected by a photomultiplier tube. All experiments were performed at room temperature. The data are expressed as a ratio of Fura-2 fluorescence from excitation at 340 nm to that from excitation at 380 nm (F340/F380). Under these conditions, eGFP and DsRed-Mito expression did not contribute significant fluorescence.
Reverse Transcription–PCR of hTrp3/6/7 Transcript.
Total RNA was extracted from DT40 cells by using Trizol (Life Technologies, Gaithersburg, MD). After DNase I treatment, 5 μg of total RNA was reverse-transcribed into first-strand cDNA by using random and oligo(dT) primers according to the Superscript Preamplification System for First Strand cDNA Synthesis instructions (Life Technologies). Aliquots of the cDNA were used as templates for PCR amplification with primers specific for the subfamily Trp3/6/7: TTC/T ATG AAG TTT GTA GCA CA (forward primer) and ATA GGA GTT GTT TAT CAT GGC T (reverse primer). Amplification conditions were 94°C (12 min), 30–35 cycles at 94°C (30 sec), 55°C (30 sec), 72°C (3 min), and 72°C (10 min). “No template” controls were run for all experiments. PCR products were separated on 1% agarose gels and stained with ethidium bromide. After purification (NucleoTrap PCR purification kit; CLONTECH), the PCR products were cloned into the pCR-XL-TOPO vector (TOPO-XL-PCR cloning kit; Invitrogen) and sequenced, or, in some cases, the purified PCR products were sequenced directly.
Results
Either hTrp3 or its vector (pcDNA3, mock-transfected cells) was transfected into both wild-type DT40 (designated T3-WT) and IP3R-KO cells (designated T3-KO) along with a construct encoding eGFP as transfection marker (see Materials and Methods). In preliminary experiments conducted to examine cotransfection efficiency of eGFP and DsRed-Mito, a cotransfection close to 100% was achieved under our conditions. Either Ca2+ or Ba2+ (see below) entry was evaluated in single eGFP-positive cells under the conditions indicated.
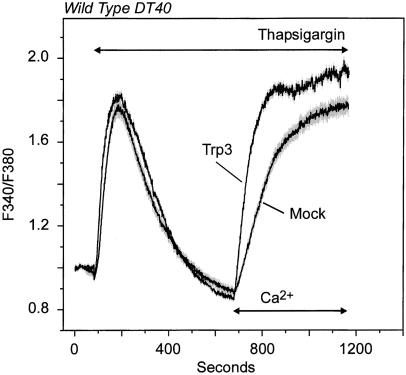
We first assessed the influence of store depletion on Ca2+ entry in hTrp3-transfected cells. As shown previously (40, 41, 44), both wild-type and IP3R-KO cells responded to thapsigargin with a transient increase in cytosolic Ca2+ as a consequence of passive depletion of endogenous stores upon blockade of the sarco-endoplasmic reticulum Ca2+/Mg2+-ATPases. The profile of the thapsigargin-induced Ca2+ rise and return to baseline in both the mock- and the hTrp3-transfected cells was similar to that of untransfected cells, indicating that the transfection did not alter Ca2+ content of the stores or the kinetics of intracellular and plasma membrane Ca2+ transport. Once Ca2+ levels returned to baseline, Ca2+ was added to the extracellular medium to evaluate activation of the CCE pathway. As for the untransfected cells (41), mock-transfected cells showed increased Ca2+ entry, which reached a quasi-steady-state level after 2–4 min. Cells transiently expressing hTrp3 showed a significantly greater Ca2+ entry after thapsigargin-induced store depletion (Fig. 1). Moreover, the initial rate of fluorescence ratio increase from Ca2+ entry into the cytosol was at least 2 times faster in hTrp3-expressing cells than in the corresponding controls. Basal Ca2+ permeability was not affected by hTrp3 expression, because Ca2+ addition to either T3-WT or T3-KO cells not exposed to thapsigargin but maintained in Ca2+-free medium resulted in no detectable increases of cytosolic Ca2+ (not shown). These results differ from previous studies in which constitutive Trp3 activity always has been observed (26, 30, 33). They also differ from previous studies that have suggested that Trp3 could be activated by a conformational coupling mechanism through the IP3R or PLC-derived products but not by store depletion alone (30, 32, 33, 35).
Figure 1.
