Abstract
Objective
To disentangle the different forms of postural tremors in Parkinson disease (PD).
Methods
In this combined observational and intervention study, we measured resting and postural tremor characteristics in 73 patients with tremulous PD by using EMG of forearm muscles. Patients were measured both “off” medication (overnight withdrawal) and after dispersible levodopa-benserazide 200/50 mg. We performed an automated 2-step cluster analysis on 3 postural tremor characteristics: the frequency difference with resting tremor, the degree of tremor suppression after posturing, and the dopamine response.
Results
The cluster analysis revealed 2 distinct postural tremor phenotypes: 81% had re-emergent tremor (amplitude suppression, frequency difference with resting tremor 0.4 Hz, clear dopamine response) and 19% had pure postural tremor (no amplitude suppression, frequency difference with resting tremor 3.5 Hz, no dopamine response). This finding was manually validated (accuracy of 93%). Pure postural tremor was not associated with clinical signs of essential tremor or dystonia, and it was not influenced by weighing.
Conclusion
There are 2 distinct postural tremor phenotypes in PD, which have a different pathophysiology and require different treatment. Re-emergent tremor is a continuation of resting tremor during stable posturing, and it has a dopaminergic basis. Pure postural tremor is a less common type of tremor that is inherent to PD, but has a largely nondopaminergic basis.
Parkinsonian tremor classically occurs at rest, but many patients (46%–93%)1–3 also have postural tremor. The exact phenomenology and etiology remain unclear.4 This is largely because postural tremor in Parkinson disease (PD) is highly heterogeneous in appearance and origin.5 We aim to disentangle the different postural tremors in PD, using a combination of clinical, electrophysiologic, and pharmacologic tremor features.
The most common postural tremor in PD is re-emergent tremor, which occurs in approximately two-thirds of patients,3,6 and has been suggested to be an “extension of the resting tremor that has reset itself after a brief latency.”7 However, other postural tremors also occur in PD.5 First, given the high prevalence of dystonic symptoms in PD8 and the clear link between idiopathic dystonia and tremor,9 some postural tremors in PD may have a dystonic origin. Second, given the increased co-occurrence of essential tremor (ET) and PD,10 it is conceivable that patients with PD with a bilateral postural arm tremor also have ET. Finally, since 8% of healthy elderly people have an enhanced physiologic tremor that is electrophysiologically similar to mild ET,11 a comparable proportion of patients with PD may also have this type of tremor.
The correct classification of postural tremors in PD is important for 2 reasons. First, similar to ET—which has been called a “family of tremors”—pooling of different postural tremors may lead to inconsistent findings across studies.12 Second, the correct postural tremor classification has clinical relevance, since different postural tremors may respond differently to pharmacologic treatments.4 Specifically, while re-emergent tremor likely responds to dopaminergic medication, other postural tremors may rather respond to β-blockers or primidone, given its phenomenologic resemblance to ET.13–15
We investigated the clinical phenomenology and dopaminergic basis of PD postural tremor by considering 3 important tremor characteristics: (1) the frequency difference with resting tremor (which is larger when 2 tremors have a different origin)16; (2) the onset of tremor after posturing (which is typically delayed in re-emergent tremor)6,17; (3) the response of tremor to levodopa. We used an automated cluster analysis, which was clinically validated, to dissect postural tremor in 2 phenotypes: re-emergent tremor and “pure postural tremor.” Furthermore, we compared clinical characteristics between both tremor phenotypes.
