Abstract
Alzheimer’s disease (AD) is one of the most common causes of dementia. Despite several decades of serious research in AD there is no standard disease modifying therapy available. Stem cells hold immense potential to regenerate tissue systems and are studied in a number of brain-related disorders. For various untreatable neurodegenerative disorders, such as Alzheimer’s disease (AD), amyotrophic lateral sclerosis (ALS) and Parkinson’s disease (PD) (current-approved drugs provide only symptomatic relief), stem cell therapy holds a great promise and provides a great research opportunity. Here we review several stem cell transplantation studies with reference to both preclinical and clinical approaches. We focus on different sources of stem cells in a number of animal models and on molecular mechanisms involved in possible treatment of neurodegenerative disorders. The clinical studies reviewed suggest safety efficacy and translational potential of stem cell therapy. The therapeutic outcome of stem cell transplantation has been promising in many studies but no unifying hypothesis exists for an underlying mechanism. Some studies reported paracrine effects exerted by these cells via release of neurotrophic factors, while other studies reported immunomodulatory effects by transplanted cells. There are also reports supporting stem cell transplantation causing endogenous cell proliferation or replacement of diseased cells at the site of degeneration. In animal models of AD, stem cell transplantation is also believed to increase expression of synaptic proteins. A number of stem cell transplantation studies point out great potential for this novel approach in preventing or halting several neurodegenerative diseases. The current challenge is to clearly define the molecular mechanism by which stem cells operate and the extent of actual contribution by the exogenous and/or endogenous cells in the rescue of disease.
Keywords: Stem cells, Alzheimer’s disease, neurodegeneration, synaptogenesis, differentiation, proliferation, therapeutics, transplantation
INTRODUCTION: THE POTENTIAL OF STEM CELL TRANSPLANTATION IN ALZHEIMER’S DISEASE
A number of neurodegenerative disorders, such as Alzheimer’s disease (AD), Amyotrophic lateral sclerosis (ALS) and Parkinson’s disease (PD) are untreatable, and they progressively worsen with age, resulting in death. The world Alzheimer Repot 2015 reported over 46 million individuals in the world’s population suffer from dementia, and this number is estimated to increase upto 131.5 million by 2050 [1]. Dementia is associated with multiple causes that include alcoholism, AD, stroke, PD and drug/medication intoxication. It is the fifth leading cause of death in the US with age of 65 years or above. In 2015 AD prevalence in USA was estimated to be close to 5.3 million, and this is expected to rise up to 11 to 16 million in 2050 [2]. In India, the number of individuals which are suffering from AD and other dementia is estimated to be approximate 3.7 million and this number is expected to double by the year 2030 [3].
The most common form of dementia, AD, is characterized by different stages of cognitive and functional impairment. Patients suffering from AD lose autonomy in their daily normal activities, and this progressively deteriorates with age. In 1901, Alois Alzheimer, a German psychiatrist, diagnosed a 51 year-old woman with a condition he called “amnestic writing disorder” [4]. Her psychosocial abnormalities included aphasia and memory impairment. Later, in 1910 when Alzheimer’s supervisor published his book Psychiatrie, he reported this case and mentioned this condition as Alzheimer’s disease [4]. Since then, extensive research has progressed worldwide to understand several aspects of the disease, ranging from its pathology, disease onset, prevalence, diagnosis and treatment in various cellular, pre-clinical and clinical studies. Currently, AD pathophysiology is based on several important hypotheses i.e., including the cholinergic hypothesis, protein misfolding, and amyloid cascade hypotheses [5–7].
The hippocampus plays a significant role in memory encoding and retrieval. Hippocampus is the first region of the brain to be affected in AD. Injury to brain tissue has not been seriously considered for treatment by cell replacement strategies as compared to the other organs e.g. skin and liver tissues. Earlier, neuroanatomists considered that the nervous system is incapable of regeneration. In 1962 Joseph Altman provided the first evidence of neurogenesis in the cerebral cortex and later, in 1963 he showed the occurrence of neurogenesis in the dentate gyrus of rat and cat hippocampus [8]. In some animals, neuronal precursors originate from the subventricular zone (SVZ) to the main olfactory bulb via specialized migratory route known as the rostral migratory stream (RMS). More recently, various strategies are being employed to activate these lesser population of stem cells by various methods [9]. Currently available FDA-approved drugs for AD provide symptomatic relief to the patients without alleviating elusive disease pathology. Alternative strategies such as herbal remedies [10–12] and cell based therapies [13, 14] are being tested in preclinical settings with the hope of halting disease progression. The underlying mechanism is either replacement of degenerating neurons or exerting neuroprotection by the paracrine effect of transplanted cells by the secretion of neurotrophic factors (Fig. 1) [15]. The efficacy of stem cells has been studied in various pre-clinical studies by transplanting these cells into the disease- specific animal models. However, there is a gap of knowledge describing the underlying molecular mechanisms involved in the rescue of disease by transplanted cells.
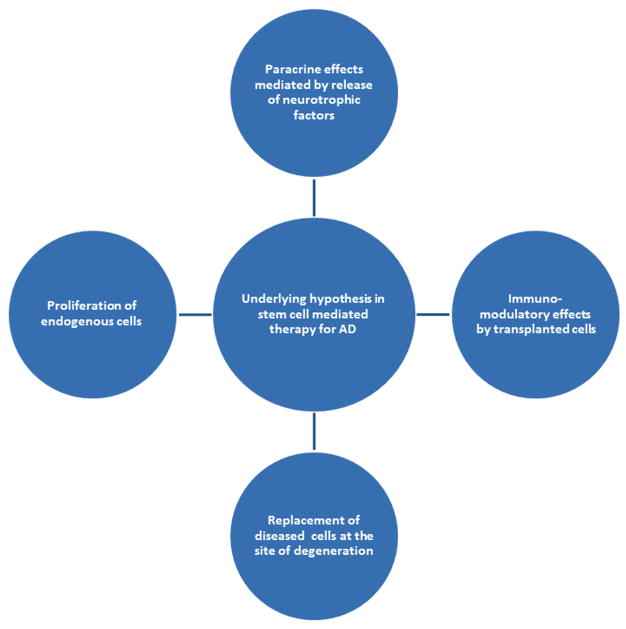
Fig. 1. Outline for underlying mechanism in stem cell mediated reversal of AD pathology.
Some underlying hypothesis may explain functional improvements in subjects of stem cell transplantation in AD. However, current experiments point to four possible explanations. A) Paracrine effects from release of neurotrophic factors by transplanted cells. B) Immunomodulatory effects by transplanted cells. C) Replacement of diseased cells by transplanted cells. D) Proliferation of endogenous cells. It is likely that all four processes operate in a coherent and synergistic manner to produce a final salutary effect(s).
PATHOPHYSIOLOGICAL FEATURES IN ALZHEIMER’S DISEASE
Several animal studies and human brain biopsies have revealed the pathological hallmarks of AD, including extracellular amyloid-β (Aβ) plaque deposits and formation of intracellular neurofibrillary tangles (NFT). NFT are misfolded structures produced by aberrant phosphorylation of microtubule-stabilizing tau proteins. The process of Aβ formation is known to play a significant role in AD etiology [16]. Amyloid plaques may trigger a pathological cascade resulting in neurofibrillary tangles and neuroinflammation causing neuritic dysfunction, which ultimately leads to neuronal death. In AD patients, excessive accumulation of amyloid plaques is likely to be due to dysregulation of activity of β-site Amyloid Precursor Protein-Cleaving Enzyme 1 (BACE1). BACE1 gives rise to Aβ from the membrane-spanning Aβ precursor protein (APP). This is the rate limiting step of Aβ production. This cleavage occurs at the N-terminus of Aβ to form soluble APPβ, and the C-terminus is further cleaved by γ-secretase complex, which yields Aβ40/42 [17]. Aβ fragments thus generated aggregate to form amyloid fibrils. Aβ40 (with 40 amino acid residue) is the predominant form but Aβ42 (with 42 residues) is more fibrillogenic than the shorter species and is involved in disease pathology.
Several environmental factors cause epigenetic changes in individuals. It plays a significant role in regulating the gene expression via modification of DNA and histone protein modification leading to genetic dysregulation thereby causing various disease pathologies. In AD, amyloid fibril-induced neuroinflammation is believed to increase expression of epigenetic factors such as. methyl-CpG-binding protein 2 and histone deacetylase 2 and their interaction further suppresses the expression of synaptic protein leading to amyloid induced memory deficiency [18].
Tau is an intracellular microtubule associated protein that plays an essential role in microtubule stabilization. Abnormal phosphorylation of tau leads to microtubule disruption. The formation of neurofibrillary tangles may be triggered by amyloid plaque. In addition, the cholinergic hypothesis postulates a reduction in neurotransmitter acetylcholine in the AD patients [19] as the primary cause of AD. Besides amyloid plaque deposition and neurofibrillary tangle formation, vascular dysfunction also appears in AD pathophysiology.
