Abstract
Objectives
This cross-sectional survey explored the characteristics and outcomes of direct oral anticoagulant (DOAC)–associated nontraumatic intracerebral hemorrhages (ICHs) by analyzing a large nationwide Japanese discharge database.
Methods
We analyzed data from 2,245 patients who experienced ICHs while taking anticoagulants (DOAC: 227; warfarin: 2,018) and were urgently hospitalized at 621 institutions in Japan between April 2010 and March 2015. We compared the DOAC- and warfarin-treated patients based on their backgrounds, ICH severities, antiplatelet therapies at admission, hematoma removal surgeries, reversal agents, mortality rates, and modified Rankin Scale scores at discharge.
Results
DOAC-associated ICHs were less likely to cause moderately or severely impaired consciousness (DOAC-associated ICHs: 31.3%; warfarin-associated ICHs: 39.4%; p = 0.002) or require surgical removal (DOAC-associated ICHs: 5.3%; warfarin-associated ICHs: 9.9%; p = 0.024) in the univariate analysis. Propensity score analysis revealed that patients with DOAC-associated ICHs also exhibited lower mortality rates within 1 day (odds ratio [OR] 4.96, p = 0.005), within 7 days (OR 2.29, p = 0.037), and during hospitalization (OR 1.96, p = 0.039).
Conclusions
This nationwide study revealed that DOAC-treated patients had less severe ICHs and lower mortality rates than did warfarin-treated patients, probably due to milder hemorrhages at admission and lower hematoma expansion frequencies.
Clinical trials have shown that 4 direct oral anticoagulants (DOACs)—dabigatran, rivaroxaban, apixaban, and edoxaban—are as efficacious and safe as warfarin for stroke prevention in patients with atrial fibrillation (AF).1 In randomized clinical trials with strict indication criteria, DOAC-treated patients are reportedly at lower risk for hemorrhagic strokes than are warfarin-treated patients.1 Mortality rates were similar among patients randomly prescribed either DOAC or warfarin according to a secondary analysis of intracerebral hemorrhages (ICHs) occurring in the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) trial2 and the Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation.3 However, because those trials featured very strict inclusion criteria, these results cannot be generalized to all patients with DOAC- or warfarin-associated ICHs. Notably, in clinical practice of stroke prevention in AF, stroke outcomes can depend on various patient baseline characteristics, suboptimal dosage, and oral anticoagulant therapy adherence.4 However, few studies have compared the characteristics and outcomes of DOAC- and warfarin-associated ICHs in clinical practice. Because DOACs are used liberally in clinical practice, we expect that the incidence of DOAC-associated ICHs will increase in coming years.5 We therefore aimed to compare the characteristics and outcomes of patients with DOAC- and warfarin-associated ICHs in clinical practice in Japan using a nationwide Diagnosis Procedure Combination (DPC) database.
Methods
Standard protocol approvals, registrations, and patient consents
This study was approved by the Kyushu University Institutional Review Board, which waived the requirement for individual informed consent.
The DPC database
The DPC is a mixed-case patient classification system that was launched in 2002 by the Japanese Ministry of Health, Labour and Welfare and is linked with a hospital financing system.6 By 2015, the DPC system had been adopted by an estimated 1,580 acute care hospitals, representing approximately half of all Japanese hospital beds and encompassing a wide variety of centers, including rural and urban, academic and nonacademic, and small and large hospitals.7 The DPC database includes data about all patients admitted to participating hospitals, including each patient's profile (e.g., age, sex); principal diagnoses and comorbidities at admission (both coded by the International Classification of Diseases and Injuries, 10th revision); complications after admission (coded similarly); procedures including surgeries, medications, and devices used during hospitalization; length of stay; discharge status; and medical expense.8 The J-ASPECT study group has analyzed the DPC database to gain new clinical insights,7,9–11 an approach we applied again for this cross-sectional survey.
Sampling strategy
Of the 1,369 training institutions certified by the Japan Neurosurgical Society, the Japanese Society of Neurology, and the Japan Stroke Society, 621 agreed to participate in the J-ASPECT study. We identified patients hospitalized for nontraumatic ICH in the deidentified discharge database using the ICD-10 diagnosis codes related to nontraumatic ICH (I61.0-9, I62.0-1, and I62.9). We further selected those patients who had been urgently hospitalized between April 1, 2010, and March 31, 2015, and were receiving a DOAC or warfarin before admission. We then extracted data about age and sex; comorbidities on admission, including those based on Charlson scores12; level of consciousness on admission according to the Japan Coma Scale (JCS), the most widely used grading scale for impaired consciousness in Japan (table e-1, links.lww.com/WNL/A284)7,9,13; concurrent preadmission use of antiplatelet drugs including aspirin, clopidogrel, ticlopidine, cilostazol, sarpogrelate, and prasugrel; hematoma removal with craniotomy, endoscopic surgery, or stereotactic aspiration; CSF drainage coded with Japanese original K-codes; use of reversal agents including vitamin K, prothrombin complex (PCC) concentrate, and fresh frozen plasma (FFP); length of hospital stay; mortality; and modified Rankin Scale (mRS) score at discharge. Patients with missing data were excluded from this survey.
Statistical analysis
We compared the characteristics of DOAC- and warfarin-associated ICHs using Wilcoxon rank sum tests for continuous variables and χ2 tests for categorical variables. Multivariable analysis was undertaken to estimate the 2 groups' odds ratios (ORs) for mortality rates within 1 day, within 7 days, and during hospitalization and for discharge mRS scores in the 0–3 (good functional outcome) or 4–6 (poor functional outcome) ranges. The ORs were adjusted for sex, age, comorbidities, admission JCS scores, concurrent antiplatelet therapies, and treatment with surgery or reversal agents. We also estimated ORs for outcomes after 1:1 propensity score matching to account for between-group differences in baseline characteristics. To match patients, we used an automated matching procedure in STATA (STATA Corp., College Station, TX) that randomly selected a DOAC-treated patient and a warfarin-treated patient within a propensity score caliper of ±0.01. Successfully matched pairs were removed, and the procedure was repeated until all patients were matched or until no further matches were available within the caliper. Propensity scores for warfarin-associated outcomes were estimated using a probit model in which the independent variables were sex, age, comorbidities, admission JCS scores, concurrent antiplatelet therapy, and treatment with surgery or reversal agents. The interaction between usage of DOAC and usage of reversal agents for ICH removal surgeries was examined. The analyses were performed using JMP 11.0 (SAS Institute, Cary, NC), SAS 9.3 (SAS Institute), STATA 12, and SPSS 12 (IBM, Armonk, NY). We defined statistical significance as p < 0.05.
Results
Patient demographics
We identified 2,245 patients with DOAC- or warfarin-associated ICHs (DOAC: 227; warfarin: 2,018). The percentages of DOAC-treated patients in each year from 2010 to 2014 were 0%, 0.4%, 3.8%, 9.6%, and 21.4%, respectively. The proportions of dabigatran, rivaroxaban, apixaban, and edoxaban among DOAC-associated cases were 23.8%, 57.8%, 18.5%, and 0%, respectively.