Methods
Study population
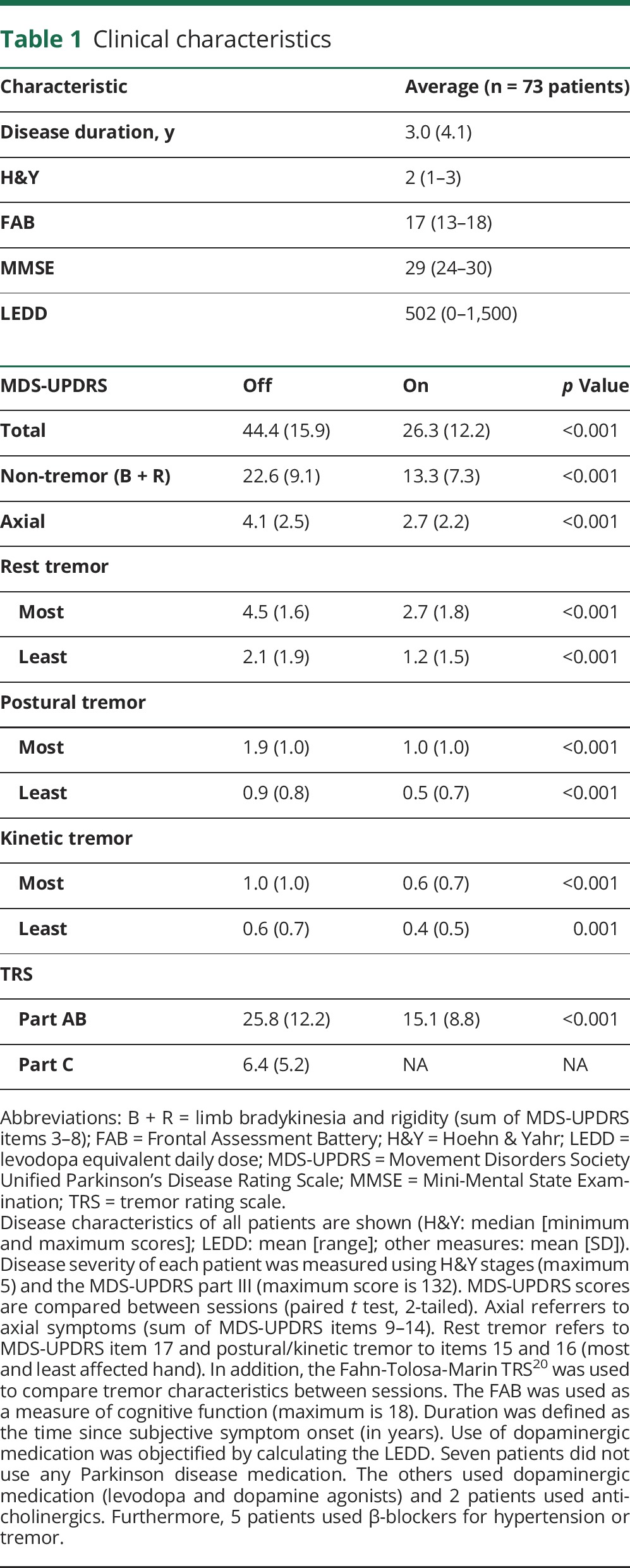
We included 77 patients (55 men; mean age 63 years; range 38–81 years) with idiopathic PD and a history of resting tremor, diagnosed according to the UK Brain Bank criteria by an experienced movement disorders specialist. Patients with neurologic comorbidity, signs of psychogenic tremor, or substantial cognitive impairment (Mini-Mental State Examination score <24 or Frontal Assessment Battery <12) were excluded. In total, 4 patients were excluded: 1 patient due to signs of psychogenic tremor and 3 due to noisy data recordings. This resulted in 73 patients for our main analyses (table 1).
Table 1.
Clinical characteristics
Standard protocol approvals, registrations, and patient consents
The study was conducted according to the standards of the 1964 Declaration of Helsinki and was approved by the local ethics committee. Before inclusion, all patients provided their written consent.
Design
Patients were measured twice on one day at the Radboud University Medical Center: first in a practically defined “off” state (after overnight fasting; >12 hours after the last dose of dopaminergic medication; >24 hours after anticholinergics or β-blockers)18 and then “on” levodopa (after 200/50 mg dispersible levodopa-benserazide19; on average 44 minutes after intake [range 32–59], when patients experience the maximal benefit of the levodopa treatment). In addition, 10 mg domperidone was given (to reduce side effects and enhance gastrointestinal absorption). Measurements took place from July 2014 until February 2016.
Clinical tremor assessment
Patients were clinically assessed using the Movement Disorders Society Unified Parkinson's Disease Rating Scale (MDS-UPDRS), Hoehn & Yahr Scale, and Fahn-Tolosa-Marin Tremor Rating Scale.20 We used item 15 of the MDS-UPDRS part III for postural tremor and part 17 for resting tremor.
Electrophysiologic tremor assessment
Patients lay on a bed during all electrophysiologic measurements, while their forearms rested on a pillow, thereby leaving the hands hanging unsupported to measure maximum tremor amplitude. Four conditions were used: rest tremor (rest) and 3 postural tremor measures (posh, post, and weight). For the postural conditions, patients received a verbal start signal, after which they (1) extended their wrists/fingers into a horizontal plane (posh), (2) lifted and stretched out both arms at the shoulder level into the horizontal plane with extension of the wrists/fingers (post), and (3) same as post but while wearing a 0.5-kg wristband (weight). Each condition lasted 1 minute and was repeated 3 times for rest, 2 times for posh/post, and once for weight. Trials were averaged per condition.
Tremor characteristics were quantified using EMG. We placed surface electrodes (sampling rate 512 Hz) on the tendons (reference) and belly (signal) of the extensor digitorum communis (EDC) and flexor carpi radialis (FCR) muscles. Preprocessing included (1) detrending to remove temporal drifts; (2) bandpass filtering between 20 and 200 Hz to only include muscle signal; (3) rectification to capture the frequency of muscle bursts; (4) high-pass filtering using a threshold of 2 Hz to remove slow frequency drifts; (5) averaging across EDC and FCR to obtain 1 EMG signal (after confirming that tremor characteristics were highly correlated between both muscles; appendix e-1 and e-2, links.lww.com/WNL/A294; figure e-1, links.lww.com/WNL/A295).