Both genetic and environmental factors contribute to etiology of AD. Genetic factors linked to autosomal dominant inherited mutations include presenilin 1 (PS1), presenilin 2 (PS2), APP and enzymes involved in amyloid processing, such as BACE1. This genetic form, also called familial AD (FAD) contributes marginally towards prevalence (no more than 5% of AD cases [20]), whereas most AD cases are sporadic, with an unknown cause. The E4 variant of APOE is largely known as a major genetic risk factor for the late onset of AD [21]. Studies suggest that there are some interactions with amyloid to cause this dramatic effect [22]. Some studies also propose that sporadic cases are the result of various environmental and epigenetic factors which lead to an etiology based upon “Latent Early-life Associated Regulation” (LEARn). LEARn describes effects resulting from exposure of stressors in early life e.g. nutritional imbalance, toxic metals (such as lead) and other stressors, which induce epigenetic alterations on disease associated gene chromatin or histones [23]. These changes remain latent as (de)methylation of promoter or chromatin modifications by (de)acetylation, (de)methylation and (de)phosphorylation. Upon one or more additional hits in the later life, expression of modified gene(s) alters sufficiently to induce pathology.
CELL TYPE CONSIDERATIONS FOR DISEASE MODIFYING THERAPIES
The requirement of a suitable cell type with particular characteristics for specific disease types is needed for proper and effective cell transplantation. Stem cells from several tissues such as bone marrow and umbilical cord blood are well characterized for their proliferation and differentiation properties and can be an optimum source for transplantation [24, 25]. Current strategies emphasize culturing of isolated cells in an optimum medium with suitable nutrient environment to obtain the desired disease phenotype. The microenvironment also provides suitable niche for selective expression of desirable markers to trigger these cells for a specialized cell type [26, 27]. Long-term culture and characterization of primary neurons isolated from rodent and human fetal tissue is essential for undertaking comparative studies. Abundant tau and amyloid-β production in human brain cultures provides a powerful cellular model for AD. In a recent study Ray et al provide a well-characterized methodology for fetal human primary brain cell culture, which is useful to test the therapeutic efficacy of drugs targeting AD [28]. Cultures of induced pluripotent stem cell (iPSC) generated from fibroblasts of FAD patients with presenilin 1 and presenilin 2 mutations were characterized after acquiring neuronal lineage [29]. Apart from increased Aβ42 expression, the iPSC model also showed variable drug response and alleviation of stress induced response by docosahexaenoic acid (DHA) treatment [30]. Likewise, RNA silencing has also been used in this cellular model. Therapeutic strategies primarily focus on targeting production of Aβ by identifying key molecular regulators of BACE1 expression. The researchers have also elucidated the role of human micro-RNA (miR)-339-5p which negatively modulates BACE1 in primary human brain cultures, and expression of miR-339-5p is reduced in AD patients [31].
PRE-CLINICAL STUDIES TO PROBE REGENERATIVE POTENTIAL OF STEM CELLS
At present, there are no consensus measures to accurately diagnose and monitor progression of AD [32]. This significantly hinders effective treatments against AD. To study AD pathologies and its targets, different animal models of AD have been established and tested in preclinical settings. These model systems range from laboratory animals like zebrafish, murid rodents and nonhuman primates to model invertebrates such as Drosophila and C elegans. Among these, rats and mice are widely used, and their transgenic counterparts are the most-established system to evaluate disease pathophysiology as well as effective treatment strategies. Several strategies have been adopted to establish AD like pathologies and induced memory impairment in these models [33]. These include predetermined brain injury, neurotoxin induced cell loss in brain and intra-cerebroventricular injection of Aβ peptides [34].
Current treatments for AD includes blocking neurotransmitter degradation, which provide temporary symptomatic relief without alleviating the pathophysiological burden of the disease [35, 36]. Therefore, alternative cell based studies for transplantation have been carried out in the belief that either these cells replace degenerating neurons or secrete trophic factors that provide a protective environment to the endogenous cells. Various neurotrophic factors are secreted by the cells to modulate the synaptic functioning in brain. In particular, BDNF is synthesized by neurons and highly expressed in cortex and hippocampus; these regions are crucial for learning and memory in brain [37].
The animal models associated with Aβ-induced memory loss have been widely studied in understanding pathophysiology of AD and testing therapeutic efficacy of various drug targets. Prakash et al. use intracerebroventricular (ICV) injection of Aβ to study the role of pioglitazone, a peroxisome proliferator-activated receptor-γ (PPAR-γ) agonist, on neurotrophic factor BDNF in a rat model of AD with neuroinflammation. Aβ-injured animals showed significant impairment in memory as well as reduced levels of BDNF, which were reversed by administration of pioglitazone [38]. Tang et al. demonstrated fibrillar Aβ40 induced neurotoxicity in rat hippocampus, characterized by congo red plaques and degenerating neurons at the site of injection. This pathological outcome was supported by impaired cognitive performance in the rats, tested in Morris water maze. Further, they have used this model to validate cell replacement efficacy of neural precursor cells derived from human embryonic stem cells. The neural precursor cells are partially differentiated, as these cells are more precisely committed to their lineage [39]. The transplanted cells were found to ameliorate Aβ-induced cognitive impairment in these rats and further survived, integrated and differentiated into GFAP and NF-200 positive neuronal cells after 16 weeks of transplantation [40].
Blurton-Jones et al. explored the role of neural stem cell transplantation in reversal of memory impairment. To study the effect of neural stem cells (NSCs) in AD pathology and cognitive functions, these cells were transplanted into aged triple transgenic mice that express mutant presenilin, tau and APP with aggressive Aβ load. Remarkably, transplanted NSCs were found to ameliorate loss in spatial learning and memory without altering Aβ and tau pathologies. Further, these cells increased synaptic density in diseased brain, which was assisted by BDNF. Loss of function studies have revealed that NSCs exert regenerative effects mediated by BDNF. It was further found that restoration of memory loss occurred when recombinant BDNF was additionally supplemented [15]. The same group recently reported that when these NSCs were genetically engineered to stably release the Aβ degrading enzyme neprilysin (NEP), they could augment synaptic plasticity as well as ameliorate underlying Aβ pathology in triple transgenic mice [41]. Neuralstem, Inc. had announced the first data on neural stem cells transplantation studies in an animal model of AD. This group reported that HK532: IGF1 (NSI-532.IGF) cells ameliorate spatial learning deficits and improved memory in AD mice. To generate human insulin-like growth factor 1 (IGF-1), a cortical neural stem cell line was engineered. IGF-1 cells also impart a wide-range of neuroprotective properties [42]. Notably, the cells, which were administered in the peri-hippocampal region showed survival up to ten weeks. Also, mice with stem cell transplantation performed better than did control mice at fourteen weeks after the surgery. It would be reasonable to conclude that such preliminary studies point toward a potentially feasible therapeutic approach to treat AD in the future, and that the therapeutic effect of stem cells upon transplantation into the brain is supported by a combination of approaches and largely mediated or at least significantly influenced by paracrine effects.
IMMUNOMODULATORY EFFECTS OF STEM CELLS TARGETING AD PATHOLOGY
Reports also suggest that transplanted stem cells exerts some immunomodulatory response at the site of injury, leading to release of cytokines that further target the underlying AD pathology. Jin et al. highlighted the phenomenon of crosstalk between transplanted cells and endogenous neuroproliferative cells by the transplantation of neural precursor cells (NPCs) in focal cerebral ischemia of rat brain. In their earlier study they found reduced infarct volume and improved behavioral outcomes upon transplantation of NPCs in middle cerebral artery occlusion model of rat. In a more recent study neurogenesis was shown by an increase in BrdU labeling and expression of neuronal migration protein doublecortin in the ipsilateral SVZ whereas not in contralateral SVZ or subgranular zone (SGZ) in young and aged rats [43]. In another study, authors have administered umbilical cord blood-derived mesenchymal stem cells (UCB-MSCs) in double transgenic mice of PS1 and APP, which substantially ameliorated loss of spatial learning and memory by microglia activation. Further, levels of Aβ peptide, hyperphosphorylation of tau and BACE1 activity were reduced significantly. This neuroprotective effect by UCB-MSCs involved modulation of neuroinflammation due to reduction in pro-inflammatory and increase in anti-inflammatory cytokines, induced by microglia activation [44]. These findings suggest that UCB-MSC may act as a therapeutic agent to ameliorate decline in cognitive functions in AD model mice.
Besides amyloid plaque deposition and neurofibrillary tangle formation, vascular dysfunction also contributes to the AD pathophysiology. Vascular endothelial growth factor (VEGF) is also implicated in AD related neurodegeneration. Therefore Garcia et al used the strategy of providing VEGF by transplantation of overexpressing bone marrow derived mesenchymal cells into the lateral ventricles of brain using stereotaxic surgery in double transgenic mouse model with APPSWE/PS1dE9 mutations [45]. Behavioral and molecular parameters were assessed for vascularization and amyloid plaque deposition. Outcomes included reducing behavioral deficit and amyloid deposition besides inducing favored neovascularization. Yang et al. have used cell based approach and transplanted differentiated neuron like cells in APP/PS1 transgenic mice. They used human mesenchymal stem cells derived from Wharton’s jelly of umbilical cord and transdifferentiated into neuron-like cells (HUMSC-NCs) by tricyclodecan-9-yl-xanthogenate (D609). Transplantation of HUMSC-NCs in a transgenic APP/PS1 mouse model significantly reduced Aβ load and improved cognitive functions via increase in microglial activation and expression of the NEP and Aβ degrading enzymes insulin-degrading enzyme (IDE). Expression of pro-inflammatory markers associated with the modulation of M2-like microglia (a type of microglia classified based on its mannose receptor and its activation by IL-4 cytokine [46]) activation was reduced whereas expression of anti-inflammatory markers, such as interleukin-4 (IL4), was found to be increased [47]. Mesenchymal stem cells derived from bone marrow of male Sprague–Dawley rats were transplanted in female rats by tail vein injection [48]. BM-MSCs were found to increase expression of nestin andcholine acetyltransferase positive cells at the injured area in the brain. Notably, these cells displayed a reduction in amyloid plaques in hippocampus.