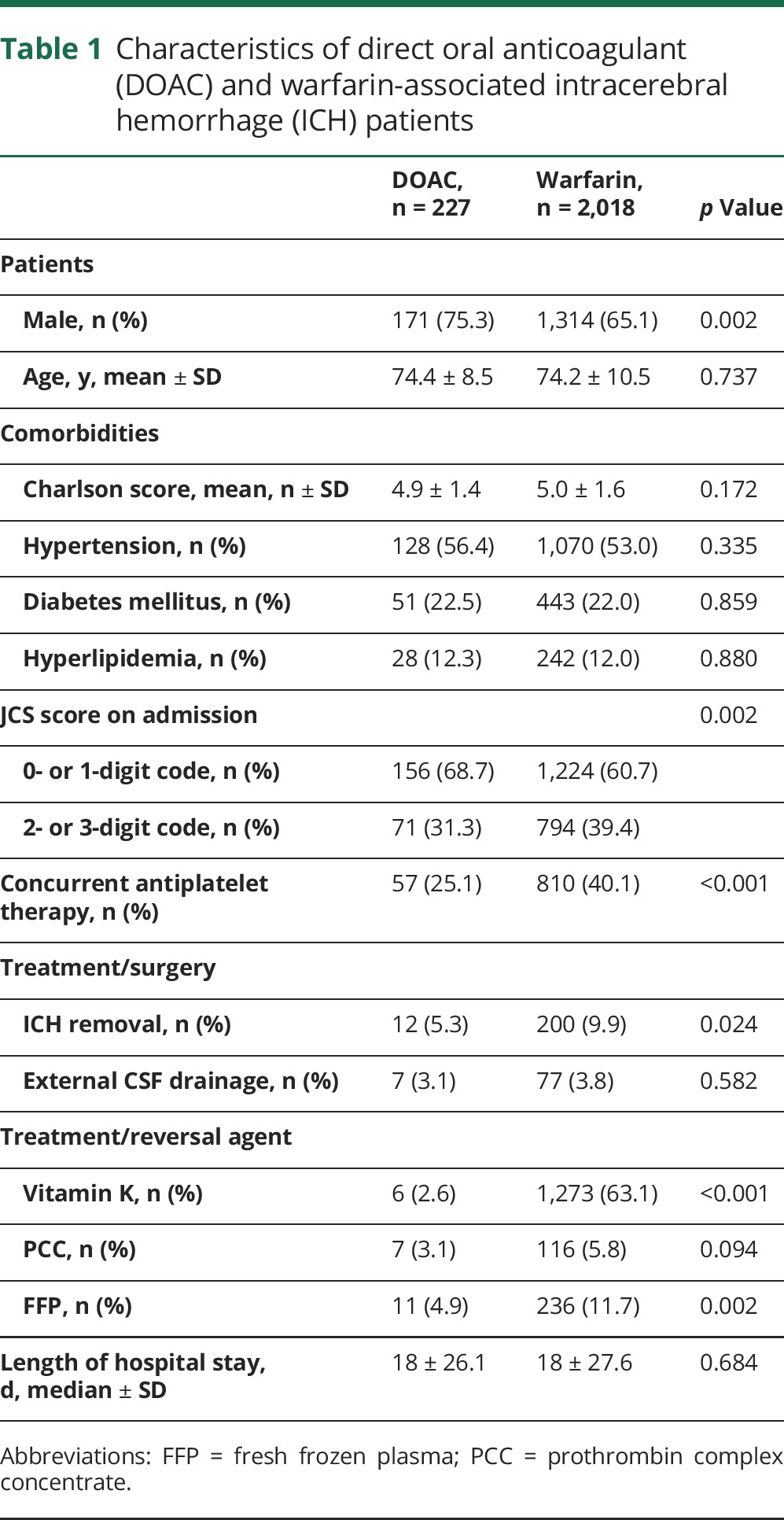
Table 1 shows the characteristics of patients with DOAC- and warfarin-associated ICHs. No significant between-group differences were noted for age (mean age in years: DOAC, 74.4; warfarin, 74.2); comorbidities such as Charlson scores (mean: 4.9, 5.0), hypertension (56.4%, 53.0%), diabetes mellitus (22.5%, 22.0%), or hyperlipidemia (12.3%, 12.0%); or median lengths of hospital stays (median days: 18.0, 18.0). The DOAC-treated group had a greater proportion of men (75.3%, 65.1%; p = 0.002) and smaller proportion of JCS 2- and 3-digit codes (moderately or severely impaired consciousness, respectively) (31.3%, 39.4%; p = 0.002). Concurrent use of antiplatelet drugs was more frequent among warfarin-treated patients (25.1%, 40.1%; p < 0.001). Overall usage rates for reversal agents, especially PCC, were quite low for both warfarin- and DOAC-treated patients. The DOAC-treated patients were less likely to be given vitamin K (2.6%, 63.1%; p < 0.001) or FFP (4.9%, 11.7%; p < 0.001); tended to be given PCC (3.1%, 5.8%; p = 0.094), the most effective early reversal agent for warfarin; and were less likely to require ICH removal surgeries (5.3%, 9.9%; p = 0.024).
Table 1.
Characteristics of direct oral anticoagulant (DOAC) and warfarin-associated intracerebral hemorrhage (ICH) patients
Considering the lack of specific antidotes for DOACs during the study period, we further analyzed the relationships between the frequency of ICH removal surgeries and the use of vitamin K, FFP, or PCC in warfarin- and DOAC-treated patients. We found that surgical ICH removal was associated with the use of vitamin K (presence, 13.6%; absence, 3.6%; p < 0.001), FFP (presence, 47.9%; absence, 4.9%; p < 0.001), or PCC (presence, 31.0%; absence, 8.6%; p < 0.001) in patients with warfarin-associated ICHs, whereas no significant associations were observed for the use of vitamin K (presence, 0%; absence, 5.3%; p = 0.558), FFP (presence, 9.1%; absence, 5.1%; p = 0.563), or PCC (presence, 14.3%; absence, 5.0%; p = 0.280) in patients with DOAC-associated ICHs. After adjusting for the use of vitamin K, PCC, or FFP, the DOAC- and warfarin-treated groups exhibited no significant difference in ORs for ICH removal surgeries (OR 1.21, p = 0.608), but the interaction between usage of DOAC and usage of reversal agents for ICH removal surgeries was marginally significant (p = 0.062) (table e-2, links.lww.com/WNL/A284). This suggested that warfarin vs DOAC tends to show different effect on ICH removal surgeries within the group based on usage of reversal agents. Table e-3 shows the hemorrhage locations of DOAC- and warfarin-associated ICHs obtained from DPC data representing 68.9% of the study cases. These data suggest no significant between-group differences in hematoma locations.
Table 2 shows mortality rates and discharge mRS scores for the DOAC- and warfarin-treated patients. The warfarin-treated patients exhibited higher mortality rates within 1 day (2.6%, 6.5%; p = 0.022), within 7 days (11.9%, 18.2%; p = 0.017), and during hospitalization (17.6%, 25.3%; p = 0.011). There were no significant differences in mean discharge mRS scores (3.30, 3.50) or the proportion of discharge mRS scores of 4–6 (52.4%, 55.1%).
Table 2.