Using FieldTrip,21 we calculated time frequency representations (TFR) between 1 and 16 Hz in steps of 0.1 seconds using a Hanning taper with a duration (time interval) of 5 seconds, resulting in a 0.2-Hz spectral resolution. Thus, we determined tremor frequency in rest and postural conditions (figure 1A). Subsequently, we repeated our TFR analyses using a Hanning taper with a duration of 2 seconds, resulting in a 0.5-Hz spectral resolution and a higher temporal resolution. This allowed us to test the effect of wrist extension on tremor power. We applied a log-transformation, given the log-linear relationship between tremor power and clinical rating scales.22,23
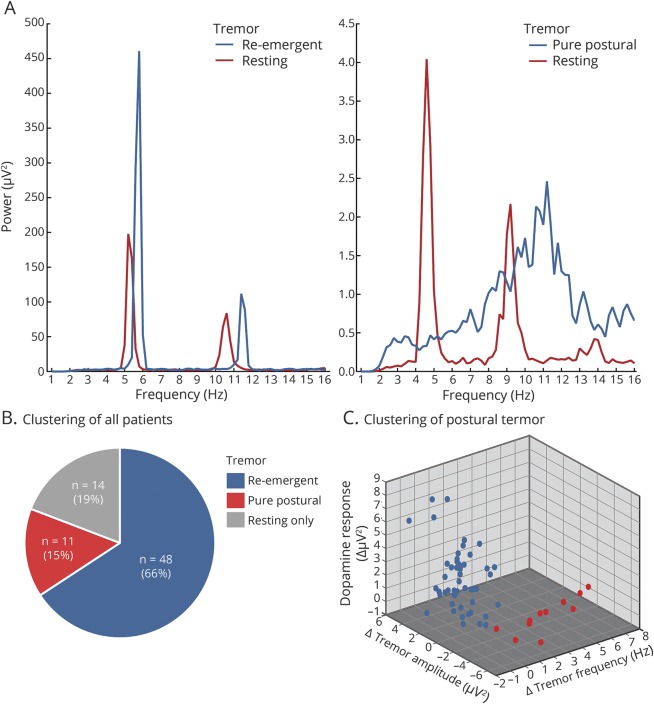
Figure 1. Example power spectra and participant classification.
Power spectrum example of a patient with a re-emergent tremor (left panel in A) and an example of a patient with pure postural tremor (right panel in A; downscaled with factor 3 for illustration purposes). (B) Overview of all patients demonstrating the number of patients without a postural tremor (gray piece) and division of patients with a postural tremor according to the 2-step clustering (blue and red pieces). (C) Values of the variables used for clustering. Blue data points indicate patients from the re-emergent tremor cluster, whereas red indicates patients from the pure postural tremor cluster.
Classification of tremor
To classify postural tremor, we selected 3 continuous variables derived from our electrophysiologic measurements. First, we calculated the difference in frequency between postural and rest tremor (Δ frequency, posh − rest). This criterion is based on the 1998 Consensus Statement of the Movement Disorders Society, which considers postural tremor separate from resting tremor if their frequencies differ more than 1.5 Hz.16 Second, we calculated the effect of wrist extension on tremor amplitude (Δ amplitude [mean power −3 until −1 second before onset posture] − [mean power 1 until 3 seconds after onset posture]). This criterion is based on the finding that PD resting tremor is suppressed by a voluntary action, and often emerges shortly afterwards during stable posturing.6,17 Third, we calculated the dopamine response of the postural tremor, i.e., the difference between the power at each participant's individual peak tremor frequency in the “off” minus “on” state. This criterion is based on reports that, on average, re-emergent tremor responds to dopamine, while some action tremors do not.15,24
Next, we used a 2-step clustering analysis (IBM [Armonk, NY] SPSS Statistics 22) to distinguish between different types of postural tremor, based on these 3 variables. This analysis identifies clusters using nonhierarchical preclustering and subsequently traditional hierarchical methods for definitive clustering. We calculated the log-likelihood of each model using the Bayesian Information Criterion (BIC) with no constraint on the number of clusters possible. Finally, we manually validated the outcome of the cluster analysis by characterizing the postural tremor in each patient, using the 3 measures above in a blinded fashion (appendix e-3, links.lww.com/WNL/A294; table e-1, links.lww.com/WNL/A296).
Statistical comparisons
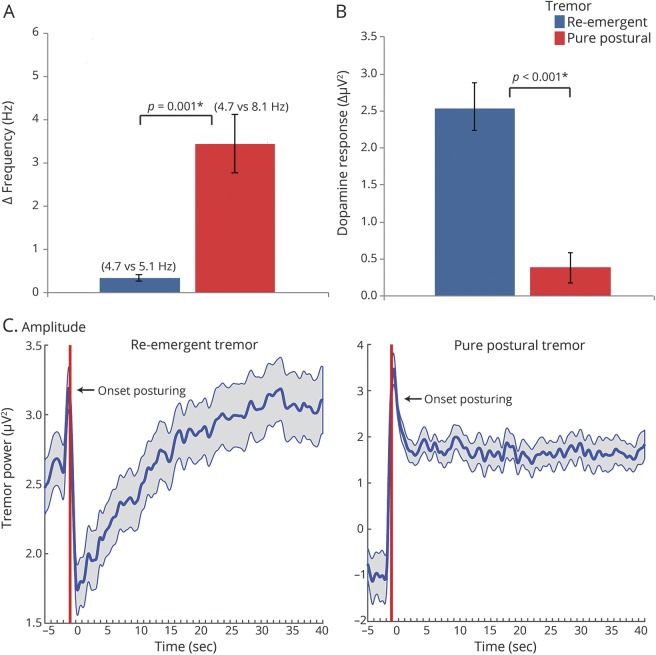
First, we compared the tremor measures outlined above between the 2 subgroups resulting from the cluster analysis, i.e., the difference between the power at each participant's individual peak tremor frequency in the “off” minus “on” state. Thus, we compared Δ frequency, Δ amplitude, and the dopamine response between subgroups using 2-sample t tests (figure 2, A and B). The transition from resting tremor to postural tremor (from −5 to +40 seconds relative to wrist extension) is shown in figure 2C.