Thus, bone marrow cells bring about their therapeutic effect by mechanisms involving anti-apoptotic activity, immunomodulation, and neurogenic properties. Furthermore, neurogenesis in the subgranular zone of dentate gyrus may act as an endogenous repair mechanism in AD via Wnt pathway in amyloid-related neurodegeneration associated with AD. Researchers also investigated the role of mesenchymal stem cells on hippocampal neurogenesis by co-culturing with the amyloid treated neural progenitor cells. Mesenchymal stem cells treatment to NPC significantly enhances the expression of GFAP, Ki67, HuD c, SOX2, and Nestin. Transplantation of these mesenchymal stem cells in Aβ-treated animals increased BrdU and HuD double positive cells in hippocampus at 2 and 4 weeks as compared to control and Aβ-treated alone animals [49]. This shows that MSC administration caused hippocampal neurogenesis and increased differentiation of NPC, which is modulated by Wnt pathway. If proven, this may provide a better therapeutic approach for treating AD patients than what is offered by anticholinesterase drugs. Zhang et al realized that neural stem cell transplantation could provide a better approach for the therapeutic treatment of AD and hypothesized that the transplantation of NSCs would ameliorate cognitive impairment by increased expression of synaptic proteins. Therefore, they isolated NSCs from mouse embryo at embryonic day 14 and transplanted these cells in both hippocampi of APP/PS1 transgenic mice. Indeed, there was enhancement in cognitive functions analyzed by better spatial learning and memory after 8th weeks of transplantation as compared to the control group. Further, the expression of synaptophysin (SYN) and GAP-43 were found to be increased significantly. Hence, these results suggest that NPC induced cognitive improvement possibly by formation of new neural circuits [50].
Studies have also been carried out to mobilize the quiescent bone marrow stem cell population into the peripheral blood by using stimulating factors. Prakash et al evaluated the effect of granulocyte colony stimulating factor (GCSF) in Aβ induced memory loss in male adult Wistar rats, and they found significant escalation in behavioral performance after GCSF elevated the progenitor population and CD34 positive cells in the brain affecting neurogenesis [51]. Shetty et al. demonstrated the efficacy of mesenchymal stem cells (MSCs) derived from umbilical cord tissue in a Parkinson disease model [52]. They have studied the comparative therapeutic efficacy of MSCs from umbilical tissue and bone marrow as well as efficacy of undifferentiated versus differentiated cells in their model and found better efficacy of differentiated MSCs into dopaminergic phenotype when transplanted. As mesenchymal stem cells lack immunomodulatory activity, these cells provide a novel cellular approach to treat some neurological disorders. Several sources of stem cells in combination with multiple approaches tested in pre-clinical AD models are further discussed in Table 1.
Table 1.
Preclinical Alzheimer’s studies of stem cell transplantation
| No | Type/Source of Stem Cell Used | Time Points | Route of Administration of Stem Cells | Results/Outcome | Animal Model Used | References |
|---|---|---|---|---|---|---|
| 1. | Neural stem cells | - | Intra-hippocampus | Ameliorated loss in spatial memory and learning by BDNF Increase in synaptic density |
Triple transgenic mice (3xTg-AD) that express PS-1, tau and APP | [15] |
| 2. | Neural precursor cells | Transplanted in 3 months and 24 months old rats | Cortical infarct cavity | Highlighted the cross-talk between transplanted and endogenous cells. Increased endogenous neurogenesis. |
Focal cerebral ischemia in rat model | [43] |
| 3. | Umbilical cord blood-mesenchymal stem cells | Transplanted at 29,31 and 33 weeks of age; MWM analysis at 33 week, 4 days | - | Improvement in spatial learning and memory by the microglial activation. Reduced expression of Aβ |
Double transgenic mice of PS1 and APP | [44] |
| 4. | Transdifferentiated human Wharton’s jelly mesenchymal stem cells into neuron-like cells | Three weeks after transplantation MWM was performed | Bilateral hippocampus injection | Improvement in cognitive functions. Reduced Aβ load by increase in microglial activation, insulin-degrading enzyme and neprilysin expression. |
AβPP/PS1 transgenic mice model | [47] |
| 5. | Mesenchymal stem cells | End point analysis done at 2 and 4 weeks after MSC transplantation | Intra-hippocampus | Increased hippocampal neurogenesis. Differentiation of NPC by Wnt signaling pathway. |
Amyloid β treated mice | [49] |
| 6. | Neural stem cell | End point analysis after 8 weeks of transplantation | Intra-hippocampus | Increased expression of synaptic protein i.e. synaptophysin and GAP-43. Ameliorate cognitive impairment |
APP + PS1 transgenic (Tg) mice | [50] |
| 7. | Umbilical cord tissue derived Mesenchymal stem cell | IHC done at at 6, 12 and 24 weeks, and at 1 year post-transplantation | Substantia nigra | Studied efficacy of undifferentiated versus differentiated. Dopaminergic differentiated MSCs showed better results. |
Parkinson Disease model | [52] |
| 8. | Mesenchymal stem cell | SC transplanted after 2 hrs of injury; Behavioural assessment has been done at 1, 3, 7, 14, 21 and 28 day after TBI; IHC done after 72 hrs of TBI |
Intravenously | Transplantation of MSCs showed immunomodulatory effects. Proinflammatory cytokines were reduced. Increased anti-inflammatory cytokine expression. |
Traumatic brain injury mode | [98] |
| 9. | Bone marrow derived mesenchymal stem cell (BM-MSC) | MWM at 2 weeks after the surgery | Intra-hippocampus | Senile plaques were reduced. Significant increased DeltaNp73 protein expression. Better performance in Morris water maze. |
APP/PS1 transgenic mice | [99] |
| 10. | Human olfactory bulb neural stem cells | NSC transplanted after 10 days of ibotenic acid administration. | Intra-hippocampus | Cells were engineered to express nerve growth factor. Enhanced cognitive abilities. |
Ibotenic acid induced AD rat model | [100] |
| 11. | Neural stem cell | NSC were transplanted in 12-months old mice; IHC performed 5 weeks post-transplantation |
- | No impact on Aβ plaques. Transplanted cells showed chemotaxis towards the plques. |
APP/PS1 double transgenic AD mice | [101] |
| 12. | Placenta derived Mesenchymal stem cell | Intravenous in mouse tail | Improved cognitive function. Prevent neuronal death Reduced inflammatory cytokines. Promoted neuronal cell differentiation |
Amyloid β1-42 peptide infused in mouse model | [102] | |
| 13. | Mesenchymal stem cell | Aβ administered at 6 week of age; MSC transplanted on post-operative day 1. | Reduction in Aβ levels Enhanced induction of autophagosome |
Amyloid beta treated animal model | [103] | |
| 14. | T-regulatory cells educated by UC-MSC | 6 months old mice were used; Behavior test performed after 2 weeks of transplantation |
Intracardiac injection | Reduced microglial activation and systemic inflammation. Ameliorate cognitive function Decreased Aβ plaques deposition |
Transgenic AβPPswe/PS1dE9 mice | [104] |
| 15. | Amniotic membrane derived mesenchymal stem cell | Pathological analysis done at 1 week post-transplantation; Behavior analysis at 12 weeks post-transplantation |
Intravenous injection | Reduced proinflammatory and increased anti-inflammatory cytokines. More activation of microglial cells. Improved spatial memory |
Tg2576 transgenic mice model of AD | [105] |
| 16. | Adipose derived stem cell | MWM was performed 3 months of stem cells injection | Intravenous transplantation | No immune response Migrated to brain by crossing blood brain barrier Rescued memory deficiency Upregulated VEGF and IL-10 expression |
Tg2576 mice model of AD | [106] |
| 17. | Bone marrow derived mesenchymal stem cell | End point analysis is upto 2 months | Intra-cerebral injection | Increased expression of dynamin 1 and synapsin1 Aβ plaque deposition reduces |
- | [107] |
| 18. | Mesenchymal stem cell derived from umbilical cord blood | Ten months old mice when stem cells were administered | Bilateral intra-hippocampal administration | Induced endogenous neprilysin expression Reduced Aβ plaques |
APP/PS1 transgenic mice model | [108] |
| 19. | Epidermal neural crest stem cell | 14 days after Aβ administration stem cells were injected | CA3 region of hippocampus | Increased granule cells in hippocampus Transplanted cells expressed neuronal markers like GFAP |
Amyloid-β1-40 injected AD rat model | [109] |
| 20. | Bone marrow derived mesenchymal stem cell | Stem cell injection after 4 months of AlCl3 induced AD | Tail vein injection | Reduced amyloid plaque in hippocampus. Increased choline acteyltransferase positive and nestin cells. |
AlCl3 induced AD female rat model | [48] |
| 21. | VEGF overexpressing bone marrow derived mesenchymal stem cell | Cells were transplanted in 6, 9 and 12 months old animal | Lateral ventricles of brain | Reduction in behavioral deficiency Reduced amyloid plaque in hippocampus. Improved neovascularization |
Double transgenic mouse model with APPswe/PS1dE9 mutations | [45] |
| 22. | Choline acetyltransferase expressing human NSC | Learning and memory test were done at 2, 4, and 6 weeks after transplantation | CA3 region in hippocampus | Differentiated into neurons Migration of transplanted cells towards injured area |
AF64A-cholinotoxin induced learning deficit rat model | [110] |
| 23 | Umbilical cord blood cells | Age of mice were 7 month old | Peripheral administration | Immunomodulation Reduced vascular amyloid β deposits |
Tg2576 AD mouse model | [111] |
POTENTIAL ADVERSE EFFECTS OF STEM CELL THERAPY
Niche provides the regulatory molecules and suitable physicochemical environment to facilitate the cells to behave in a particular fashion [53]. These cells are exploited for therapeutic purposes, by isolating them from their niche which can pose some unexpected or undesirable outcomes such as tumorigenicity, which has a major concern. Very few studies have reported the potential adverse effect of these stem cells upon transplantation. In one of the studies, investigators evaluated the long term safety efficacy of 253G1-NSs (neural stem cells). The 253G1-NSs were transplanted to treat spinal cord injury (SCI) in SCID-NOD mice. These transplanted cells were found to have temporary improvement of motor function assessed by rota rod experiment for upto 47 days of post transplantation; however, this was followed by gradual deterioration in motor functioning [54]. It has also been shown to be involved in enhanced proliferation of grafted cells and tumor formation. The proportion of nestin positive cells have been found to be increased from 47 days and 103 post-transplantation which suggests tumor formation in the long term by the grafted cells. In an 18-years old patient with spinal cord injury at T10-T11, an olfactory mucosal cells were transplanted after three years. This led to severe back pain and paraplegia after 8 years. Further imaging revealed a mass formation of an intramedullary spinal cord [55].
