Abstract
Aims
Heart failure with preserved ejection fraction (HFpEF) has several pathophysiological aspects, including stiffness and/or congestion of multiple organs. Poor prognosis is expected in heart failure patients with liver stiffness, which has recently been assessed by non‐alcoholic fatty liver disease fibrosis score (NFS; based on aspartate aminotransferase to alanine aminotransferase ratio, platelet counts, and albumin). We aimed to investigate the impact of NFS on prognosis of HFpEF patients, with consideration for the peripheral collagen markers such as procollagen type III peptide (PIIIP), type IV collagen 7S, and hyaluronic acid.
Methods and results
We performed a prospective observational study. Consecutive 492 hospitalized HFpEF patients were divided into four groups based on their NFS: first–fourth quartiles (n = 123). The fourth quartile group had the highest levels of PIIIP, type IV collagen 7S, hyaluronic acid, and B‐type natriuretic peptide (P<0.001 each). In addition, there were significant positive correlations between PIIIP, type IV collagen 7S, hyaluronic acid, B‐type natriuretic peptide, and NFS (P < 0.001 each). In the follow‐up period (mean 1107 days), 93 deaths occurred. All‐cause mortality increased in all four quartiles (8.1%, 12.2%, 23.6%, and 31.7%, P < 0.001). In the multivariable Cox proportional hazard analysis, NFS was an independent predictor of all‐cause mortality in the HFpEF patients.
Conclusions
NFS, a novel indicator of liver fibrosis, correlates with circulating systemic markers of fibrosis and congestion and is associated with higher all‐cause mortality in HFpEF patients. NFS can be calculated simply and may be a useful tool to assess liver stiffness and prognosis in HFpEF patients.
Keywords: Heart failure with preserved ejection fraction, Liver stiffness, Collagen, Procollagen type III peptide, Type IV collagen 7S
Introduction
Heart failure (HF) with preserved ejection fraction (HFpEF) is a common and increasing public health problem.1, 2, 3 It has several pathophysiological aspects including cardiac hypertrophy and interstitial fibrosis1, 2, 3, 4, 5 and/or impaired functional reserve of multiple organs, such as lungs, vessels, skeletal muscle, kidney, and liver.1, 2, 6 The abdominal compartment (e.g. liver, splanchnic vasculature, and gut) has recently been focused to contribute significantly to deranged cardiac as well as renal function in HF patients.1, 7 Congestive hepatopathy8, 9 due to HF causes functional abnormalities of the liver,10 and increased liver stiffness, measured by transient elastography, indicates higher mortality.11, 12, 13, 14, 15 Liver dysfunction, such as the elevation of serum bilirubin, alkaline phosphatase, gamma‐glutamyl transferase, aspartate aminotransferase (AST), and alanine aminotransferase (ALT), is frequent in HF related to reduced arterial perfusion and passive congestion and is associated with disease severity and prognosis.7, 9 In many HFpEF patients, fluid retention is less apparent in the periphery but occurs in the abdominal cavity.1 Liver congestion due to increased central venous pressure might directly contribute to a state of impaired natriuresis.7 Liver congestion may be mutually associated with liver stiffness, resulting in fibrosis (e.g. nutmeg liver), and adverse prognosis.8, 9, 10 In addition, elevated central venous pressure and right atrial pressure may contribute to cholestatic abnormalities and impairment of hepatocyte function and liver reserve in HF patients.8, 9, 10
On the other hand, not only previous imaging tests,11, 12, 13, 14, 15 but also a simple score to evaluate liver fibrosis is desirable and required in HF patients. Recently, in patients with chronic liver failure, non‐alcoholic fatty liver disease (NAFLD) fibrosis score (NFS) has begun to be used to assess liver fibrosis based on simple laboratory testing (age, body mass index, presence of diabetes, AST to ALT ratio, platelet counts, and albumin) in patients with NAFLD.16, 17, 18 We hypothesized that NFS, which is measured simply and quickly, indicates liver congestion and fibrosis, and impaired functional reserve of the liver, and is associated with adverse prognosis in HFpEF patients.
Therefore, the aims of the present study were to verify the value of NFS as an assessment tool for liver fibrosis and higher mortality in patients with HFpEF, and its underlying clinical and pathophysiological background [e.g. echocardiographic and haemodynamic parameters, systemic peripheral collagen markers such as procollagen type III peptide (PIIIP), type IV collagen 7S, and hyaluronic acid, which are established biochemical markers of liver fibrosis].19, 20
Methods
Subjects and study protocol
This was a prospective observational study that enrolled consecutive symptomatic HF patients hospitalized for treatment of decompensated HFpEF and discharged from the Fukushima Medical University Hospital between 2009 and 2014. Symptomatic HF was defined based on the Framingham criteria,21 and left ventricular ejection fraction (LVEF) was more than 50%.22 Blood samples and echocardiography were obtained at hospital discharge. All the patients underwent testing of hepatitis B surface antigen and hepatitis C antibodies, and their medical histories were checked for chronic liver disease (cirrhosis, hepatic tumours, bile duct disease, etc.). Patients with distinct liver diseases, severe valvular disease, acute coronary syndrome, advanced cancer, and/or undergoing dialysis were excluded. The patient flow is described in Figure 1 . Liver fibrosis was estimated by NFS [−1.675 + 0.037 × age (years) + 0.094 × body mass index (kg/m2) + 1.13 × diabetes mellitus (if presence, given 1) + 0.95 × AST (U/L) to ALT (U/L) ratio − 0.013 × platelet count (10−9/L) − 0.66 × albumin (mg/L)].16, 17, 18 These patients were divided into four groups based on NFS: first (NFS < −1.13, n = 123), second (−1.13 ≤ NFS < 0.20, n = 123), third (0.20 ≤ NFS < 1.56, n = 123), and fourth quartiles (1.56 ≤ NFS, n = 123).16, 17, 18
Figure 1.