Store depletion induces Ca2+ entry in hTrp3-transfected wild-type DT40 cells. Fura-2-loaded wild-type DT40 cells transfected with either hTrp3 or its vector (Mock), along with a construct encoding eGFP as transfection marker (see Materials and Methods), were incubated in a nominally Ca2+-free medium and then exposed to 2 μM thapsigargin to deplete intracellular Ca2+ stores. After cytosolic Ca2+ returned to basal levels, Ca2+ (1.5 mM) was re-added to the medium. Shown are average curves ± SEM (in gray) from three independent experiments, each of them performed on at least four single cells. Measurements were performed on positively transfected cells, which were selected by their green fluorescence when excited at 488 nm (eGFP-positive cells).
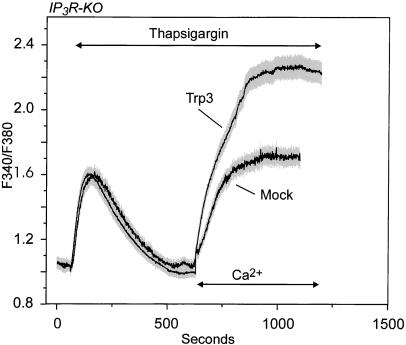
We next expressed hTrp3 in IP3R-KO cells (T3-KO), and, again, the thapsigargin-induced Ca2+ entry appeared elevated (Fig. 2). This indicates that the ability of hTrp3 to couple to intracellular store depletion does not require the presence of an IP3R. Surprisingly, the steady-state intracellular Ca2+ concentration, [Ca2+]i, was actually somewhat higher (P < 0.05) in the T3-KO cells than in the T3-WT cells. This could indicate either more channels or more efficient signaling to the channels, or, alternatively, it could reflect differences in the degree of feedback regulation of the channels by Ca2+. Thus, we determined the initial rates of [Ca2+]i increase after addition of Ca2+ to T3-KO and T3-WT cells. The values were 0.46 ± 0.01 ratio units per min for the T3-WT cells and 0.28 ± 0.02 ratio units per min for T3-KO cells. Thus, the initial rate of Ca2+ entry suggests that hTrp3 was actually more active in the wild-type cells than in the IP3R-KO cells.
Figure 2.
Store depletion induces Ca2+ entry in hTrp3-transfected IP3R-KO DT40 cells. The procedure was the same as for Fig. 1, except that hTrp3 was transfected into the IP3R-KO line of DT40. Shown are average curves ± SEM (in gray) from three independent experiments, each of them performed on at least four single cells.
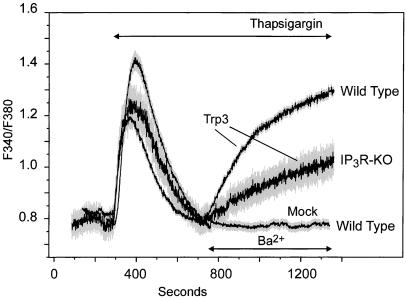
To avoid potential complications of differing Ca2+-dependent regulation of Ca2+ channels or possible variations in cellular Ca2+ metabolism and to assess hTrp3 function in the absence of endogenous CCE, we carried out experiments examining the entry of Ba2+ into the cells rather than Ca2+. hTrp3, a nonselective cation channel, will readily pass Ba2+. In addition, Ba2+ cannot be removed from cytosol because Ba2+ is a poor substrate for endoplasmic reticulum or plasma membrane calcium pumps (45), thus providing a reliable way to monitor unidirectional cation entry.