Outcomes of direct oral anticoagulant (DOAC) and warfarin-associated intracerebral hemorrhage patients
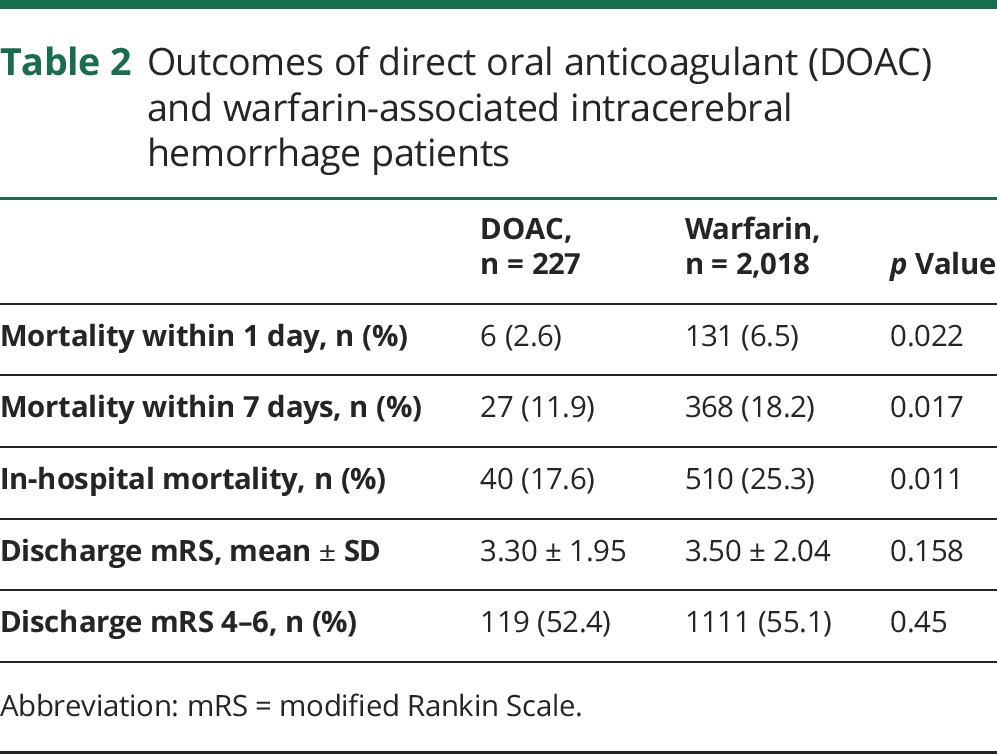
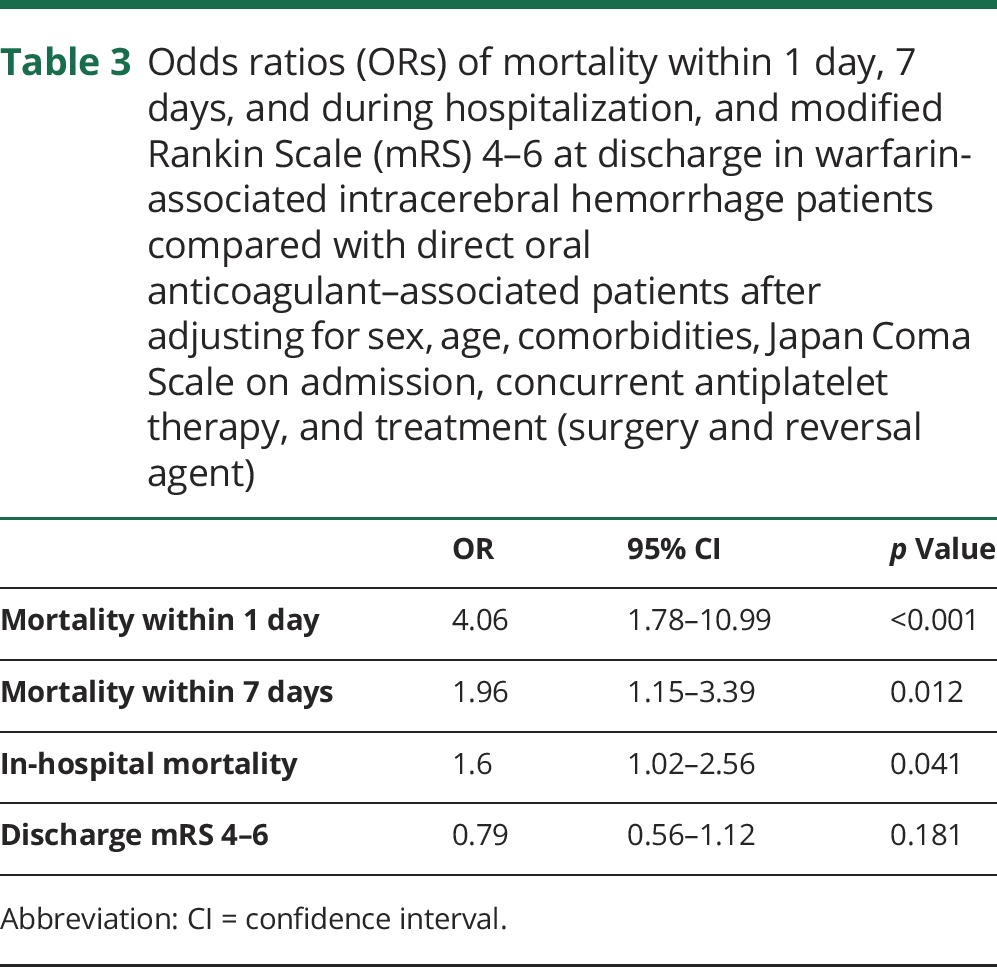
Table 3 shows the 2 groups' ORs for mortality within 1 day, within 7 days, and during hospitalization and for discharge mRS scores of 4–6 after adjusting for sex, age, comorbidities, admission JCS scores, concurrent antiplatelet therapy, and treatment with surgery or reversal agents. The warfarin-treated patients exhibited higher ORs for mortality within 1 day (OR 4.06, p < 0.001), within 7 days (OR 1.96, p = 0.012), and during hospitalization (OR 1.60, p = 0.041). The 2 groups' ORs for discharge mRS scores of 4–6 were not significantly different.
Table 3.
Odds ratios (ORs) of mortality within 1 day, 7 days, and during hospitalization, and modified Rankin Scale (mRS) 4–6 at discharge in warfarin-associated intracerebral hemorrhage patients compared with direct oral anticoagulant–associated patients after adjusting for sex, age, comorbidities, Japan Coma Scale on admission, concurrent antiplatelet therapy, and treatment (surgery and reversal agent)
Propensity score analysis
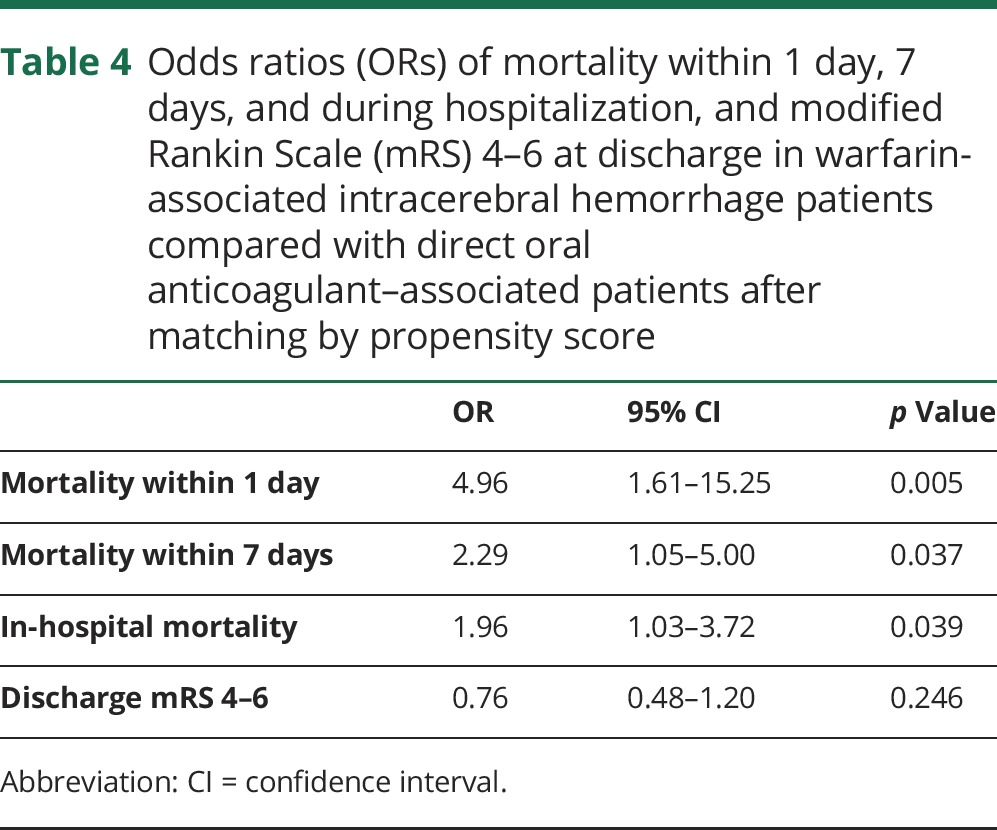
Table 4 shows the 2 groups' ORs for mortality within 1 day, within 7 days, and during hospitalization and for discharge mRS score of 4–6 after matching patients by propensity scores (n = 221 vs n = 221). Table e-4 (links.lww.com/WNL/A284) shows the propensity score–adjusted data for sex, age, comorbidities, admission JCS scores, concurrent antiplatelet therapies, and treatment with surgery or reversal agents. The warfarin-treated patients exhibited higher ORs for mortality within 1 day (OR 4.96, p = 0.005), within 7 days (OR 2.29, p = 0.037), and during hospitalization (OR 1.96, p = 0.039). The 2 groups did not significantly differ in ORs for discharge mRS scores of 4–6.
Table 4.
Odds ratios (ORs) of mortality within 1 day, 7 days, and during hospitalization, and modified Rankin Scale (mRS) 4–6 at discharge in warfarin-associated intracerebral hemorrhage patients compared with direct oral anticoagulant–associated patients after matching by propensity score
We also applied propensity score analysis to the outcomes for DOAC- and warfarin-treated patients. The propensity score–matched patients tended to have similar characteristics. Compared to the warfarin-treated patients, the dabigatran- (n = 51 vs n = 51) and apixaban-treated patients (n = 42 vs n = 42) did not exhibit significantly different mortality rates or likelihoods of discharge mRS scores of 4–6 (table e-5 and e-6). The rivaroxaban-treated patients (n = 127 vs n = 127), however, exhibited lower mortality rates within 1 day (OR = 11.22, p = 0.023), though this was not the case for mortality rates within 7 days or during hospitalization or for the likelihood of discharge mRS scores of 4–6 (table e-7).
