Abstract
Aims
Cardiac affection constitutes a major limiting condition in systemic sarcoidosis. The primary objective of this study was to investigate the persistence rate of cardiac sarcoid involvement by cardiovascular magnetic resonance (CMR) imaging in patients diagnosed with cardiac sarcoidosis (CS). Moreover, we examined the additional insights into myocardial damage's characteristics gained by somatostatin receptor scintigraphy.
Methods and results
In a pilot study, we had previously identified cardiac involvement—diagnosed by CMR imaging—to be present in 29 of 188 patients (15.4%) with histologically proven, extra‐CS. Out of these initial 29 CS‐positive patients, 27 patients (49.9 ± 11.8 years, 59.3% male) were presently re‐examined and underwent a second CMR study and complementary standard clinical testing. Somatostatin receptor scintigraphy using the ligand 68Ga‐DOTATOC was additionally performed when clinically indicated (17 patients). Within a median follow‐up period of 2.6 years, none of the initial 29 patients deceased or experienced aborted sudden cardiac death. However, two patients developed third‐degree atrioventricular block that required device therapy. Among the 27 re‐examined CS patients, pathological CMR findings persisted in 14 of 27 patients (51.9%). CS remission was primarily due to a resolution of acute inflammatory processes. 68Ga‐DOTATOC positron emission tomography/computed tomography (PET/CT) identified one patient with regions of raised tracer uptake that concorded with acute inflammatory changes, as assessed by CMR; this patient received no immunosuppressive medication at the time of PET/CT execution.
Conclusions
Within follow‐up, CS persisted in barely half the patients, and the patients were not afflicted with cardiac death. Additional 68Ga‐DOTATOC PET/CT allowed for visualization of acute myocardial inflammation.
Keywords: Cardiac sarcoidosis, Cardiovascular magnetic resonance, 68Ga‐DOTATOC PET/CT
Introduction
Sarcoidosis is a multisystem, non‐caseating granulomatous disorder of unknown aetiology, whose epidemiology underlies geographic and ethnic variations.1, 2 Cardiac sarcoidosis (CS) may occur isolatedly without any concurrent extracardiac presentation or in the context of multiorgan sarcoid affection. The clinical presentation of sarcoidal heart involvement is manifold and comprehends the whole range from clinically silent courses up to non‐ischaemic cardiomyopathy and conduction system defects, depending on the location of cardiac granulomatous infiltration.3 Cardiac affection is described to be the second leading cause of death by sarcoidosis worldwide and the most common one in Japan.4, 5 In turn, up to 65% of cardiac deceases are due to sudden cardiac death in consequence of conduction blocks or malignant ventricular arrhythmias.6 In keeping with this, the necessity of establishing accurate diagnostic strategies arises that allow for both feasible baseline diagnosis and follow‐up monitoring.
In a preceding, prospectively conducted trial comprising 188 patients with histologically proven sarcoidosis, we observed a prevalence of cardiovascular magnetic resonance (CMR)‐based cardiac affection of 15.4%.7 In order to assess the middle‐term persistence rate of CMR findings, we presently took aim at performing longitudinal investigation of CS‐positive patients by CMR re‐examination and correlated the hereby obtained results with standard clinical testing.
Moreover, we assessed the additional insights into myocardial damage's characteristics gained by somatostatin receptor (SSTR) scintigraphy. As demonstrated previously,8, 9 receptors for somatostatin are present in the inflammatory lesions of patients with sarcoidosis and located on, e.g. granuloma epithelioid cells, macrophages, and giant cells. SSTR‐directed positron emission tomography (PET) imaging might complement CMR by visualizing the aforementioned inflammatory cell's distribution within damaged myocardium.10 In a sub‐analysis, we, therefore, retrospectively identified patients who additionally underwent PET/computed tomography (CT) using the SSTR ligand 68Ga‐DOTA‐d‐Phe1‐Tyr3‐octreotide (68Ga‐DOTATOC) and correlated PET results with CMR findings and clinical parameters.
Materials and methods
Study population
We disposed of the data previously obtained by CMR imaging in 188 Caucasian patients comprising study population that was composed of patients linked to the University Hospital's Department of Pneumology (Bonn, Germany) and those recruited by a nationwide acting registered sarcoidosis self‐help association.7 This initial examination revealed that CS was present in 29 out of 188 patients, i.e. in 15.4% of total study population. These CS‐positive patients were presently prospectively followed up by CMR re‐examination after a median period of 2.6 years. At the time of CMR re‐examination, complementary standard clinical testing by 12‐lead electrocardiogram (ECG), 24 h Holter monitoring, transthoracic echocardiography, and laboratory and pulmonary function testing was performed.
Contrary to the prospective approach of CMR re‐conduction, additional nuclear imaging by use of 68Ga‐DOTATOC PET/CT was performed subsequent to CMR re‐examination and only in cardiac symptomatic patients (chest pain, palpitations, dizziness, and syncopal episodes) at the discretion of the treating physician. The hereby obtained observational data on 68Ga‐DOTATOC PET/CT were retrospectively analysed.
The study was approved by the ethics committee of the University of Bonn and was according to the principles expressed in the Declaration of Helsinki. Written informed consent was obtained from all patients.
