Abstract
INTRODUCTION
Although Aβ is cleared from brain to CSF and the peripheral circulation, mechanisms for its removal from blood remain unresolved. Primates have uniquely evolved a highly effective peripheral clearance mechanism for pathogens, immune adherence, in which erythrocyte complement receptor 1 (CR1) plays a major role.
METHODS
Multidisciplinary methods were employed to demonstrate immune adherence capture of Aβ by erythrocytes and its deficiency in Alzheimer’s disease (AD).
RESULTS
Aβ was shown to be subject to immune adherence at every step in the pathway. Aβ dose-dependently activated serum complement. Complement-opsonized Aβ was captured by erythrocytes via CR1. Erythrocytes, Aβ, and hepatic Kupffer cells co-localized in human liver. Significant deficits in erythrocyte Aβ were found in AD and MCI patients.
DISCUSSION
CR1 polymorphisms elevate AD risk and >80% of human CR1 is vested in erythrocytes to subserve immune adherence. The present results suggest that this pathway is pathophysiologically relevant in AD.
Keywords: Alzheimer’s disease, amyloid β peptide, complement, complement receptor 1, immune adherence, blood, erythrocyte, human
1. Background
Multiple studies have made clear that amyloid β peptide (Aβ) can move from brain to the peripheral circulation [e.g., 1–3] and from the peripheral circulation to brain [e.g., 4,5]. As such, the disposition of circulating Aβ may be pathophysiologically important. For example, failure to clear Aβ from blood could lead to an unfavorable concentration gradient for movement of Aβ out of the brain [3]. Moreover, the propensity of fluid-phase Aβ to form insoluble fibrils and to activate complement and other inflammatory mediators could well play a role in the co-localization of inflammatory mediators with the vascular abnormalities that are observed in Alzheimer’s disease (AD) [reviewed in 6,7]. Mackic and colleagues [8,9] have provided critical data on serum and organ levels of Aβ after its intravenous inoculation into the bloodstream of non-human primates (NHPs). However, the mechanisms by which Aβ is purged from the circulation in primates still remain unclear.
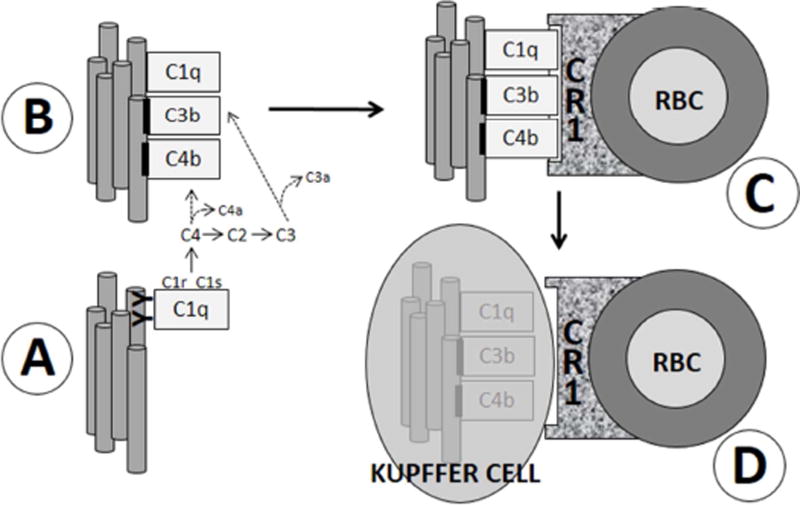
Originally elucidated by Nelson et al. [10] in 1953, complement-dependent, erythrocyte-mediated clearance of circulating immune complexes (“immune adherence”) has been investigated in detail for over 60 years, and is now considered a primary mechanism for pathogen removal in humans [reviewed in 11,12]. Fig. 1 illustrates some of the major steps in this pathway, several points of which may be worth emphasizing.
Fig. 1. Simplified schematic of classical pathway complement activation and immune adherence.
(A) An epitope on pathogens (gray tubes) is bound by circulating antibodies (YY) specific to it. C1, the first component of the classical complement pathway, then binds to closely-apposed antibodies, forming an immune complex (IC). Notably, like certain bacterial and tumor antigens [24], Aβ has been shown to bind C1 [23] and to induce activation of the C1r and C1s proteases without antibody mediation [20–23]. C1s-mediated activation of the classical complement pathway ensues, including generation of C4b, C3b, and iC3b, which become covalently fixed to the antigen (black bars). C1q also remains bound to the antigen. The antigen and/or IC is therefore said to be “opsonized” by complement (B). C) Primate (but not subprimate) erythrocytes (RBC) express cell-surface CR1, which has C4b, C3b, and C1q as ligands. Antigen/complement complexes thus become bound to erythrocytes. D) Erythrocytes then ferry the complex through the bloodstream until they reach specialized macrophages, Kupffer cells, lining the hepatic sinusoids. Kupffer cells recognize the complement tag via cell surface CRIg receptors and strip off and degrade the opsonized antigen [11,12,43].
First, in humans immune adherence hinges on the expression of complement receptor 1 (CR1) by erythrocytes, a phenomenon that is unique to primates. Subprimate erythrocytes do express complement receptors (e.g., Crry), but not CR1, so that their capacity to capture complement-opsonized immune complexes appears to be significantly limited compared to human immune adherence mechanisms [13].
Second, polymorphisms in the CR1 gene have been consistently shown to be among the top genetic risk factors for AD [14–18], and >80% of human CR1 is devoted to the erythrocyte compartment [11, 12]. Taken together with the unique expression of CR1 by primate erythrocytes, these findings make erythrocytes perhaps the most parsimonious site for CR1 to impact AD risk.
Third, although human erythrocytes only express some 200–1500 CR1 molecules per red cell [11], the sheer number of erythrocytes (2–3 × 1013) in the bloodstream, compared to circulating and fixed macrophages, makes this an extremely powerful and efficient pathway for pathogen clearance. For example, pathogens experimentally infused into non-human primates that have been immunized against the pathogen are typically eliminated by immune adherence mechanisms in 10–20 min [19].
Fourth, immune adherence research has focused on the clearance of immune complexes [11,12]. However, our research [20,21] and that of others [22,23] has shown that Aβ, like certain bacterial and viral antigens [24], does not require immune complex formation in order to activate complement or to be bound by complement opsonins that serve as ligands for immune adherence. Thus, Aβ (and other antibody-independent complement activators) may have been overlooked as a substrate for immune adherence pathways.
Our laboratory first suggested that immune adherence might play a role in peripheral Aβ clearance and that erythrocyte Aβ levels were significantly deficient in a small sample of AD and mild cognitive impairment (MCI) patients compared to nondemented elderly controls (ND) [25]. In the present study, we provide new and more definitive, multidisciplinary evidence of immune adherence reactions with Aβ, as well as confirmation, in a 140 patient cohort, of AD and MCI deficits in erythrocyte Aβ capture.
2. Methods
2.01 Human subjects
Under Institutional Review Board-approved protocols and consents, human intravenous (IV) blood samples were obtained prospectively from well-annotated, well-matched AD, MCI, and nondemented elderly (ND) subjects evaluated and diagnosed at a National Institute on Aging Alzheimer’s Disease Center, Banner Sun Health Research Institute, using standard NIA AD Center criteria. The AD group (N = 59) had a mean age of 80.1 ± 1.0 years (range 61–94 years), and consisted of 61% males and 39% females. The MCI group (N = 19) had a mean age of 80.1 ± 1.3 years (range 62–90 years) and consisted of 68% males and 32% females. The ND group (N = 62) had a mean age of 80.4 ± 1.5 years (range 50–95 years) and consisted of 48% males and 52% females. Routine autopsy samples of AD, Parkinson’s disease, and control liver were obtained from the Tissue Bank at Banner Sun Health Research Institute.
2.02 Non-human primates
Non-human primate (NHP) IV blood samples were obtained from two 16 year-old male Cynomolgus macaque monkeys and one 19 year-old Cynomolgus macaque under Institutional Animal Care and Use Committee-approved protocols.