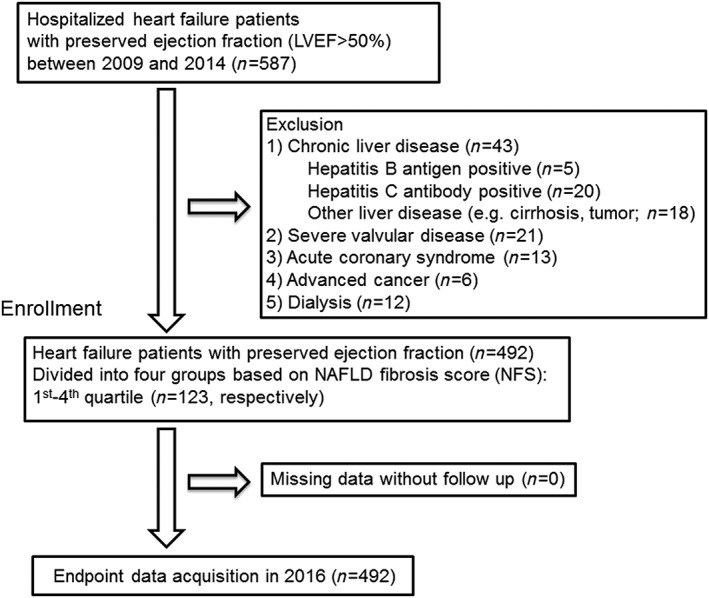
Patient flowchart.
Diabetes was defined as recent use of insulin or antidiabetic drugs, a fasting blood glucose value of ≥126 mg/dL, and/or a haemoglobin A1c value of ≥6.5%. Hypertension was defined as recent use of antihypertensive drugs or systolic blood pressure of ≥140 mmHg and/or diastolic blood pressure of ≥90 mmHg. Dyslipidaemia was defined as recent use of cholesterol‐lowering drugs, a triglyceride value of ≥150 mg/dL, a low‐density lipoprotein cholesterol value of ≥140 mg/dL, and/or a high‐density lipoprotein cholesterol value of <40 mg/dL. Chronic kidney disease was defined as an estimated glomerular filtration rate of <60 mL/min/1.73 m2, measured by the Modification of Diet in Renal d as haemoglobin levels of <12.0 g/dL in females and <13.0 g/dL in males.22 Atrial fibrillation was identified by an electrocardiogram performed during hospitalization and/or medical records including past history.
We compared the clinical features and results from laboratory tests and echocardiography among the four groups. All patients were followed up until 2016 for all‐cause mortality as the primary outcome of our study (Figure 1 ). Cardiac death was adjudicated by independent experienced cardiologists and included death due to worsened HF in accordance with the Framingham criteria,21 ventricular fibrillation documented by electrocardiogram or other implantable devices, and acute coronary syndrome. Non‐cardiac death included death due to respiratory failure, renal failure, liver failure, infection, sepsis, stroke, cancer, or digestive haemorrhage. Status and date of death were obtained from the patients' medical records or their referring cardiologists. Survival time was calculated from the date of hospitalization until the date of death or last follow‐up. Those who administered the survey were blind to the analyses, and written informed consent was obtained from all study subjects. The study protocol was approved by the ethical committee of Fukushima Medical University. The investigation conforms to the principles outlined in the Declaration of Helsinki, and reporting of the study conforms to STROBE along with references to STROBE and the broader EQUATOR guidelines.23
Measurement of procollagen type III peptide, type IV collagen 7S, and hyaluronic acid
The level of serum PIIIP was measured by immunoradiometric assay (RIA‐gnost PIIIP c.t., CISbio Bioassays, Codolet, France). Serum type IV collagen 7S was measured by radioimmunoassay (type IV collagen 7S kit, SCETI MEDICAL LABO K.K., Tokyo, Japan). Serum hyaluronic acid was measured by latex agglutination‐turbidimetric immunoassay (LPIA Ace HA., LSI Medience Co., Tokyo, Japan). These assays were blindly performed by a laboratory company (LSI Medience Co., Tokyo, Japan).
Echocardiography
Echocardiography was performed blindly by an experienced echocardiographer using the standard techniques.22 Echocardiographic parameters included left ventricular dimension, LVEF, left atrial volume, the ratio of early transmitral flow velocity to mitral annular velocity (mitral valve E/e′), dimension and area of right ventricle and atrial, right ventricular fractional area change, inferior vena cava diameter, systolic pulmonary artery pressure (SPAP), tissue Doppler‐derived tricuspid lateral annular systolic velocity (tricuspid valve S′), and the ratio of the peak transtricuspid velocity during early diastole to the peak tricuspid valve annular velocity during early diastole (tricuspid valve E/e′).24 The LVEF was calculated using Simpson's method. The right ventricular fractional area change, defined as (end diastolic area − end systolic area)/end diastolic area × 100, was a measure of right ventricular systolic function.24 SPAP was calculated by adding the right atrial pressure (estimated by the diameter and collapsibility of the inferior vena cava) to the systolic trans tricuspid pressure gradient. Tricuspid valve E/e′ was calculated by transtricuspid Doppler flow and tissue Doppler imaging.24 All measurements were performed using ultrasound systems (ACUSON Sequoia, Siemens Medical Solutions USA, Inc., Mountain View, CA, USA).
Haemodynamic measurements
Right catheterizations were performed within 1 year prior to admission till discharge. All catheterizations were performed in a resting supine position under fluoroscopic guidance. Pulmonary capillary wedge pressure, mean pulmonary artery pressure, mean right atrial pressure, and cardiac output were measured using a 7F Swan‐Ganz catheter (Edwards Lifesciences, Irvine, CA, USA).25 We used the thermodilution method for the measurement of cardiac output.
Statistical analysis
Normally distributed data are presented as mean ± standard deviation, and non‐normally distributed data are log transformed (e.g. BNP, C‐reactive protein, and hyaluronic acid). Categorical variables are expressed as numbers and percentages. The χ2 test was used for comparisons of categorical variables. We used the analysis of variance followed by Tukey's post hoc test. Multivariable regression analysis was used to determine each element of NFS related to the score. Correlations between NFS and markers of liver fibrosis and haemodynamic parameters (e.g. PIIIP, type IV collagen 7S, hyaluronic acid, BNP, cardiac index, pulmonary capillary wedge pressure, mean pulmonary artery pressure, and right atrial pressure) were assessed using Pearson's correlation analysis. The Kaplan–Meier method was used for presenting the all‐cause mortality, and the log‐rank test was used for initial comparisons. Univariable and multivariable Cox proportional hazard analyses were used to analyse predictors of all‐cause mortality to adjust confounding factors. Hazard ratio and 95% confidence interval are presented. To minimize potential confounding, including those that are generally known risk factors for mortality and are not included in NFS elements, we considered the following clinical factors: NFS; sex; presence of New York Heart Association class III or IV; ischaemic aetiology; atrial fibrillation; chronic kidney disease; anaemia; BNP; hyponatraemia (<135 mEq/L); and usage of renin–angiotensin–aldosterone system inhibitors, β‐blockers, and diuretics. Among these factors, those that independently predicted mortality with a value of P < 0.05 were selected in the final adjusted model. We constructed two models: in the first, we analysed NFS as a categorical variable model (fourth, third, second vs. first quartile), and in the second, we analysed NFS as a continuous variable model. A value of P < 0.05 was considered statistically significant for all comparisons. These analyses were performed using a statistical software package (SPSS ver. 21.0, IBM, Armonk, NY, USA).