Electrocardiography
A 12‐lead surface ECG was systematically obtained at the time of CMR re‐conduction. Pathological electrocardiographic findings comprehended those described to be general risk stratifiers for sudden cardiac death,11 those integrated in the Heart Rhythm Society's (HRS's) expert consensus statement on the diagnosis and management of CS‐associated arrhythmias,12 and those exposed in the revised Japanese Ministry of Health and Welfare Guidelines (JMHWG).13
All study participants underwent 24 h Holter monitoring, recorded by the SpiderView™ Ambulatory Electrocardiographic Recorder (SORIN GROUP, Munich, Germany). Holter ECG data were analysed for conduction block occurrence, as well as supraventricular and ventricular arrhythmic burden. Moreover, we examined heart rate variability as an established predictor of increased mortality and elevated risk of cardiac events in the general population.14 T‐wave alternans that displays heterogeneity in ventricular repolarization and thus predicts ventricular arrhythmias in patients at risk for sudden cardiac death was additionally registered.15 Both 12‐lead ECG and Holter recording were analysed by experienced cardiologists, blinded to the patients' clinical background information.
Transthoracic echocardiography
In addition to CMR follow‐up, each CS‐positive patient underwent standardized two‐dimensional (2D) transthoracic echocardiography for evaluation of left and right ventricular functional parameters in accordance with the recommendations of the American Society of Echocardiography.16 Standard echocardiographic views were obtained with the patient in the left lateral decubitus position in 2D and colour tissue Doppler imaging modes and by use of a commercially available ultrasound scanner (Vivid 7, General Electric Medical Health, Waukesha, WI, USA; iE 33, Philips Medical Systems, Koninklijke N.V., Hamburg, Germany). Pathological echocardiographic results were pre‐defined in line with the HRS consensus statement12 and the revised JMHWG.13 Accordant with electrocardiographic analysis, all echocardiographic studies were performed by versed cardiologists.
Laboratory and pulmonary function testing
All CS‐positive patients underwent additional laboratory and lung function assessment at the time of CMR re‐conduction. Blood sampling comprised a full blood count and C‐reactive protein for inflammatory measurement, soluble interleukin‐2 receptor, and serum angiotensin converting enzyme for evaluation of sarcoid activity, as well as plasma N terminal pro‐brain natriuretic peptide as a biomarker for cardiac failure. In line with the European Respiratory Society's guidelines,17 standardized pulmonary function testing comprehended spirometry, body plethysmography, and assessment of diffusing capacity for carbon monoxide.
Cardiovascular magnetic resonance imaging
CMR images were acquired ECG‐gated during repeated breath‐holding on a clinical 1.5 T scanner (Intera 1.5T, Philips Healthcare, Best, The Netherlands) by use of cardiac‐dedicated phased‐array coils. The CMR protocol comprised functional imaging, T2 imaging, and assessment of relative and late gadolinium enhancement (LGE). Functional imaging was performed using steady‐state free‐precession cine sequences in short axis (SA), horizontal long axis (HLA), vertical long axis, and left ventricular outflow tract orientation. Fat‐suppressed T2‐weighted black‐blood turbo spin echo images in SA and HLA were obtained and allowed for visualization of myocardial oedema. Presence of myocardial hyperaemia as evidence of inflammatory myocardial tissue damage was detected by assessment of early relative contrast enhancement using T1‐weighted multislice imaging in transverse orientation before and immediately after injection of a single dose of extracellular contrast agent (0.1 mmol/kg gadolinium‐DTPA).18 Subsequently, a second single dose of extracellular contrast agent was injected, allowing for delayed contrast enhancement imaging (detection of myocardial scars) by the use of T1‐weighted inversion‐recovery gradient echo sequence. The 17‐segment model of standardized myocardial nomenclature was applied for description of left ventricular delayed contrast enhancement distribution.19 CMR studies were conducted in conformity with the recommendations on standardized CMR imaging20 and independently analysed by two experienced radiologists, blinded to the patients' clinical data. In case of discordant results, the studies were re‐assessed to reach consensus.
68 Ga‐DOTATOC positron emission tomography/computed tomography
68Ga‐DOTATOC, combined with high‐resolution PET and integrated CT, was employed for functional molecular cardiac imaging to assess inflammatory myocardial activity. It targets the SSTR, which has been shown to be overexpressed by activated lymphocytes, macrophages, and epithelioid cells. 68Ga‐DOTATOC was conducted on a Biograph 2 PET/CT scanner (Siemens Medical Solutions, Erlangen, Germany). After a mean time interval of 65.1 ± 28.0 min after intravenous administration of 2 MBq/kg 68Ga‐DOTATOC (mean, 118.2 ± 24.7 MBq 68Ga‐DOTATOC), static image acquisition was performed. Emission scans (three‐dimensional mode) were obtained of the body trunk (4 min emission time per bed position) followed by one additional bed position of the cardiac region (20 min emission time). A low‐dose CT (no intravenous contrast, 16 mA·s, 130 kV) was obtained for attenuation correction. PET data were reconstructed iteratively (AWOSEM, four iterations, eight subsets) using scatter and attenuation correction. After reconstruction, images were smoothed by a 5 mm Gaussian filter.
68 Ga‐DOTATOC PET/CT and CMR data were co‐registered using syngoMMWP software (version VE50A, Siemens Healthineers, Erlangen, Germany). Co‐registration was performed using an automatic rigid algorithm provided by the software, and image fusion was manually corrected by visual confirmation.
PET/CT images were analysed in a multidisciplinary setting of nuclear medicine physicians and radiologists. In case of discordance, studies were re‐assessed to reach consensus.
Statistical analysis
In case of continuous variables, data were expressed as mean values ± standard deviation; categorical variables were given as total numbers and percentages. Comparison between two groups was performed by use of Mann–Whitney U‐test or Student's t‐test, as appropriate. When comparing more than two groups, the non‐parametric Kruskal–Wallis test was employed. Categorical variables were analysed by Pearson's χ2 test or Fisher's exact test. Correlation analysis was performed by use of Pearson correlation coefficient (Pearson's r). A two‐tailed P‐value of <0.05 was considered statistically significant. Statistics were performed using SPSS software version 23.0 (Armonk, NY, USA).