Discussion
Our objective in this study was to compare the characteristics and outcomes of DOAC- and warfarin-associated ICHs in clinical practice. Administrative databases of hospital admissions are increasingly used for clinical outcome studies. Despite several limitations, using nationwide databases provides large sample sizes and produces more generalizable results than randomized trials because it eliminates potential selection and referral biases.10 The J-ASPECT study is the first nationwide survey on the clinical practice of stroke care in Japan that uses data obtained from hospitals subject to the DPC-based payment system.10 This study had a larger sample of clinical patients than did previous reports comparing DOAC- and warfarin-associated ICHs.5,14–16 Furthermore, we added a propensity score analysis and a multivariable analysis to compare the clinical outcomes from warfarin-associated ICHs to DOAC-associated ICHs collectively and the ICHs associated with each individual DOAC separately, with adjustments for key variables such as concurrent antiplatelet therapies17–19 and reversal agents.17,20,21 Compared to the warfarin-associated ICHs, the DOAC-associated ICHs were of lesser severity in the univariate analysis and were associated with lower in-hospital mortality after matching with propensity scores derived from clinical data. Our results are therefore robust and provide important clinical insights into optimizing oral anticoagulation therapy for stroke prevention in patients with AF.
Few studies have compared the clinical and radiologic characteristics and outcomes of DOAC- and warfarin-associated ICHs,5,15,16 and they have featured small sample sizes of DOAC-associated ICHs.5,15,16 These studies found that patients with DOAC-associated ICHs had lower mortality rates,15 smaller hematomas,5,15,16 and less hematoma expansion.5,15 In a case series study of DOAC-associated ICHs,22,23 none showed small hematoma expansion. In animal model studies, dabigatran treatment results in lower ICH volumes and less hematoma expansion than does warfarin treatment.24,25 These reports support our observation that DOAC-associated ICHs were less severe and were associated with lower mortality rates.
Several reports have explored the mechanisms behind DOAC-associated ICHs having smaller volumes and less hematoma expansion than warfarin-associated ICHs.5,22,23,26 Complexes of tissue factor VII are essential for the first reaction in the extrinsic coagulation cascade.27 Warfarin inhibits vitamin K–dependent coagulation proteins II, VII, IX, and X, while DOAC never inhibits factor VII function.28 Furthermore, DOAC half-lives are shorter than warfarin's half-life.28
Our univariate analysis showed that warfarin-treated patients were more likely to undergo ICH removal surgeries, but this difference did not survive adjustments for the use of reversal agents. However, it is difficult to interpret these contradictory results, because the interaction between ICH removal surgeries and reversal agent usage was marginally significant. The appropriateness of surgery for most patients with spontaneous ICHs remains controversial, but it is recommended for patients with cerebellar hemorrhages who are deteriorating neurologically or who have brainstem compression or hydrocephalus from ventricular obstruction.29 Initial hematoma volume is the most powerful predictor of neurologic deterioration, functional outcome, and mortality in both spontaneous and oral anticoagulant–associated ICHs, whereas the level of consciousness is highly predictive in infratentorial ICHs.30,31 The proportion of cerebellar hemorrhages in this study is notably similar to that of a previous Japanese study that showed that prior treatment with antiplatelets, warfarin, or both is predictive of cerebellar hemorrhages, hematoma enlargement, and early death in Japanese patients with ICHs.32 In line with previous studies comparing patients with DOAC- and warfarin-associated ICHs,5,16,33 we found no significant between-group difference in ICH locations. However, the DPC data excluded information about the eloquence of the ICH lesions and the size and expansion of hematomas. In terms of lesion eloquence, our study revealed that DOAC-associated ICHs were associated with similar functional outcomes. Given the strong effect of initial hemorrhage locations on functional outcomes following DOAC- and warfarin-associated ICHs regardless of hematoma size or expansion,34 we doubt there were any between-group differences in the proportions of eloquent area ICHs in our sample. Therefore, the localization and eloquence of the lesions does not seem to explain the different rates of ICH removal surgeries for our warfarin- and DOAC-treated groups. Regarding the size and expansion of hematomas, past studies5,15–20 have shown that DOAC-associated ICHs have smaller hemorrhage volumes and lower chances of hematoma expansion, which may explain our observation. A previous study comparing the effects of different reversal agents on hematoma growth and outcomes in patients with warfarin-related ICHs found that PCC was associated with a reduced incidence and extent of hematoma growth relative to FFP and vitamin K.35 The present study, however, revealed that reversal strategies are not as widely applied in clinical practice in Japan as they are in Western countries,16,33 with PCC being used less frequently for warfarin-treated patients and specific reversal agents for DOACs such as idarucizumab being unavailable during the study period.17,20,21 Despite such overall low usage, more frequent usage in specific scenarios, especially PCC for patients who underwent removal surgeries for warfarin-associated ICHs, suggests that such strategies are mainly indicated for patients with large initial hematoma volumes and a high risk of neurologic deterioration. Furthermore, our study revealed that patients with warfarin-associated ICHs who were treated with reversal agents were more likely to require removal surgeries. This suggests that among patients with warfarin-associated ICHs, those who require surgical removal of large ICHs are more likely to use reversal agents.
Despite the release of DOACs, issues with warfarin such as overuse for low-risk patients and underuse for high-risk patients persist, as does discordance between guidelines and clinical practice. Our results are inconsistent with those of comparative studies of DOAC- or warfarin-associated ICHs in clinical settings in Western countries.5,14,33 Alonso et al.14 found no significant medication-related differences in mortality for any type of intracranial hemorrhage (intracerebral, subdural, or subarachnoid). However, the results of subtype analyses should be interpreted cautiously because of imprecise estimates and small numbers.14 Considering the prognosis differences between ICH subtypes, we focused on 227 DOAC-associated ICHs while excluding other hemorrhage subtypes. In contrast, the Registry of Acute Stroke Under New Oral Anticoagulants33 showed that DOAC-associated ICHs were associated with higher mortality rates, more unfavorable outcomes, and greater hematoma expansion frequencies. However, these conclusions were derived from comparisons with both warfarin-associated ICHs in that study and those in previously published retrospective observational studies. Another comparative study showed slightly higher mortality rates in patients with DOAC-associated ICHs but did not adjust for confounding factors with multivariate analyses or propensity score matching.5 Another explanation for the disparity between our study and that of those 3 studies5,14,33 may be the differences in patient ethnicity. The median case-fatality at 1 month for patients with ICHs is lower in Japan than in other countries,36 and there are slight differences in the efficacy and safety of DOACs and warfarin between Asian and non-Asian populations.37,38 Resolving these discrepancies will require multinational observational collaborative studies and randomized controlled trials comparing DOACs and warfarin.
Each DOAC exhibits a unique dose-dependent efficacy and safety profile.1 We therefore used propensity score analysis to separately compare the outcomes for each DOAC to those of warfarin. Compared to warfarin-treated patients, rivaroxaban-treated patients exhibited significantly lower 1-day mortality rates, while dabigatran- and apixaban-treated patients did not. These differences may be explained by the sample sizes for each DOAC. In Japan, dabigatran, rivaroxaban, and apixaban were launched in March 2011, April 2012, and February 2013, respectively, and edoxaban was approved for additional indications in September 2014. The timing of these launches may account for the proportions of each DOAC in this study. Larger sample sizes are needed to compare the characteristics and outcomes of each DOAC with those of warfarin.
The DPC database lacks several types of data. First, the database does not include laboratory data. The prothrombin time–international normalized ratio (PT-INR) is a particularly important factor in assessing the influence of hemorrhagic stroke.17 In this respect, it is interesting to note that the Japanese Fushimi AF Registry reported that over 90% of patients had PT-INR values within the optimal range.39 Given this observation, it is unlikely that high PT-INR values alone could account for our observation of warfarin-associated ICHs being more severe, requiring removal more often, and being associated with higher mortality rates. Second, it does not contain vital signs data. Notably, a meta-analysis of 5 randomized controlled trials also showed that early intensive blood pressure reduction did not significantly reduce the mortality rate.40 Admittedly, these missing data are unmeasured confounders for this study, so there is a need for further research that combines DPC database information with laboratory and radiologic data.