Results
In the multiple regression analysis to determine NFS, the elements of the score were as follows: platelet count, β = −0.571, P < 0.001; age, β = 0.362, P < 0.001; diabetes, β = 0.279, P < 0.001; AST/ALT ratio, β = 0.253, P < 0.001; albumin, β = −0.223, P < 0.001; and body mass index, β = 0.185, P < 0.001. In addition, there were significant positive correlations between NFS and systemic fibrosis markers and central venous pressure (PIIIP, R = 0.429, P < 0.001; type IV collagen 7S, R = 0.343, P < 0.001; hyaluronic acid, R = 0.376, P < 0.001; BNP, R = 0.283, P < 0.001; and right atrial pressure, R = 0.216, P = 0.038). In contrast, there were no significant correlations between NFS and cardiac index, pulmonary capillary wedge pressure, and mean pulmonary artery pressure.
The clinical features of the present study's subjects are summarized in Table 1. The fourth quartile group had a higher prevalence of ischaemic aetiology, diabetes, atrial fibrillation, chronic kidney disease, and anaemia, as well as higher usage of renin–angiotensin–aldosterone system inhibitors and diuretics. Subjects in the fourth quartile group also had a higher mean age and tended to have a higher body mass index. Comparisons of the laboratory data and echocardiographic and haemodynamic parameters among the four groups are shown in Table 2. The fourth quartile group had the lowest platelet count, as well as the lowest levels of albumin and cholinesterase. In addition, the fourth quartile group had the highest levels of BNP, C‐reactive protein, PIIIP, type IV collagen 7S, and hyaluronic acid. In contrast, total bilirubin, direct bilirubin, AST, ALT, alkaline phosphatase, gamma‐glutamyl transferase, and sodium did not differ significantly among the four groups. With regard to the echocardiographic parameters, although left and right ventricular systolic function, left ventricular dimension, and SPAP did not differ among the four groups, left atrial volume, right atrial area, and inferior vena cava diameter were larger in the fourth quartile than in the first quartile. Among the haemodynamic parameters (n = 290), right atrial pressure was higher in the fourth quartile than in the first to third quartiles. In contrast, cardiac index, pulmonary capillary wedge pressure, and mean pulmonary artery pressure did not differ among the four groups.
Table 1.
Comparisons of clinical features among non‐alcoholic fatty liver disease fibrosis score quartiles (N = 492)
| First quartile, NAFLD fibrosis < −1.13 (n = 123) | Second quartile, −1.13 ≤ NAFLD fibrosis < 0.20 (n = 123) | Third quartile, 0.20 ≤ NAFLD fibrosis < 1.56 (n = 123) | Fourth quartile, 1.56 ≤ NAFLD fibrosis (n = 123) | P‐value | |
|---|---|---|---|---|---|
| NAFLD fibrosis score | −2.28 ± 1.24†† | −0.47 ± 0.39**, †† | 0.87 ± 0.38**, †† | 2.82 ± 1.31** | <0.001 |
| Age (years) | 59.5 ± 15.8†† | 69.7 ± 12.2**, †† | 73.3 ± 10.7** | 76.5 ± 9.9** | <0.001 |
| Male gender (n, %) | 59 (48.0) | 59 (48.0) | 61 (49.6) | 66 (53.7) | 0.786 |
| Body mass index (kg/cm2) | 23.4 ± 4.1 | 22.8 ± 4.3 | 23.4 ± 4.2 | 24.3 ± 4.3 | 0.061 |
| NYHA class III or IV (n, %) | 1 (0.8) | 2 (1.6) | 5 (4.1) | 5 (4.1) | 0.258 |
| Ischaemic aetiology (n, %) | 9 (7.3) | 9 (7.3) | 25 (20.3) | 32 (26.0) | <0.001 |
| Co‐morbidity | |||||
| Hypertension (n, %) | 81 (65.9) | 103 (83.7) | 108 (87.8) | 100 (81.3) | <0.001 |
| Diabetes (n, %) | 12 (9.8) | 31 (25.2) | 53 (43.1) | 73 (59.3) | <0.001 |
| Dyslipidaemia (n, %) | 97 (78.9) | 90 (73.2) | 94 (76.4) | 95 (77.2) | 0.759 |
| Atrial fibrillation (n, %) | 35 (28.5) | 53 (43.1) | 52 (42.3) | 60 (48.8) | 0.010 |
| CKD (n, %) | 30 (24.4) | 58 (47.2) | 83 (67.5) | 84 (68.3) | <0.001 |
| Anaemia (n, %) | 48 (39.0) | 61 (49.6) | 80 (65.0) | 94 (76.4) | <0.001 |
| Medications | |||||
| RAS inhibitors (n, %) | 69 (56.1) | 89 (72.4) | 91 (74.0) | 95 (77.2) | 0.001 |
| β‐blockers (n, %) | 77 (62.6) | 78 (63.4) | 83 (67.5) | 80 (65.0) | 0.862 |
| Diuretics (n, %) | 64 (52.0) | 77 (62.6) | 76 (61.8) | 94 (76.4) | 0.001 |
CKD, chronic kidney disease; NAFLD, non‐alcoholic fatty liver disease; NYHA, New York Heart Association; RAS, renin–angiotensin–aldosterone system.
P < 0.05 vs. the first quartile.
P < 0.01 vs. the first quartile.
P < 0.05 vs. the fourth quartile.
P < 0.01 vs. the fourth quartile.
Table 2.