Results
Patient characteristics
Demographic and clinical characteristics of patients undergoing CMR follow‐up are specified in Table 1. Twenty‐seven patients were prospectively enrolled and examined for persistence and degree of cardiac sarcoid affection. The total patient number diverged from the original 29 CS‐positive patients as identified by primary CMR analysis for the following reasons: two patients developed third‐degree atrioventricular block in the meantime, which required permanent pacemaker placement; of these two patients, only one patient received a CMR‐compatible device that permitted CMR re‐conduction. A further patient was excluded from follow‐up owing to renal impairment that contradicted gadolinium application.
Table 1.
Patient demographic and clinical characteristics at the time of cardiovascular magnetic resonance re‐conduction
|
All patients n = 27 | |
|---|---|
| Age (years) | 49.9 ± 11.8 |
| Male gender, n (%) | 16 (59.3%) |
| Organ of sarcoidosis' histological confirmation, n (%) | |
| Lung | 15 (55.6%) |
| Lymph node | 7 (25.9%) |
| Skin | 3 (11.1%) |
| Kidney | 1 (3.7%) |
| Central nervous system | 1 (3.7%) |
| Pulmonary function parameters | |
| FEV1 (L) | 3.0 ± 1.0 |
| FEV1 (% predicted) | 92.9 ± 19.3 |
| RV (L) | 2.1 ± 0.5 |
| RV (% predicted) | 105.6 ± 22.1 |
| R tot (kPa·s/L) | 0.24 ± 0.10 |
| R tot (% predicted) | 79.2 ± 35.1 |
| IVC (L) | 3.9 ± 1.2 |
| IVC (% predicted) | 94.4 ± 15.8 |
| TLC (L) | 6.0 ± 1.3 |
| TLC (% predicted) | 96.0 ± 11.5 |
| DLco (mmol/min/kPa) | 7.0 ± 2.2 |
| DLco (% predicted) | 72.6 ± 15.4 |
| DLco/VA (mmol/min/kPa/L) | 1.4 ± 0.2 |
| DLco/VA (% predicted) | 88.6 ± 14.2 |
| Capillary blood gas analysis | |
| pO2 (mmHg) | 78.2 ± 8.8 |
| pCO2 (mmHg) | 33.9 ± 4.0 |
| sO2 (%) | 95.6 ± 2.2 |
| Cardiovascular risk factors, n (%) | |
| Arterial hypertension | 11 (40.7%) |
| Diabetes mellitus | 4 (14.8%) |
| Dyslipidaemia | 12 (44.4%) |
| Obesity | 9 (33.3%) |
| BMI (kg/m2) | 29.3 ± 6.4 |
| Familial disposition | 10 (37.0%) |
| Continued nicotine consumption | 12 (44.4%) |
| Pack‐years | 6.0 ± 9.5 |
| Cardiac symptomatology, n (%)a | |
| Palpitation | 15 (55.6%) |
| Chest pain | 15 (55.6%) |
| Dizziness | 10 (37.0%) |
| Syncopal episodes | 0 (0.0%) |
| NYHA functional class, n (%) | |
| No limitations | 1 (3.7%) |
| I | 6 (22.2%) |
| II | 14 (51.9%) |
| III | 5 (18.5%) |
| IV | 1 (3.7%) |
| Cardiovascular medication use at CMR re‐conduction, n (%) | |
| Beta‐blocker | 8 (29.6%) |
| Bisoprolol | 5 (18.5%) |
| Metoprolol | 2 (7.4%) |
| Carvedilol | 1 (3.7%) |
| ACE inhibitor | 9 (33.3%) |
| Angiotensin II receptor blocker | 1 (3.7%) |
| Diuretics | 9 (33.3%) |
| Ivabradine | 2 (7.4%) |
| Amiodarone | 1 (3.7%) |
| Immunosuppressant use at CMR re‐conduction, n (%) | |
| Steroidal monotherapy | 10 (37.0%) |
| Azathioprine, combined with steroids | 2 (7.4%) |
| Methotrexate, combined with steroids | 1 (3.7%) |
| Immunosuppressant use since the initial, CS‐confirming CMR conduction, n (%) | |
| Steroidal monotherapy | 11 (40.7%) |
| Azathioprine, combined with steroids | 4 (14.8%) |
| Methotrexate, combined with steroids | 1 (3.7%) |
Data are presented as mean ± SD or total number and percentage (in parentheses), as appropriate.
ACE, angiotensin converting enzyme; BMI, body mass index; CMR, cardiovascular magnetic resonance; CS, cardiac sarcoidosis; DLco, diffusion capacity of the lung for carbon monoxide; FEV1, forced expiratory volume in first second of expiration; IVC, inspiratory vital capacity; NYHA, New York Heart Association; pCO2, carbon dioxide partial pressure; pO2, oxygen partial pressure; R tot, total resistance; RV, residual volume; sO2, oxygen saturation; TLC, total lung capacity; VA, alveolar volume.
Symptomatology refers to any time occurrence since the initial, CS‐confirming CMR conduction.
Overall, study population showed a slight predominance of men (59.3%) and was middle aged (49.9 ± 11.8 years); all patients were of Caucasian origin. Histological confirmation and principal manifestation of sarcoidosis were mainly pulmonary (55.6%). Pulmonary function testing revealed no substantial restrictive pattern, with values for inspiratory vital and total lung capacity within the normal range (94.4 ± 15.8% and 96.0 ± 11.5% of predicted, respectively). Mean diffusing capacity for carbon monoxide showed no restriction for pulmonary gas exchange (diffusion capacity of the lung for carbon monoxide/alveolar volume, 88.6 ± 14.2% of predicted). Table 1 presents the patients' cardiovascular risk profile; of note, no patient had a history of concomitant coronary heart disease. Eight patients were asymptomatic for cardiac symptomatology that comprised chest pain, palpitations, dizziness, and syncopal episodes. At the time of CMR re‐conduction, 13 patients (48.1%) were under medical immunosuppressive treatment: 37.0% received steroidal monotherapy; in 7.4% and 3.7% of cases, azathioprine and methotrexate were added, respectively. Out of these 13 patients, in four patients, medical immunosuppressive treatment was primarily due to cardiac sarcoid affection, whereas the remaining nine patients received immunosuppressants in consequence of their extracardiac sarcoid manifestation.