Figure 2. Tremor subgroup comparison.
The difference between re-emergent and pure postural tremor for Δ frequency (A; postural minus rest tremor frequency), dopamine response (B; mean tremor power in “off” minus “on”), and the effect of wrist extension on tremor amplitude (C; log-transformed tremor power at individual tremor frequency ± 1.5 Hz to accommodate slight changes in tremor frequency).
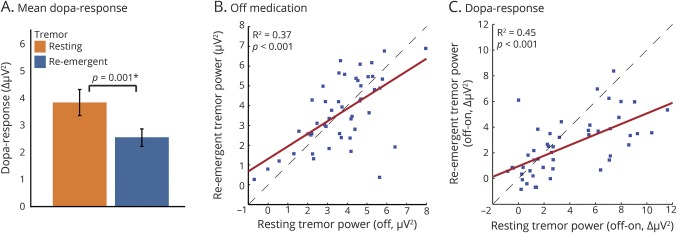
Second, we tested the hypothesis that in group 1 resting tremor and postural tremor share the same pathophysiology. Thus, in these patients we correlated the (log-transformed) power and dopamine response of rest tremor vs postural tremor (figure 3, B and C), while controlling for “off”-state tremor power (partial correlation). We also tested for differences in the dopamine response between postural and rest tremor, using repeated-measures analysis of variance.
Figure 3. Rest vs re-emergent tremor.
(A) Dopamine response (on the y-axis, tremor power “off” minus “on,” ± SEM) for resting and re-emergent tremor, with a larger dopamine response for rest tremor than for re-emergent tremor (same patient group). (B, C) Correlation of (log-transformed) tremor power (B) and dopamine response (log-transformed tremor power “off” minus “on” corrected for absolute “off” state tremor power, C) between resting tremor (x-axis) and re-emergent tremor (y-axis). The dashed lines indicate a reference line when x = y.
Third, we tested several hypotheses regarding the pathophysiology underlying postural tremor in group 2. We first tested whether weighing (post vs weight condition, paired t test) decreased tremor frequency, since this would point towards a peripheral rather than a central origin.11 Next, we tested whether the occurrence of specific clinical characteristics (i.e., dystonia, history of ET, disease severity) differed between both subgroups.
Results
Prevalence and classification of postural tremor
Out of the 73 included patients, 60 patients (82%) had a postural tremor (indicated by a peak in the power spectrum). One of the 60 patients had a very subtle rest tremor of the thumb and not the EDC/FCR muscle, so we excluded him from further analyses.
The cluster analysis revealed 2 separate groups of patients with very distinct postural tremors. The 2 clusters significantly differed on each of the 3 tremor characteristics (table 2). In cluster 1 (n = 48; 81%), there was a small but significant difference between the frequency of resting tremor and postural tremor (figure 3A); tremor amplitude significantly decreased after posturing (average duration from posture onset to time point where postural tremor power exceeded resting tremor power6: ±15 seconds, figure 2C), and levodopa significantly reduced tremor power (figure 2B). In cluster 2 (n = 11; 19%), there was a much larger difference between the frequency of resting tremor and postural tremor (figure 2A); there was no amplitude reduction upon posturing (figure 2C), and no dopamine response (figure 2B). Based on these findings, cluster 1 can be classified as re-emergent tremor6 and cluster 2 as pure postural tremor.16 It should be noted that patients with re-emergent tremor had a variable dopamine response (figure 1C). Thus, re-emergent tremor was not dopamine-responsive in all patients.
Table 2.