Laboratory, echocardiographic, and haemodynamic data
| First quartile (n = 123) | Second quartile (n = 123) | Third quartile (n = 123) | Fourth quartile (n = 123) | P‐value | |
|---|---|---|---|---|---|
| Laboratory data | |||||
| Platelet count (×103/μL) | 254.7 ± 110.4†† | 192.3 ± 49.5**, †† | 167.6 ± 50.7** | 151.8 ± 54.9** | <0.001 |
| Log BNP | 2.0 ± 0.6† | 2.3 ± 0.5*, † | 2.4 ± 0.5*, † | 2.5 ± 0.5* | <0.001 |
| Log C‐reactive protein | −0.8 ± 0.7 | −0.6 ± 0.7† | −0.5 ± 0.8 | −0.4 ± 0.8** | 0.001 |
| Albumin (g/dL) | 4.0 ± 0.5†† | 3.7 ± 0.6**, †† | 3.6 ± 0.5**, †† | 3.2 ± 0.6** | <0.001 |
| Total bilirubin (mg/dL) | 0.9 ± 0.4 | 0.9 ± 0.4 | 0.9 ± 0.5 | 0.9 ± 0.5 | 0.965 |
| Direct bilirubin (mg/dL) | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.2 ± 0.2 | 0.113 |
| AST (U/L) | 35.0 ± 6.4 | 32.3 ± 6.9 | 61.7 ± 17.2 | 91.5 ± 44.0 | 0.175 |
| ALT (U/L) | 34.6 ± 5.4 | 35.7 ± 5.6 | 46.2 ± 17.9 | 46.2 ± 16.9 | 0.302 |
| ALP (U/L) | 244.0 ± 126.2 | 256.1 ± 151.0 | 269.4 ± 111.9 | 263.8 ± 106.0 | 0.569 |
| γ‐GTP (U/L) | 49.7 ± 4.7 | 69.9 ± 11.4 | 57.6 ± 5.9 | 48.0 ± 6.7 | 0.166 |
| Cholinesterase (U/L) | 315.0 ± 78.5†† | 279.7 ± 85.0†, †† | 249.4 ± 65.2**, †† | 205.9 ± 75.1**, †† | <0.001 |
| Sodium (mEq/L) | 139.3 ± 3.2 | 139.4 ± 3.1 | 138.6 ± 4.4 | 138.6 ± 4.4 | 0.225 |
| PIIIP (U/mL) | 0.6 ± 0.3†† | 0.7 ± 0.4† | 0.8 ± 0.4** | 0.9 ± 0.3** | <0.001 |
| Type IV collagen 7S (ng/mL) | 4.3 ± 1.5†† | 4.8 ± 1.7* †† | 5.2 ± 2.2**, † | 6.1 ± 2.4** | <0.001 |
| Log hyaluronic acid | 1.6 ± 0.3†† | 1.8 ± 0.4* †† | 1.9 ± 0.5**, †† | 2.1 ± 0.5** | <0.001 |
| Echocardiographic data | |||||
| Interventricular septal wall thickness (mm) | 11.7 ± 3.5 | 11.8 ± 3.4 | 11.4 ± 2.6 | 11.4 ± 2.1 | 0.672 |
| LV end‐diastolic diameter (mm) | 45.2 ± 8.6 | 47.5 ± 9.3 | 46.1 ± 10.2 | 47.5 ± 8.5 | 0.175 |
| LV end‐systolic diameter (mm) | 29.8 ± 7.3 | 30.1 ± 8.8 | 30.7 ± 9.5 | 31.2 ± 7.8 | 0.221 |
| LV posterior wall (mm) | 11.3 ± 2.6 | 11.3 ± 2.3 | 11.6 ± 2.2 | 11.1 ± 1.9 | 0.660 |
| LVEF (%) | 63.1 ± 9.5 | 61.8 ± 9.5 | 61.7 ± 8.9 | 60.9 ± 8.8 | 0.217 |
| Left atrial volume (mL) | 72.9 ± 70.4† | 89.3 ± 64.0 | 88.0 ± 60.0 | 92.7 ± 58.0* | 0.043 |
| Mitral valve E/e′ | 13.8 ± 8.7 | 15.3 ± 9.9 | 14.5 ± 8.6 | 16.8 ± 9.7 | 0.204 |
| RV area‐diastolic (mL) | 17.5 ± 7.5 | 17.6 ± 7.4 | 16.5 ± 6.0 | 17.0 ± 7.1 | 0.829 |
| RV area‐systolic (mL) | 10.7 ± 5.8 | 10.7 ± 5.6 | 9.5 ± 4.3 | 10.1 ± 5.1 | 0.611 |
| RV‐FAC (%) | 43.2 ± 17.0 | 41.8 ± 13.4 | 43.9 ± 15.2 | 43.6 ± 13.6 | 0.885 |
| Right atrial diameter long (mm) | 50.5 ± 9.5†† | 54.2 ± 11.6* | 55.4 ± 13.4* | 56.9 ± 14.6** | 0.027 |
| Right atrial diameter short (mm) | 36.5 ± 9.0 | 36.7 ± 9.5 | 38.1 ± 10.9 | 35.6 ± 10.8 | 0.664 |
| Right atrial end systolic area (cm2) | 17.1 ± 7.2†† | 18.9 ± 9.6 | 19.4 ± 10.8* | 20.6 ± 11.3** | 0.033 |
| SPAP (mmHg) | 36.3 ± 23.3 | 33.2 ± 18.8 | 31.9 ± 16.7 | 32.9 ± 16.3 | 0.478 |
| Tricuspid valve S′ (cm/s) | 9.4 ± 3.7 | 9.7 ± 3.8 | 8.9 ± 3.5 | 9.9 ± 3.5 | 0.796 |
| Tricuspid valve E/e′ | 5.5 ± 3.3 | 4.3 ± 2.9 | 6.3 ± 4.6 | 5.1 ± 2.3 | 0.188 |
| Inferior vena cava (mm) | 14.1 ± 4.0†† | 14.3 ± 3.9† | 15.1 ± 5.3 | 16.3 ± 5.7** | 0.006 |
| Haemodynamic data (n = 290) | |||||
| Cardiac output (L /min) | 4.6 ± 2.0 | 4.5 ± 1.6 | 4.1 ± 1.1 | 4.5 ± 1.5 | 0.212 |
| Cardiac index (L /min/m2) | 2.9 ± 1.2 | 2.8 ± 0.9 | 2.7 ± 0.7 | 2.9 ± 0.9 | 0.521 |
| Pulmonary capillary wedge pressure (mmHg) | 13.1 ± 7.1 | 13.5 ± 7.2 | 13.6 ± 6.8 | 13.7 ± 7.3 | 0.959 |
| Mean pulmonary artery pressure (mmHg) | 24.1 ± 15.0 | 23.6 ± 11.1 | 23.5 ± 10.0 | 22.4 ± 9.0 | 0.217 |
| Right atrial pressure (mmHg) | 6.8 ± 3.8†† | 6.9 ± 4.4† | 7.6 ± 5.3* | 8.6 ± 5.0** | 0.031 |
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; LV, left ventricular; LVEF, left ventricular ejection fraction; PIIIP, procollagen type III peptide; RV, right ventricular; RV‐FAC, right‐ventricular fractional area change; SPAP, systolic pulmonary artery pressure.