In mock-transfected wild-type and IP3R-KO cells, the addition of Ba2+ after thapsigargin-induced store depletion resulted in negligible or undetectable cation influx, confirming poor permeability of the endogenous, store-dependent cation entry pathway to Ba2+. Additionally, hTrp3 expression did not affect basal Ba2+ permeability, because Ba2+ addition to either T3-WT or T3-KO cells maintained in Ca2+-free medium resulted in no measurable Ba2+ entry (not shown). However, thapsigargin treatment significantly augmented Ba2+ permeability in T3-WT and T3-KO cells (Fig. 3), indicating that hTrp3 mediates Ba2+ entry into these cells in response to Ca2+ store depletion. In agreement with the initial rate measurements from the Ca2+ experiments, both the initial rate and the maximal fluorescence ratio increase from Ba2+ entry were at least two times higher in T3-WT than in T3-KO cells (0.15 ± 0.03 vs. 0.06 ± 0.02 ratio units per min, respectively, P < 0.01). As mentioned above, previous studies have suggested a role of the IP3R in the activation of Trp3 by a conformational coupling mechanism, whereby the IP3R itself acts to sense Ca2+ content in the stores and to gate the channel (34, 35). Although other interpretations are possible, it seems likely that hTrp3 coupling to IP3Rs in the T3-WT cells accounts for the improved response to store depletion exhibited by these cells compared with T3-KO. The simplest interpretation is that Trp3 forms two differently regulated channels, one regulated through IP3R and one by another mechanism. However, it is possible that a single channel exists in a conformation that works better with IP3R than without. Nonetheless, it is clear that store-operated influx of Ba2+ occurs independently of IP3Rs.
Figure 3.
Store depletion induces Ba2+ entry in hTrp3-transfected wild-type and IP3R-KO DT40 cells. Ba2+ influx was measured in Fura-2-loaded DT40 cells transfected with either hTrp3 (wild type, IP3R-KO) or its vector (pcDNA3, Mock, wild type only) as described in Fig. 1 legend. The cells were maintained in a nominally Ca2+-free medium, exposed to 2 μM thapsigargin, and then Ba2+ (10 mM) was added where indicated. Shown are average curves ± SEM (in gray) from four independent experiments, each of them performed on at least four single cells.
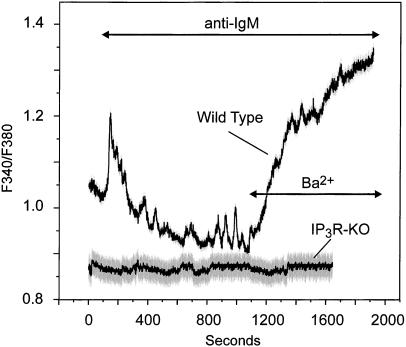
Although we have demonstrated clearly the activation of hTrp3 in DT40 cells by store depletion, we considered the possibility that activation of the agonist-PLC pathway might induce even more efficient activation. However, activation of T3-WT cells with an anti-IgM directed against the B cell receptor induced a Ba2+ entry similar to that with thapsigargin (Fig. 4). In IP3R-KO cells, anti-IgM stimulation did not induce a Ba2+ entry in untransfected (not shown) or in hTrp3-transfected cells (Fig. 4), indicating that the activation of hTrp3 by agonist absolutely depends on IP3Rs, presumably because this is the mechanism for depletion of Ca2+ stores.
Figure 4.
Agonist activation of the B cell receptor induces Ba2+ entry in hTrp3-transfected wild-type but not IP3R-KO DT40 cells. Ba2+ influx was measured in Fura-2-loaded, hTrp3-transfected wild-type or hTrp3-transfected IP3R-KO cells as described in Fig. 1 legend. The cells were maintained in a nominally Ca2+-free medium, exposed to 2 μg/ml anti-IgM, and then Ba2+ (10 mM) was added where indicated. Shown are average curves ± SEM (in gray) from experiments performed on six single cells. The data are representative of three independent experiments (nine cells).
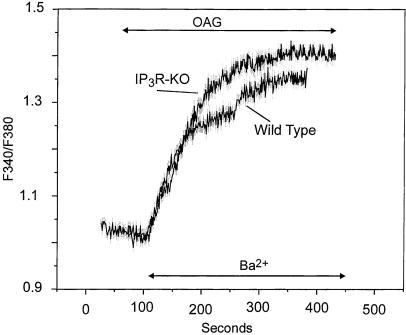
Although we suggest that the lower hTrp3 response in the IP3R knockout cells reflects the absence of IP3Rs for coupling to the channels, there also could be a difference in the level of expression of hTrp3 in these two cell lines. Members of the Trp3/6/7 group of Trp channels are known to be activated by diacylglycerols in a membrane-delimited fashion (31). Thus, we examined the effect of the membrane-permeant diacylglycerol 1-oleoyl-2-acetyl-sn-glycerol (OAG) on hTrp3-induced Ba2+ influx in both T3-WT and T3-KO cells. OAG treatment of both mock- and hTrp3-transfected cells maintained in Ca2+-free medium did not affect either cytosolic Ca2+ levels or the Ca2+ content of the stores (not shown). Addition of OAG to both T3-WT and T3-KO cells significantly stimulated Ba2+ influx (Fig. 5), whereas it had no effect on the cation entry pathway in mock-transfected cells (not shown). In the T3-WT cells, OAG-induced Ba2+ entry was comparable to that obtained upon either store depletion or receptor stimulation. Also, OAG-induced Ba2+ entry was similar in T3-WT and T3-KO cells (Fig. 5; 0.21 ± 0.03 vs. 0.19 ± 0.02 ratio units per min, respectively). Thus, in the T3-KO cells, OAG-induced Ba2+ influx was both faster and higher than that induced by thapsigargin (0.19 ± 0.02 vs. 0.06 ± 0.02 ratio units per min, respectively). Because diacylglycerols are believed to act directly on the channels, this result is consistent with the interpretation that, in the T3-KO cells, a portion of the expressed hTrp3 channels is uncoupled from regulation by intracellular stores, presumably from a lack of available IP3Rs.