The Yamanaka study of induced pluripotent stem cells opens up a possible window for untreatable diseases [56] as well as for stem cell clinical trials; even though the use of iPSCs also carries a risk for tumors formation. The generation of iPSCs involves retroviral transduction by the factors i.e. Oct3/4, Sox2, Klf4 and c-Myc [57]. The retroviral transduction of c-Myc is believed to increase a risk for tumorigenicity, hindering its clinical application. Further, the approach has also shown elimination of the c-Myc factor for iPSC generation, which is relatively safer than the earlier approach.
Therefore, an evaluation of safety efficacy of stem cells would provide us better therapeutic approach and its clinical application [58, 59].
THE PUTATIVE LINK OF BDNF AND CREB BEHIND STEM CELL MEDIATED REGENERATION
In brain BDNF and CREB (cAMP response element-binding protein) are believed to play a major role in complex memory formation, consolidation and retention [60–62]. It is also reported in both in-vivo and in-vitro studies that Aβ induced toxicity leads to downregulation of BDNF and its major regulatory molecule CREB. Hota et al studied the phosphorylation of CREB to investigate the molecular mechanism of bacoside action. Administration of Bacopa monniera leaf extractin hypobaric hypoxia induced rat model increased learning ability and ameliorated cognitive dysfunction [63]. Tota et al. investigated the effect of angiotensin II on spatial memory and BDNF expression in Sprague-Dawley male rats. Spatial memory was reduced as assessed by Morris water maze after angiotensin ICV administration, and no change was observed in BDNF expression [64]. In an in-vitro study, Sharma et al. have investigated the role of CREB binding protein (CREB-BP) in neuronal differentiation. Their deletion construct p-CREB-BP were transfected into NT2 cells and expression profile for neuronal genes i.e. SHH, Wnt, Notch and their mutant counterparts were evaluated. Defects in neuronal differentiation due to aberrant interaction of CREB-BP with their transcriptional regulatory proteins were investigated by CHIP-PCR and co-immunoprecipitation. Cells that are lacking in CREB, BROMO and HAT domains were found to show more proliferation and less differentiation whereas cells expressing CREB-BP showed less proliferation and more differentiation [65]. In 2009, Verma et al. suggested the role of dichlorvos in memory impairment by muscarinic receptor induced signal transduction and phosphorylation of CREB. Dichlorvos belongs to the organophosphate compounds which are widely used to as insecticide and may act as cholinesterase inhibitor [66]. Low doses of dichlorvos impaired the signal transduction linked to the adenylyl cyclase pathway and reduced CREB phosphorylation, leading to neurobehavioral impairment [67].
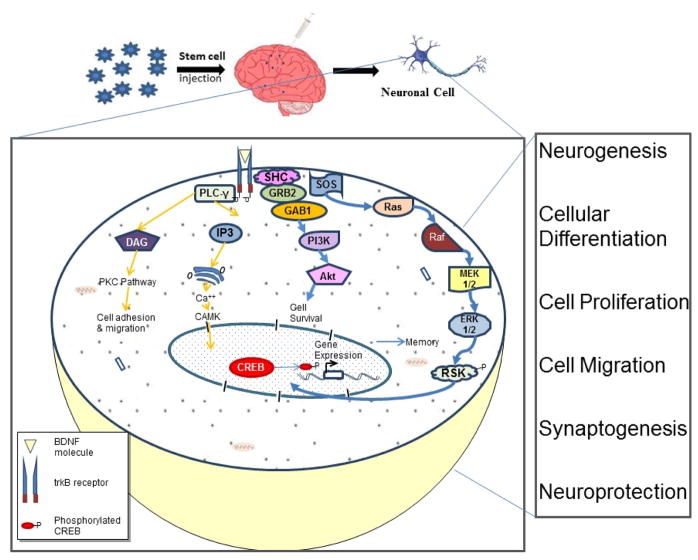
Neurotrophic factors such as BDNF, NGF and GDNF, which have been earlier shown to rescue hypoxia induced ischemic rat brain upon intravenous transplantation of UCB cells. This indicates an intrinsic role for neurotrophic factors, rather than direct differentiation, being significant in the stem cell mediated recovery [68]. It is pertinent to note that the role of BDNF has been well described in AD literature. BDNF levels are decreased when compared to healthy controls in the postmortem brains of AD patients [69–71]. Mature BDNF and its mRNA expression have also been shown to be confined to hippocampus and parietal cortex region of the brain [72–74]. BDNF is believed to exert neuroprotective effect in several neurodegenerative diseases. This may include pathologies characterized by Aβ-induced neuronal cell death. Several studies have shown the complete reversal of neuroprotective effects driven by BDNF in neuronal culture death induced by Aβ in specific and dose-dependent manner [75–80]. BDNF induced neuroprotective effect has shown incorporation of the Trkβ receptor [76]. Moreover, specific Aβ42 induced neuronal cell death has been shown to be reversed by BDNF, in addition to other neurotrophins like IGF-1 and GDNF [75].
Since CREB is a DNA binding protein and acts as a transcription factor for several genes, including c-fos, tyrosine hydroxylase, several neuronal peptides, and, importantly, neurotrophin BDNF, it is possible that an association exists between the role of BDNF expression and its regulation by CREB in rescuing learning and memory deficits [81, 82]. The function of CREB in the formation of spatial memory; conversion of this memory into long term memory and in neuronal plasticity is well documented [83]. It is well known that gene expression has a major role in memory consolidation as well as long term potentiation [83, 84]. These expression profiles are possibly activated through CREB and involvement of Ca++, protein kinase A (PKA) and by the activation of cAMP, but need additional studies [85–87]. The activated PKA would phosphorylate CREB protein which further regulates gene expression of several proteins [86, 88, 89]. Recently, Suzuki et al. have shown the effect of CREB on both short as well as long term memory. They have reported the increase in long term memory (LTM) as well as long term potential in hippocampus CA1 region in gain-of function CREB mice in which mice express dominant active CREB protein. In addition, they reported short term memory (STM) improvement in response to fear conditioning and spatial clues, which was related with enhanced BDNF levels in these mice. Therefore, up-regulation of BDNF and CREB expression may mutually trigger enhancement of LTM and STM, suggesting that CREB mediated BDNF expression plays intrinsic role in memory consolidation and retrieval. (Fig. 2) [90].
Fig. 2. Schematic showing the plausible mechanism behind stem cell mediated cognitive improvement in Alzheimer’s disease.
We propose that the therapeutic effect of stem cells upon transplantation into the brain is largely mediated by the paracrine effects. The increase in neurotrophic factors, such as BDNF, results in increased CREB phosphorylation which in turn activates the genes that regulate cognitive functions and memory by involving one or other phenomena, such as neuroprotection, cell proliferation, differentiation, cell migration, synaptogenesis and neurogenesis.
CLINICAL STUDIES FOR TREATING NEURODE-GENERATIVE DISORDERS
Although a number of pre-clinical studies have been launched, very few clinical studies have been carried out so far. Venkataramana et al suggested the safety and effectiveness of autologous bone-marrow derived mesenchymal stem cells when transplanted unilaterally in PD patients. Notably, no adverse effects of these stem cells were seen, paving the way for additional studies in future [91]. In 2012, human retinal stem cells were used to treat PD patients. Authors isolated human retinal stem cells from retinal pigmented epithelium tissue from post-mortem eyes and cultured in-vitro to differentiate into dopaminergic neurons. These cells were then transplanted by stereotaxic operation into the post-commissural putamen of 12 PD patients. Interestingly, PET analysis showed a trend of increased dopamine release during the 6 month study [92]. In a current study of a phase I open-label clinical trial, authors evaluated the safety efficacy of intrathecal and intravenous transplantation of autologous bone marrow cells in children with cerebral palsy. Eighteen children with cerebral palsy, who had transplantation, were evaluated for motor and cognitive functions and MRI was done after the sixth month showing it was a safe procedure [93].