P < 0.05 vs. the first quantile.
P < 0.01 vs. the first quartile.
P < 0.05 vs. the fourth quartile.
P < 0.01 vs. the fourth quartile.
During the follow‐up period (mean 1096 days), there were 33 cardiac deaths, including 17 due to worsening HF, 13 due to ventricular fibrillation, and 3 due to acute coronary syndrome, and 60 non‐cardiac deaths (respiratory failure and/or pneumonia, n = 17; infection/sepsis, n = 13; stroke, n = 11; renal failure/liver failure/multiple organ failure, n = 9; digestive haemorrhage, n = 3; aneurysm, n = 2; and other causes, n = 5). As shown in Figure 2 , all‐cause mortality increased in all four quartiles (P < 0.001).
Figure 2.
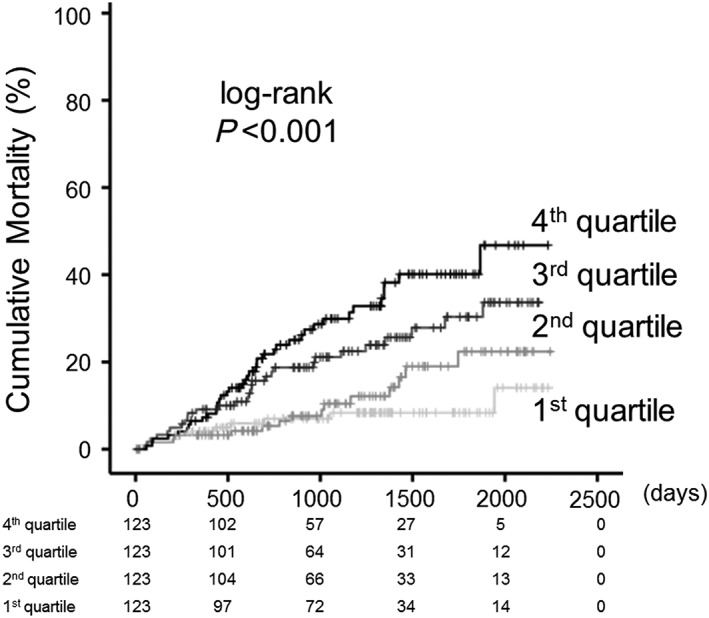
Cumulative all‐cause mortality stratified by non‐alcoholic fatty liver disease fibrosis score. Kaplan–Meier analysis for all‐cause mortality in the first–fourth quartiles in heart failure with preserved ejection fraction patients.
The Cox proportional hazard model was used to examine the prognostic value of the NFS in HFpEF patients (Table 3). In the multivariable Cox proportional hazard analysis, a high NFS score was determined to be an independent predictor of all‐cause mortality in HFpEF patients after adjusting for other confounding factors (Table 3).
Table 3.
Cox proportional hazard model of all‐cause mortality in heart failure with preserved ejection fraction
| All‐cause mortality (n = 93/492) | Hazard ratio | 95% confidence interval | P‐value |
|---|---|---|---|
| Non‐alcoholic fatty liver disease fibrosis score: categorical variable model | |||
| Fourth vs. first quartile unadjusted | 4.354 | 2.172–8.729 | <0.001 |
| Fourth vs. first quartile adjusteda | 2.784 | 1.343–5.775 | 0.006 |
| Third vs. first quartile unadjusted | 2.992 | 1.458–6.139 | 0.003 |
| Third vs. first quartile adjusteda | 2.014 | 1.005–4.246 | 0.046 |
| Second vs. first quartile unadjusted | 1.533 | 0.689–3.412 | 0.296 |
| Second vs. first quartile adjusteda | 1.235 | 0.550–2.774 | 0.609 |
| Non‐alcoholic fatty liver disease fibrosis score: continuous variable model | |||
| Unadjusted model | 1.285 | 1.176–1.404 | <0.001 |
| Adjusted modela | 1.132 | 1.020–1.256 | 0.020 |
Adjusted for sex, New York Heart Association class III or IV, ischaemic aetiology, atrial fibrillation, chronic kidney disease, anaemia, BNP, hyponatraemia, renin–angiotensin–aldosterone system inhibitors, β‐blockers, and diuretics.
Discussion
The present study is the first to present that NFS, a marker of liver fibrosis, was correlated with central venous pressure (right atrial pressure, inferior vena cava diameter, right atrial area, left atrial volume, and BNP) and circulating markers of systemic fibrosis (PIIIP, type IV collagen 7S, and hyaluronic acid) and is associated with higher mortality in HFpEF patients. In contrast, higher NFS was not associated with left ventricular hypertrophy and impaired right and left ventricular systolic function and lower cardiac index in HFpEF patients.
HF sometimes consists of not only congestion but also reduced arterial flow called hypoxic hepatopathy. Hypoxia causes centrilobular necrosis in the liver and leads to the elevation of transaminase.26 Increased central venous pressure causes hepatocyte atrophy and perisinusoidal oedema in the liver.8, 10 Sinusoidal damage leads to impaired clearance of aspartate aminotransaminase.27 Portal hypertension and liver fibrosis cause a reduced platelet count and protein synthesis.7, 9 Increased pressure within the hepatic sinusoid favours bile duct damage by disrupting endothelial cells and the interhepatocyctic tight junctions that separate the extravascular space from the bile canaliculus. Further, stagnant flow favours thrombosis within sinusoids, hepatic venules, and portal tracts, thereby contributing to liver fibrosis.9, 28 Centrilobular liver cell necrosis can extend to peripheral areas if HF persists and worsens and is followed by the deposition and spread of connective tissue bridging one central vein to another, ultimately leading to liver cirrhosis.9 Concordant with these findings, increased central venous pressure and atrial volume overload, rather than decreased cardiac output or increased pulmonary artery pressure, were assumed in high‐NFS groups in the current HFpEF patients.