2.03 Initial processing of blood samples
Serum from human and non-human primate subjects was obtained by drawing blood into Becton-Dickinson (Franklin Lakes, NJ) Serum Vacutainer tubes. After clotting for 30 minutes, the samples were centrifuged at 1,100 × g for 10 minutes at 4°C and serum was withdrawn and stored at −80°C. Plasma and erythrocytes were derived from blood drawn into Becton-Dickenson EDTA 2K Vacutainer tubes. The plasma/erythrocyte samples were immediately spun at 1,100 × g for 10 minutes at 4°C. Plasma and buffy coat were removed and the remaining erythrocytes were washed with 5 volumes of TBS. Plasma was stored at −80°C. Erythrocytes were used immediately or processed to erythrocyte membranes by lysis in 5 volumes of double-distilled (dd) H2O with 1x Protease Inhibitor Cocktail (PIC) (Roche, Basel, Switzerland) for 30 minutes at 4°C. Membranes were then pelleted in an ultracentrifuge by spinning for 40 minutes at 40,000 × g, washed in 5 volumes ddH2O with 1x PIC, centrifuged again at 40,000 × g, and either used immediately or flash-frozen and stored at −80°C for subsequent experimen ts.
2.04 Aβ preparation
Human synthetic Aβ40 and Aβ42 (Bachem, Torrence, CA, or Genscript, Piscataway, NJ) were solubilized in 100% DMSO at 10 mg/ml, gradually diluted in ddH2O to 2 mg/ml, and then brought to a 1 mg/ml concentration in 0.1M Tris buffer (pH 7.4). The 1 mg/ml Aβ42 stock solution was aggregated overnight at room temperature with agitation. The 1 mg/ml Aβ40 stock solution was aggregated for 3 days at 37°C without agitation.
2.05 Complement activation
To assess dose-dependent Aβ complement activation, Aβ40 or Aβ42 was diluted with 0.1M Tris buffer to various concentrations, then incubated with an equal volume of normal human serum (NHS) (Complement Technology, Tyler, TX) that had been diluted 1:5 in veronal-buffered saline containing calcium and magnesium (pH 7.4) for 1 hour at 37°C. To demonstrate specificity, 10 mM EDTA, which blocks complement activation, was added to Aβ/NHS control wells immediately before the incubation. To halt further activation, 10mM EDTA was also added to all samples following incubation. Complement activation was assessed by measurement of C3a or sC5b-9 formation using Quidel (San Diego, CA) Human C3a or Human sC5b-9 ELISA kits. All assays were performed according to the protocols provided by the manufacturer. Conventional assays of complement activation, particularly with antibody-independent activators, typically require very high concentrations of activator under in vitro conditions, as here and in virtually all previous studies of complement interactions with Aβ [c.f., 20–23,25,26].
2.06 Binding of Aβ by complement opsonins
NHS was incubated with Aβ42, as above, to permit complement activation, generation of complement opsonins, and their covalent binding to Aβ. Aβ/NHS solutions were then run on conventional, reducing, SDS/PAGE Western blots using anti-Aβ antibody 6E10 (Biolegend, San Diego, CA) or an antibody directed at C3b (Quidel) or iC3b (Quidel). In our hands, the iC3b antibody also reacts with purified C3 and its two major chains, C3α and C3β, which are produced under SDS/reducing conditions. As a control to block complement activation and opsonization of Aβ, 10 mM EDTA was added to NHS prior to incubation with Aβ.
Opsonization of Aβ was also studied in vivo. Here, a 19 year-old male Cynomolgus macaque was anesthetized with telazol (5 mg/kg, intramuscular) and infused IV with 61 µg/kg Aβ42 diluted in 0.9% sterile saline solution. Femoral artery blood samples were taken 20 minutes later and processed for plasma, as described above. After a 1:1 dilution with sterile ddH2O, the plasma preparations were incubated with either monoclonal anti-C3 antibody (Abcam, Cambridge, MA), which binds C3 and C3 split products (e.g., C3b, iC3b), or monoclonal anti-Aβ/APP 4G8 antibody (Covance, Princeton, NJ). Incubations were overnight at 4°C with gentle rocking. The samples were then subjected to immunoprecipitation (IP) using Thermo Scientific (Rockford, IL) spin columns. To prepare each column, 50 µl of protein G sepharose 4 fast flow (GE Healthcare Life Sciences, Pittsburgh, PA) was added and the columns were pre-washed with 200 µl of IP wash buffer (Thermo Scientific). Samples were loaded into the columns and incubated for 3 hours at 4°C with gentle rocking to harvest immune complexes. The columns were washed three times with 200 µl of 1X IP lysis/wash buffer (Thermo Scientific), and were additionally washed with 100 µl of 1X conditioning buffer (Thermo Scientific). Aβ and C3b protein complexes were eluted with 50 µl of elution buffer (Thermo Scientific) and incubated for 5 minutes at room temperature before centrifugation at 1,000 × g, 4°C. To demonstrate complement opsonization of Aβ, the IP-bound fractions were boiled 5 minutes with Laemmli buffer containing 5% β-mercaptoethanol, then loaded into 4–20% Tris-glycine precast gels (Bio-Rad, Hercules, CA). Following electrophoresis, proteins were transferred to a PVDF membrane using the Trans-blot Turbo Transfer System (Bio-Rad) at 25 V for 30 minutes and blocked with Blotto blocking buffer (Thermo Scientific) overnight at 4°C. Membranes containing protein complexes that had been immunoprecipitated with the anti-Aβ antibody were immunoblotted with a 1:5,000 dilution of mouse monoclonal anti-C3b antibody directed at a neoepitope specific to C3b (Quidel) or with mouse monoclonal anti-Aβ/APP antibody 4G8 at a 1:1,000 dilution. Conversely, membranes with protein samples that had been immunoprecipitated with the anti-C3 antibody were immunoblotted with a 1:1,000 dilution of mouse monoclonal 4G8 or with mouse monoclonal anti-C3b neo-epitope-specific antibody at a 1:5,000 dilution. After four 5 minute washes with 1X PBS containing 0.1% Tween 20, the blots were incubated with a 1:10,000 dilution of anti-mouse Alexa Fluor 680 secondary antibody (Molecular Probes, Life Technologies, Grand Island, NY) and imaged using the Odyssey imaging system (LI-COR Biosciences, Lincoln, NE). Blots were then stripped and incubated with a mouse monoclonal antibody specific for β actin (Santa Cruz Biotechnology, Dallas, TX) as a control.
2.07 Erythrocyte capture of Aβ in vitro
To assess the ability of erythrocytes to take up complement-opsonized Aβ in blood via CR1-dependent mechanisms, two strategies were employed. First, 300 µl of NHS was incubated at 37°C for 1 hour with 300 µl of 20 µg/ml Aβ42 to permit complement activation and opsonization, then diluted to various concentrations in 1X TBS. The resulting NHS/Aβ42 solutions were incubated with 600 µl of packed erythrocytes (in TBS) at 37°C for 1 hour. After incubation, the mixtures were spun at 100 × g in a microfuge for 10 minutes, the supernatant was removed, and the erythrocytes were processed to erythrocyte membranes as described above in Section 2.03. For ELISA assay, 200 µl of the erythrocyte membranes were solubilized in 160 µl ddH2O containing PIC and 40 µl of 1% SDS for 30 minutes at room temperature. The SDS-solubilized membranes were then mixed 1:5 with Wako (Richmond, VA) ELISA sample buffer and assayed using Wako Human Aβ40 ELISA kits (#298-62301) or Wako Human Aβ42 ELISA kits (#298-62401). The manufacturer’s protocols were followed throughout.
A second strategy for demonstrating specificity to complement mechanisms employed a modified tip plate adhesion assay previously used to characterize erythrocyte CR1 binding to its ligands [27]. Here, 96-well Costar high-binding microplates (Corning, Corning, NY) were coated for 1 hour with aggregated Aβ42, diluted to various concentrations in 10 mM carbonate buffer (pH 9.6), then blocked using 0.5% PEG 3350 in 2/3 TBST (10 mM Tris pH 7.2, 100 mM NaCl, 0.05% Tween 20). Wells were subsequently exposed for 30 minutes, room temperature, to NHS to permit complement activation and binding (Complement Technology). To demonstrate that Aβ binding is mediated by complement opsonization, parallel wells were incubated with heat-inactivated NHS, C1q-depleted NHS (Complement Technology), C4-depleted NHS (Complement Technology), or EDTA + NHS, all of which block various stages of complement activation. Heat inactivation was for 30 minutes at 56°C. All sera were diluted 1:32 in Veronal buffered saline (with Mg++ and Ca++) (Complement Technology).
To evaluate binding, packed erythrocytes were diluted (1:2,667) to 375 ppm with adhesion buffer (8 mM Tris pH 7.4, 100 mM NaCl, 140 mM dextrose, 0.45 mM CaCl2, 0.17 mM MgCl2). From the diluted erythrocyte sample, 200 µl (~700,000 erythrocytes) was added to each well and incubated for 60 minutes at room temperature. The wells were subjected to gentle, continuous washing/aspiration using 2 ml of adhesion buffer followed by 4 ml of PBS per well.