Results obtained by cardiovascular magnetic resonance imaging
Presence of LGE defined cardiac sarcoid affection; early relative gadolinium enhancement and regional oedema were suspective of cardiac sarcoid involvement. Overall, aforesaid CMR findings were present in 14 out of 27 patients (51.9%). Differentiation of these persistently CS‐positive patients as a function of the disease's activity showed that 50.0% of patients had chronic, non‐viable‐fibrotic changes as described by LGE (Figures 1 and 2): 7.1% of patients presented isolated acute inflammatory changes, suspective of sarcoidal origin, and 42.9% of patients presented both acute and chronic cardiac lesions. Localization analysis of late contrast enhancement per segment identified the mid‐inferoseptal ventricular region to be preferentially affected (Table 2). Table 3 displays results obtained for LGE, early relative gadolinium enhancement, and T2‐weighted imaging at baseline and follow‐up CMR for the whole study population.
Figure 1.

CMR baseline (A, B) and follow‐up examination (C, D) in axial (A), horizontal long axis (C), and short axis orientation (B, D) in an asymptomatic patient without abnormalities in standard clinical testing. CMR demonstrates mid‐myocardial and subepicardial late gadolinium enhancement within the septal wall (arrows). Data correspond to Case 14 in Table 3. CMR, cardiac magnetic resonance.
Figure 2.
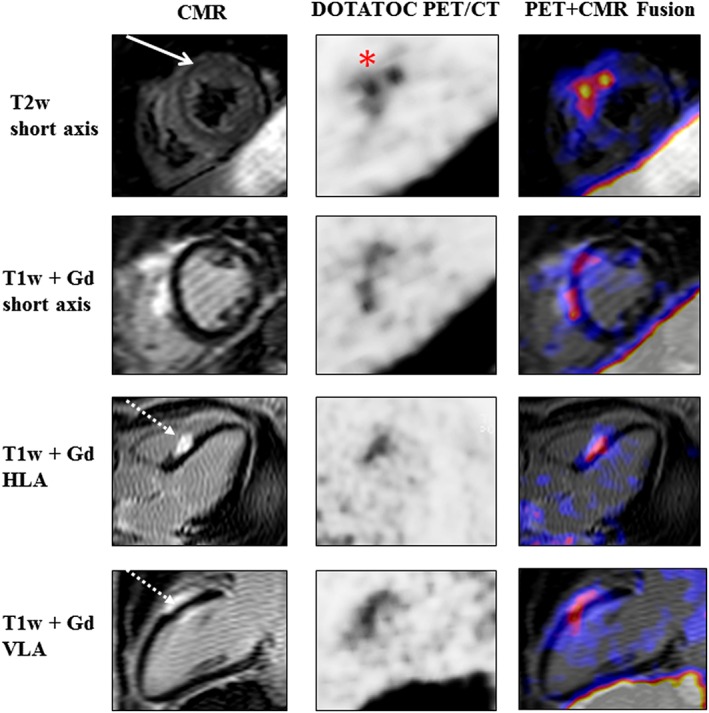
CMR, 68Ga‐DOTATOC PET/CT, and 68Ga‐DOTATOC PET/CMR fused images (from left to right) of a 48‐year‐old woman with histologically proven pulmonary sarcoidosis and previously CMR‐confirmed cardiac affection. Follow‐up CMR illustrates mid‐myocardial oedema (full‐line arrow) alongside the mid‐ventricular anteroseptal myocardium and mid‐myocardial/subepicardial late gadolinium enhancement (dotted‐line arrow) at the mid‐ventricular septal level. Radionuclide imaging visualizes the highest focal 68Ga‐DOTATOC uptake alongside the mid‐anteroseptal myocardium (red asterisk). CMR, cardiovascular magnetic resonance; Gd, gadolinium; HLA, horizontal long axis; PET, positron emission tomography; T1w, T1 weighted; T2w, T2 weighted; VLA, vertical long axis.
Table 2.
Cardiac magnetic resonance‐derived cardiac functional parameters and dimensions, pathology's activity, and its localization
|
All patients n = 27 |
CMR‐based diagnosis of cardiac sarcoidosisa
n = 14 |
CMR‐based exclusion of cardiac sarcoidosis n = 13 |
P‐value | |
|---|---|---|---|---|
| CMR‐assessed cardiac functional parameters and dimensions | ||||
| LVEF (%) | 58.8 ± 10.3 | 57.0 ± 11.4 | 60.7 ± 9.0 | 0.36 |
| LVEDV (mL) | 141.8 ± 48.5 | 150.1 ± 59.2 | 132.9 ± 33.6 | 0.37 |
| IVSD (mm) | 9.8 ± 1.6 | 9.5 ± 1.4 | 10.3 ± 1.7 | 0.20 |
| CMR‐assessed pathology's activity, n (%) | ||||
| Only non‐viable tissue damage, as assessed by late gadolinium enhancement | 7 (50.0%) | |||
| Both acute inflammatory and non‐viable tissue damage, as assessed by early relative gadolinium enhancement, T2 imaging, and late gadolinium enhancement | 6 (42.9%) | |||
| Only acute inflammatory tissue damage, as assessed by early relative gadolinium enhancement | 1 (7.1%) | |||
| CMR‐assessed localization of LGE according to AHA's segmentation and nomenclature,19 n (%) | ||||
| 1 basal anterior | 3 (8.3%) | |||
| 2 basal anteroseptal | 3 (8.3%) | |||
| 3 basal inferoseptal | 3 (8.3%) | |||
| 4 basal inferior | 2 (5.6%) | |||
| 5 basal inferolateral | 3 (8.3%) | |||
| 6 basal anterolateral | 2 (5.6%) | |||
| 7 mid‐anterior | 4 (11.1%) | |||
| 8 mid‐anteroseptal | 4 (11.1%) | |||
| 9 mid‐inferoseptal | 5 (13.9%) | |||
| 10 mid‐inferior | 0 (0.0%) | |||
| 11 mid‐inferolateral | 2 (5.6%) | |||
| 12 mid‐anterolateral | 4 (11.1%) | |||
| 13 apical anterior | 1 (2.8%) | |||
| 14 apical septal | 0 (0.0%) | |||
| 15 apical inferior | 0 (0.0%) | |||
| 16 apical lateral | 0 (0.0%) | |||
| 17 apex | 0 (0.0%) | |||
Data are presented as mean ± SD or total number and percentage (in parentheses), as appropriate.