Although end-of-life decisions are an important factor in mortality, we had no information about such decisions. Previous studies comparing patients with DOAC- and warfarin-associated ICHs revealed no between-group differences in end-of-life decisions and found that mortality due to end-of-life decisions was low (i.e., 6%).5 In Japan, the proportion of end-of-life decisions is reportedly much lower than in other countries.41 Collectively, these facts lead us to suspect a minor effect of end-of-life decision on mortality in our patients.
This is the largest Japan-wide study of DOAC-associated ICHs in clinical practice, and it revealed that DOAC-associated ICHs were less lethal than warfarin-associated ICHs, probably due to lower hemorrhage severity at admission and lower hematoma expansion frequencies.
Acknowledgment
The authors thank the J-ASPECT study collaborators for their contributions. The names of the 621 participating hospitals and their representatives are listed in the online-only data supplement. All contributors were involved in data collection. The authors also thank Drs. Manabu Hasegawa, Tomoatsu Tsuji, and Yasuhiro Nishijima for discussion, Profs. Takamasa Kayama and Nobuo Hashimoto for supervision of the collaboration with the Japan Neurosurgical Society, and Arisa Ishitoko for secretarial assistance.
Glossary
- AF
atrial fibrillation
- DOAC
direct oral anticoagulant
- DPC
Diagnosis Procedure Combination
- FFP
fresh frozen plasma
- ICD-10
International Classification of Diseases–10
- ICH
intracerebral hemorrhage
- JCS
Japan Coma Scale
- mRS
modified Rankin Scale
- OR
odds ratio
- PCC
prothrombin complex
- PT-INR
prothrombin time–international normalized ratio
Contributor Information
Collaborators: J-ASPECT Study Collaborators, Masayoshi Takigami, Kenji Kamiyama, Kiyohiro Houkin, Shougo Nishi, Tetsuyuki Yoshimoto, Sadao Kaneko, Koji Oka, Yusuke Nakagaki, Hiroshi Ooyama, Kyousuke Kamada, Kenichi Makino, Naoki Tokumitsu, Kazuhiro Sako, Naoki Tokumitsu, Susumu Suzuki, Nozomi Suzuki, Teruo Kimura, Naoto Izumi, Kazumi Nitta, Masahumi Ootaki, Masanori Isobe, Mikio Nishiya, Takaaki Yamazaki, Syouji Mabuchi, Kuniaki Ogasawara, Naohiko Kubo, Yukihiko Shimizu, Keiichi Saito, Tatumi Yamanome, Akinori Yabuta, Akira Suzuki, Atsuo Yoshino, Junichi Harashina, Mitsuyuki Fujitsuka, Hiroyuki Masaoka, Masaaki Takami, Hirotoshi Ohtaka, Teruyuki Hirano, Yosiaki Shiokawa, Takaharu Okada, Ichiro Suzuki, Michihiro Kohno, Jou Haraoka, Yoshinori Arai, Noriyoshi Kawamura, Akira Isoshima, Masaharu Yasue, Mitsuhiko Hokari, Takayoshi Kobayashi, Kensuke Kawai, Taketoshi Maehara, Hajime Arai, Takakazu Kawamata, Yoshikazu Okada, Makoto Noguchi, Haruhiko Hoshino, Hirofumi Hiyama, Kensaku Yoshida, Mitsuyuki Fujitsuka, Osamu Utsugi, Yasuaki Takeda, Kouichi Tamaki, Hirohide Karasudani, Takao Urabe, Shiro Kobayashi, Michio Nakamura, Yorio Koguchi, Junichi Ono, Sumio Suda, Hiromu Hadeishi, Toshio Fukutake, Kenji Wakui, Hirokazu Tanno, Naoki Ishige, Takashi Ohasi, Naoaki Sato, Hideki Sakai, Yasuaki Nishimura, Takayuki Watanabe, Takashi Matsumoto, Naoki Koketsu, Yuichi Hirose, Manabu Doyu, Toshinori Hasegawa, Naoto Kuwayama, Shinichi Terao, Nobuhiko Mizutani, Noriyuki Suzaki, Satoshi Okuda, Keizo Yasui, Yukio Seki, Yasuhiro Hasegawa, Akira Ikeda, Youtarou Takeuchi, Sigeki Ohara, Yoshio Araki, Toshihiko Wakabayashi, Hisashi Tanaka, Junpei Yoshimoto, Makoto Sugiura, Ogura Koichiro, Nozomu Kobayashi, Toshio Yokoe, Kenichi Murao, Tomonori Yamada, Amami Kato, Toshiho Ohtsuki, Akatsuki Wakayama, Jun Takahashi, Hiroharu Kataoka, Toshiki Yoshimine, Yoshikazu Nakajima, Hidehuku Gi, Ryunosuke Uranishi, Yusaku Nakamura, Kazunori Yamanaka, Kazumi Ohmori, Hiroyuki Matsumoto, Yoshitugu Oiwa, Yosihiko Uemura, Hiroaki Fujiwara, Yoshiyasu Iwai, Masashi Morikawa, Kazuyuki Tane, Kazuo Hashikawa, Toshiyuki Fujinaka, Shunichi Yoneda, Kohsuke Yamashita, Masahiko Kitano, Shinsuke Tominaga, Kazuhito Nakamura, Katsuhiko Kono, Kenji Ohata, Hirokatsu Taniguchi, Takanori Hazama, Toshihiko Kuroiwa, Yoji Tamura, Kazusige Maeno, Motohiro Arai, Masaaki Iwase, Kenji Hashimoto, Keisuke Yamada, Takashi Turuno, Tsutomu Ichinose, Shinichiro Kurokawa, Takeshi Matsuyama, Toshiaki Fujita, Takamichi Yuguchi, Yoshihumi Teramoto, Hiroto Kakita, Takayuki Matsuo, Tsuyoshi Izumo, Nobutoshi Ryu, Wataru Haraguchi, Naoki Kitagawa, Makio Kaminogo, Seisaburo Sakamoto, Yosiharu Tokunaga, Ei-Ichirou Urasaki, Junichi Kuratsu, Akira Takada, Tadashi Terasaki, Toru Nishi, Isao Fuwa, Hisami Ooshima, Shigeo Yamashiro, Makoto Yoshikawa, Hiromasa Tsuiki, Kazunari Koga, Hiroshi Egami, Hirofumi Nagatomi, Tadao Kawamura, Makoto Goda, Yu Takeda, Kunihiko Mitsuo, Takamitu Hikawa, Masaki Morisige, Yuu Takeda, Yutaka Yamaguchi, Shiro Miyata, Shunro Uchinokura, Tomokazu Goya, Hideo Takeshima, Kazutaka Yatsushiro, Hajime Ohta, Tatsui Nagadou, Kazuho Hirahara, Souichi Obara, Hiroshi Seto, Shunichi Tanaka, Koichi Moroki, Kazunori Arita, Shogo Ishiuchi, Toshimitsu Uchihara, Susumu Mekaru, Tomoaki Nagamine, Naoki Tomiyama, Jin Momoji, Satoshi Yamamoto, Koji Idomari, Atushi Kimoto, Tsutomu Kadekaru, Hirosi Syamoto, Osamu Sasaki, Makoto Minagawa, Yukihiko Fujii, Hideaki Takahashi, Kiyoshi Onda, Hiroyuki Arai, Shigekazu Takeuchi, Hiroshi Abe, Osamu Fukuda, Mitsuo Kouno, Tetsuro Tamura, Yukio Horie, Michiya Kubo, Hiroaki Hondo, Tadao Miyamori, Hisashi Takada, Toru Masuoka, Naoki Shirasaki, Hisashi Nitta, Makoto Kimura, Yasuo Katsuki, Yutaka Hayashi, Hisato Minamide, Shigeru