Clinical and electrophysiologic features per tremor subgroup
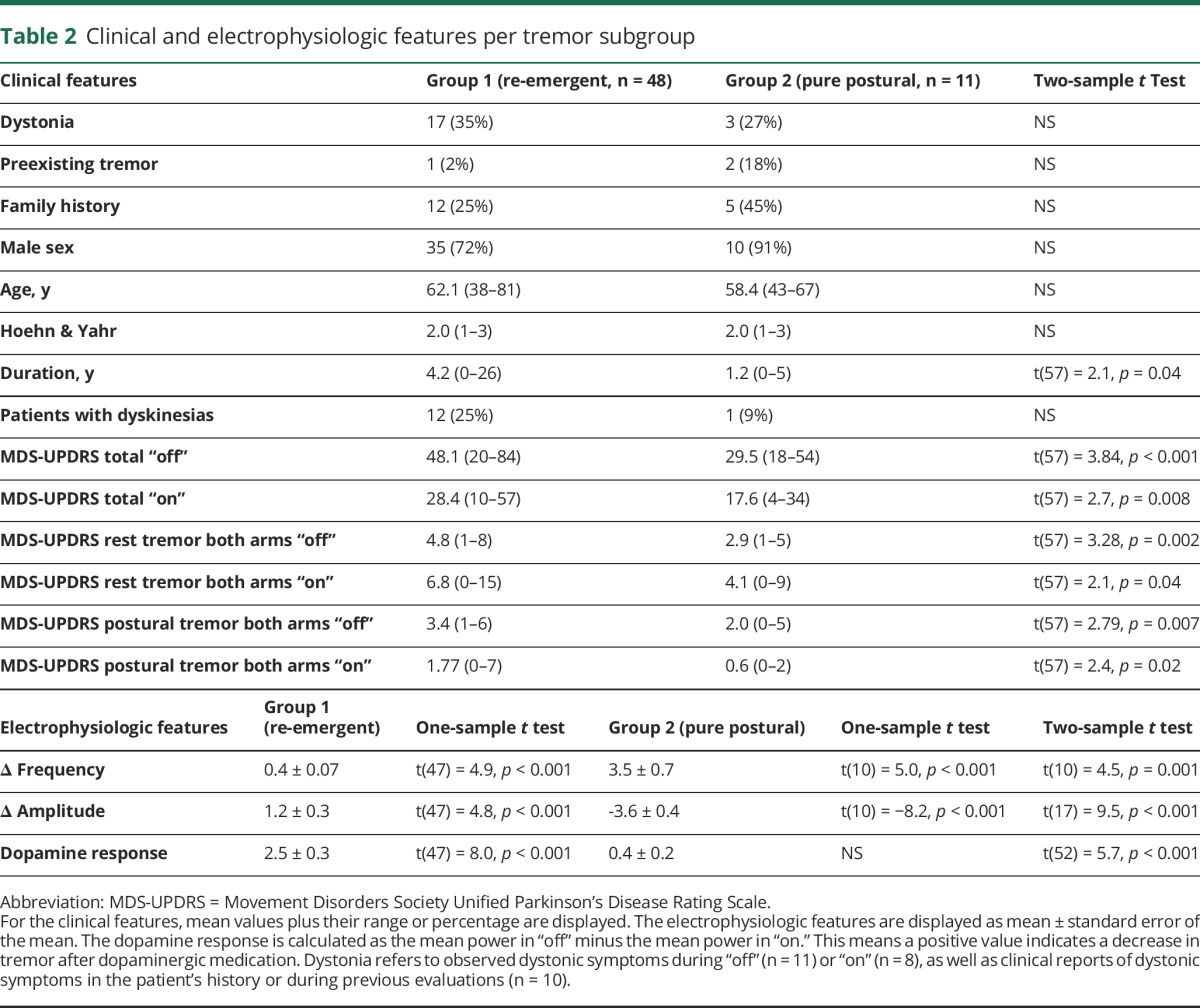
The evidence for a 2-cluster model was very strong: the Δ BIC was >10 for a 2-cluster model compared to a one-cluster model, and compared to any n-cluster model (with n > 3). This means that our model was >99% more likely than any other model.25 The Δ BIC for a 2-cluster model compared to a 3-cluster model was 5.0, meaning that our model was 75%–95% more likely.25 Exploration of the 3-cluster model revealed that this procedure divided cluster 1 (re-emergent tremor) into 2 subgroups: a dopamine-resistant group (n = 25; dopamine response 0.71 ± 0.17) and a dopamine-responsive group (n = 24; dopamine response 4.35 ± 0.35; 2-sample t test: p < 0.001) (see appendices e-1–e-3, links.lww.com/WNL/A294). This fits with the idea that the classical PD tremor is sometimes dopamine-resistant.26
The automated clustering was manually validated: 46 patients were classified as re-emergent and 13 as pure postural tremor (93% accordance with 2-cluster model; statistical comparisons described above were replicated). Differences could be explained by the strict cutoff values used for manual classification (see appendices e-1–e-3).
Comparison between re-emergent tremor and rest tremor
Within group 1, we compared resting tremor and re-emergent tremor characteristics. The power and dopamine response were highly correlated (power: R2 = 0.37; p < 0.001, figure 3B; dopamine response: R2 = 0.45; p < 0.001, figure 3C), which may suggest a common mechanism underlying both tremor types. However, there were also important differences: the dopamine response of resting tremor was significantly higher than that of re-emergent tremor (interaction between tremor type and levodopa; F1,47 = 11.7, p = 0.001; figure 3A). Furthermore, re-emergent tremor frequency was slightly (but significantly) higher than resting tremor frequency (4.7 vs 5.1 Hz, p < 0.001).
The nature of pure postural tremor in PD
First, we tested whether pure postural tremor may have a peripheral origin. Postural tremor frequency was stable across conditions (wrist extension [posh; frequency 8.1 ± 0.6 Hz] vs lifting of the arms [post; frequency 8.5 ± 0.7 Hz]; paired t test: t[10] = 1.4, p = 0.19). Importantly, weighing did not significantly alter tremor frequency (weight: 8.1 ± 0.5 Hz; difference between post vs weight: t[10] = 1.2, p = 0.27). This suggests that pure postural tremor has a central rather than a peripheral origin.11
Second, we tested whether pure postural tremor was related to specific clinical symptoms. There were no significant differences in the occurrence of dystonic posturing or a (family) history of ET (table 2). This indicates that pure postural tremor is not ET co-occurring in PD, or a form of dystonic tremor. The total MDS-UPDRS score and disease duration were significantly lower in the pure postural group (table 2). This suggests that pure postural tremor is a feature of early PD, or that a more severe tremor (such as re-emergent tremor) can “swamp” subtler postural tremors. Accordingly, the MDS-UPDRS postural and rest tremor scores were lower in the pure postural group (table 2).