Figure 5.
OAG stimulation of Ba2+ entry in hTrp3-transfected DT40 cells. Ba2+ influx was measured in Fura-2-loaded wild type or IP3R-KO cells transfected with hTrp3. The cells were maintained in a nominally Ca2+-free medium and then exposed to 100 μM of the membrane-permeant diacylglycerol OAG. When indicated, Ba2+ (10 mM) was added to the medium. Shown are average curves ± SEM (in gray) from experiments performed on six single cells. The data are representative of four independent experiments (12 cells).
Because the expressed hTrp3 channels did not behave identically to the endogenous store-operated channels, we used reverse transcription (RT)–PCR to see whether an avian Trp3 transcript was expressed in DT40 cells. By RT–PCR with cDNA prepared from wild-type DT40 total RNA as template, and primers designed based on two short peptides known to be conserved among the subfamily Trp3/6/7 in human and mouse, we amplified a single PCR fragment of 879 bp. This PCR product was sequenced and found to encode a continuous reading frame of 293 aa sharing 94.5%, 85.5%, and 77.6% sequence identity with human Trp7, Trp3, and Trp6, respectively. Because the sequence of avian Trp3/6 is not known, we cannot exclude the possibility that other Trps exist in DT40 cells.
Discussion
A number of interesting observations have been made in the course of this study. This is a clear demonstration that Trp3, a member of the Trp3/6/7 subfamily of Trp channel proteins, can function as a store-operated channel. It also demonstrates that Trp3 can be activated in an IP3R-independent manner. Note that we cannot rule out the possibility that Trp3 expressed in DT40 cells serves to regulate an endogenous SOC activity rather than forming a component of a SOC channel itself. However, this seems unlikely because no Ba2+-permeable SOC is seen in the nontransfected cells.
A more fundamental question that arises is: why does Trp3 behave as a store-operated channel in DT40 cells but not in other expression environments? We cannot provide a definitive answer for this question at present. It has been suggested that the failure of expressed channels to exhibit store-operated behavior may result from the degree of overexpression (21). Thus, it may be that this mammalian gene, driven by a cytomegalovirus promoter, and with a nonavian 5′ untranslated sequence, does not express in avian cells to the same levels as in, for example, HEK293 cells. We hypothesize that the mode of coupling of Trp channels depends on the level of expression.
Regardless of the explanation, we believe that the ability of hTrp3 to show store-operated regulation suggests that Trp3, and/or its close relatives, Trp6 or Trp7, is likely an SOC in the native environment. This subfamily of channel proteins produces nonselective cation channels when expressed exogenously (26, 27). Thus, it is possible that, under conditions of endogenous expression, Trp3 associates with other subunits, forming heterotetrameric channels with greater calcium or divalent cation selectivity. In support of this idea, DT40 B cells were shown to express an endogenous member of this subfamily (Trp7), yet no entry of Ba2+ occurs in the nontransfected cells upon Ca2+ store depletion. Alternatively, because it appears that Trp7, rather than Trp3, is present in DT40, it may be that the minimal differences in the sequences of these two proteins impart properties that lead to different ion selectivities. Alternatively, there is now considerable evidence for store-operated, nonselective cation channels in other cell types (14–18), and it seems very possible that one or more members of the Trp3/6/7 subfamily may serve as a component of this important calcium entry channel. Although not characterized electrophysiologically, the channels formed by hTrp3 in DT40 B lymphocytes are clearly less Ca2+-selective than the endogenous channels; they readily pass Ba2+, whereas the endogenous channels do not. We expect that the endogenous channel in this hematopoietic cell line may be Icrac-like, i.e., highly Ca2+-selective, and the failure of the channels to pass significant quantities of Ba2+ is consistent with this. However, no electrophysiological characterization of the endogenous channels in these cells has been published as yet. The failure of endogenous channels to pass Ba2+ results in an excellent expression environment for examining the store-dependent regulation of Ba2+-permeable hTrp3 channels.