To further demonstrate that Aβ binding to erythrocytes is dependent on CR1, suspensions of erythrocytes were blocked, prior to exposure to Aβ-coated plates, with 2.0 µl of 0.2 mg/ml anti-CR1 antibody J3D3 (Becton Coulter, Indianapolis, IN) or with 25 µl of 1 mg/ml recombinant C3b (Complement Technology) for 30 minutes, room temperature. Erythrocytes that remained bound to the plate were imaged with brightfield illumination at 100x using an inverted Olympus IX71 microscope (Olympus, Center Valley, PA) and quantified using investigator-independent ImageJ software. Erythrocyte counts were normalized to wells coated with anti-CR1 antibody to adjust for any minor differences in the number of erythrocytes/sample.
2.08 Erythrocyte capture of Aβ in vivo
Following telazol (5 mg/kg) intramuscular anesthesia, two 16 year-old male Cynomolgus macaques received 183 µg/kg or 366 µg/kg Aβ40, respectively, through an IV catheter inserted into the saphenous vein. IV blood samples were taken from the same catheter at various intervals from baseline to 60 minutes after Aβ infusion. After Aβ infusion and after each withdrawal, the cannula was thoroughly flushed in order to prevent contamination of subsequent samples. The blood samples were centrifuged at 1,100 × g for 10 min at 4°C to isolate erythrocytes. The erythrocytes were then lysed and their membranes solubilized and assayed using Wako Human Aβ40 ELISA kits, as described above.
2.09 Electron microscopy of liver samples
Liver samples from rapid (<4 hours) autopsies of AD, Parkinson’s disease, and ND patients were dissected and processed using standard immunohistochemical and ultrastructural methods, as previously described in detail by our laboratory [26]. Antibodies directed at CD68 (Abcam, Cambridge, UK), a marker for Kupffer cells, and anti-Aβ antibody 4G8 (Biolegend) were employed. Like most antibodies to Aβ, 4G8 also reacts with amyloid precursor protein (APP).
2.10 Aβ capture by AD, MCI, and ND erythrocytes
Erythrocyte samples from AD, MCI, and ND subjects were processed to erythrocyte membranes, as above, solubilized in SDS, and stored at −80°C. Plasma and the solubilized membranes were subsequently assayed for Aβ42 using a Covance (Princeton, NJ) BetaMark Aβ42 ELISA kit (now marketed by Biolegend).
2.11 Statistics
Parametric (ANOVA) and Pearson Correlation statistics were used throughout. P values are two-tailed.
3. Results
3.01 Aβ is an antibody-independent activator of complement
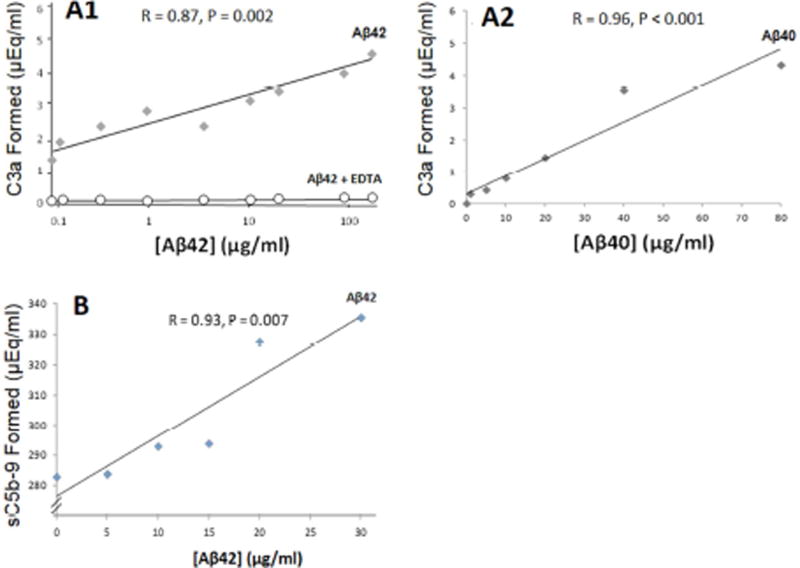
To be cleared by erythrocyte/CR1-mediated mechanisms, pathogens must first activate the complement cascade. Here, we show that such activation occurs for Aβ and is significantly dose dependent and complete through C5b-9 (R = 0.93, P = 0.007), the terminal step of the classical and alternative pathways, as well as C3a (Aβ40: R = 0.96, P < 0.001; Aβ42: R = 0.87, P = 0.002), the step in which C3b/iC3b complement opsonins are generated (Fig. 2). Addition of 10 mM EDTA, a standard inhibitor of complement activation, reduced activation to background (i.e., serum only).
Fig. 2. Antibody-independent activation of the complement cascade.
A1) Aggregated Aβ42 was incubated with NHS, then assayed by ELISA for production of C3a, a cleavage product generated from C3 following C3 activation. A significant dose-dependent response was obtained. Incubation of Aβ and serum with 10 mM EDTA, which blocks complement activation, abolished the response to Aβ and gave only background readings. A2) Aβ40 gave similar results. B) These findings were also extended to the terminal step in classical and alternative pathway activation, formation of C5b-9, the membrane attack complex, and its soluble form, sC5b-9. Significant dose-dependent activation was observed in all experiments.
3.02 Complement activation by Aβ results in its opsonization
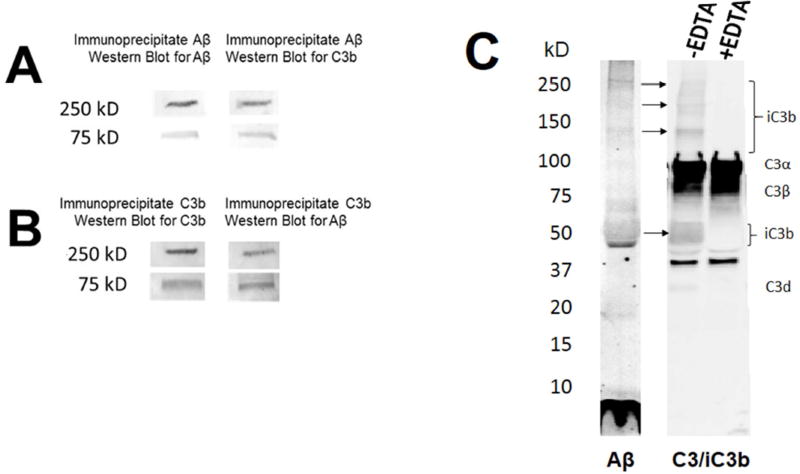
Substances that activate complement generate cleavage fragments of C3, including C3b, iC3b, and C4b, which bind back, covalently, to the activator and are said to “opsonize” it. Here, we show that Aβ intravenously-infused into a non-human primate can be retrieved by immunoprecipitation with an antibody to C3b, and, conversely, that putative C3 opsonins can be retrieved by immunoprecipitation with an antibody to Aβ. In both cases, two major bands were observed for the opsonins and Aβ, both of which co-localized at ~75 kD and >250 kD (Fig. 3A,3B). Importantly, these two bands were not observed with EDTA treatment, which abolishes complement activation and opsonization. These in vivo findings in a non-human primate extend two previous in vitro studies using human blood wherein co-localized bands for Aβ and C3b were also detected at high molecular weights on Western blots [22,28], consistent with the fact that only Aβ aggregates, particularly Aβ fibrils, activate complement [21]. In addition, co-localization at the same molecular weights after immunoprecipitation and the reducing/denaturing conditions of the Western blot strongly suggests that Aβ and C3b were covalently bound, a characteristic feature of complement opsonization.
Fig. 3. Complement opsonization of Aβ in blood.
A) In blood samples from a non-human primate inoculated with Aβ, immunoprecipitation with an anti-Aβ antibody retrieved two major bands of Aβ immunoreactivity at ~75 kD and >250 kD (left lane) and two major bands of putative C3b immunoreactivity at the same molecular weights (right lane). B) Likewise, immunoprecipitation with an anti-C3b antibody retrieved two major bands of Aβ immunoreactivity at ~75 kD and >250 kD (left lane) and two major bands of putative C3b immunoreactivity (right lane) at the same molecular weights. C) Western blot of Aβ incubated with NHS using an antibody directed against Aβ (left lane) and a Western blot of the same solution using an antibody that reacts with C3 and iC3b (right two lanes). C3 is abundantly present whether complement activation has occurred or not. In SDS/PAGE gels under reducing conditions, its two major, disulfide-linked chains, C3α and C3β, therefore dominate the gel, and, as endogenous constituents, are not affected by EDTA. By contrast, generation and covalent binding of iC3b to activating substrates such as Aβ requires complement activation and is sensitive to EDTA. Thus, putative immunoreactivity for iC3b and its fragments (brackets) is present when complement activation is permitted (−EDTA), and absent when activation is inhibited (+EDTA).