Munemoto, Shunsuke Shiraga, Kiyonobu Ikeda, Mitsutoshi Nakada, Yutaka Hayashi, Syuji Sato, Taketo Hatano, Osamu Yamamura, Masanori Kabuto, Takahiro Sakuma, Jyunya Hayashi, Hiroyuki Kinouchi, Hidehito Koizumi, Mikito Uchida, Syougo Imae, Hiroshi Ozawa, Osamu Nishizaki, Manabu Fujita, Masakazu Suga, Shinji Iwata, Kanehisa Kohno, Takeharu Kunieda, Kiichiro Zenke, Mutsuo Fujisawa, Hiromichi Sadashima, Hikaru Mizobuchi, Satoru Hayashi, Masanori Morimoto, Takeshi Kohno, Tetsuya Ueba, Hiroyuki Nishimura, Naoki Ikawa, Yuzo Matsumoto, Seiji Kannuki, Masahiro Kagawa, Naoki Hayashi, Takashi Tamiya, Atsushi Shindo, Kimihiro Yoshino, Tetsuya Masaoka, Ichiro Nakahara, Akira Nakamizo, Yuji Okamoto, Shigenari Kin, Haruki Takahashi, Satoshi Suzuki, Koji Iihara, Katsuyuki Hirakawa, Shinji Nagata, Akio Ookura, Koichirou Matsukado, Hidenori Yoshida, Yoshiro Kaneko, Hiroshi Nakane, Isao Inoue, Maeda Yoshihisa, Kei Hisada, Tsutomu Hitotsumatsu, Terukazu Kuramoto, Kouichi Kuramoto, Junya Hayashi, Yoshihisa Matumoto, Hiromichi Ooishi, Toru Inoue, Masani Nonaka, Motohiro Morioka, Masahiro Mizoguchi, Haruhisa Tsukamoto, Hiroshi Sugimori, Shuji Sakata, Hiroshi Takashima, Shin-Ichiro Ishihara, Kenji Suzuyama, Nobuaki Momozaki, Masayuki Miyazono, Masafumi Morimoto, Itaro Hattori, Satoshi Ozaki, Nobuo Hirota, Yasunori Takemoto, Yasuhiko Mochimatsu, Makoto Takagi, Isao Yamamoto, Kenji Nakayama, Yoshinori Uchida, Hiroshi Tanaka, Katsumi Sakata, Kawahara Nobutaka, Motohiro Nomura, Hitoshi Ozawa, Kotaro Tsumura, Makoto Inaba, Michiyuki Maruyama, Tatsuro Mori, Tomoaki Terada, Takahisa Mori, Masato Sugitani, Yuichiro Tanaka, Masaru Yamada, Mitsunori Matsumae, Keiichirou Onitsuka, Kosuke Miyahara, Tatsuya Takahashi, Sumio Endou, Atsuhiro Kojima, Hidekazu Takahashi, Hiroyuki Kaidu, Akira Tsunoda, Chikashi Maruki, Kyoichi Nomura, Toru Matsui, Takamitsu Fujimaki, Hidetoshi Ooigawa, Masahiko Tanaka, Masatsugu Uchida, Hiroshi Wanihuti, Kouichi Katoh, Hirochiyo Wada, Akio Hyodo, Ken Asakura, Shigeyoshi Nakajima, Takao Kanzawa, Hideyuki Kurihara, Sigehiro Ohmori, Yoshinao Mitsugi, Hiroshi Kusunoki, Satoshi Magarisawa, Shinichi Okabe, Yuji Kujiraoka, Shin Tsuruoka, Mikihiko Takeshita, Tetsuya Yamamoto, Akira Matsumura, Kazuya Uemura, Hitoshi Tabata, Makoto Sonobe, Masashi Nakatsukasa, Ryoji Yoshida, Norifumi Shimoeda, Hideo Kunimine, Masayuki Ishihara, Mikio Teduka, Nozomu Murai, Waro Taki, Nobukuni Murakami, Minoru Kidooka, Yoshihiro Iwamoto, Hiroshi Tenjin, Kouji Shiga, Masahiko Takamasu, Nobuhito Mori, Shigeru Kose, Eiji Kohmura, Haruo Yamashita, Keigo Matsumoto, Naoya Takeda, Takayuki Sakaki, Hiroji Miyake, Eiichiro Mabuchi, Masayuki Yokota, Hideyuki Ohnishi, Yosihiro Kuga, Mitsuru Kimura, Osamu Narumi, Masaaki Saiki, Norio Nakajima, Minoru Asahi, Junji Koyama, Yoshio Sakagami, Shinya Noda, Junichi Iida, Toyohisa Fujita, Hiroyuki Nakase, Hidehiro Hirabayashi, Toru Hoshida, Takayoshi Fujimoto, Naoyuki Nakao, Yoshiyuki Tanaka, Fuminori Ozaki, Yoshinari Nakamura, Kazuhito Miki, Teruyuki Habu, Takashi Watanabe, Hiroki Ohkuma, Seiko Hasegawa, Hiromu Konno, Atsuhito Takemura, Atsuya Okubo, Hitoshi Saito, Tatsuya Ishikawa, Taizen Nakase, Hiroaki Shimizu, Toshio Sasajima, Masayuki Sasou, Yoichi Watanabe, Taku Sato, Kiyoshi Saito, Satoshi Taira, Masahiro Satoh, Zenichiro Watanabe, Takayuki Koizumi, Yasuhiro Suzuki, Shoji Mashiyama, Tomoyoshi Oikawa, Yukihiko Sonoda, Rei Kondo, Shinjiro Saito, Atsuo Shinoda, Eiichiro Kamatsuka, Keiten So, Toshihiko Kinjo, Toru Sasaki, Kenji Ito, Yohei Kudoh, Kazuhiko Sato, Hidenori Endo, Hiroaki Shimizu, Hiroshi Karibe, Furukawaseiryou Byouin, Kou Takahashi, Masayuki Nakajima, Kazuyoshi Watanabe, Kazuhiko Nozaki, Motohiro Takayama, Taro Komuro, Hisao Hirai, Fumio Suzuki, Hidenori Suzuki, Hiroto Murata, Fumitaka Miya, Kenji Kanamaru, Akira Tamura, Kiyoshi Harada, Seiji Fukazawa, Seiya Takehara, Yoshihiko Watanabe, Teiji Nakayama, Haruhiko Sato, Hiroshi Nagura, Shinji Amano, Chiharu Tanoi, Katsuhiro Kuroda, Satoru Morooka, Takafumi Wataya, Masashi Kitagawa, Kazuo Koide, Tetsuya Tanigawara, Toru Iwama, Junki Ito, Shinji Noda, Kazuyuki Kohno, Kazuo Kitazawa, Yoshikazu Kusano, Toshiki Takemae, Masanobu Hokama, Hiroki Sato, Yoshihisa Nishiyama, Tatsuya Seguchi, Sumio Kobayashi, Yoshihiko Inui, Yoji Ohigashi, Shinsuke Muraoka, Masaki Miyatake, Kensuke Hayashida, Shinichi Nakagawa, Atsushi Inoue, Keiichi Sakai, Shuhei Yamaguchi, Tatsuya Mizoue, Fusao Ikawa, Gen Ishida, Hideki Irie, Takato Kagawa, Yoichiro Namba, Hiroyuki Nakashima, Koji Tokunaga, Isao Date, Koji Abe, Masaaki Uno, Masaki Chin, Sen Yamagata, Hidemichi Sasayama, Soichiro Takao, Hideyuki Yoshida, Koji Muneda, Akira Watanebe, Kunihiko Harada, Syouichi Kato, Yasuhiro Hamada, Michiyasu Suzuki, Takafumi Nishizaki, Katsuhiro Yamashita, Takaharu Nakamura, Shinichi Wakabayashi, Takahito Okazaki, Kaoru Kurisu, Masayasu Matsumoto, Naohisa Hosomi, Atsushi Tominaga, Katsuzo Kiya, Masaaki Shibukawa, Syuichi Oki, Toshinori Nakahara, Shinji Okita, Tsuyosi Torii, Minoru Nakagawa, Kenjirou Fujiwara, Takashi Matsuoka, Syuhei Nishimura, Osamu Hamasaki, Naoyuki Isobe, Junichiro Satomi, Shinji Nagahiro, Masahito Agawa, Hirofumi Oka, Kunikazu Yoshimura, Sei Haga, Katsuyuki Asaoka, Toshitaka Nakamura, Tsutomu Kato, Nobuaki Kobayasi, Satoshi Minoshima, Nobuhiro Mikuni, Jun Niwa, Rokuya Tanikawa, Akinori Yamamura, Noriaki Watabe, Jyunkou Sasaki, Yasunari Otawara, Kazuyuki Miura, Teiji Tominaga, Tatsuya Sasaki, Takayuki Sugawara, Masayuki