Discussion
We investigated the nature of postural tremor in PD. The data show 2 distinct postural tremor phenotypes: re-emergent tremor (81%) and pure postural tremor (19%). Re-emergent tremor had a slightly higher frequency than resting tremor, a delayed onset after posturing, and on average a clear dopamine response (although individual responses varied considerably). Pure postural tremor had a higher frequency than resting tremor, occurred immediately after posturing, and did not respond to levodopa. Furthermore, pure postural tremor was not associated with dystonic symptoms or a history of ET. Taken together, we propose that both postural tremor types are inherent to PD, have distinct but central origins, and require different treatment.
The prevalence of re-emergent tremor (81% of patients with postural tremor) is similar to estimates from previous electrophysiologic studies (67%–75%),6,27 although one study reported a much lower prevalence (38%).28 It is, however, higher than estimates from clinical studies (30%–34%)3,29—which report a higher prevalence of non-re-emergent tremor (58%–59%).28,29 This discrepancy may be explained by reduced ability to clinically detect subtle tremor suppression and frequency changes, by differences in patient selection (previous studies included all PD phenotypes rather than tremor-dominant patients),3,29 and medication status (most previous studies were performed “on” dopaminergic medication).3,29
Re-emergent tremor intensity, and its response to levodopa, was highly correlated with that of resting tremor. This suggests that both tremor types are manifestations of a single underlying tremor mechanism. Accordingly, it has been suggested to label both tremors as tremor of stability,4 as both tremors occur in motorically stable contexts (at rest or during stable posturing) and are only transiently interrupted by voluntary movements.17 The reason why voluntary movements interrupt resting tremor remains a topic for future research.
There were also 2 relevant differences between both tremor types: re-emergent tremor had a slightly higher frequency and was less responsive to levodopa than resting tremor. Previous studies in smaller samples have shown nonsignificant trends in the same direction.6,28 Speculatively, these findings may be explained by the fact that a voluntary movement increases neural excitability within the cerebello-thalamo-cortical motor circuit, resulting in faster synaptic transmission (higher frequency). Also, the increased somatosensory input during posturing may produce reverberations within the cerebello-thalamo-cortical tremor circuit,30 making it less dependent from dopaminergic influences from the basal ganglia4,31 (reduced dopamine response).
Pure postural tremor had a much higher frequency than resting tremor and no dopamine response. This indicates that (partly) distinct neural mechanisms are involved in both tremors. The frequency difference fits with previous work, which described similar frequencies for PD resting tremor (5 Hz) and action tremor (9 Hz) as shown here.32 The absent dopamine response also fits with previous findings, which showed that the cerebellar pathway,33 and possibly serotonin depletion in the raphe,34 has a specific role in postural tremor—although these studies did not differentiate between postural tremor types.
We systematically tested several possible hypotheses regarding the nature of pure postural tremor in PD.5 First, postural tremor frequency was not lowered by weighing, making it unlikely that is has a peripheral (non-neuronal) origin. This fits previous observations that high-frequency action tremor in PD continued at the same frequency after anesthesia of the limb, suggestive of a central origin.32 However, 8% of healthy patients have enhanced physiologic tremor of central origin, with a frequency of 9–12 Hz (young patients) or 5–7 Hz (elderly). The prevalence of pure postural tremor in our sample was considerably higher (19% vs 8%), and the frequency was higher than in healthy elderly (8.1 vs 5–7 Hz). Thus, it is unlikely that pure postural tremor in PD reflects enhanced physiologic tremor of central origin. Second, the onset of pure postural tremor preceded the onset of PD symptoms in only 2 patients with pure postural tremor (18%), and in one patient with re-emergent tremor (2%), without a significant group difference. This makes it unlikely that pure postural tremor in our sample reflects co-occurring ET—which has been suggested to occur more often in PD than expected.10 Future research may test whether the tremor stability index, which differentiates ET from PD resting tremor,35 can also distinguish pure postural tremor from re-emergent tremor. Third, dystonic symptoms were as frequent in patients with pure postural tremor as in patients with re-emergent tremor. Taken together, we propose that pure postural tremor is inherent to PD, that (like rest and re-emergent tremor) it has a central origin, and that nondopaminergic systems may be involved in its pathophysiology.
Our findings show that levodopa is effective for treating a good portion of patients with re-emergent tremor, but not pure postural tremor. Besides dopaminergic medication, other pharmacologic treatments are sometimes used for treating PD tremor, including β-blockers, anticholinergics, and atypical neuroleptics (e.g., clozapine).36 Although a positive effect of these drugs on PD tremor has been described, most studies did not distinguish between rest and postural tremor,37–39 and even if they did,40 no distinction between pure postural and re-emergent tremor was made. As nondopaminergic systems may be involved in pure postural tremor, it may respond better to nondopaminergic treatments such as β-blockers. Future studies may focus on alternative treatment strategies for postural tremor, considering the distinct phenotypes described here.