Binding of Aβ to a second complement opsonin, iC3b, was also demonstrated— here, in human blood samples exposed to Aβ in vitro. Under the reducing/denaturing conditions of the present experiment, iC3b is cleaved to 39, 63, and 75 kD fragments, and, like the C3b from which it derives, iC3b remains covalently bound to activators through a thioester bond. After incubation of Aβ42 with NHS to permit complement activation, Western blots for Aβ exhibited bands for Aβ monomer and multiple Aβ oligomer species (Fig. 3C, left lane), consistent with pre-aggregation of the peptide. Blots of the same solution that were immunoreacted for C3/iC3b (Fig. 3C, right lanes) showed bands parallel to those for Aβ at ~48–60 kD and >110 kD. These bands putatively represent iC3b fragments covalently bound to Aβ since they are absent in samples treated with EDTA, which blocks iC3b formation and opsonization, B) they remain present even under SDS/reducing conditions, consistent with the covalent binding of complement opsonins, and C) they are shifted up from the normal molecular weights for iC3b fragments to match corresponding bands for Aβ. As expected, there was also heavy labelling of bands corresponding to fragments of C3, one of the most abundant proteins in blood. For example, under SDS/reducing conditions, the two disulfide-linked chains that comprise C3 (C3α and C3β) are observed (Fig. 3C, right lanes). Because C3 is endogenous in NHS and does not require complement activation for its generation, immunoreactive bands at the normal molecular weights for C3α and C3β remain present in the blot regardless of whether or not EDTA is employed.
A faint band at approximately 34 kD was also detected, and may correspond to C3d, another C3 fragment generated by further cleavage of iC3b by the protease, Factor I. The band is at the correct molecular weight for C3d, and was abolished with EDTA treatment. Alternatively, the slow kinetics of C3d formation may not be consistent with the time scale of the present experiment [29]. Finally, the absence of any complement immunoreactivity associated with monomeric Aβ, despite the large amounts that were present, confirms our previous finding that Aβ monomer does not activate complement and that increasing fibrilization of Aβ enhances complement activation [21].
3.03 Erythrocytes capture Aβ through complement-dependent processes
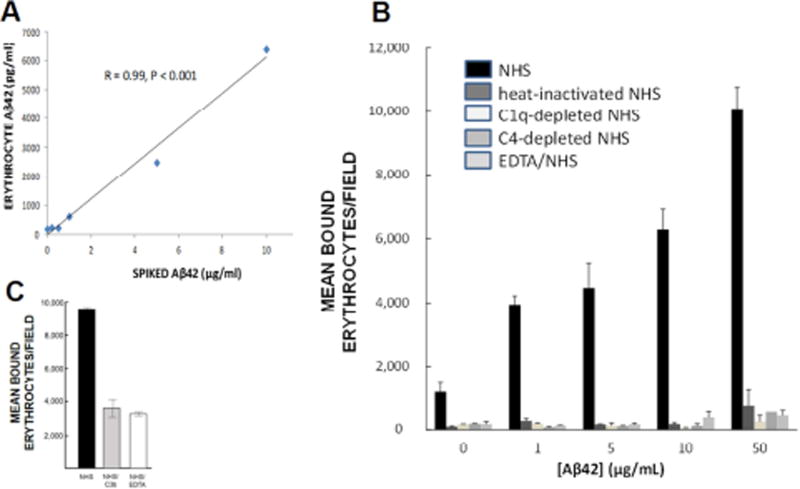
Once opsonized by complement, circulating pathogens in primates are bound by erythrocytes via CR1 expressed at the erythrocyte surface. When incubated with erythrocytes, Aβ that had been exposed to NHS to permit complement activation and opsonization was captured by the erythrocytes in a significant dose-dependent fashion (R = 0.99, P < 0.001) (Fig. 4A). To confirm this result using a second technique, and to assess its specificity with respect to CR1 and the different potential ligands for CR1 (i.e., C1q, C4b, C3b), we next performed erythrocyte tip plate adhesion assays that have been previously employed to assess CR1/ligand interactions [27]. Here, Aβ42 was coated to the bottom of wells, exposed to NHS (to permit complement activation and opsonization) or, as controls, exposed to C1-depleted NHS, C4-depleted NHS, EDTA-treated NHS, or heat-inactivated NHS, all of which inhibit complement activation and opsonization at different stages. Treated wells were then washed and incubated with erythrocytes, followed by washing to remove non-adherent cells. Incubation of Aβ42 with NHS produced significant dose-dependent adherence of erythrocytes to Aβ-coated wells (R = 0.91, P = 0.03) (Fig. 4B). By contrast, incubation of Aβ42 with heat-inactivated NHS or EDTA-treated NHS, which eliminate both alternative and classical pathway complement reactions and provide an estimate of non-complement-mediated mechanisms, reduced erythrocyte binding to Aβ-coated wells to background. C1-depleted serum, which abolishes classical complement pathway reactions but still permits C3b opsonization of Aβ via the alternative or lectin pathways, also reduced erythrocyte adherence to background. Likewise, C4-depleted serum, which eliminates classical pathway generation of C4b and C3b opsonins, reduced erythrocyte adherence to background (Fig. 4B). These findings show that erythrocyte/Aβ binding is predominantly mediated by classical complement pathway-dependent mechanisms (i.e., immune adherence) and depends on the generation of appropriate CR1 ligands.
Fig. 4. Erythrocyte binding and capture of Aβ.
A) Following complement activation, Aβ is opsonized, tagging it for immune adherence reactions with erythrocytes. Here, NHS was incubated with various concentrations of Aβ42 to permit complement activation and opsonization, then incubated with erythrocytes from the same subject. Erythrocytes captured the Aβ42 in a significant dose-dependent manner. B) When Aβ42 was incubated with NHS, permitting complement activation and opsonization of the Aβ, significant dose-dependent binding to erythrocytes was observed (R = 0.91, P = 0.03). By contrast, binding was reduced to background with heat-inactivated NHS, which abolishes complement reactions. To control for the possibility that heat inactivation might also somehow inhibit non-complement-mediated erythrocyte capture of Aβ, we also included use of C1-depleted and C4-depleted NHS, which are specific to complement reactions, as well as EDTA treatment, a standard inhibitor of complement reactions. These conditions also abolished erythrocyte binding to Aβ, showing that complement mediation and formation of CR1 ligands is a primary mechanism for Aβ binding to erythrocytes. C) An excess of C3b, one of the ligands for CR1, was used to block erythrocyte C3b/CR1 binding sites, resulting in 62% inhibition of erythrocyte adhesion to Aβ42-coated plates.
Specificity to CR1-mediated mechanisms was also explored by pre-incubating erythrocytes with anti-CR1 antibody J3D3 or recombinant C3b. J3D3 only blocks three of the four CR1 binding sites for complement-opsonized ligands (to our knowledge, no available antibody blocks all four sites), but nonetheless reduced erythrocyte/Aβ adhesion by 60% (F = 30.9, P = 0.03) (not shown). Likewise, recombinant C3b still permits CR1 binding to other complement opsonins (e.g., the CR1 LHR-A binding site preferentially captures C4b-opsonized substrates), but nonetheless reduced erythrocyte/Aβ adhesion by 62% (F = 52.1, P < 0.001) (Fig. 4C).