AHA, American Heart Association; CMR, cardiovascular magnetic resonance; IVSD, diastolic interventricular septal thickness; LGE, late gadolinium enhancement; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction.
Detection of cardiac sarcoidosis relied on presence of LGE; in case of early relative gadolinium enhancement or pathological T2‐weighted imaging, cardiac sarcoid involvement was suspected.
Table 3.
Results obtained for late gadolinium enhancement, early relative gadolinium enhancement, and T2‐weighted imaging at baseline and follow‐up cardiac magnetic resonance
| Case | Age | CMR baseline T2/rel. Enh/LGE | CMR follow‐up T2/rel. Enh/LGE | Immunosuppressant use since baseline CMR |
|---|---|---|---|---|
| 1 | 59 | pos/neg/pos | neg/neg/neg | pos |
| 2 | 55 | neg/pos/neg | neg/neg/neg | pos |
| 3 | 47 | neg/pos/neg | neg/neg/neg | neg |
| 4 | 42 | neg/pos/neg | neg/neg/neg | pos |
| 5 | 50 | neg/pos/neg | pos/pos/pos | pos |
| 6 | 47 | neg/pos/neg | neg/neg/neg | pos |
| 7 | 62 | neg/pos/neg | neg/neg/neg | pos |
| 8 | 46 | neg/pos/neg | neg/neg/neg | neg |
| 9 | 58 | neg/neg/pos | neg/neg/pos | neg |
| 10 | 25 | neg/neg/pos | neg/neg/pos | pos |
| 11 | 52 | neg/neg/pos | neg/neg/pos | neg |
| 12 | 52 | neg/neg/pos | neg/neg/pos | pos |
| 13 | 31 | neg/neg/pos | neg/neg/pos | pos |
| 14 | 61 | neg/neg/pos | neg/neg/pos | pos |
| 15 | 51 | neg/pos/neg | neg/pos/neg | neg |
| 16 | 47 | neg/neg/pos | neg/neg/pos | neg |
| 17 | 67 | neg/pos/neg | neg/neg/neg | pos |
| 18 | 50 | pos/neg/neg | neg/neg/neg | neg |
| 19 | 61 | pos/neg/neg | pos/neg/pos | neg |
| 20 | 22 | neg/neg/pos | pos/neg/pos | neg |
| 21 | 47 | pos/neg/neg | pos/neg/pos | neg |
| 22 | 51 | neg/neg/pos | neg/neg/neg | pos |
| 23 | 42 | neg/neg/pos | neg/neg/neg | pos |
| 24 | 37 | pos/pos/neg | pos/pos/pos | pos |
| 25 | 71 | pos/pos/neg | pos/neg/pos | pos |
| 26 | 54 | neg/neg/pos | neg/neg/neg | pos |
| 27 | 65 | pos/neg/neg | neg/neg/neg | neg |
CMR, cardiovascular magnetic resonance; LGE, late gadolinium enhancement; neg, negative; pos, positive; rel. Enh, early relative gadolinium enhancement; T2, T2‐weighted imaging.
The comparison of CMR‐assessed left ventricular functional parameters and dimensions in CS‐positive and CS‐negative patients is displayed in Table 2. Overall, mean values for left ventricular ejection fraction (LVEF), left ventricular end‐diastolic volume, and diastolic interventricular septal thickness were within the normal range (58.8 ± 10.3%, 141.8 ± 48.5 mL, and 9.8 ± 1.6 mm, respectively). None of the aforementioned CMR‐derived parameters differed significantly between CS‐positive and CS‐negative patients (P = 0.36, P = 0.37, and P = 0.20, respectively).
Table 3 displays CMR baseline and CMR follow‐up data. Remission of early relative enhancement or regional oedema was observed in seven cases, of which five patients were under immunosuppressive treatment since baseline CMR. Case 1 is visualized in Figure 3 : interestingly, former positive LGE, T2, and early relative gadolinium enhancement were not detectable in CMR follow‐up examination. This patient was under immunosuppressants since baseline CMR. This course is consistent with the observations made by Nagai et al. who reported a similar case with remission of LGE findings under immunosuppressive treatment.21
Figure 3.