Systemic fibrosis, fibrogenesis, and collagen turnover may be one plausible link between HFpEF and NAFLD.5, 16, 19, 29, 30, 31, 32 Extracellular matrix alterations, which contain synthesis and degradation of collagen, occur not only in the myocardium but also in the arterial wall, kidneys, lungs, and liver in systemic disease (e.g. HF and chronic liver disease).29, 30 Collagen‐dependent ventricular stiffness is increased in HFpEF.1, 2, 3, 4, 5 The cardiac extracellular matrix is predominantly composed of fibrillar collagen type I (85%) and type III (11%).29 Myocardial fibrosis occurs when synthesis of fibrillary collagen types I and III predominates over unchanges or decreased degradation.32 An excess of highly cross‐linked thick collagen type I fibres to the detriment of poorly cross‐linked thin collagen type III fibres is typical of adverse fibrosis.32 On the other hand, type IV collagens are relatively specifically observed in the liver and partially observed in the basement membrane of the myocytes, in the perivascular, and in the pericellular space in the heart.29 Unlike type I and type III collagens, which are processed by proteolysis, type IV collagen is deposited intact in the matrix and the serum component of type IV collagen reflects matrix degradation.20
Circulating markers of plausible systemic fibrosis and collagen turnover, such as procollagen type III collagen aminoterminal peptide as metabolites of PIIIP,33, 34, 35, 36 type IV collagen,36, 37 carboxy‐terminal telopeptide of collagen type I,33, 35 and growth differentiation factor 15,38 are associated with cardiac remodelling and adverse prognosis in HF patients.33, 34, 35, 38 Elevated circulating levels of type III collagen is associated with increased aortic fibrosis and stiffness,39 increased myocardial collagen types I and III,33 systolic and diastolic dysfunction of right and left ventricles in hypertensive patients,35, 40 and diastolic dysfunction in HFpEF patients39, 41, 42 and is partly associated with adverse prognosis in HFpEF patients.34 On the other hand, it has been recently reported that type IV collagen 7S, which is a relatively specific fibrosis marker in the liver, is correlated with pulmonary capillary wedge pressure and right ventricular and atrial pressure, but not with cardiac index,37 and associated with higher 1 year incidence of death or HF hospitalization in patients with HF.37 Concordant with these findings, in the present study, there were significant associations between type IV collagen 7S, PIIIP, and hyaluronic acid, which are established markers of liver fibrosis,16, 17, 19, 20, 43, 44 and the NFS. We assumed that higher NFS may indicate higher central venous pressure with resultant liver fibrosis (type IV collagen 7S, hyaluronic acid, and PIIIP) and impaired liver functional reserve and partly reflected by cardiac fibrosis (PIIIP) in HFpEF patients. The NFS16, 17, 18 can be used as an alternative marker for the measurement of liver fibrosis and/or reserve. This score can be calculated only by parameters measured in daily medical care. These parameters, such as AST, ALT, albumin, and platelet count, can be measured quickly even in outpatient clinics, by emergency medical services and more with point‐of‐care testing.
HFpEF and NAFLD may share other common pathophysiologies,1, 2, 45, 46, 47 namely elevated renin–angiotensin–aldosterone system, oxidative stress and inflammation, insulin resistance, impaired adiponectin, increased visceral fat, and increased systemic organ fibrosis associated with collagen turnover. Hence, NFS may suggest severity of pathophysiology of HFpEF, as well as NAFLD.
Study strengths and limitations
Our study has several strengths. For instance, the present study is the first to show the association of high NFS with high all‐cause mortality in HF patients, taking into consideration echocardiographic and haemodynamic parameters and peripheral collagen markers. In addition, HF diagnoses were made, and detailed causes of death were determined by our experienced cardiologists. Furthermore, there were no patients who dropped out.
The current study also has several limitations. First, as a prospective cohort study of a single centre with a relatively small number of patients, the study may be somewhat underpowered. Second, it remains unclear whether NFS only reflects liver fibrosis and/or congestion, with these together resulting in liver stiffness. Although HF patients with distinct liver disease were excluded, we cannot completely deny the presence of any liver disease. The relationship between NFS and other evaluations of fibrosis, such as liver biopsy, which is not generally performed in HF patients, or imaging (e.g. computed tomography) should be assessed in further studies. Third, time of catheterization is not simultaneous with evaluation of NFS at all. Hence, changes of haemodynamics did not necessarily reflect NFS. Fourth, we have used only variables on hospitalization in this study, without taking into consideration changes in medical parameters and post‐discharge treatment. Fifth, although we used multivariable Cox proportional hazard regression analyses, the effects of differences in clinical background among the four groups may not have been completely adjusted. Therefore, the present results should be viewed as preliminary, and further studies with a larger population are needed.
Conclusions
NFS, a novel indicator of liver fibrosis, is correlated with central venous pressure and peripheral collagen markers and is associated with higher mortality in the present HFpEF patients. NFS can be calculated simply and may be a useful tool to assess liver fibrosis and prognosis in HFpEF patients.
Conflict of interest
None declared.
Funding
This study was supported in part by a Grant‐in‐Aid for Scientific Research (no. 16K09447) from the Japan Society for the Promotion of Science.
Acknowledgements
The authors acknowledge the efforts of Ms Kumiko Watanabe, Hitomi Kobayashi, and Tomiko Miura for their outstanding technical assistance.
Yoshihisa, A. , Sato, Y. , Yokokawa, T. , Sato, T. , Suzuki, S. , Oikawa, M. , Kobayashi, A. , Yamaki, T. , Kunii, H. , Nakazato, K. , Saitoh, S. , and Takeishi, Y. (2018) Liver fibrosis score predicts mortality in heart failure patients with preserved ejection fraction. ESC Heart Failure, 5: 262–270. doi: 10.1002/ehf2.12222.