3.04 Erythrocyte capture of Aβ in vivo
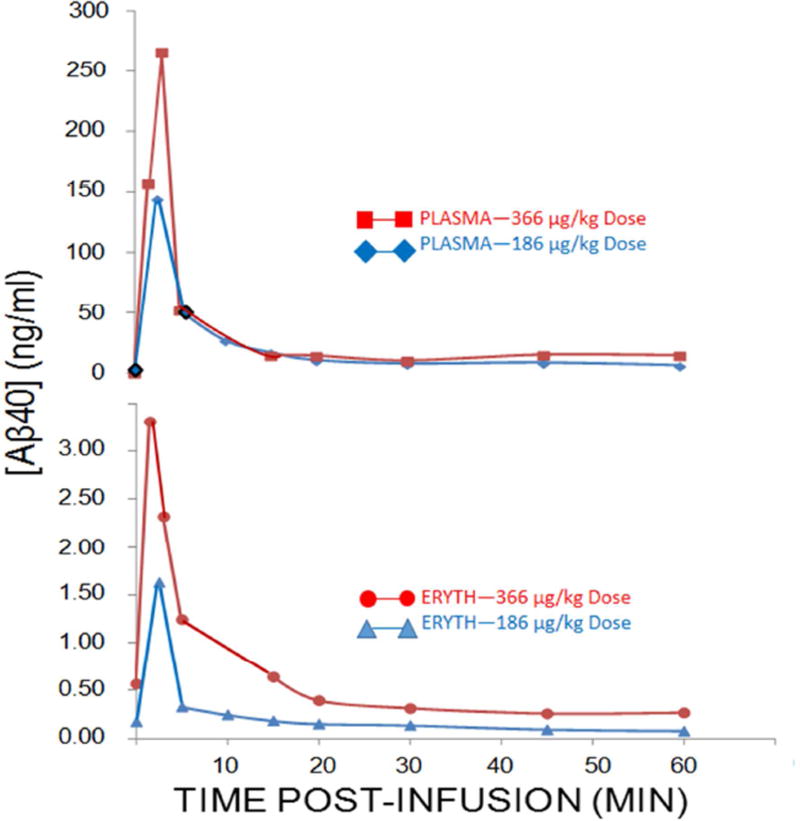
To extend the above findings to in vivo conditions, two Cynomolgus monkeys were infused with either 183 µg/kg Aβ40 or 366 µg/kg Aβ40. Saphenous vein blood samples were taken at baseline and at intervals from 2–60 minutes thereafter. Plasma and erythrocyte Aβ40 levels were tightly correlated (R = 0.98, P < 0.001 and R = 0.85, P = 0.004 for the 186 µg/kg and 366 µg/kg Aβ doses, respectively) (Fig. 5), with an immediate spike at 2.5 minutes and a return to near baseline within 20 minutes. These kinetics are comparable to previous studies of immune adherence with bacterial pathogens wherein >90% of plasma and erythrocyte clearance is observed within the first 10–20 minutes after intravenous injection [19]. Previous studies in monkeys by Mackic and colleagues [8,9] reported that some 97% of infused, radiolabeled Aβ40 was sequestered in other organs, including brain, with only ~3–4% retrievable in plasma. Our studies, using a direct ELISA assay of Aβ40, gave almost identical results, including the spike and rapid fall in plasma Aβ in the first 20 minutes after infusion. Clearance of Aβ through the erythrocyte pathway appeared to operate on demand, such that the higher, 366 µg/kg dose of Aβ was reduced to near baseline as quickly as the lower, 188 µg/kg dose. Although erythrocyte Aβ40 levels were typically only ~1–3% of plasma levels at any given time, the erythrocyte immune adherence pathway is clearly capable of clearing normal circulating levels of Aβ. For example, from 2.5 to 20 minutes after infusion of 366 µg/kg Aβ, 3 ng/ml Aβ was removed from the erythrocyte compartment, which is some 6-fold greater than typical blood Aβ levels in the monkeys (and humans).
Fig. 5. Plasma and erythrocyte Aβ concentrations after infusion of Aβ40 into a non-human primate.
Consistent with previous studies [8,9], plasma levels of Aβ40 spiked almost immediately after inoculation and rapidly declined over the next 15–20 minutes (top panel). Erythrocyte levels followed a nearly identical pattern and were significantly correlated with plasma levels at both doses of Aβ (bottom panel). Clearance from the erythrocyte pathway was rapid, also consistent with previous studies [19], and was capable of reducing the high dose of Aβ from nearly 10-fold normal levels to normal levels in 15–20 minutes (bottom panel).
3.05 Erythrocyte-mediated clearance of circulating Aβ to the liver
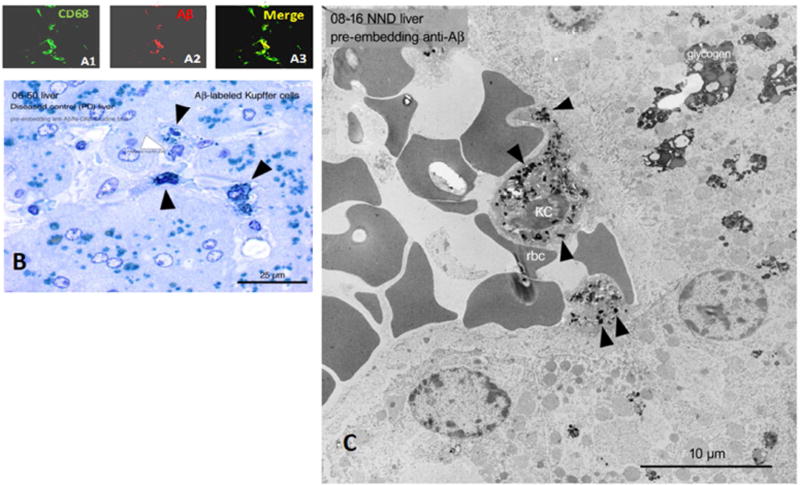
In primates, specialized macrophages called Kupffer cells line the hepatic sinusoids, where they recognize and strip off complement-tagged pathogens from erythrocytes [11,12]. Low power, high power, and electron micrographs of human AD, Parkinson’s disease, and ND liver reveal Aβ immunoreactivity in Kupffer cells co-localized with Aβ-immunoreactive erythrocytes within the sinusoid (Fig. 6). Notably, Aβ clearance to Kupffer cells was observed in all patient diagnostic conditions, suggesting that this pathway is normally used for peripheral clearance of Aβ, as it is for other toxins.
Fig. 6. Localization of Aβ42 to hepatic Kupffer cells, the final step in immune adherence.
A1–A3) Confocal microscopy of a 20 µm section of AD liver showing co-localization of CD68 immunoreactivity, a marker for Kupffer cells (KC), and Aβ immunoreactivity. B) Aβ-immunostained, toluidine blue-counterstained semi-thin section of Parkinson’s disease liver, showing Aβ-immunoreactive Kupffer cells. C) Electron micrograph of ND liver, again showing typical Kupffer cell localization with apposed erythrocytes and cytoplasmic Aβ42 (arrowheads). Deletion of primary antibodies in all experiments gave uniformly negative results (not shown). We note that, like most anti-Aβ antibodies, the 4G8 antibody employed here also reacts with APP. However, the punctate, granular, intracytoplasmic labelling in these micrographs appears to be more characteristic of Aβ than its precursor, APP.
3.06 Pathophysiologic significance of immune adherence in AD
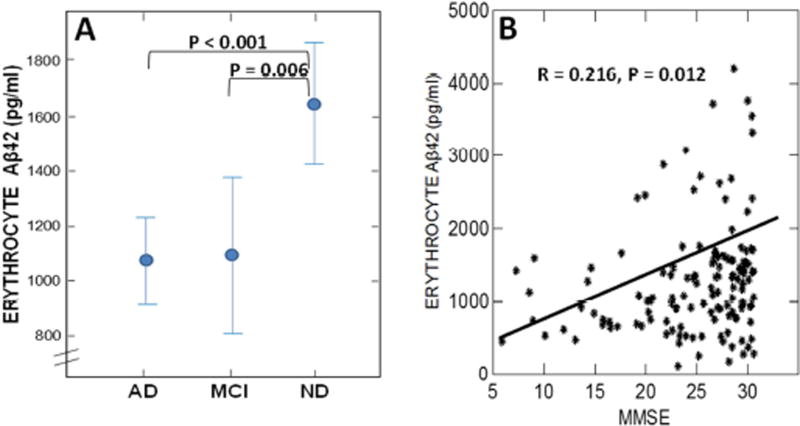
We previously reported AD and MCI deficits in erythrocyte Aβ levels in a small (N = 36), retrospective set of AD, MCI, and ND subjects [25]. Here, we confirm this finding in a prospective set of samples from 140 well-annotated, well-matched AD, MCI, and ND subjects. As in our previous study [25], AD (F = 18.1, P < 0.001) and MCI (F = 7.5, P = 0.006) groups each exhibited significantly lower levels of erythrocyte Aβ compared to the ND group (Fig. 7A) and there was a significant correlation of erythrocyte-captured Aβ with Mini Mental Status Scores (MMSE) (R = 0.216, P = 0.012) (Fig. 7B). A major point left unclear by the present replication, however, is whether or not MCI patients are developing or have fully evolved deficits in erythrocyte capture of Aβ. In our original study, MCI patients exhibited levels of erythrocyte Aβ that were significantly intermediate between AD and ND patients [25], whereas in the present study MCI deficits were similar to those of AD patients. In either case, the findings are consistent with the growing consensus that AD treatment strategies need to be inaugurated as early as possible.