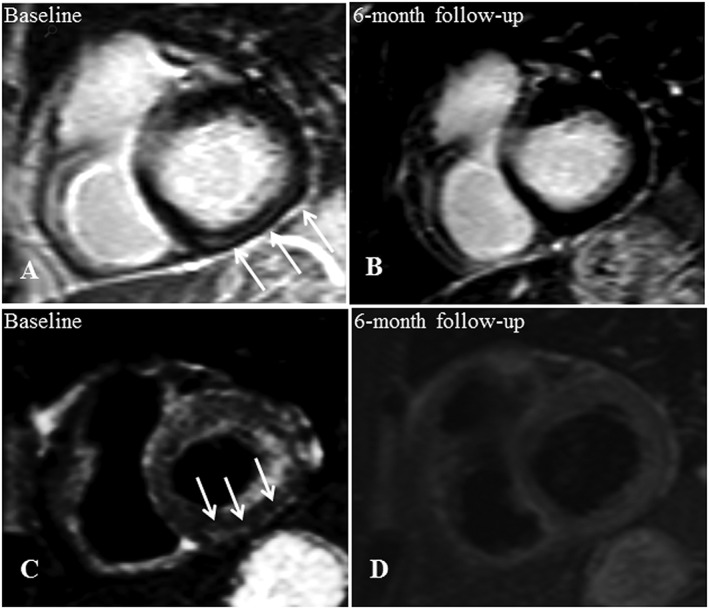
Baseline CMR (A, C) and 6 month follow‐up (B, D) of a 59‐year‐old cardiac asymptomatic patient. This woman suffered from hepatic sarcoidosis that had been histologically proven by liver biopsy. The initial LGE image in short axis view shows a striatal mid‐myocardial enhancement within the basal inferior wall (A) with corresponding oedema in the T2 black‐blood image (C). Both LGE (B) and oedema (D) were not detectable within the follow‐up examination. Data correspond to those of Case 1 in Table 3. CMR, cardiovascular magnetic resonance; LGE, late gadolinium enhancement.
Association of standard clinical testing parameters with cardiac magnetic resonance results
Table 4 summarizes the distribution of standard evaluation parameters as a function of follow‐up CMR results. Conventional transthoracic echocardiography failed to recognize CS persistence. Neither reduction in LVEF < 40% (enlisted as a diagnostic criterion within the current HRS expert consensus statement12) nor regional wall motion abnormalities or wall thickening (as specified by the revised JMHWG13) showed significant distributive differences between persistently CS‐positive and CS‐negative patients.
Table 4.
Demographic and clinical data, as a function of results at cardiac magnetic resonance re‐conduction
|
All patients n = 27 |
CMR‐based diagnosis of cardiac sarcoidosisa
n = 14 |
CMR‐based exclusion of cardiac sarcoidosis n = 13 |
P‐value | |
|---|---|---|---|---|
| Age (years) | 49.9 ± 11.8 | 48.8 ± 15.0 | 51.2 ± 7.4 | 0.60 |
| Male gender, n (%) | 16 (59.3%) | 7 (50.0%) | 9 (69.2%) | 0.31 |
| Cardiac symptomatology, n (%)b | ||||
| Palpitation | 15 (55.6%) | 6 (42.9%) | 9 (69.2%) | 0.17 |
| Chest pain | 15 (55.6%) | 6 (46.2%) | 9 (69.2%) | 0.23 |
| Dizziness | 10 (37.0%) | 4 (30.8%) | 6 (46.2%) | 0.42 |
| Syncopal episodes | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| NYHA functional class, n (%) | ||||
| No limitations | 1 (3.7%) | 1 (7.1%) | 0 (0.0%) | 0.33 |
| I | 6 (22.2%) | 4 (28.6%) | 2 (15.4%) | 0.41 |
| II | 14 (51.9%) | 5 (35.7%) | 9 (69.2%) | 0.08 |
| III | 5 (18.5%) | 3 (21.4%) | 2 (15.4%) | 0.69 |
| IV | 1 (3.7%) | 1 (7.1%) | 0 (0.0%) | 0.33 |
| Laboratory parameters | ||||
| Leucocytes, g/L | 7.0 ± 2.3 | 6.8 ± 2.7 | 7.2 ± 1.9 | 0.62 |
| Haemoglobin, g/dL | 14.3 ± 1.2 | 14.2 ± 1.3 | 14.4 ± 1.2 | 0.67 |
| Thrombocytes, g/L | 255.4 ± 66.6 | 251.1 ± 53.4 | 260.2 ± 80.4 | 0.74 |
| C‐reactive protein, mg/dL | 4.4 ± 5.4 | 4.0 ± 6.1 | 4.9 ± 4.9 | 0.70 |
| sIL‐2R, U/mL | 838.6 ± 621.0 | 723.1 ± 354.7 | 973.3 ± 831.2 | 0.32 |
| ACE, U/L | 39.2 ± 30.7 | 36.9 ± 30.2 | 41.6 ± 32.3 | 0.70 |
| NT‐proBNP, pg/mL | 110.7 ± 152.0 | 64.9 ± 32.0 | 153.00 ± 203.1 | 0.15 |
| Pulmonary function parameters | ||||
| FEV1 (L) | 3.0 ± 1.0 | 3.0 ± 1.1 | 3.2 ± 1.0 | 0.56 |
| FEV1 (% predicted) | 92.9 ± 19.3 | 89.6 ± 19.8 | 97.9 ± 18.9 | 0.28 |
| RV (L) | 2.1 ± 0.5 | 2.2 ± 0.6 | 2.0 ± 0.4 | 0.32 |
| RV (% predicted) | 105.6 ± 22.1 | 110.4 ± 26.7 | 99.1 ± 15.3 | 0.20 |
| R tot (kPa·s/L) | 0.24 ± 0.10 | 0.2 ± 0.1 | 0.3 ± 0.1 | 0.06 |
| R tot (% predicted) | 79.2 ± 35.1 | 67.1 ± 23.4 | 92.9 ± 40.0 | 0.06 |
| IVC (L) | 3.9 ± 1.2 | 3.8 ± 1.1 | 4.0 ± 1.2 | 0.62 |
| IVC (% predicted) | 94.4 ± 15.8 | 92.6 ± 17.3 | 97.3 ± 14.2 | 0.46 |