An additional and surprising finding coming from this study is that the mechanism of store-dependent activation of expressed hTrp3 channels partly, but not wholly, depends on the presence of IP3Rs. It is difficult at present to know which signaling mechanism underlies the IP3R-independent response. One possibility is that ryanodine receptors may fulfill this function (46). However, there appear to be few or no functional ryanodine receptors expressed in this cell line (47). A clear alternative is the suggested diffusible messenger for CCE, termed calcium influx factor (9, 10). Interestingly, there is recent evidence for such a mode of signaling in vascular smooth muscle cells (48), which is one of the cell types that expresses the nonselective cation channel version of CCE (18).
In summary, expression of the human Trp3 channel protein in avian DT40 B lymphocytes results in a divalent, cation-permeable channel that is activated by Ca2+ store depletion. This regulation apparently occurs through both IP3R-dependent and IP3R-independent mechanisms. The ability to observe these distinct modes of store-dependent regulation of expressed hTrp3 channels in DT40 cells should provide a starting point for eventually unraveling the molecular details of this elusive signaling pathway.
Abbreviations
- CCE
capacitative calcium entry
- hTrp3
human transient receptor potential 3
- SOC
store-operated Ca2+ channel(s)
- IP3
inositol trisphosphate
- IP3R
IP3 receptor
- Icrac
calcium release-activated calcium current
- OAG
1-oleoyl-2-acetyl-sn-glycerol
- IP3R-KO
DT40 B lymphocytes with all three IP3Rs knocked out
- T3-WT
wild-type DT40 transiently transfected with hTrp3
- T3-KO
IP3R-KO transiently transfected with hTrp3
- PLC
phospholipase C
- eGFP
enhanced green fluorescent protein
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Putney J W, Jr, Bird G S J. Endocr Rev. 1993;14:610–631. doi: 10.1210/edrv-14-5-610. [DOI] [PubMed] [Google Scholar]
- 2.Clapham D E. Cell. 1995;80:259–268. doi: 10.1016/0092-8674(95)90408-5. [DOI] [PubMed] [Google Scholar]
- 3.Tsien R W, Tsien R Y. Annu Rev Cell Biol. 1990;6:715–760. doi: 10.1146/annurev.cb.06.110190.003435. [DOI] [PubMed] [Google Scholar]
- 4.Berridge M J. Nature (London) 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- 5.Putney J W., Jr Cell Calcium. 1986;7:1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 6.Putney J W., Jr . Capacitative Calcium Entry. Austin, TX: Landes; 1997. [Google Scholar]
- 7.Irvine R F. FEBS Lett. 1990;263:5–9. doi: 10.1016/0014-5793(90)80692-c. [DOI] [PubMed] [Google Scholar]
- 8.Berridge M J. Biochem J. 1995;312:1–11. doi: 10.1042/bj3120001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Randriamampita C, Tsien R Y. Nature (London) 1993;364:809–814. doi: 10.1038/364809a0. [DOI] [PubMed] [Google Scholar]
- 10.Csutora P, Su Z, Kim H Y, Bugrim A, Cunningham K W, Nuccitelli R, Keizer J E, Hanley M R, Blalock J E, Marchase R B. Proc Natl Acad Sci USA. 1999;96:121–126. doi: 10.1073/pnas.96.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parekh A B, Penner R. Physiol Rev. 1997;77:901–930. doi: 10.1152/physrev.1997.77.4.901. [DOI] [PubMed] [Google Scholar]