Fig. 7. Pathophysiologic significance of erythrocyte capture of Aβ in AD.
(A) Confirming our previous results [25], erythrocyte capture of Aβ42 is significantly deficient in both AD and MCI patients. (B) Also in concert with our previous findings [25], there was a significant correlation of erythrocyte Aβ42 levels with cognitive status (MMSE) score. Although the data clearly exhibit too much scatter to make erythrocyte Aβ a definitive prognostic for AD, they do strongly suggest that it has pathophysiologic relevance to clinical AD progression.
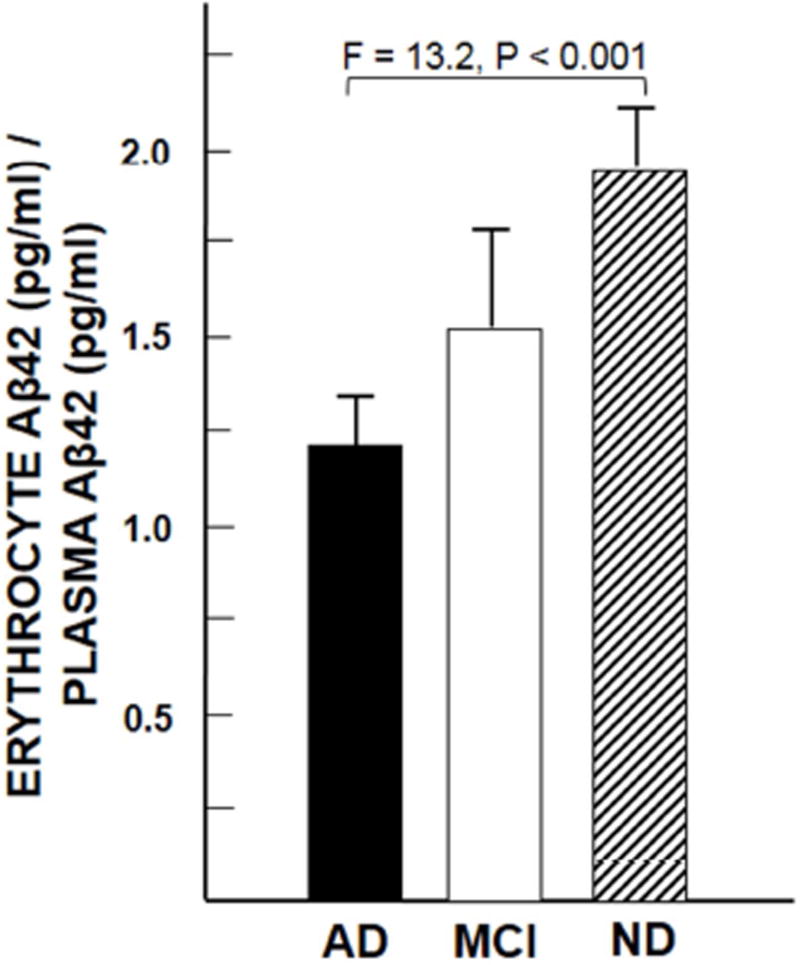
Finally, as in our previous research [25], plasma Aβ42 levels tended to be higher in the AD group (mean ± SEM = 1,284 ± 190 pg/ml) compared to the ND group (mean ± SEM = 995 ± 88 pg/ml), but did not differ significantly, consistent with the mixed results for plasma Aβ reported by other investigators [30–33]. By contrast, the fraction of Aβ42 captured in the erythrocyte compartment relative to the amount available in the plasma compartment was significantly lower for the AD group than the ND group (F = 13.2, P < 0.001) (Fig. 8), again confirming an AD deficit in erythrocyte clearance. As shown in the figure, it may also be notable that the amount of Aβ42 in the erythrocyte compartment in all the patient groups was at least equal to or higher than (P = 0.07) the amount of Aβ42 in the plasma compartment, consistent with the hypothesis that erythrocyte clearance of Aβ, immune adherence, is a major player in handling circulating loads of Aβ.
Fig. 8. Erythrocyte capture of Aβ relative to the amount of Aβ available in the plasma compartment.
Note that in each group mean erythrocyte Aβ42 levels tend to be higher than those in plasma.
4. Discussion
The present research demonstrates for the first time that all steps in the classical immune adherence pathway are fulfilled with respect to clearance of circulating Aβ, and confirms our previous finding that this mechanism is deficient in AD and MCI patients compared to ND patients [25]. Aβ inoculated into human serum dose-dependently activated complement, forming complement-opsonized complexes. When incubated with human erythrocytes, complement-opsonized Aβ was captured in a dose-dependent manner. Specificity to complement- and CR1-dependent mechanisms rather than to nonspecific binding was demonstrated by abolition of Aβ/erythrocyte adherence after heat inactivation of complement, EDTA treatment, or depletion of classical pathway components. Since these manipulations eliminate formation of the ligands for CR1, they indirectly demonstrate that erythrocyte binding of Aβ is most likely to be dependent on CR1. This conclusion was directly demonstrated by inhibiting erythrocyte CR1 binding to Aβ by blocking CR1 binding sites with anti-CR1 antibody and recombinant C3b, a CR1 ligand. At the terminal end of the immune adherence pathway, Aβ-immunoreactive Kupffer cells and apposed erythrocytes could be localized to the hepatic sinusoids by electron microscopy. In primates, Kupffer cells are specialized to capture complement-opsonized complexes carried by erythrocytes [11,12].
Several lines of evidence suggest that erythrocyte CR1-dependent mechanisms play a pathophysiologically-relevant role in Aβ clearance. First, our experiments show that peripheral erythrocyte capture of Aβ is highly dependent on complement reactions with Aβ, and, moreover, is dependent on interactions of complement-opsonized Aβ with the receptor for complement-opsonized ligands expressed on erythrocytes, CR1. Although these findings do not necessarily exclude other mechanisms for Aβ capture by erythrocytes, they strongly suggest that complement/CR1-mediation is predominant.
Recent, non-overlapping genome-wide association studies (GWAS) provide a second important connection of the erythrocyte immune adherence pathway to AD pathophysiology. Namely, multiple studies have found single nucleotide polymorphisms (SNPs) in CR1 to be among the top genetic risk factors for AD [14–18]. Perhaps because AD is a brain disorder, an underlying CR1 mechanism in brain has understandably been sought in previous studies [34–40]. Although the findings from these endeavors clearly confirm a role for CR1 in AD, the cell types expressing CR1 in brain—and even the presence of CR1 in brain—remain controversial. While it is possible that the CR1 in brain is modified in some way that conceals, removes, or alters epitopes to conventional CR1 antibodies [41], the fact remains that the vast majority of human CR1 indisputably resides in the erythrocyte compartment, where CR1 expression is unequivocally and universally detected [11,12]. The most parsimonious underlying basis for CR1 as an AD risk factor is therefore likely to be its role in peripheral clearance of Aβ, where CR1 expression by erythrocytes has evolved to amplify pathogen clearance in primates and is most abundantly expressed toward that end. Consistent with this view, AD and MCI patients exhibited significant deficits in CR1-mediated erythrocyte capture of Aβ, a finding we previously reported [25] and confirm in this report with a much larger sample. Deficits in erythrocyte clearance of pathogens have also been reported in several other human disorders, including leprosy, lupus, and malaria [11,12]. No consensus mechanism for this association with disease—whether by erythrocyte immunosenescence, decreased erythrocyte CR1 expression, impaired binding of CR1 to its ligands, or other mechanisms—has been accorded, and we are exploring these possibilities in the context of AD and the reported polymorphisms of CR1 [Johansson et al., in submission].
In addition to peripheral clearance of Aβ, complement opsonization of Aβ in the brain itself is also likely to have pathophysiologic significance—though not necessarily through CR1 mechanisms. Our laboratory, in fact, first reported co-localization of complement opsonins with Aβ in brain [20], a finding that has been recently extended by Hong et al. [42]. The latter demonstrated that opsonized Aβ associated with synapses helps target the synapses for microglial engulfment via microglial expression of another complement receptor, CR3. We have also shown direct attack of neurites in the vicinity of Aβ plaques by the terminal component of complement, C5b-9, the membrane attack complex [26].