| TLC (L) | 6.0 ± 1.3 | 6.0 ± 1.2 | 6.0 ± 1.5 | 0.96 |
| TLC (% predicted) | 96.0 ± 11.5 | 96.7 ± 11.5 | 95.6 ± 11.5 | 0.82 |
| DLco (mmol/min/kPa) | 7.0 ± 2.2 | 6.7 ± 2.0 | 7.4 ± 2.3 | 0.43 |
| DLco (% predicted) | 72.6 ± 15.4 | 69.7 ± 14.9 | 76.6 ± 15.4 | 0.26 |
| DLco/VA (mmol/min/kPa/L) | 1.4 ± 0.2 | 1.3 ± 0.2 | 1.4 ± 0.2 | 0.37 |
| DLco/VA (% predicted) | 88.6 ± 14.2 | 86.0 ± 14.0 | 91.7 ± 14.0 | 0.31 |
| Capillary blood gas analysis | ||||
| pO2 (mmHg) | 78.2 ± 8.8 | 78.1 ± 7.7 | 78.3 ± 10.2 | 0.95 |
| pCO2 (mmHg) | 33.9 ± 4.0 | 34.5 ± 2.8 | 33.2 ± 5.0 | 0.42 |
| sO2 (%) | 95.6 ± 2.2 | 95.93 ± 0.9 | 95.3 ± 3.0 | 0.61 |
| Echocardiographic pathologies, n (%) | ||||
| LVEF < 40% | 3 (10.3%) | 3 (21.4%) | 0 (0.0%) | 0.09 |
| LVEDV (mL) | 116.9 ± 66.1 | 130.4 ± 85.6 | 101.0 ± 27.2 | 0.29 |
| Diastolic dysfunction ≥ I° | 13 (48.1%) | 7 (50.0%) | 6 (54.5%) | 0.82 |
| Regional abnormal wall motion | 2 (7.4%) | 1 (7.1%) | 1 (8.3%) | 0.91 |
| Wall thickening | 11 (40.7%) | 7 (50.0%) | 4 (33.3%) | 0.39 |
| Ventricular aneurysm | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Mitral valve regurgitation ≥ I° | 16 (59.3%) | 9 (64.3%) | 7 (58.3%) | 0.76 |
| ECG pathologies, n (%) | ||||
| Heart rate (b.p.m.) | 72.2 ± 12.7 | 69.3 ± 12.7 | 75.0 ± 12.1 | 0.26 |
| Advanced atrioventricular block | 2 (7.4%) | 1 (7.1%) | 1 (7.7%) | 0.62 |
| Abnormal Q‐wave | 3 (11.1%) | 1 (7.1%) | 2 (15.4%) | 0.32 |
| QRS fragmentation | 5 (18.5%) | 2 (14.3%) | 3 (23.1%) | 0.34 |
| T‐wave negativity, n (%) | 13 (48.1%) | 7 (50.0%) | 6 (46.2%) | 0.31 |
| Complete RBBB | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Incomplete LBBB | 2 (7.4%) | 2 (14.3%) | 0 (0.0%) | 0.16 |
| Complete LBBB | 2 (7.4%) | 1 (7.1%) | 1 (7.7%) | 0.62 |
| QTc interval duration (ms) | 428.8 ± 33.5 | 423.2 ± 29.7 | 431.9 ± 36.8 | 0.52 |
| Premature atrial contractions | 1 (3.7%) | 1 (7.1%) | 0 (0.0%) | 0.33 |
| Premature ventricular contractions | 3 (11.1%) | 2 (14.3%) | 1 (7.7%) | 0.59 |
| Holter monitoring pathologies, n (%) | ||||
| Heart rate (b.p.m.) | 76.7 ± 10.1 | 73.4 ± 9.9 | 80.3 ± 9.5 | 0.11 |
| Premature atrial contractions | 115.5 ± 230.4 | 137.8 ± 295.4 | 91.1 ± 139.9 | 0.64 |
| Atrial fibrillation | 0 (0%) | 0 (0%) | 0 (0%) | |
| Premature ventricular contractions | 659.6 ± 2207.4 | 1122.4 ± 3010.0 | 154.7 ± 464.6 | 0.30 |
| Sustained ventricular tachycardia | 0 (0%) | 0 (0%) | 0 (0%) | |
| T‐wave alternans | 15.2 ± 7.6 | 14.9 ± 8.3 | 15.6 ± 7.2 | 0.83 |
| Heart rate variability measures | ||||
| Time‐domain analysis | ||||
| SDNN (ms) | 128.9 ± 36.5 | 131.2 ± 40.3 | 126.3 ± 33.6 | 0.76 |
| SDANN (ms) | 114.5 ± 32.0 | 116.6 ± 35.8 | 112.2 ± 28.9 | 0.75 |
| RMSSD (ms) | 26.1 ± 13.9 | 27.5 ± 13.4 | 24.6 ± 14.9 | 0.63 |
| PNN50% | 5.9 ± 9.1 | 6.3 ± 8.2 | 5.5 ± 10.5 | 0.83 |
| Frequency‐domain analysis | ||||
| HF (ms2) | 185.1 ± 246.9 | 219.8 ± 321.3 | 147.2 ± 132.1 | 0.49 |
| LF (ms2) | 647.2 ± 693.4 | 650.2 ± 861.7 | 643.9 ± 490.9 | 0.98 |
| VLF (ms2) | 2152.3 ± 1862.0 | 2458.5 ± 2418.8 | 1818.4 ± 978.1 | 0.42 |
| LF/HF | 4.6 ± 3.6 | 3.7 ± 2.7 | 5.6 ± 4.3 | 0.21 |
| Total power (ms2) | 2914.8 ± 2204.3 | 3065.8 ± 2772.9 | 2750.0 ± 1474.6 | 0.74 |
Data are presented as mean ± SD or total number and percentage (in parentheses), as appropriate.