There are very few reports registered at www.clinicaltrials.gov of stem cells transplantation in AD patients, and their outcomes are largely unavailable. In 2011 Medipost Co Ltd. completed an open level, phase I safety and efficacy trial on Korean AD patients, but they did not post their outcome measures. Human umbilical cord blood derived MSCs were transplanted in AD patients at two different doses (3 million and 6 million) and endpoint analysis was measured by ADAS-cog scoring, PET imaging, and Aβ and tau levels in CSF [94]. Another group in China is currently recruiting AD patients in phase I/II trial in a similar study design with 30 probable AD participants, where patients are intravenously administered with 20 million human UCB-MSCs [95]. Medipost Co Ltd. has recently started a double blinded, placebo controlled, phase I/IIa trial in Korea where patients with mild to moderate AD will be subjected to repeated intraventricular administrations of UCB-MSCs and will be evaluated 24 weeks after first dose of transplantation [96].
CONCLUSION
Stem cells have promising translational significance as evident by emerging scientific data showing therapeutic benefits in several neurodegenerative disorders. The intrinsic pathways through which these cells exert their therapeutic effects still remain a challenge requiring thorough investigation. There are several studies describing the underlying pathways ranging from proliferation, differentiation, immunomodulation to cell replacement and paracrine effects at the site of neurodegeneration. Pre-clinical studies have shown variable effects depending on the types and sources of stem cells. Several studies explain this on the basis of paracrine effects either mediated by neurotrophic factors or endogenous cell proliferation. In animal models of AD, stem cell transplantation has been shown to increase the expression of synaptic protein markers. Transplantation of mesenchymal stem cells has shown decrease in Aβ load due to microglial expression and escalation of Aβ degrading enzymes. A combinatorial approach, wherein stem cells are tagged with neurotransmitters or Aβ modifying enzymes may exhibit a substantial therapeutic outcome in AD. There is also insufficient literature to explain the actual relative contributions of exogenous cells and endogenous cells towards rescue of function after stem cell transplantation. There is absence of comparative studies involving different sources and types of stem cells i.e. undifferentiated versus differentiated cells in animal models of AD. Nevertheless, a few clinical studies have paved the way for clinical translation but such innovative treatments also carry substantial risk for tumor formation [55]. A thorough investigation is needed on the sources, types, stages, doses and routes of stem cell transplantation in AD model to validate their optimum therapeutic outcome. Moreover, the different stages of AD progression and other related pathologies may play a critical role in the outcome of the cell transplantation. Hence understanding the etiology of AD and its other pathologies is of paramount significance for successful clinical translation of stem cell related therapies [97].
Acknowledgments
We sincerely thank assistance from Bryan Maloney, and grant supports from the NIH/NIA R01-AG051086, R21-AG4687100, P30-AG010133 and ISDH Spinal Cord & Brain Injury Board to DKL.
LIST OF ABBREVIATIONS
- AD
Alzheimer’s disease
- APP
Aβ precursor protein
- Aβ
Amyloid Beta
- BACE1
β-Site Amyloid Precursor Protein-Cleaving Enzyme 1
- BDNF
Brain derived neurotrophic growth factor
- BM-MSCs
Bone-marrow derived mesenchymal stem cells
- Brdu
Bromodeoxyuridine
- CHIP
Chromatin Immunoprecipitation
- CREB
cAMP response element-binding protein
- CREBBP
CREB binding protein
- DHA
Docosahexaenoic acid
- FAD
Familial Alzheimer’s Disease
- GCSF
Granulocyte colony stimulating factor
- GFAP
Glial fibrillary acidic protein
- ICV
Intracerebroventricular
- IDE
Insulin-degrading enzyme
- IHC
Immunohistochemistry iPSC-Induced pluripotent stem cells
- LTM
Long term memory
- MRI
Magnetic resonance imaging
- NEP
Neprilysin
- NFT
Neurofibrillary tangles
- NPC
Neural precursor cells
- NPC
Neural progenitor cells
- NSC
Neural stem cells
- MWM
Morris water maze
- PD
Parkinson Disease
- PET
Positron emission tomography
- PPAR-γ
peroxisome proliferator-activated receptor-γ
- PS1
Presilin1
- RMS
Rostral migratory stream
- SC
Stem Cells
- STM
Short term memory
- SVZ
Subventricular zone
- SYN
Synaptophysin
- TBI
Traumatic Brain Injury
- UCB-MSC
Umbilical cord blood derived mesenchymal stem cells
- VEGF
Vascular endothelial growth factor
Footnotes
AUTHOR’S CONTRIBUTION
PB and AB contributed in writing of manuscript. AA participated in the concept of review, it’s designing and editing. DKL has done concept designing, editing, rephrasing of manuscript and manuscript writing.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
References
- 1.Martin A, Guerchet Maëlenn, Ali Gemma-Claire, Wu Yu-Tzu. Matthew World Alzheimer Report. Vol. 2015 Alzheimer’s Disease International; 2015. [Google Scholar]
- 2.2015 Alzheimer’s disease facts and figures. Alzheimers Dement. 2015;11(3):332–84. doi: 10.1016/j.jalz.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 3.ARSI. Alzheimer’s and Related Disorders Society of India (ARDSI) 2010. [Google Scholar]
- 4.Graeber MB, Kosel S, Egensperger R, Banati RB, Muller U, Bise K, et al. Rediscovery of the case described by Alois Alzheimer in 1911: historical, histological and molecular genetic analysis. Neurogenetics. 1997;1(1):73–80. doi: 10.1007/s100480050011. [DOI] [PubMed] [Google Scholar]
- 5.Cummings JL, Back C. The cholinergic hypothesis of neuropsychiatric symptoms in Alzheimer’s disease. Am J Geriatr Psychiatry. 1998;6(2 Suppl 1):S64–78. doi: 10.1097/00019442-199821001-00009. [DOI] [PubMed] [Google Scholar]
- 6.Soto C. Unfolding the role of protein misfolding in neurodegenerative diseases. Nat Rev Neurosci. 2003;4(1):49–60. doi: 10.1038/nrn1007. [DOI] [PubMed] [Google Scholar]
- 7.Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science (New York, NY) 1992;256(5054):184–5. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 8.Altman J. Autoradiographic investigation of cell proliferation in the brains of rats and cats. Anatomical Res. 1963;145:573–91. doi: 10.1002/ar.1091450409. [DOI] [PubMed] [Google Scholar]
- 9.Si YC, Li Q, Xie CE, Niu X, Xia XH, Yu CY. Chinese herbs and their active ingredients for activating xue (blood) promote the proliferation and differentiation of neural stem cells and mesenchymal stem cells. Chinese Med. 2014;9(1):13. doi: 10.1186/1749-8546-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saraf MK, Prabhakar S, Khanduja KL, Anand A. Bacopa monniera attenuates scopolamine-induced impairment of spatial memory in mice. Evid Based Complement Alternat Med. 2011;2011:236186. doi: 10.1093/ecam/neq038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prabhakar S, Saraf MK, Banik A, Anand A. Bacopa monniera selectively attenuates suppressed superoxide dismutase activity in diazepam induced amnesic mice. Ann Neurosci. 2011;18(1):8–13. doi: 10.5214/ans.0972.7531.1118104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saraf MK, Prabhakar S, Anand A. Neuroprotective effect of Bacopa monniera on ischemia induced brain injury. Pharmacol Biochem Behav. 2010;97(2):192–7. doi: 10.1016/j.pbb.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 13.Singh T, Prabhakar S, Gupta A, Anand A. Recruitment of stem cells into the injured retina after laser injury. Stem Cells Develop. 2012;21(3):448–54. doi: 10.1089/scd.2011.0002. [DOI] [PubMed] [Google Scholar]
- 14.Muthaian R, Minhas G, Anand A. Pathophysiology of stroke and stroke-induced retinal ischemia: emerging role of stem cells. J Cell Physiol. 2012;227(3):1269–79. doi: 10.1002/jcp.23048. [DOI] [PubMed] [Google Scholar]
- 15.Blurton-Jones M, Kitazawa M, Martinez-Coria H, Castello NA, Muller FJ, Loring JF, et al. Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc Natl Acad Sci USA. 2009;106(32):13594–9. doi: 10.1073/pnas.0901402106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilcock GK, Esiri MM. Plaques, tangles and dementia. A quantitative study. J Neurol Sci. 1982;56(2–3):343–56. doi: 10.1016/0022-510x(82)90155-1. [DOI] [PubMed] [Google Scholar]