Immune adherence has historically been studied in the context of immune complexes (i.e., antigen-antibody complexes) that activate and are bound by complement opsonins, as opposed to antigens such as Aβ that directly activate and are bound by complement in the absence of antibody. Although Aβ is a relatively potent antibody-independent complement activator [20–23], antibody-dependent classical pathway activation is typically much more powerful [e.g., 43]. As such, circulating endogenous anti-Aβ antibodies, which are common in human subjects [44–48], as well as anti-Aβ antibodies introduced by immunotherapy [reviewed in 49], should theoretically enhance complement activation and peripheral clearance of Aβ. We have, in a subsequent paper [Crane et al., in submission], explored these hypotheses as a means to better understand the putatively beneficial mechanisms and adverse consequences of Aβ immunization.
In summary, immune adherence, the clearance of pathogens through an erythrocyte CR1-mediated process, appears to be an important mechanism for removing Aβ from the circulation in humans, and deficiencies in this mechanism are likely to be pathologically relevant to AD.
RESEARCH IN CONTEXT.
Systematic Review
Although there are data on peripheral amyloid β peptide (Aβ) levels after intravenous Aβ inoculation in monkeys, the mechanisms for removing Aβ from the blood in primates remain unclear.
Interpretation
Our findings demonstrate that circulating Aβ is subject to a highly-efficient, well-studied pathway for clearing complement-opsonized antigens: immune adherence. This mechanism is unique to primates, deficient in Alzheimer’s disease (AD), and dependent on erythrocyte complement receptor 1 (CR1), single-nucleotide polymorphisms in which are a consistent risk factor for AD. Failure to remove peripheral Aβ is likely to provide an unfavorable concentration gradient for its clearance from brain, and to have deleterious effects on the vasculature and other organ systems in which it becomes sequestered.
Future Directions
Peripheral Aβ clearance by immune adherence should be enhanced by Aβ antibodies introduced in the course of Aβ immunotherapy, providing an additional explanation for why this treatment strategy helps remove Aβ from brain.
Acknowledgments
Experiments on human erythrocyte Aβ uptake were supported by the National Institute on Aging of the National Institutes of Health under award number RO1AG07367. Experiments on human CR1 were supported by the National Institute on Aging of the National Institutes of Health under award number RO1AG039750. Studies with non-human primates were supported by SRI International. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank Patrick McGeer, M.D., Ph.D., James Gaio, Ph.D., Michael Pangburn, Ph.D., John Atkinson, Ph.D., Douglas Walker, Ph.D., and Lih-Fen Lue, Ph.D., for technical and other advice, as well as Thomas Beach, M.D., Ph.D., and Lucia Sue for provision of postmortem tissue samples.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST: None
References
- 1.Kanekiyo T, Liu CC, Shinohara M, Li J, Bu G. LRP1 in brain vascular smooth muscle cells mediates local clearance of Alzheimer’s amyloid-beta. J Neurosci. 2012;32:16458–65. doi: 10.1523/JNEUROSCI.3987-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Castellano JM, Deane R, Gottesdiener AJ, Verghese PB, Stewart FR, West T, et al. Low-density lipoprotein receptor overexpression enhances the rate of brain-to-blood Abeta clearance in a mouse model of beta-amyloidosis. Proc Natl Acad Sci U S A. 2012;109:15502–7. doi: 10.1073/pnas.1206446109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeMattos RB, Bales KR, Cummins DJ, Dodart JC, Paul SM, Holtzman DM. Peripheral anti-A beta antibody alters CNS and plasma A beta clearance and decreases brain A beta burden in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2001;98:8850–5. doi: 10.1073/pnas.151261398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deane R, Du Yan S, Submamaryan RK, LaRue B, Jovanovic S, Hogg E, et al. RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. Nat Med. 2003;9:907–13. doi: 10.1038/nm890. [DOI] [PubMed] [Google Scholar]
- 5.Do TM, Bedussi B, Chasseigneaux S, Dodacki A, Yapo C, Chacun H, et al. Oatp1a4 and an L-thyroxine-sensitive transporter mediate the mouse blood-brain barrier transport of amyloid-beta peptide. J Alzheimers Dis. 2013;36:555–61. doi: 10.3233/JAD-121891. [DOI] [PubMed] [Google Scholar]
- 6.Grammas P. Neurovascular dysfunction, inflammation and endothelial activation: implications for the pathogenesis of Alzheimer’s disease. J Neuroinflammation. 2011;8:26. doi: 10.1186/1742-2094-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Broussard GJ, Mytar J, Li RC, Klapstein GJ. The role of inflammatory processes in Alzheimer’s disease. Inflammopharmacology. 2012;20:109–26. doi: 10.1007/s10787-012-0130-z. [DOI] [PubMed] [Google Scholar]
- 8.Mackic JB, Bading J, Ghiso J, Walker L, Wisniewski T, Frangione B, et al. Circulating amyloid-beta peptide crosses the blood-brain barrier in aged monkeys and contributes to Alzheimer’s disease lesions. Vascul Pharmacol. 2002;38:303–13. doi: 10.1016/s1537-1891(02)00198-2. [DOI] [PubMed] [Google Scholar]
- 9.Mackic JB, Weiss MH, Miao W, Kirkman E, Ghiso J, Calero M, et al. Cerebrovascular accumulation and increased blood-brain barrier permeability to circulating Alzheimer’s amyloid beta peptide in aged squirrel monkey with cerebral amyloid angiopathy. J Neurochem. 1998;70:210–5. doi: 10.1046/j.1471-4159.1998.70010210.x. [DOI] [PubMed] [Google Scholar]
- 10.Nelson RA., Jr The immune-adherence phenomenon; an immunologically specific reaction between microorganisms and erythrocytes leading to enhanced phagocytosis. Science. 1953;118:733–7. doi: 10.1126/science.118.3077.733. [DOI] [PubMed] [Google Scholar]
- 11.Birmingham DJ, Hebert LA. CR1 and CR1-like: the primate immune adherence receptors. Immunol Rev. 2001;180:100–11. doi: 10.1034/j.1600-065x.2001.1800109.x. [DOI] [PubMed] [Google Scholar]
- 12.Hess C, Schifferli JA. Immune adherence revisited: novel players in an old game. News Physiol Sci. 2003;18:104–8. doi: 10.1152/nips.01425.2002. [DOI] [PubMed] [Google Scholar]
- 13.Molina H, Wong W, Kinoshita T, Brenner C, Foley S, Holers VM. Distinct receptor and regulatory properties of recombinant mouse complement receptor 1 (CR1) and Crry, the two genetic homologues of human CR1. J Exp Med. 1992;175:121–9. doi: 10.1084/jem.175.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lambert JC, Heath S, Even G, Campion D, Sleegers K, Hiltunen M, et al. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nat Genet. 2009;41:1094–9. doi: 10.1038/ng.439. [DOI] [PubMed] [Google Scholar]
- 15.Harold D, Abraham R, Hollingworth P, Sims R, Gerrish A, Hamshere ML, et al. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nat Genet. 2009;41:1088–93. doi: 10.1038/ng.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pedraza O, Allen M, Jennette K, Carrasquillo M, Crook J, Serie D, et al. Evaluation of memory endophenotypes for association with CLU, CR1, and PICALM variants in black and white subjects. Alzheimers Dement. 2014;10:205–13. doi: 10.1016/j.jalz.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keenan BT, Shulman JM, Chibnik LB, Raj T, Tran D, Sabuncu MR, et al. A coding variant in CR1 interacts with APOE-epsilon4 to influence cognitive decline. Hum Mol Genet. 2012;21:2377–88. doi: 10.1093/hmg/dds054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin C, Li W, Yuan J, Xu W, Cheng Z. Association of the CR1 polymorphism with late-onset Alzheimer’s disease in Chinese Han populations: a meta-analysis. Neurosci Lett. 2012;527:46–9. doi: 10.1016/j.neulet.2012.08.032. [DOI] [PubMed] [Google Scholar]