We propose that PD harbors 2 distinct postural tremor phenotypes. Re-emergent tremor is a continuation of resting tremor during stable posturing, and it has a dopaminergic basis. Pure postural tremor is a second, less common tremor that is inherent to PD, but has a largely nondopaminergic basis.
Glossary
- BIC
Bayesian Information Criterion
- EDC
extensor digitorum communis
- ET
essential tremor
- FCR
flexor carpi radialis
- MDS-UPDRS
Movement Disorders Society Unified Parkinson's Disease Rating Scale
- PD
Parkinson disease
- TFR
time frequency representations
Footnotes
Editorial, page 581
Author contributions
M.F. Dirkx collected and analyzed the data and wrote a substantial part of the paper. Dr. Zach collected the data, analyzed a part of the data, and wrote a part of the paper. Dr. Hallett contributed to the construction of study design. Dr. Bloem contributed to construction of the study design. Dr. Helmich contributed to the construction of the study design and wrote a substantial part of the paper.
Study funding
M.D., R.C.H., and the study expenses were funded by a grant of the Dutch Brain Foundation (grant F2013[1]-15 to R.C.H). H.Z. was supported by the Erwin Schroedinger grant of the Austrian Science Fund (FWF: J-3723).
Disclosure
M. Dirkx and H. Zach report no disclosures relevant to the manuscript. B. Bloem reports personal fees from Nutricia, personal fees from Glaxo-Smith-Kline, personal fees from UCB, personal fees from Adamas, personal fees from TEVA, personal fees from Zambon, personal fees from AbbVie, grants from National Parkinson Foundation, grants from The Netherlands Organisation for Scientific Research, grants from Hersenstichting, grants from Michael J Fox foundation, and grants from Stichting Internationaal Parkinson Fonds, all outside the submitted work. M. Hallett and R. Helmich report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Gigante AF, Bruno G, Iliceto G, et al. Action tremor in Parkinson's disease: frequency and relationship to motor and non-motor signs. Eur J Neurol 2014;22:223–228. [DOI] [PubMed] [Google Scholar]
- 2.Koller WC, Vetere-Overfield B, Barter R. Tremors in early Parkinson's disease. Clin Neuropharmacol 1989;12:293–297. [DOI] [PubMed] [Google Scholar]
- 3.Louis ED, Levy G, Côte LJ, Mejia H, Fahn S, Marder K. Clinical correlates of action tremor in Parkinson disease. Arch Neurol 2001;58:1630–1634. [DOI] [PubMed] [Google Scholar]
- 4.Helmich RC, Hallett M, Deuschl G, Toni I, Bloem BR. Cerebral causes and consequences of parkinsonian resting tremor: a tale of two circuits? Brain 2012;135:3206–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hallett M, Deuschl G. Are we making progress in the understanding of tremor in Parkinson's disease? Ann Neurol 2010;68:780–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jankovic J, Schwartz KS, Ondo W. Re-emergent tremor of Parkinson's disease. J Neurol Neurosurg Psychiatr 1999;67:646–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hallett M. Parkinson's disease tremor: pathophysiology. Parkinsonism Relat Disord 2011;18:S85–S86. [DOI] [PubMed] [Google Scholar]
- 8.Jankovic J, Tintner R. Dystonia and parkinsonism. Parkinsonism Relat Disord 2001;8:109–121. [DOI] [PubMed] [Google Scholar]
- 9.Defazio G, Conte A, Gigante AF, Fabbrini G, Berardelli A. Is tremor in dystonia a phenotypic feature of dystonia? Neurology 2015;84:1053–1059. [DOI] [PubMed] [Google Scholar]
- 10.Thenganatt MA, Jankovic J. The relationship between essential tremor and Parkinson's disease. Parkinsonism Relat Disord 2016;22(suppl 1):S162–S165. [DOI] [PubMed] [Google Scholar]
- 11.Elble RJ. Characteristics of physiologic tremor in young and elderly adults. Clin Neurophysiol 2003;114:624–635. [DOI] [PubMed] [Google Scholar]
- 12.Louis ED. Essential tremors: a family of neurodegenerative disorders? Arch Neurol 2009;66:1202–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deuschl G, Raethjen J, Hellriegel H, Elble RJ. Treatment of patients with essential tremor. Lancet Neurol 2011;10:148–161. [DOI] [PubMed] [Google Scholar]
- 14.Caligiuri MP, Lohr JB. Worsening of postural tremor in patients with levodopa-induced dyskinesia. Clin Neuropharmacol 1993;16:244–250. [DOI] [PubMed] [Google Scholar]
- 15.Raethjen J, Pohle S, Govindan R, Morsnowski A, Wenzelburger R, Deuschl G. Parkinsonian action tremor: interference with object manipulation and lacking levodopa response. Exp Neurol 2005;194:151–160. [DOI] [PubMed] [Google Scholar]