- 12.Vaca L, Kunze D L. Am J Physiol. 1994;267:C920–C925. doi: 10.1152/ajpcell.1994.267.4.C920. [DOI] [PubMed] [Google Scholar]
- 13.Lückhoff A, Clapham D E. Biophys J. 1994;67:177–182. doi: 10.1016/S0006-3495(94)80467-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang H, Inazu M, Weir B, Buchanan M, Daniel E. Eur J Pharmacol. 1994;251:119–125. doi: 10.1016/0014-2999(94)90391-3. [DOI] [PubMed] [Google Scholar]
- 15.Worley J F, III, McIntyre M S, Spencer B, Dukes I D. J Biol Chem. 1994;269:32055–32058. [PubMed] [Google Scholar]
- 16.Krause E, Pfeiffer F, Schmid A, Schulz I. J Biol Chem. 1996;271:32523–32528. doi: 10.1074/jbc.271.51.32523. [DOI] [PubMed] [Google Scholar]
- 17.Wayman C P, Wallace P, Gibson A, McFadzean I. Eur J Pharmacol. 1999;376:325–329. doi: 10.1016/s0014-2999(99)00400-8. [DOI] [PubMed] [Google Scholar]
- 18.Trepakova E S, Gericke M, Hirakawa Y, Weisbrod R M, Cohen R A, Bolotina V M. J Biol Chem. 2001;276:7782–7790. doi: 10.1074/jbc.M010104200. [DOI] [PubMed] [Google Scholar]
- 19.Birnbaumer L, Zhu X, Jiang M, Boulay G, Peyton M, Vannier B, Brown D, Platano D, Sadeghi H, Stefani E, et al. Proc Natl Acad Sci USA. 1996;93:15195–15202. doi: 10.1073/pnas.93.26.15195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Warnat J, Philipp S, Zimmer S, Flockerzi V, Cavalié A. J Physiol (London) 1999;518:631–638. doi: 10.1111/j.1469-7793.1999.0631p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yue L, Peng J-B, Hediger M A, Clapham D E. Nature (London) 2001;410:705–709. doi: 10.1038/35070596. [DOI] [PubMed] [Google Scholar]
- 22.Zhu X, Chu P B, Peyton M, Birnbaumer L. FEBS Lett. 1995;373:193–198. doi: 10.1016/0014-5793(95)01038-g. [DOI] [PubMed] [Google Scholar]
- 23.Wes P D, Chevesich J, Jeromin A, Rosenberg C, Stetten G, Montell C. Proc Natl Acad Sci USA. 1995;92:9652–9656. doi: 10.1073/pnas.92.21.9652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu X, Jiang M, Peyton M, Boulay G, Hurst R, Stefani E, Birnbaumer L. Cell. 1996;85:661–671. doi: 10.1016/s0092-8674(00)81233-7. [DOI] [PubMed] [Google Scholar]
- 25.Harteneck C, Plant T D, Schultz G. Trends Neurosci. 2000;23:159–166. doi: 10.1016/s0166-2236(99)01532-5. [DOI] [PubMed] [Google Scholar]
- 26.Zitt C, Obukhov A G, Strübing C, Zobel A, Kalkbrenner F, Lückhoff A, Schultz G. J Cell Biol. 1997;138:1333–1341. doi: 10.1083/jcb.138.6.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hurst R S, Zhu X, Boulay G, Birnbaumer L, Stefani E. FEBS Lett. 1998;422:333–338. doi: 10.1016/s0014-5793(98)00035-0. [DOI] [PubMed] [Google Scholar]
- 28.Hofmann T, Schaefer M, Schultz G, Gudermann T. J Mol Med. 2000;78:14–25. doi: 10.1007/s001099900070. [DOI] [PubMed] [Google Scholar]
- 29.Preuss K-D, Nöller J K, Krause E, Göbel A, Schulz I. Biochem Biophys Res Commun. 1997;240:167–172. doi: 10.1006/bbrc.1997.7528. [DOI] [PubMed] [Google Scholar]
- 30.Zhu X, Jiang M, Birnbaumer L. J Biol Chem. 1998;273:133–142. doi: 10.1074/jbc.273.1.133. [DOI] [PubMed] [Google Scholar]
- 31.Hofmann T, Obukhov A G, Schaefer M, Harteneck C, Gudermann T, Schultz G. Nature (London) 1999;397:259–262. doi: 10.1038/16711. [DOI] [PubMed] [Google Scholar]