- 17.Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, et al. Beta-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science (New York, NY) 1999;286(5440):735–41. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- 18.Bie B, Wu J, Yang H, Xu JJ, Brown DL, Naguib M. Epigenetic suppression of neuroligin 1 underlies amyloid-induced memory deficiency. Nat Neurosci. 2014;17(2):223–31. doi: 10.1038/nn.3618. [DOI] [PubMed] [Google Scholar]
- 19.Davies P, Maloney AJ. Selective loss of central cholinergic neurons in Alzheimer’s disease. Lancet. 1976;2(8000):1403. doi: 10.1016/s0140-6736(76)91936-x. [DOI] [PubMed] [Google Scholar]
- 20.Thies W, Bleiler L. 2013 Alzheimer’s disease facts and figures. Alzheimers Dement. 2013;9(2):208–45. doi: 10.1016/j.jalz.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 21.Tai LM, Ghura S, Koster KP, Liakaite V, Maienschein-Cline M, Kanabar P, et al. APOE-modulated Abeta-induced neuroinflammation in Alzheimer’s disease: current landscape, novel data, and future perspective. J Neurochem. 2015;133(4):465–88. doi: 10.1111/jnc.13072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wisniewski T, Frangione B. Apolipoprotein E: a pathological chaperone protein in patients with cerebral and systemic amyloid. Neurosci Lett. 1992;135(2):235–8. doi: 10.1016/0304-3940(92)90444-c. [DOI] [PubMed] [Google Scholar]
- 23.Lahiri DK, Maloney B, Basha MR, Ge YW, Zawia NH. How and when environmental agents and dietary factors affect the course of Alzheimer’s disease: the “LEARn” model (latent early-life associated regulation) may explain the triggering of AD. Curr Alzheimer Res. 2007;4(2):219–28. doi: 10.2174/156720507780362164. [DOI] [PubMed] [Google Scholar]
- 24.Banik A, Prabhakar S, Kalra J, Anand A. An enriched population of CD45, CD34 and CD117 stem cells in human umbilical cord blood for potential therapeutic regenerative strategies. Curr Neurovasc Res. 2014;11(4):312–20. doi: 10.2174/1567202611666140902144836. [DOI] [PubMed] [Google Scholar]
- 25.Jindal N, Minhas G, Prabhakar S, Anand A. Characterization of Linve CD34 and CD117 cell population reveals an increased expression in bone marrow derived stem cells. Curr Neurovasc Res. 2014;11(1):68–74. doi: 10.2174/1567202610666131209110035. [DOI] [PubMed] [Google Scholar]
- 26.Abburi C, Prabhakar S, Kalra J, Huria A, Anand A. Vascular endothelial growth factor (VEGF) induced proliferation of human fetal derived ciliary epithelium stem cells is mediated by jagged-N cadherin pathway. Curr Neurovasc Res. 2013;10(2):93–102. doi: 10.2174/1567202611310020002. [DOI] [PubMed] [Google Scholar]
- 27.Abburi C, Anand A. Ciliary epithelium: an underevaluated target for therapeutic regeneration. Critl Rev Eukaryotic Gene Exp. 2012;22(2):87–95. doi: 10.1615/.v22.i2.10. [DOI] [PubMed] [Google Scholar]
- 28.Ray B, Chopra N, Long JM, Lahiri DK. Human primary mixed brain cultures: preparation, long-term maintenance, characterization and application to neuroscience research. Mol Brain. 2014;7(1):63. doi: 10.1186/s13041-014-0063-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yagi T, Ito D, Okada Y, Akamatsu W, Nihei Y, Yoshizaki T, et al. Modeling familial Alzheimer’s disease with induced pluripotent stem cells. Hum Mol Genet. 2011;20(23):4530–9. doi: 10.1093/hmg/ddr394. [DOI] [PubMed] [Google Scholar]
- 30.Kondo T, Asai M, Tsukita K, Kutoku Y, Ohsawa Y, Sunada Y, et al. Modeling Alzheimer’s disease with iPSCs reveals stress phenotypes associated with intracellular Abeta and differential drug responsiveness. Cell Stem Cell. 2013;12(4):487–96. doi: 10.1016/j.stem.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 31.Long JM, Ray B, Lahiri DK. MicroRNA-339–5p down-regulates protein expression of beta-site amyloid precursor protein-cleaving enzyme 1 (BACE1) in human primary brain cultures and is reduced in brain tissue specimens of Alzheimer disease subjects. J Biol Chem. 2014;289(8):5184–98. doi: 10.1074/jbc.M113.518241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cure S, Abrams K, Belger M, Dell’agnello G, Happich M. Systematic literature review and meta-analysis of diagnostic test accuracy in Alzheimer’s disease and other dementia using autopsy as standard of truth. J Alzheimers Dis. 2014;42(1):169–82. doi: 10.3233/JAD-131559. [DOI] [PubMed] [Google Scholar]
- 33.Anand A, Banik A, Thakur K, Masters CL. The animal models of dementia and Alzheimer’s disease for pre-clinical testing and clinical translation. Curr Alzheimer Res. 2012;9(9):1010–29. doi: 10.2174/156720512803569055. [DOI] [PubMed] [Google Scholar]
- 34.Banik A, Anand APD. Preclinical non-human models to combat dementia. Ann Neurosci. 2013;20(1):24–29. doi: 10.5214/ans.0972.7531.200109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Monacelli F, Rosa G. Cholinesterase inhibitors: cardioprotection in Alzheimer’s disease. J Alzheimers Dis. 2014;42(4):1071–7. doi: 10.3233/JAD-141089. [DOI] [PubMed] [Google Scholar]
- 36.Birks J. Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst Rev. 2006;(1):CD005593. doi: 10.1002/14651858.CD005593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ernfors P, Wetmore C, Olson L, Persson H. Identification of cells in rat brain and peripheral tissues expressing mRNA for members of the nerve growth factor family. Neuron. 1990;5(4):511–26. doi: 10.1016/0896-6273(90)90090-3. [DOI] [PubMed] [Google Scholar]
- 38.Prakash A, Kumar A. Role of nuclear receptor on regulation of BDNF and neuroinflammation in hippocampus of beta-amyloid animal model of Alzheimer’s disease. Neurotox Res. 2014;25(4):335–47. doi: 10.1007/s12640-013-9437-9. [DOI] [PubMed] [Google Scholar]
- 39.Seaberg RM, van der Kooy D. Stem and progenitor cells: the premature desertion of rigorous definitions. Trends Neurosci. 2003;26(3):125–31. doi: 10.1016/S0166-2236(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 40.Tang J, Xu H, Fan X, Li D, Rancourt D, Zhou G, et al. Embryonic stem cell-derived neural precursor cells improve memory dysfunction in Abeta(1–40) injured rats. Neurosci Res. 2008;62(2):86–96. doi: 10.1016/j.neures.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 41.Blurton-Jones M, Spencer B, Michael S, Castello NA, Agazaryan AA, Davis JL, et al. Neural stem cells genetically-modified to express neprilysin reduce pathology in Alzheimer transgenic models. Stem Cell Res Ther. 2014;5(2):46. doi: 10.1186/scrt440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.ONK Oral presentation titled “Peri-hippocampal stem cell transplantation rescues cognitive decline in Alzheimer’s disease”. Congress of Neurological Surgeons Annual Meeting; Boston, MA. 2014. [Google Scholar]
- 43.Jin K, Xie L, Mao X, Greenberg MB, Moore A, Peng B, et al. Effect of human neural precursor cell transplantation on endogenous neurogenesis after focal cerebral ischemia in the rat. Brain Res. 2011;1374:56–62. doi: 10.1016/j.brainres.2010.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee HJ, Lee JK, Lee H, Carter JE, Chang JW, Oh W, et al. Human umbilical cord blood-derived mesenchymal stem cells improve neuropathology and cognitive impairment in an Alzheimer’s disease mouse model through modulation of neuroinflammation. Neurobiol Aging. 2012;33(3):588–602. doi: 10.1016/j.neurobiolaging.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 45.Garcia KO, Ornellas FL, Martin PK, Patti CL, Mello LE, Frussa-Filho R, et al. Therapeutic effects of the transplantation of VEGF overexpressing bone marrow mesenchymal stem cells in the hippocampus of murine model of Alzheimer’s disease. Front Aging Neurosci. 2014;6:30. doi: 10.3389/fnagi.2014.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cherry JD, Olschowka JA, O’Banion MK. Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. J Neuroinflamm. 2014;11:98. doi: 10.1186/1742-2094-11-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang H, Xie Z, Wei L, Yang H, Yang S, Zhu Z, et al. Human umbilical cord mesenchymal stem cell-derived neuron-like cells rescue memory deficits and reduce amyloid-beta deposition in an AbetaPP/PS1 transgenic mouse model. Stem Cell Res Therap. 2013;4(4):76. doi: 10.1186/scrt227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salem AM, Ahmed HH, Atta HM, Ghazy MA, Aglan HA. Potential of bone marrow mesenchymal stem cells in management of Alzheimer’s disease in female rats. Cell Biol Intern. 2014;38(12):1367–83. doi: 10.1002/cbin.10331. [DOI] [PubMed] [Google Scholar]