- 19.Taylor RP, Martin EN, Reinagel ML, Nardin A, Craig M, Choice Q, et al. Bispecific monoclonal antibody complexes facilitate erythrocyte binding and liver clearance of a prototype particulate pathogen in a monkey model. J Immunol. 1997;159:4035–44. [PubMed] [Google Scholar]
- 20.Rogers J, Cooper NR, Webster S, Schultz J, McGeer PL, Styren SD, et al. Complement activation by beta-amyloid in Alzheimer disease. Proc Natl Acad Sci USA. 1992;89:10016–20. doi: 10.1073/pnas.89.21.10016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Webster S, Bradt B, Rogers J, Cooper N. Aggregation state-dependent activation of the classical complement pathway by the amyloid beta peptide. J Neurochem. 1997;69:388–98. doi: 10.1046/j.1471-4159.1997.69010388.x. [DOI] [PubMed] [Google Scholar]
- 22.Bradt BM, Kolb WP, Cooper NR. Complement-dependent proinflammatory properties of the Alzheimer’s disease beta-peptide. J Exp Med. 1998;188:431–8. doi: 10.1084/jem.188.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang H, Burdick D, Glabe CG, Cotman CW, Tenner AJ. beta-Amyloid activates complement by binding to a specific region of the collagen-like domain of the C1q A chain. J Immunol. 1994;152:5050–9. [PubMed] [Google Scholar]
- 24.Cooper NR, Jensen FC, Welsh RM, Jr, Oldstone MB. Lysis of RNA tumor viruses by human serum: direct antibody-independent triggering of the classical complement pathway. J Exp Med. 1976;144:970–84. doi: 10.1084/jem.144.4.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rogers J, Li R, Mastroeni D, Grover A, Leonard B, Ahern G, et al. Peripheral clearance of amyloid beta peptide by complement C3-dependent adherence to erythrocytes. Neurobiol Aging. 2006;27:1733–9. doi: 10.1016/j.neurobiolaging.2005.09.043. [DOI] [PubMed] [Google Scholar]
- 26.Webster S, Lue LF, Brachova L, Tenner AJ, McGeer PL, Terai K, et al. Molecular and cellular characterization of the membrane attack complex, C5b-9, in Alzheimer’s disease. Neurobiol Aging. 1997;18:415–21. doi: 10.1016/s0197-4580(97)00042-0. [DOI] [PubMed] [Google Scholar]
- 27.Tas SW, Klickstein LB, Barbashov SF, Nicholson-Weller A. C1q and C4b bind simultaneously to CR1 and additively support erythrocyte adhesion. J Immunol. 1999;163:5056–63. [PubMed] [Google Scholar]
- 28.Watson DM, Roher AE, Kim KS, Spiegel K, Emmerling MR. Complement labeling of aggregated Aβ1–42 by normal human serum involves the classical and alternative pathways. In: Iqbal K, Winblad B, Nishimura T, Takeda M, Wisniewski HM, editors. Alzheimer’s Disease: Biology, Diagnostics and Therapeutics. John Wiley and Sons; 1997. pp. 365–73. [Google Scholar]
- 29.Vik DP, Fearon DT. Neutrophils express a receptor for iC3b, C3dg, and C3d that is distinct from CR1, CR2, and CR3. J Immunol. 1985;134:2571–9. [PubMed] [Google Scholar]
- 30.Kuo YM, Kokjohn TA, Kalback W, Luehrs D, Galasko DR, Chevallier N, et al. Amyloid-beta peptides interact with plasma proteins and erythrocytes: implications for their quantitation in plasma. Biochem Biophys Res Commun. 2000;268(3):750–6. doi: 10.1006/bbrc.2000.2222. [DOI] [PubMed] [Google Scholar]
- 31.Mayeux R, Tang MX, Jacobs DM, Manly J, Bell K, Merchant C, et al. Plasma amyloid beta-peptide 1–42 and incipient Alzheimer’s disease. Ann Neurol. 1999;46(3):412–6. doi: 10.1002/1531-8249(199909)46:3<412::aid-ana19>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 32.Mehta PD, Pirttila T, Mehta SP, Sersen EA, Aisen PS, Wisniewski HM. Plasma and cerebrospinal fluid levels of amyloid beta proteins 1–40 and 1–42 in Alzheimer disease. Arch Neurol. 2000;57(1):100–5. doi: 10.1001/archneur.57.1.100. [DOI] [PubMed] [Google Scholar]
- 33.Tamaoka A, Fukushima T, Sawamura N, Ishikawa K, Oguni E, Komatsuzaki Y, et al. Amyloid beta protein in plasma from patients with sporadic Alzheimer’s disease. J Neurol Sci. 1996;141(1–2):65–8. doi: 10.1016/0022-510x(96)00143-8. [DOI] [PubMed] [Google Scholar]
- 34.Gasque P, Chan P, Mauger C, Schouft MT, Singhrao S, Dierich MP, et al. Identification and characterization of complement C3 receptors on human astrocytes. J Immunol. 1996;156:2247–55. [PubMed] [Google Scholar]
- 35.Hazrati LN, Van Cauwenberghe C, Brooks PL, Brouwers N, Ghani M, Sato C, et al. Genetic association of CR1 with Alzheimer’s disease: a tentative disease mechanism. Neurobiol Aging. 2012;33:2949. doi: 10.1016/j.neurobiolaging.2012.07.001. e5-e12. [DOI] [PubMed] [Google Scholar]
- 36.Singhrao SK, Neal JW, Rushmere NK, Morgan BP, Gasque P. Differential expression of individual complement regulators in the brain and choroid plexus. Lab Invest. 1999;79:1247–59. [PubMed] [Google Scholar]
- 37.Zanjani H, Finch CE, Kemper C, Atkinson J, McKeel D, Morris JC, et al. Complement activation in very early Alzheimer disease. Alzheimer Dis Assoc Disord. 2005;19:55–66. doi: 10.1097/01.wad.0000165506.60370.94. [DOI] [PubMed] [Google Scholar]
- 38.Karch CM, Jeng AT, Nowotny P, Cady J, Cruchaga C, Goate AM. Expression of novel Alzheimer’s disease risk genes in control and Alzheimer’s disease brains. PLoS One. 2012;7:e50976. doi: 10.1371/journal.pone.0050976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Allen M, Kachadoorian M, Carrasquillo MM, Karhade A, Burgess JD, et al. Late-onset Alzheimer’s disease risk variants mark brain regulatory loci. Neurol Genet. 2015:e15. doi: 10.1212/NXG.0000000000000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holton P, Ryten M, Nalls M, Trabzuni D, Weale ME, et al. Initial assessment of the pathologenic mechanisms of the recently identified Alzheimer risk loci. Ann Hum Genet. 2013;77:85–105. doi: 10.1111/ahg.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fonseca MI, Chu S, Pierce AL, Brubaker WD, Hauuhart RE, et al. Analysis of the putative role of CR1 in Alzheimer’s disease: genetic association, expression, and function. PLoS One. 2016;11(2):e0149792. doi: 10.1371/journal.pone.0149792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hong S, Beja-Glasser VF, Nfonoyim BM, Frouin A, Li S, et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science. 2016;352:712–6. doi: 10.1126/science.aad8373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gras GS, Dormont D. Antibody-dependent and antibody-independent complement-mediated enhancement of human immunodeficiency virus type 1 infection in a human, Epstein-Barr virus-transformed B-lymphocytic cell line. J Virol. 1991;65:541–5. doi: 10.1128/jvi.65.1.541-545.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim I, Lee J, Hong HJ, Jung ES, Ku YH, Jeong IK, et al. A relationship between Alzheimer’s disease and type 2 diabetes mellitus through the measurement of serum amyloid-beta autoantibodies. J Alzheimers Dis. 2010;19:1371–6. doi: 10.3233/JAD-2010-1332. [DOI] [PubMed] [Google Scholar]
- 45.Maetzler W, Berg D, Synofzik M, Brockmann K, Godau J, Melms A, et al. Autoantibodies against amyloid and glial-derived antigens are increased in serum and cerebrospinal fluid of Lewy body-associated dementias. J Alzheimers Dis. 2011;26:171–9. doi: 10.3233/JAD-2011-110221. [DOI] [PubMed] [Google Scholar]
- 46.Maetzler W, Langkamp M, Lerche S, Godau J, Brockmann K, Gaenslen A, et al. Lowered serum amyloid-beta1–42 autoantibodies in individuals with lifetime depression. J Alzheimers Dis. 2012;32:95–100. doi: 10.3233/JAD-2012-120625. [DOI] [PubMed] [Google Scholar]
- 47.Piazza F, Greenberg SM, Savoiardo M, Gardinetti M, Chiapparini L, Raicher I, et al. Anti-amyloid beta autoantibodies in cerebral amyloid angiopathy-related inflammation: implications for amyloid-modifying therapies. Ann Neurol. 2013;73:449–58. doi: 10.1002/ana.23857. [DOI] [PubMed] [Google Scholar]
- 48.Sohn JH, So JO, Hong HJ, Kim JW, Na DR, Kim M, et al. Identification of autoantibody against beta-amyloid peptide in the serum of elderly. Front Biosci (Landmark Ed) 2009;14:3879–83. doi: 10.2741/3496. [DOI] [PubMed] [Google Scholar]
- 49.Wisniewski T, Goni F. Immunotherapeutic approaches for Alzheimer’s disease. Neuron. 2015;85:1162–76. doi: 10.1016/j.neuron.2014.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]