References
- 1. Sharma K, Kass DA. Heart failure with preserved ejection fraction: mechanisms, clinical features, and therapies. Circ Res 2014; 115: 79–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Komajda M, Lam CS. Heart failure with preserved ejection fraction: a clinical dilemma. Eur Heart J 2014; 35: 1022–1032. [DOI] [PubMed] [Google Scholar]
- 3. Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 2013; 62: 263–271. [DOI] [PubMed] [Google Scholar]
- 4. Zile MR, Baicu CF, Ikonomidis JS, Stroud RE, Nietert PJ, Bradshaw AD, Slater R, Palmer BM, Van Buren P, Meyer M, Redfield MM, Bull DA, Granzier HL, LeWinter MM. Myocardial stiffness in patients with heart failure and a preserved ejection fraction: contributions of collagen and titin. Circulation 2015; 131: 1247–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weber KT, Sun Y, Bhattacharya SK, Ahokas RA, Gerling IC. Myofibroblast‐mediated mechanisms of pathological remodelling of the heart. Nat Rev Cardiol 2013; 10: 15–26. [DOI] [PubMed] [Google Scholar]
- 6. Fu M, Zhou J, Thunstrom E, Almgren T, Grote L, Bollano E, Schaufelberger M, Johansson MC, Petzold M, Swedberg K, Andersson B. Optimizing the management of heart failure with preserved ejection fraction in the elderly by targeting comorbidities (OPTIMIZE‐HFPEF). J Card Fail 2016; 22: 539–544. [DOI] [PubMed] [Google Scholar]
- 7. Verbrugge FH, Dupont M, Steels P, Grieten L, Malbrain M, Tang WH, Mullens W. Abdominal contributions to cardiorenal dysfunction in congestive heart failure. J Am Coll Cardiol 2013; 62: 485–495. [DOI] [PubMed] [Google Scholar]
- 8. Samsky MD, Patel CB, DeWald TA, Smith AD, Felker GM, Rogers JG, Hernandez AF. Cardiohepatic interactions in heart failure: an overview and clinical implications. J Am Coll Cardiol 2013; 61: 2397–2405. [DOI] [PubMed] [Google Scholar]
- 9. Moller S, Bernardi M. Interactions of the heart and the liver. Eur Heart J 2013; 34: 2804–2811. [DOI] [PubMed] [Google Scholar]
- 10. Nikolaou M, Parissis J, Yilmaz MB, Seronde MF, Kivikko M, Laribi S, Paugam‐Burtz C, Cai D, Pohjanjousi P, Laterre PF, Deye N, Poder P, Cohen‐Solal A, Mebazaa A. Liver function abnormalities, clinical profile, and outcome in acute decompensated heart failure. Eur Heart J 2013; 34: 742–749. [DOI] [PubMed] [Google Scholar]
- 11. Taniguchi T, Sakata Y, Ohtani T, Mizote I, Takeda Y, Asano Y, Masuda M, Minamiguchi H, Kanzaki M, Ichibori Y, Nishi H, Toda K, Sawa Y, Komuro I. Usefulness of transient elastography for noninvasive and reliable estimation of right‐sided filling pressure in heart failure. Am J Cardiol 2014; 113: 552–558. [DOI] [PubMed] [Google Scholar]
- 12. Colli A, Pozzoni P, Berzuini A, Gerosa A, Canovi C, Molteni EE, Barbarini M, Bonino F, Prati D. Decompensated chronic heart failure: increased liver stiffness measured by means of transient elastography. Radiology 2010; 257: 872–878. [DOI] [PubMed] [Google Scholar]
- 13. Hakui H, Yamada T, Tamaki S, Morita T, Furukawa Y, Iwasaki Y, Kawasaki M, Kikuchi A, Kondo T, Ishimi M, Sato Y, Seo M, Ozaki T, Ikeda I, Fukuhara E, Sakata Y, Fukunami M. Usefulness of cardiac metaiodobenzylguanidine imaging to improve prognostic power of the model for end‐stage liver disease scoring system in patients with mild‐to‐moderate chronic heart failure. Am J Cardiol 2016; 117: 1947–1952. [DOI] [PubMed] [Google Scholar]
- 14. Jalal Z, Iriart X, De Ledinghen V, Barnetche T, Hiriart JB, Vergniol J, Foucher J, Thambo JB. Liver stiffness measurements for evaluation of central venous pressure in congenital heart diseases. Heart 2015; 101: 1499–1504. [DOI] [PubMed] [Google Scholar]
- 15. Hopper I, Kemp W, Porapakkham P, Sata Y, Condon E, Skiba M, Farber L, Porapakkham P, Williams TJ, Menahem S, Roberts S, Krum H. Impact of heart failure and changes to volume status on liver stiffness: non‐invasive assessment using transient elastography. Eur J Heart Fail 2012; 14: 621–627. [DOI] [PubMed] [Google Scholar]
- 16. Musso G, Gambino R, Cassader M, Pagano G. Meta‐analysis: natural history of non‐alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non‐invasive tests for liver disease severity. Ann Med 2011; 43: 617–649. [DOI] [PubMed] [Google Scholar]
- 17. McPherson S, Stewart SF, Henderson E, Burt AD, Day CP. Simple non‐invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non‐alcoholic fatty liver disease. Gut 2010; 59: 1265–1269. [DOI] [PubMed] [Google Scholar]
- 18. Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, Enders F, Saksena S, Burt AD, Bida JP, Lindor K, Sanderson SO, Lenzi M, Adams LA, Kench J, Therneau TM, Day CP. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007; 45: 846–854. [DOI] [PubMed] [Google Scholar]
- 19. Sanyal AJ, American Gastroenterological Association . AGA technical review on nonalcoholic fatty liver disease. Gastroenterology 2002; 123: 1705–1725. [DOI] [PubMed] [Google Scholar]
- 20. Grigorescu M. Noninvasive biochemical markers of liver fibrosis. J Gastrointestin Liver Dis 2006; 15: 149–159. [PubMed] [Google Scholar]
- 21. McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med 1971; 285: 1441–1446. [DOI] [PubMed] [Google Scholar]
- 22. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Authors/Task Force M, Document R . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 23. von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, Initiative S. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007; 335: 806–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010; 23: 685–713; quiz 786‐8. [DOI] [PubMed] [Google Scholar]
- 25. Dragu R, Rispler S, Habib M, Sholy H, Hammerman H, Galie N, Aronson D. Pulmonary arterial capacitance in patients with heart failure and reactive pulmonary hypertension. Eur J Heart Fail 2015; 17: 74–80. [DOI] [PubMed] [Google Scholar]
- 26. Birrer R, Takuda Y, Takara T. Hypoxic hepatopathy: pathophysiology and prognosis. Intern Med 2007; 46: 1063–1070. [DOI] [PubMed] [Google Scholar]