- 49.Oh SH, Kim HN, Park HJ, Shin JY, Lee PH. Mesenchymal stem cells increase hippocampal neurogenesis and neuronal differentiation by enhancing the Wnt signaling pathway in Alzheimer’s disease model. Cell Transplan. 2015;24(6):1097–109. doi: 10.3727/096368914X679237. [DOI] [PubMed] [Google Scholar]
- 50.Zhang W, Wang GM, Wang PJ, Zhang Q, Sha SH. Effects of neural stem cells on synaptic proteins and memory in a mouse model of Alzheimer’s disease. J Neurosci Res. 2014;92(2):185–94. doi: 10.1002/jnr.23299. [DOI] [PubMed] [Google Scholar]
- 51.Prakash A, Medhi B, Chopra K. Granulocyte colony stimulating factor (GCSF) improves memory and neurobehavior in an amyloid-beta induced experimental model of Alzheimer’s disease. Pharmacol Biochem Behav. 2013;110:46–57. doi: 10.1016/j.pbb.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 52.Shetty P, Thakur AM, Viswanathan C. Dopaminergic cells, derived from a high efficiency differentiation protocol from umbilical cord derived mesenchymal stem cells, alleviate symptoms in a Parkinson’s disease rodent model. Cell Biol Intern. 2013;37(2):167–80. doi: 10.1002/cbin.10029. [DOI] [PubMed] [Google Scholar]
- 53.Chen S, Lewallen M, Xie T. Adhesion in the stem cell niche: biological roles and regulation. Development (Cambridge, England) 2013;140(2):255–65. doi: 10.1242/dev.083139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nori S, Okada Y, Nishimura S, Sasaki T, Itakura G, Kobayashi Y, et al. Long-term safety issues of iPSC-based cell therapy in a spinal cord injury model: oncogenic transformation with epithelial-mesenchymal transition. Stem Cell Rep. 2015;4(3):360–73. doi: 10.1016/j.stemcr.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dlouhy BJ, Awe O, Rao RC, Kirby PA, Hitchon PW. Autograft-derived spinal cord mass following olfactory mucosal cell transplantation in a spinal cord injury patient: Case report. J Neurosurg Spine. 2014;21(4):618–22. doi: 10.3171/2014.5.SPINE13992. [DOI] [PubMed] [Google Scholar]
- 56.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 57.Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, et al. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26(1):101–6. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- 58.Jarocha D, Milczarek O, Kawecki Z, Wendrychowicz A, Kwiatkowski S, Majka M. Preliminary study of autologous bone marrow nucleated cells transplantation in children with spinal cord injury. Stem Cells Trans Med. 2014;3(3):395–404. doi: 10.5966/sctm.2013-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mazzini L, Gelati M, Profico DC, Sgaravizzi G, Projetti Pensi M, Muzi G, et al. Human neural stem cell transplantation in ALS: initial results from a phase I trial. J Transl Med. 2015;13(1):17. doi: 10.1186/s12967-014-0371-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Song JH, Yu JT, Tan L. Brain-derived neurotrophic factor in Alzheimer’s disease: risk, mechanisms, and therapy. Mol Neurobiol. 2015;52(3):1477–93. doi: 10.1007/s12035-014-8958-4. [DOI] [PubMed] [Google Scholar]
- 61.Dominguez G, Dagnas M, Decorte L, Vandesquille M, Belzung C, Beracochea D, et al. Rescuing prefrontal cAMP-CREB pathway reverses working memory deficits during withdrawal from prolonged alcohol exposure. Brain Struct Funct. 2014 doi: 10.1007/s00429-014-0941-3. [DOI] [PubMed] [Google Scholar]
- 62.Saraf MK, Anand A, Prabhakar S. Scopolamine induced amnesia is reversed by Bacopa monniera through participation of kinase-CREB pathway. Neurochem Res. 2010;35(2):279–87. doi: 10.1007/s11064-009-0051-4. [DOI] [PubMed] [Google Scholar]
- 63.Hota SK, Barhwal K, Baitharu I, Prasad D, Singh SB, Ilavazhagan G. Bacopa monniera leaf extract ameliorates hypobaric hypoxia induced spatial memory impairment. Neurobiol Dis. 2009;34(1):23–39. doi: 10.1016/j.nbd.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 64.Tota S, Goel R, Pachauri SD, Rajasekar N, Najmi AK, Hanif K, et al. Effect of angiotensin II on spatial memory, cerebral blood flow, cholinergic neurotransmission, and brain derived neurotrophic factor in rats. Psychopharmacology (Berl) 2013;226(2):357–69. doi: 10.1007/s00213-012-2913-8. [DOI] [PubMed] [Google Scholar]
- 65.Sharma N, Jadhav SP, Bapat SA. CREBBP re-arrangements affect protein function and lead to aberrant neuronal differentiation. Differentiation. 2010;79(4–5):218–31. doi: 10.1016/j.diff.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 66.Raheja G, Gill KD. Altered cholinergic metabolism and muscarinic receptor linked second messenger pathways after chronic exposure to dichlorvos in rat brain. Toxicol Indust Health. 2007;23(1):25–37. doi: 10.1177/0748233707072490. [DOI] [PubMed] [Google Scholar]
- 67.Verma SK, Raheja G, Gill KD. Role of muscarinic signal transduction and CREB phosphorylation in dichlorvos-induced memory deficits in rats: an acetylcholine independent mechanism. Toxicology. 2009;256(3):175–82. doi: 10.1016/j.tox.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 68.Yasuhara T, Hara K, Maki M, Xu L, Yu G, Ali MM, et al. Mannitol facilitates neurotrophic factor up-regulation and behavioural recovery in neonatal hypoxic-ischaemic rats with human umbilical cord blood grafts. J Cell Mol Med. 2010;14(4):914–21. doi: 10.1111/j.1582-4934.2008.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fumagalli F, Racagni G, Riva MA. The expanding role of BDNF: a therapeutic target for Alzheimer’s disease? Pharmacogenom J. 2006;6(1):8–15. doi: 10.1038/sj.tpj.6500337. [DOI] [PubMed] [Google Scholar]
- 70.Siegel GJ, Chauhan NB. Neurotrophic factors in Alzheimer’s and Parkinson’s disease brain. Brain Res Brain Res Rev. 2000;33(2–3):199–227. doi: 10.1016/s0165-0173(00)00030-8. [DOI] [PubMed] [Google Scholar]
- 71.Fahnestock M, Garzon D, Holsinger RM, Michalski B. Neurotrophic factors and Alzheimer’s disease: are we focusing on the wrong molecule? J Neural Transm Supplemen. 2002;(62):241–52. doi: 10.1007/978-3-7091-6139-5_22. [DOI] [PubMed] [Google Scholar]
- 72.Peng S, Wuu J, Mufson EJ, Fahnestock M. Precursor form of brain-derived neurotrophic factor and mature brain-derived neurotrophic factor are decreased in the pre-clinical stages of Alzheimer’s disease. JNeurochem. 2005;93(6):1412–21. doi: 10.1111/j.1471-4159.2005.03135.x. [DOI] [PubMed] [Google Scholar]
- 73.Michalski B, Fahnestock M. Pro-brain-derived neurotrophic factor is decreased in parietal cortex in Alzheimer’s disease. Brain research Mol Brain Res. 2003;111(1–2):148–54. doi: 10.1016/s0169-328x(03)00003-2. [DOI] [PubMed] [Google Scholar]
- 74.Holsinger RM, Schnarr J, Henry P, Castelo VT, Fahnestock M. Quantitation of BDNF mRNA in human parietal cortex by competitive reverse transcription-polymerase chain reaction: decreased levels in Alzheimer’s disease. Brain Res Mol Brain Res. 2000;76(2):347–54. doi: 10.1016/s0169-328x(00)00023-1. [DOI] [PubMed] [Google Scholar]
- 75.Kitiyanant N, Kitiyanant Y, Svendsen CN, Thangnipon W. BDNF-, IGF-1- and GDNF-secreting human neural progenitor cells rescue amyloid beta-induced toxicity in cultured rat septal neurons. Neurochem Res. 2012;37(1):143–52. doi: 10.1007/s11064-011-0592-1. [DOI] [PubMed] [Google Scholar]
- 76.Arancibia S, Silhol M, Mouliere F, Meffre J, Hollinger I, Maurice T, et al. Protective effect of BDNF against beta-amyloid induced neurotoxicity in vitro and in vivo in rats. Neurobiol Dis. 2008;31(3):316–26. doi: 10.1016/j.nbd.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 77.Holback S, Adlerz L, Iverfeldt K. Increased processing of APLP2 and APP with concomitant formation of APP intracellular domains in BDNF and retinoic acid-differentiated human neuroblastoma cells. J Neurochem. 2005;95(4):1059–68. doi: 10.1111/j.1471-4159.2005.03440.x. [DOI] [PubMed] [Google Scholar]
- 78.Tong L, Balazs R, Thornton PL, Cotman CW. Beta-amyloid peptide at sublethal concentrations downregulates brain-derived neurotrophic factor functions in cultured cortical neurons. J Neurosci. 2004;24(30):6799–809. doi: 10.1523/JNEUROSCI.5463-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zeng Y, Zhao D, Xie CW. Neurotrophins enhance CaMKII activity and rescue amyloid-beta-induced deficits in hippocampal synaptic plasticity. J Alzheimer’s Dis. 2010;21(3):823–31. doi: 10.3233/JAD-2010-100264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li N, Liu GT. The novel squamosamide derivative FLZ enhances BDNF/TrkB/CREB signaling and inhibits neuronal apoptosis in APP/PS1 mice. Acta Pharmacol Sin. 2010;31(3):265–72. doi: 10.1038/aps.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee Y, Kim J, Jang S, Oh S. Administration of phytoceramide enhances memory and upregulates the expression of pCREB and BDNF in Hippocampus of mice. Biomol Ther. 2013;21(3):229–33. doi: 10.4062/biomolther.2013.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.MF . MSc Thesis. 2012. The effects of CREB-mediated BDNF expression on memory- and anxiety-related behaviours in the adult mouse. [Google Scholar]