- 32.Ma H-T, Patterson R L, van Rossum D B, Birnbaumer L, Mikoshiba K, Gill D L. Science. 2000;287:1647–1651. doi: 10.1126/science.287.5458.1647. [DOI] [PubMed] [Google Scholar]
- 33.McKay R R, Szmeczek-Seay C L, Lièvremont J-P, Bird G S J, Zitt C, Jüngling E, Lückhoff A, Putney J W., Jr Biochem J. 2000;351:735–746. [PMC free article] [PubMed] [Google Scholar]
- 34.Kiselyov K, Xu X, Mozhayeva G, Kuo T, Pessah I, Mignery G, Zhu X, Birnbaumer L, Muallem S. Nature (London) 1998;396:478–482. doi: 10.1038/24890. [DOI] [PubMed] [Google Scholar]
- 35.Boulay G, Brown D M, Qin N, Jiang M, Dietrich A, Zhu M X, Chen Z, Birnbaumer M, Mikoshiba K, Birnbaumer L. Proc Natl Acad Sci USA. 1999;96:14955–14960. doi: 10.1073/pnas.96.26.14955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Z, Tang J, Tikunova S, Johnson J D, Chen Z, Qin N, Dietrich A, Stefani E, Birnbaumer L, Zhu M X. Proc Natl Acad Sci USA. 2001;98:3168–3173. doi: 10.1073/pnas.051632698. . (First Published February 27, 2001; 10.1073/pnas.051632698) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zubov A I, Kaznacheeva E V, Alexeeno V A, Kiselyov K, Muallem S, Mozhayeva G. J Biol Chem. 1999;274:25983–25985. doi: 10.1074/jbc.274.37.25983. [DOI] [PubMed] [Google Scholar]
- 38.Kaznacheyeva E, Zubov A, Gusev K, Bezprozvanny I, Mozhayeva G. Proc Natl Acad Sci USA. 2001;98:148–153. doi: 10.1073/pnas.98.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang L, Saffen D. J Biol Chem. 2001;276:13331–13339. doi: 10.1074/jbc.M008914200. [DOI] [PubMed] [Google Scholar]
- 40.Sugawara H, Kurosaki M, Takata M, Kurosaki T. EMBO J. 1997;16:3078–3088. doi: 10.1093/emboj/16.11.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Broad L M, Braun F-J, Lièvremont J-P, Bird G S J, Kurosaki T, Putney J W., Jr J Biol Chem. 2001;276:15945–15952. doi: 10.1074/jbc.M011571200. [DOI] [PubMed] [Google Scholar]
- 42.Buerstedde J-M, Reynaud C A, Humphries E H, Olson W, Ewert D L, Weill J C. EMBO J. 1990;9:921–927. doi: 10.1002/j.1460-2075.1990.tb08190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buerstedde J-M, Takeda S. Cell. 1991;67:179–188. doi: 10.1016/0092-8674(91)90581-i. [DOI] [PubMed] [Google Scholar]
- 44.Ma H-T, Venkatachalam K, Li H S, Montell C, Kurosaki T, Patterson R L, Gill D L. J Biol Chem. 2001;276:18888–18896. doi: 10.1074/jbc.M100944200. . (First Published March 19, 2001; 10.1074/jbc.M100944200) [DOI] [PubMed] [Google Scholar]
- 45.Byron K L, Taylor C W. J Physiol (London) 1995;485:455–468. doi: 10.1113/jphysiol.1995.sp020742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kiselyov K, Shin D M, Wang Y, Pessah I N, Allen P D, Muallem S. Mol Cell. 2000;6:421–431. doi: 10.1016/s1097-2765(00)00041-1. [DOI] [PubMed] [Google Scholar]
- 47.Bultynck G, De Smet P, Weidema A F, Ver Heyen M, Maes K, Callewaert G, Missiaen L, Parys J B, De Smedt H. J Physiol (London) 2000;525:681–693. doi: 10.1111/j.1469-7793.2000.t01-1-00681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trepakova E S, Csutora P, Hunton D L, Marchase R B, Cohen R A, Bolotina V M. J Biol Chem. 2000;275:26158–26163. doi: 10.1074/jbc.M004666200. [DOI] [PubMed] [Google Scholar]