- 27. Kamimoto Y, Horiuchi S, Tanase S, Morino Y. Plasma clearance of intravenously injected aspartate aminotransferase isozymes: evidence for preferential uptake by sinusoidal liver cells. Hepatology 1985; 5: 367–375. [DOI] [PubMed] [Google Scholar]
- 28. Cogger VC, Fraser R, Le Couteur DG. Liver dysfunction and heart failure. Am J Cardiol 2003; 91: 1399. [DOI] [PubMed] [Google Scholar]
- 29. Chalikias GK, Tziakas DN. Biomarkers of the extracellular matrix and of collagen fragments. Clin Chim Acta 2015; 443: 39–47. [DOI] [PubMed] [Google Scholar]
- 30. Lopez B, Gonzalez A, Diez J. Circulating biomarkers of collagen metabolism in cardiac diseases. Circulation 2010; 121: 1645–1654. [DOI] [PubMed] [Google Scholar]
- 31. Lopez B, Ravassa S, Gonzalez A, Zubillaga E, Bonavila C, Berges M, Echegaray K, Beaumont J, Moreno MU, San Jose G, Larman M, Querejeta R, Diez J. Myocardial collagen cross‐linking is associated with heart failure hospitalization in patients with hypertensive heart failure. J Am Coll Cardiol 2016; 67: 251–260. [DOI] [PubMed] [Google Scholar]
- 32. Heymans S, Gonzalez A, Pizard A, Papageorgiou AP, Lopez‐Andres N, Jaisser F, Thum T, Zannad F, Diez J. Searching for new mechanisms of myocardial fibrosis with diagnostic and/or therapeutic potential. Eur J Heart Fail 2015; 17: 764–771. [DOI] [PubMed] [Google Scholar]
- 33. Klappacher G, Franzen P, Haab D, Mehrabi M, Binder M, Plesch K, Pacher R, Grimm M, Pribill I, Eichler HG, Dietmar Glogar H. Measuring extracellular matrix turnover in the serum of patients with idiopathic or ischemic dilated cardiomyopathy and impact on diagnosis and prognosis. Am J Cardiol 1995; 75: 913–918. [DOI] [PubMed] [Google Scholar]
- 34. Krum H, Elsik M, Schneider HG, Ptaszynska A, Black M, Carson PE, Komajda M, Massie BM, McKelvie RS, McMurray JJ, Zile MR, Anand IS. Relation of peripheral collagen markers to death and hospitalization in patients with heart failure and preserved ejection fraction: results of the I‐PRESERVE collagen substudy. Circ Heart Fail 2011; 4: 561–568. [DOI] [PubMed] [Google Scholar]
- 35. Plaksej R, Kosmala W, Frantz S, Herrmann S, Niemann M, Stork S, Wachter R, Angermann CE, Ertl G, Bijnens B, Weidemann F. Relation of circulating markers of fibrosis and progression of left and right ventricular dysfunction in hypertensive patients with heart failure. J Hypertens 2009; 27: 2483–2491. [DOI] [PubMed] [Google Scholar]
- 36. Sato Y, Kataoka K, Matsumori A, Sasayama S, Yamada T, Ito H, Takatsu Y. Measuring serum aminoterminal type III procollagen peptide, 7S domain of type IV collagen, and cardiac troponin T in patients with idiopathic dilated cardiomyopathy and secondary cardiomyopathy. Heart 1997; 78: 505–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nagao K, Tamura A, Morimoto T, Shimamura K, Yukawa H, Ito H, Hayashi F, Makita T, Takemura G, Sato Y, Inada T, Kimura T, Tanaka M. Liver fibrogenesis marker, 7S domain of collagen type IV in patients with acutely decompensated heart failure: correlates, prognostic value and time course. Int J Cardiol 2017; 236: 483–487. [DOI] [PubMed] [Google Scholar]
- 38. Izumiya Y, Hanatani S, Kimura Y, Takashio S, Yamamoto E, Kusaka H, Tokitsu T, Rokutanda T, Araki S, Tsujita K, Tanaka T, Yamamuro M, Kojima S, Tayama S, Kaikita K, Hokimoto S, Ogawa H. Growth differentiation factor‐15 is a useful prognostic marker in patients with heart failure with preserved ejection fraction. Can J Cardiol 2014; 30: 338–344. [DOI] [PubMed] [Google Scholar]
- 39. Bonapace S, Rossi A, Cicoira M, Franceschini L, Golia G, Zanolla L, Marino P, Zardini P. Aortic distensibility independently affects exercise tolerance in patients with dilated cardiomyopathy. Circulation 2003; 107: 1603–1608. [DOI] [PubMed] [Google Scholar]
- 40. Poulsen SH, Andersen NH, Heickendorff L, Mogensen CE. Relation between plasma amino‐terminal propeptide of procollagen type III and left ventricular longitudinal strain in essential hypertension. Heart 2005; 91: 624–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Martos R, Baugh J, Ledwidge M, O'Loughlin C, Murphy NF, Conlon C, Patle A, Donnelly SC, McDonald K. Diagnosis of heart failure with preserved ejection fraction: improved accuracy with the use of markers of collagen turnover. Eur J Heart Fail 2009; 11: 191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cicoira M, Rossi A, Bonapace S, Zanolla L, Golia G, Franceschini L, Caruso B, Marino PN, Zardini P. Independent and additional prognostic value of aminoterminal propeptide of type III procollagen circulating levels in patients with chronic heart failure. J Card Fail 2004; 10: 403–411. [DOI] [PubMed] [Google Scholar]
- 43. Guha IN, Parkes J, Roderick P, Chattopadhyay D, Cross R, Harris S, Kaye P, Burt AD, Ryder SD, Aithal GP, Day CP, Rosenberg WM. Noninvasive markers of fibrosis in nonalcoholic fatty liver disease: validating the European Liver Fibrosis Panel and exploring simple markers. Hepatology 2008; 47: 455–460. [DOI] [PubMed] [Google Scholar]
- 44. Murawaki Y, Ikuta Y, Koda M, Kawasaki H. Serum type III procollagen peptide, type IV collagen 7S domain, central triple‐helix of type IV collagen and tissue inhibitor of metalloproteinases in patients with chronic viral liver disease: relationship to liver histology. Hepatology 1994; 20(4 Pt 1): 780–787. [DOI] [PubMed] [Google Scholar]
- 45. Brea A, Puzo J. Non‐alcoholic fatty liver disease and cardiovascular risk. Int J Cardiol 2013; 167: 1109–1117. [DOI] [PubMed] [Google Scholar]
- 46. Bhatia LS, Curzen NP, Calder PC, Byrne CD. Non‐alcoholic fatty liver disease: a new and important cardiovascular risk factor? Eur Heart J 2012; 33: 1190–1200. [DOI] [PubMed] [Google Scholar]
- 47. Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med 2010; 363: 1341–1350. [DOI] [PubMed] [Google Scholar]