- 83.Silva AJ, Kogan JH, Frankland PW, Kida S. CREB and memory. Ann Rev Neurosci. 1998;21:127–48. doi: 10.1146/annurev.neuro.21.1.127. [DOI] [PubMed] [Google Scholar]
- 84.Martin SJ, Grimwood PD, Morris RG. Synaptic plasticity and memory: an evaluation of the hypothesis. Ann Rev Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- 85.Jensen KF, Ohmstede CA, Fisher RS, Sahyoun N. Nuclear and axonal localization of Ca2+/calmodulin-dependent protein kinase type Gr in rat cerebellar cortex. Proc Natl Acad Sci USA. 1991;88(7):2850–3. doi: 10.1073/pnas.88.7.2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nakamura Y, Okuno S, Sato F, Fujisawa H. An immunohistochemical study of Ca2+/calmodulin-dependent protein kinase IV in the rat central nervous system: light and electron microscopic observations. Neuroscience. 1995;68(1):181–94. doi: 10.1016/0306-4522(95)00092-w. [DOI] [PubMed] [Google Scholar]
- 87.Matthews RP, Guthrie CR, Wailes LM, Zhao X, Means AR, McKnight GS. Calcium/calmodulin-dependent protein kinase types II and IV differentially regulate CREB-dependent gene expression. Mol Cell Biol. 1994;14(9):6107–16. doi: 10.1128/mcb.14.9.6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chrivia JC, Kwok RP, Lamb N, Hagiwara M, Montminy MR, Goodman RH. Phosphorylated CREB binds specifically to the nuclear protein CBP. Nature. 1993;365(6449):855–9. doi: 10.1038/365855a0. [DOI] [PubMed] [Google Scholar]
- 89.Bito H, Deisseroth K, Tsien RW. CREB phosphorylation and dephosphorylation: a Ca(2+)- and stimulus duration-dependent switch for hippocampal gene expression. Cell. 1996;87(7):1203–14. doi: 10.1016/s0092-8674(00)81816-4. [DOI] [PubMed] [Google Scholar]
- 90.Suzuki A, Fukushima H, Mukawa T, Toyoda H, Wu LJ, Zhao MG, et al. Upregulation of CREB-mediated transcription enhances both short- and long-term memory. J Neurosci. 2011;31(24):8786–802. doi: 10.1523/JNEUROSCI.3257-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Venkataramana NK, Kumar SK, Balaraju S, Radhakrishnan RC, Bansal A, Dixit A, et al. Open-labeled study of unilateral autologous bone-marrow-derived mesenchymal stem cell transplantation in Parkinson’s disease. Transl Res. 2010;155(2):62–70. doi: 10.1016/j.trsl.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 92.Yin F, Tian ZM, Liu S, Zhao QJ, Wang RM, Shen L, et al. Transplantation of human retinal pigment epithelium cells in the treatment for Parkinson disease. CNS Neurosci Ther. 2012;18(12):1012–20. doi: 10.1111/cns.12025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mancias-Guerra C, Marroquin-Escamilla AR, Gonzalez-Llano O, Villarreal-Martinez L, Jaime-Perez JC, Garcia-Rodriguez F, et al. Safety and tolerability of intrathecal delivery of autologous bone marrow nucleated cells in children with cerebral palsy: an open-label phase I trial. Cytotherapy. 2014;16(6):810–20. doi: 10.1016/j.jcyt.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 94.The Safety and The Efficacy Evaluation of NEUROSTEM®-AD in Patients With Alzheimer’s Disease. ClinicalTrialsgov Identifier: NCT01297218. 2014 http://clinicaltrials.gov/ct2/show/NCT01297218.
- 95.Safety and Efficiency of Umbilical Cord-derived Mesenchymal Stem Cells(UC-MSC) in Patients With Alzheimer’s Disease (SEMAD) Clinical Trialsgov Identifier: NCT01547689. 2014 May; https://clinicaltrials.gov/ct2/show/NCT01547689.
- 96.Safety and Exploratory Efficacy Study of NEUROSTEM® Versus Placebo in Patients With Alzheimer’s Disease. clinicaltrialsgov identifier: nct02054208. 2014 Feb; https://clinicaltrials.gov/ct2/show/NCT02054208.
- 97.Banik A, Brown RE, Bamburg J, Lahiri DK, Khurana D, Friedland RP, et al. Translation of pre-clinical studies into successful clinical trials for alzheimer’s disease: what are the roadblocks and how can they be overcome? J Alzheimers Dis. 2015;47(4):815–43. doi: 10.3233/JAD-150136. [DOI] [PubMed] [Google Scholar]
- 98.Zhang R, Liu Y, Yan K, Chen L, Chen XR, Li P, et al. Anti-inflammatory and immunomodulatory mechanisms of mesenchymal stem cell transplantation in experimental traumatic brain injury. J Neuroinflamm. 2013;10(1):106. doi: 10.1186/1742-2094-10-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wen SR, Qi HP, Ren YJ, Liu GJ, Gong FC, Zhong H, et al. Expression of deltaNp73 in hippocampus of APP/PS1 transgenic mice following GFP-BMSCs transplantation. Neurol Res. 2011;33(10):1109–14. doi: 10.1179/1743132811Y.0000000051. [DOI] [PubMed] [Google Scholar]
- 100.Marei HE, Farag A, Althani A, Afifi N, AAE, Lashen S, et al. Human Olfactory Bulb Neural Stem Cells expressing hNGF Restore cognitive deficit in Alzheimer’s disease rat model. J Cell Physiol. 2015;230(1):116–30. doi: 10.1002/jcp.24688. [DOI] [PubMed] [Google Scholar]
- 101.Zhang W, Wang PJ, Gu GJ, Li MH, Gao XL. Effects of neural stem cells transplanted into an animal model of Alzheimer disease on Abeta plaques. Zhonghua yi xue za zhi. 2013;93(45):3636–9. [PubMed] [Google Scholar]
- 102.Yun HM, Kim HS, Park KR, Shin JM, Kang AR, il Lee K, et al. Placenta-derived mesenchymal stem cells improve memory dysfunction in an Abeta1–42-infused mouse model of Alzheimer’s disease. Cell Death Dis. 2013;4:e958. doi: 10.1038/cddis.2013.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shin JY, Park HJ, Kim HN, Oh SH, Bae JS, Ha HJ, et al. Mesenchymal stem cells enhance autophagy and increase beta-amyloid clearance in Alzheimer disease models. Autophagy. 2014;10(1):32–44. doi: 10.4161/auto.26508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yang H, Yang H, Xie Z, Wei L, Bi J. Systemic transplantation of human umbilical cord derived mesenchymal stem cells-educated T regulatory cells improved the impaired cognition in AbetaPP-swe/PS1dE9 transgenic mice. PloS One. 2013;8(7):e69129. doi: 10.1371/journal.pone.0069129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kim KS, Kim HS, Park JM, Kim HW, Park MK, Lee HS, et al. Long-term immunomodulatory effect of amniotic stem cells in an Alzheimer’s disease model. Neurobiol Aging. 2013;34(10):2408–20. doi: 10.1016/j.neurobiolaging.2013.03.029. [DOI] [PubMed] [Google Scholar]
- 106.Kim S, Chang KA, Kim J, Park HG, Ra JC, Kim HS, et al. The preventive and therapeutic effects of intravenous human adipose-derived stem cells in Alzheimer’s disease mice. PloS One. 2012;7(9):e45757. doi: 10.1371/journal.pone.0045757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bae JS, Jin HK, Lee JK, Richardson JC, Carter JE. Bone marrow-derived mesenchymal stem cells contribute to the reduction of amyloid-beta deposits and the improvement of synaptic transmission in a mouse model of pre-dementia Alzheimer’s disease. Curr Alzheimer Res. 2013;10(5):524–31. [PubMed] [Google Scholar]
- 108.Kim JY, Kim DH, Kim JH, Lee D, Jeon HB, Kwon SJ, et al. Soluble intracellular adhesion molecule-1 secreted by human umbilical cord blood-derived mesenchymal stem cell reduces amyloid-beta plaques. Cell Death Differen. 2012;19(4):680–91. doi: 10.1038/cdd.2011.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Esmaeilzade B, Nobakht M, Joghataei MT, Rahbar Roshandel N, Rasouli H, Samadi Kuchaksaraei A, et al. Delivery of epidermal neural crest stem cells (EPI-NCSC) to hippocamp in Alzheimer’s disease rat model. Iranian Biomed. 2012;J16(1):1–9. doi: 10.6091/IBJ.1029.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Park D, Joo SS, Kim TK, Lee SH, Kang H, Lee HJ, et al. Human neural stem cells overexpressing choline acetyltransferase restore cognitive function of kainic acid-induced learning and memory deficit animals. Cell Transplant. 2012;21(1):365–71. doi: 10.3727/096368911X586765. [DOI] [PubMed] [Google Scholar]
- 111.Nikolic WV, Hou H, Town T, Zhu Y, Giunta B, Sanberg CD, et al. Peripherally administered human umbilical cord blood cells reduce parenchymal and vascular beta-amyloid deposits in Alzheimer mice. Stem Cells Devel. 2008;17(3):423–39. doi: 10.1089/scd.2008.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]