- 16.Deuschl G, Bain P, Brin M. Consensus statement of the Movement Disorder Society on tremor: Ad Hoc Scientific Committee. Mov Disord 1998;13(suppl 3):2–23. [DOI] [PubMed] [Google Scholar]
- 17.Papengut F, Raethjen J, Binder A, Deuschl G. Rest tremor suppression may separate essential from Parkinsonian rest tremor. Parkinsonism Relat Disord 2013;19:693–697 Elsevier. [DOI] [PubMed] [Google Scholar]
- 18.Langston JW, Widner H, Goetz CG, et al. Core Assessment Program for Intracerebral Transplantations (CAPIT). Mov Disord 1992;7:2–13. [DOI] [PubMed] [Google Scholar]
- 19.Albanese A, Bonuccelli U, Brefel C, et al. Consensus statement on the role of acute dopaminergic challenge in Parkinson's disease. Mov Disord 2001;16:197–201. [DOI] [PubMed] [Google Scholar]
- 20.Fahn S, Tolosa E, Marin C. Clinical rating scale for tremor. In: Parkinson's Disease and Movement Disorders. Baltimore: Urban & Schwarzenberg; 1988:225–234. [Google Scholar]
- 21.Oostenveld R, Fries P, Maris E, Schoffelen J-M. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci 2011;2011:156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elble RJ. Tremor amplitude is logarithmically related to 4- and 5-point tremor rating scales. Brain 2006;129:2660–2666. [DOI] [PubMed] [Google Scholar]
- 23.Haubenberger D, Abbruzzese G, Bain PG, et al. Transducer-based evaluation of tremor. Mov Disord 2016;31:1327–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sturman MM. Effects of subthalamic nucleus stimulation and medication on resting and postural tremor in Parkinson's disease. Brain 2004;127:2131–2143. [DOI] [PubMed] [Google Scholar]
- 25.Raftery AE. Bayesian model selection in social research. Sociological Methodol 1995;25:111. [Google Scholar]
- 26.Helmich R, Dirkx M. Pathophysiology and management of parkinsonian tremor. Semin Neurol 2017;37:127–134. [DOI] [PubMed] [Google Scholar]
- 27.Schwingenschuh P, Ruge D, Edwards MJ, et al. Distinguishing SWEDDs patients with asymmetric resting tremor from Parkinson's disease: a clinical and electrophysiological study. Mov Disord 2010;25:560–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mailankody P, Thennarasu K, Nagaraju BC, Yadav R, Pal PK. Re-emergent tremor in Parkinson's disease: a clinical and electromyographic study. J Neurol Sci 2016;366:33–36. [DOI] [PubMed] [Google Scholar]
- 29.Belvisi D, Conte A, Bologna M, et al. Re-emergent tremor in Parkinson's disease. Parkinsonism Relat Disord 2017;36:41–46. [DOI] [PubMed] [Google Scholar]
- 30.Volkmann J, Joliot M, Mogilner A, et al. Central motor loop oscillations in parkinsonian resting tremor revealed by magnetoencephalography. Neurology 1996;46:1359–1370. [DOI] [PubMed] [Google Scholar]
- 31.Dirkx MF, Ouden den H, Aarts E, et al. The cerebral network of Parkinson's tremor: an effective connectivity fMRI study. J Neurosci 2016;36:5362–5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lance JW, Schwab RS, Peterson EA. Action tremor and the cogwheel phenomenon in Parkinson's disease. Brain 1963;86:95–110. [DOI] [PubMed] [Google Scholar]
- 33.Ni Z, Pinto AD, Lang AE, Chen R. Involvement of the cerebellothalamocortical pathway in Parkinson disease. Ann Neurol 2010;68:816–824. [DOI] [PubMed] [Google Scholar]
- 34.Loane C, Wu K, Bain P, Brooks DJ, Piccini P, Politis M. Serotonergic loss in motor circuitries correlates with severity of action-postural tremor in PD. Neurology 2013;80:1850–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.di Biase L, Brittain J-S, Shah SA, et al. Tremor stability index: a new tool for differential diagnosis in tremor syndromes. Brain 2017;140:1977–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Connolly BS, Lang AE. Pharmacological treatment of Parkinson disease. JAMA 2014;311:1670. [DOI] [PubMed] [Google Scholar]
- 37.Katzenschlager R, Sampaio C, Costa J, Lees A. Anticholinergics for symptomatic management of Parkinson's disease. Cochrane Database Syst Rev 2003;CD003735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crosby NJ, Deane KHO, Clarke CE. Beta-blocker therapy for tremor in Parkinson's disease. Cochrane Database Syst Rev 2003;15:CD003361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Friedman JH, Koller WC, Lannon MC, Busenbark K, Swanson-Hyland E, Smith D. Benztropine versus clozapine for the treatment of tremor in Parkinson's disease. Neurology 1997;48:1077–1081. [DOI] [PubMed] [Google Scholar]
- 40.Henderson JM, Yiannikas C, Morris JG, Einstein R, Jackson D, Byth K. Postural tremor of Parkinson's disease. Clin Neuropharmacol 1994;17:277–285. [DOI] [PubMed] [Google Scholar]