Ezura, Syuichi Ishikawa, Sunao Takemura, Masahisa Kawakami, Satoshi Ihara, Yasushi Shibata, Takashi Saegusa, Toshihiko Iuchi, Chiaki Ito, Sumio Isimaru, Osamu Okuda, Kazunari Yoshida, Takekazu Akiyama, Sadao Suga, Masateru Katayama, Masahiko Kasai, Akihiro Oikawa, Naohisa Miura, Takahiro Ota, Atsumi Takenobu, Toshihiro Kumabe, Sachio Suzuki, Takashi Kumagai, Keiichi Nishimaki, Kazuhiro Hongo, Hiroaki Shigeta, Atsushi Sato, Satoshi Kuroda, Sotaro Higashi, Hirofumi Oyama, Kazuyoshi Hattori, Yoichi Uozumi, Norimoto Nakahara, Nobukazu Hashimoto, Toshikazu Ichihashi, Katsunobu Takenaka, Yuko Nonaka, Shinichi Shirakami, Shu Imai, Yoshinari Okumura, Ryo Tamaki, Kazuhiro Yokoyama, Susumu Miyamoto, Yoshinori Akiyama, Kenji Hashimoto, Kazuo Yamamoto, Tsugumichi Ichioka, Kazutomo Nakazawa, Misao Nishikawa, Tsuyoshi Inoue, Manabu Kinoshita, Shinichi Yoshimura, Minoru Saitoh, Hideo Aihara, Hajimu Miyake, Kotaro Ogihara, Tsukasa Nishiura, Shigeki Nishino, Yasuyuki Miyoshi, Tadashi Arisawa, Shigeru Daido, Shoji Tsuchimoto, Kimihisa Kinoshita, Kiyoshi Yuki, Keisuke Migita, Keiichi Akatsuka, Hirosuke Fujisawa, Junkoh Yamamoto, Satoshi Inoha, Hitonori Takaba, Tadahisa Shono, Hitoshi Tsugu, Shuji Hayashi, Tatsuya Abe, Susumu Nakashima, Takehisa Tuji, Keizo Yamamoto, Akihiko Kaga, Reizou Kanemaru, Koji Takasaki, Junichi Imamura, Masahiro Noha, Saburo Watanabe, Nobuyuki Sakai, Yasuhisa Yoshida, Hiroaki Minami, Tomoyoshi Okumura, Shinjitsu Nishimura, Shinichi Numazawa, Yasunari Niimi, Kiyoshi Kazekawa, Masanori Tsutsumi, Kouzou Fukuyama, Makoto Ichinose, Yasuhiro Fujimoto, Youichi Hashimoto, Takeshi Matsuoka, Takamitsu Uchizawa, Tomohiko Sato, Hiroaki Sawaura, Satoshi Utsuki, Chiaki Takahashi, Kazumasa Yamatani, Toshiyuki Tsukada, Ryoichi Hayashi, Masakazu Kitahara, Yukinari Kakizawa, Yasumasa Yamamoto, Takashi Yoshida, Yasunobu Goto, Takashi Tominaga, Shigeru Miyake, Nozomi Mori, Naoki Shinohara, Yasushi Ejima, Mayumi Mori, Hitoshi Miyake, Hiromichi Koga, Kenichi Matsumoto, Kazuya Morimoto, Yoshimasa Niiya, Tsuneo Shishido, Mamoru Murakami, Takaaki Yoshida, Masahito Hara, Tatsuya Nakamura, Takuya Kawai, Takashi Inoue, Isao Sasaki, and Naoko Fujimura
Author contributions
Ryota Kurogi drafted the manuscript. Koji Iihara was involved in conceptualizing and designing the study and in obtaining funding. Jyoji Nakagawara, Kazunori Toyoda, Kuniaki Ogasawara, Junichi Ono, Yoshiaki Shiokawa, Toru Aruga, Shigeru Miyachi, Izumi Nagata, Shinya Matsuda, Shinichi Yoshimura, Kazuo Okuchi, and Akifumi Suzuki were involved in data acquisition. Ryota Kurogi, Kunihiro Nishimura, Michikazu Nakai, Akiko Kada, Satoru Kamitani, Daisuke Onozuka, Keisuke Ido, Ai Kurogi, Nobutaka Mukae, Ataru Nishimura, Koichi Arimura, Akihito Hagihara, and Koji Iihara were involved in analyzing and interpreting the data. Jyoji Nakagawara, Kazunori Toyoda, Kuniaki Ogasawara, Junichi Ono, Yoshiaki Shiokawa, Toru Aruga, Shigeru Miyachi, Izumi Nagata, Shinya Matsuda, Shinichi Yoshimura, Kazuo Okuchi, Akifumi Suzuki, Takanari Kitazono, and Koji Iihara were involved in study supervision.
Study funding
This work was supported by Grants-in-Aid from the Japanese Ministry of Health, Labour and Welfare and a KAKENHI grant (25293314; principal investigator: Koji Iihara) from the Japan Society for the Promotion of Science. This research is partially supported by the Practical Research Project for Life-Style related Diseases including Cardiovascular Diseases and Diabetes Mellitus managed by the Japan Agency for Medical Research and Development. The funding sources had no role in the study design, data collection and analysis, manuscript preparation, or decision to publish.
Disclosure
R. Kurogi reports no disclosures relevant to the manuscript. K. Nishimura reports receiving lecture fees from Bristol-Myers Squibb. M. Nakai, A. Kada, S. Kamitani, and J. Nakagawara report no disclosures relevant to the manuscript. K. Toyoda reports receiving honoraria for lectures from Bayer, Daiichi-Sankyo, Boehringer-Ingelheim, and Bristol-Myers Squibb. K. Ogasawara reports receiving grants from Nihon Medi-Physics and Bristol-Myers Squibb. J. Ono, Y. Shiokawa, T. Aruga, and S. Miyachi report no disclosures relevant to the manuscript. I. Nagata reports receiving honoraria for lectures from Bristol-Myers Squibb. S. Matsuda reports receiving honoraria for lectures from Chugai Pharmaceutical. S. Yoshimura reports receiving grants from Shionogi & Co., Terumo, Takeda, and Bristol-Meyers Squibb and honoraria for lectures from Mitsubishi Tanabe Pharma, Sanofi, Bristol-Myers Squibb, Boehringer-Ingelheim, Otsuka Pharmaceutical, Bayer, Daiichi Sankyo, and Pfizer. K. Okuchi reports no disclosures relevant to the manuscript. A. Suzuki is a member of the medical advisory committee to the Akita Pilot Study undertaken by Bayer. F. Nakamura reports receiving grants from Asahi Kasei. D. Onozuka, K. Ido, A. Kurogi, N. Mukae, A. Nishimura, and K. Arimura report no disclosures relevant to the manuscript. T. Kitazono reports receiving speaker fees from Bayer Yakuhin and Daiichi Sankyo, consulting fees from Chugai Pharmaceutical, and grants from Mitsubishi Tanabe Pharma, Takeda Pharmaceutical, Eizai, Merck Sharp & Dohme, Astellas Pharma, Daiichi Sankyo, and Chugai Pharmaceutical. A. Hagihara reports no disclosures relevant to the manuscript. K. Iihara reports receiving grants from Otsuka Pharmaceutical, Nihon Medi-Physics, and AstraZeneca. Go to Neurology.org/N for full disclosures.
References
- 1.Chan NC, Paikin JS, Hirsh J, Lauw MN, Eikelboom JW, Ginsberg JS. New oral anticoagulants for stroke prevention in atrial fibrillation: impact of study design, double counting and unexpected findings on interpretation of study results and conclusions. Thromb Haemost 2014;111:798–807. [DOI] [PubMed] [Google Scholar]
- 2.Hart RG, Diener HC, Yang S, et al. Intracranial hemorrhage in atrial fibrillation patients during anticoagulation with warfarin or dabigatran: the RE-LY trial. Stroke 2012;43:1511–1517. [DOI] [PubMed] [Google Scholar]
- 3.Hankey GJ, Stevens SR, Piccini JP, et al. Intracranial hemorrhage among patients with atrial fibrillation anticoagulated with warfarin or rivaroxaban: the rivaroxaban once daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation. Stroke 2014;45:1304–1312. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen E, White CM, Patel MR, et al. Doses of apixaban and rivaroxaban prescribed in real-world United States cardiology practices compared to registration trials. Curr Med Res Opin 2016;32:1277–1279. [DOI] [PubMed] [Google Scholar]
- 5.von der Brelie C, Doukas A, Naumann R, et al. Clinical and radiological course of intracerebral haemorrhage associated with the new non-vitamin K anticoagulants. Acta Neurochir 2017;159:101–109. [DOI] [PubMed] [Google Scholar]
- 6.Yasunaga H, Ide H, Imamura T, Ohe K. Impact of the Japanese Diagnosis Procedure Combination-based payment system on cardiovascular medicine-related costs. Int Heart J 2005;46:855–866. [DOI] [PubMed] [Google Scholar]
- 7.Iihara K, Nishimura K, Kada A, et al. The impact of comprehensive stroke care capacity on the hospital volume of stroke interventions: a nationwide study in Japan: J-ASPECT study. J Stroke Cerebrovasc Dis 2014;23:1001–1018. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura K. Diagnosis Procedure Combination database would develop nationwide clinical research in Japan. Circ J 2016;80:2289–2290. [DOI] [PubMed] [Google Scholar]
- 9.Iihara K, Nishimura K, Kada A, et al. Effects of comprehensive stroke care capabilities on in-hospital mortality of patients with ischemic and hemorrhagic stroke: J-ASPECT study. PLoS One 2014;9:e96819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishimura A, Nishimura K, Kada A, Iihara K; J-ASPECT study group. Status and future perspectives of utilizing big data in neurosurgical and stroke research. Neurol Med Chir 2016;56:655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Onozuka D, Hagihara A, Nishimura K, et al. Prehospital antiplatelet use and functional status on admission of patients with non-haemorrhagic moyamoya disease: a nationwide retrospective cohort study (J-ASPECT study). BMJ Open 2016;6:e009942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 13.Shigematsu K, Nakano H, Watanabe Y. The eye response test alone is sufficient to predict stroke outcome: reintroduction of Japan Coma Scale: a cohort study. BMJ Open 2013;3:e002736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alonso A, Bengtson LG, MacLehose RF, Lutsey PL, Chen LY, Lakshminarayan K. Intracranial hemorrhage mortality in atrial fibrillation patients treated with dabigatran or warfarin. Stroke 2014;45:2286–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hagii J, Tomita H, Metoki N, et al. Characteristics of intracerebral hemorrhage during rivaroxaban treatment: comparison with those during warfarin. Stroke 2014;45:2805–2807. [DOI] [PubMed] [Google Scholar]
- 16.Wilson D, Charidimou A, Shakeshaft C, et al. Volume and functional outcome of intracerebral hemorrhage according to oral anticoagulant type. Neurology 2016;86:360–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mittal MK, LacKamp A. Intracerebral hemorrhage: perihemorrhagic edema and secondary hematoma expansion: from bench work to ongoing controversies. Front Neurol 2016;7:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oldgren J, Wallentin L, Alexander JH, et al. New oral anticoagulants in addition to single or dual antiplatelet therapy after an acute coronary syndrome: a systematic review and meta-analysis. Eur Heart J 2013;34:1670–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toyoda K, Yasaka M, Iwade K, et al. Dual antithrombotic therapy increases severe bleeding events in patients with stroke and cardiovascular disease: a prospective, multicenter, observational study. Stroke 2008;39:1740–1745. [DOI] [PubMed] [Google Scholar]
- 20.Kaatz S, Kouides PA, Garcia DA, et al. Guidance on the emergent reversal of oral thrombin and factor Xa inhibitors. Am J Hematol 2012;87(suppl 1):S141–S145. [DOI] [PubMed] [Google Scholar]
- 21.Pollack CV Jr. Managing bleeding in anticoagulated patients in the emergency care setting. J Emerg Med 2013;45:467–477. [DOI] [PubMed] [Google Scholar]
- 22.Akiyama H, Uchino K, Hasegawa Y. Characteristics of symptomatic intracranial hemorrhage in patients receiving non-vitamin K antagonist oral anticoagulant therapy. PLoS One 2015;10:e0132900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Komori M, Yasaka M, Kokuba K, et al. Intracranial hemorrhage during dabigatran treatment. Circ J 2014;78:1335–1341. [DOI] [PubMed] [Google Scholar]
- 24.Lauer A, Cianchetti FA, Van Cott EM, et al. Anticoagulation with the oral direct thrombin inhibitor dabigatran does not enlarge hematoma volume in experimental intracerebral hemorrhage. Circulation 2011;124:1654–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Won SY, Schlunk F, Dinkel J, et al. Imaging of contrast medium extravasation in anticoagulation-associated intracerebral hemorrhage with dual-energy computed tomography. Stroke 2013;44:2883–2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tempaku A. Intracranial hemorrhage during administration of a novel oral anticoagulant. J Rural Med 2016;11:69–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morrison SA, Jesty J. Tissue factor-dependent activation of tritium-labeled factor IX and factor X in human plasma. Blood 1984;63:1338–1347. [PubMed] [Google Scholar]
- 28.Ieko M, Naitoh S, Yoshida M, Takahashi N. Profiles of direct oral anticoagulants and clinical usage-dosage and dose regimen differences. J Intensive Care 2016;4:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hemphill JC III, Greenberg SM, Anderson CS, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2015;46:2032–2060. [DOI] [PubMed] [Google Scholar]
- 30.Berwaerts J, Dijkhuizen RS, Robb OJ, Webster J. Prediction of functional outcome and in-hospital mortality after admission with oral anticoagulant-related intracerebral hemorrhage. Stroke 2000;31:2558–2562. [DOI] [PubMed] [Google Scholar]
- 31.Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G. Volume of intracerebral hemorrhage: a powerful and easy-to-use predictor of 30-day mortality. Stroke 1993;24:987–993. [DOI] [PubMed] [Google Scholar]
- 32.Toyoda K, Yasaka M, Nagata K, et al. Antithrombotic therapy influences location, enlargement, and mortality from intracerebral hemorrhage: The Bleeding with Antithrombotic Therapy (BAT) Retrospective Study. Cerebrovasc Dis 2009;27:151–159. [DOI] [PubMed] [Google Scholar]
- 33.Purrucker JC, Haas K, Rizos T, et al. Early clinical and radiological course, management, and outcome of intracerebral hemorrhage related to new oral anticoagulants. JAMA Neurol 2016;73:169–177. [DOI] [PubMed] [Google Scholar]
- 34.Rost NS, Smith EE, Chang Y, et al. Prediction of functional outcome in patients with primary intracerebral hemorrhage: the FUNC score. Stroke 2008;39:2304–2309. [DOI] [PubMed] [Google Scholar]
- 35.Huttner HB, Schellinger PD, Hartmann M, et al. Hematoma growth and outcome in treated neurocritical care patients with intracerebral hemorrhage related to oral anticoagulant therapy: comparison of acute treatment strategies using vitamin K, fresh frozen plasma, and prothrombin complex concentrates. Stroke 2006;37:1465–1470. [DOI] [PubMed] [Google Scholar]
- 36.van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol 2010;9:167–176. [DOI] [PubMed] [Google Scholar]
- 37.Senoo K, Lau YC, Dzeshka M, Lane D, Okumura K, Lip GY. Efficacy and safety of non-vitamin K antagonist oral anticoagulants vs. warfarin in Japanese patients with atrial fibrillation: meta-analysis. Circ J 2015;79:339–345. [DOI] [PubMed] [Google Scholar]
- 38.Yasaka M, Lip GY. Stroke prevention in Asian patients with atrial fibrillation. Stroke 2014;45:1608–1609. [DOI] [PubMed] [Google Scholar]
- 39.Akao M, Chun YH, Esato M, et al. Inappropriate use of oral anticoagulants for patients with atrial fibrillation. Circ J 2014;78:2166–2172. [DOI] [PubMed] [Google Scholar]
- 40.Ma J, Li H, Liu Y, You C, Huang S, Ma L. Effects of intensive blood pressure lowering on intracerebral hemorrhage outcomes: a meta-analysis of randomized controlled trials. Turk Neurosurg 2015;25:544–551. [DOI] [PubMed] [Google Scholar]
- 41.Makino J, Fujitani S, Twohig B, Krasnica S, Oropello J. End-of-life considerations in the ICU in Japan: ethical and legal perspectives. J Intensive Care 2014;2:9. [DOI] [PMC free article] [PubMed] [Google Scholar]