ACE, angiotensin converting enzyme; b.p.m., beats per minute; CMR, cardiovascular magnetic resonance; DLco, diffusion capacity of the lung for carbon monoxide; ECG, electrocardiography; FEV1, forced expiratory volume in first second of expiration; HF, high frequency; IVC, inspiratory vital capacity; LBBB, left bundle branch block; LF, low frequency; LF/HF ratio, low frequency/high frequency ratio; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; NT‐proBNP, N terminal pro‐brain natriuretic peptide; NYHA, New York Heart Association; pCO2, carbon dioxide partial pressure; PNN50%, percent NN intervals >50 ms different from the prior interval; pO2, oxygen partial pressure; QTc, corrected QT; R tot, total resistance; RBBB, right bundle branch block; RMSSD; root‐mean square successive difference of R‐R intervals; RV, residual volume; SDANN, standard deviation of the average NN intervals; SDNN = mean standard deviation of NN intervals; sIL‐2R, soluble interleukin‐2 receptor; sO2, oxygen saturation; TLC, total lung capacity; VA, alveolar volume; VLF, very low frequency.
Detection of cardiac sarcoidosis relied on presence of late gadolinium enhancement; in case of early relative gadolinium enhancement or pathological T2‐weighted imaging, cardiac sarcoid involvement was suspected.
Symptomatology refers to any time occurrence since the initial, CS‐confirming CMR conduction.
In like manner, findings obtained by 12‐lead ECG, 24 h Holter monitoring, laboratory, and pulmonary function testing did not show correlation with CMR results. Cardiac symptomatology, as occurred in the period between the initial, CS‐confirming CMR, and the follow‐up examination, was balanced between groups and not indicative of CS degree or persistence.
Results obtained by 68 Ga‐DOTATOC positron emission tomography/computed tomography
Observational data on 68Ga‐DOTATOC‐directed PET/CT imaging were available for 17 out of 27 followed‐up patients:
Out of 17 patients who underwent PET/CT, five subjects presented acute inflammatory injury in CMR imaging (T2‐weighted imaging and early relative gadolinium enhancement). Out of these five patients, only one patient presented PET/CT pathologies: as depicted in Figure 2 , the highest focal 68Ga‐DOTATOC uptake correlated with the area of myocardial oedema.
Further five out of 17 patients who underwent 68Ga‐DOTATOC PET/CT presented isolated LGE in CMR. All of them presented negative PET/CT findings. Out of these five patients, three subjects were under immunosuppressive treatment, while the remaining two patients offered less extensive LGE that only comprised one ventricular segment.19
The remaining seven subjects were all CMR negative for CS and concordantly negative in PET/CT imaging.
Discussion
The main findings of our study are as follows: (i) within a time frame of 2.6 years, cardiac sarcoid involvement persisted in barely half the patients, as confirmed by CMR re‐examination. (ii) Although two patients developed advanced heart block, no deaths occurred during follow‐up. (iii) 68Ga‐DOTATOC PET/CT revealed areas of increased tracer uptake to be consistent with acute inflamed myocardium. (iv) The diagnostic value of standard clinical testing, as recommended by the current agreements,12, 13 was found to be modest.
Over a median time interval of 2.6 years, Greulich and colleagues performed longitudinal follow‐up in a cohort that comprised 155 patients with systemic sarcoidosis and clinically suspected cardiac affection.22 During this period, three patients died, and four patients experienced aborted sudden cardiac death among the CS population. Almost all of these events occurred for cardiac reasons and were best predicted by presence of LGE. Consistent with Greulich's trial, we presently performed CS patients' follow‐up over a median period of 2.6 years. Although two of our 29 initially CS‐positive patients developed advanced heart block in the meantime, no deaths were registered. Thus, our results are contrary to Greulich's observations and relativize both the risk of CS persistence and the fatality that CS has been ascribed to. This discrepancy in event rates may foremost be originated by differences in study population selection: we consciously included all sarcoidal patients irrespective of any cardiac symptomatology at the time of baseline CMR study in terms of an actual screening for CS. Out of these original 188 sarcoidosis patients, CMR detected cardiac affection in 29 patients, 10 of which were clinically inapparent (with regard to chest pain, palpitations, dizziness, and syncopal episodes). At the time of CMR re‐conduction, eight out of 27 patients still did not present any clinical cardiac symptomatology. By contrast, Greulich and colleagues focused on patients with clinically suspected cardiac sarcoid affection. Likewise, Blankstein et al. related 18F‐fluorodeoxyglucose (18F‐FDG) PET/CT imaging findings to adverse events in 118 patients with clinical suspected or known CS during a follow‐up period of 1.5 years.23 Adverse cardiac events (sustained ventricular tachycardia or death) were reported in 26% of study population, and abnormal PET/CT results were present in 60% of study population. This high number of adverse events may be ascribed to the pronounced clinical symptomatology of study population; e.g. 40% already had an implantable cardiac defibrillator prior to PET/CT, mean ejection fraction was 47 ± 16%, and JMHWG criteria turned positive in 34% of study population. In this line of thought, a recent review that included eight studies on prevalence and prognosis of clinically silent CS reported a more favourable course in clinical silent CS in five out of these eight studies: within a mean follow‐up of 23 months, no adverse cardiac event like ventricular arrhythmia or death was reported.24 It illustrates that differences in cardiac symptomatology of study population may influence adverse events' occurrence.
Detection of CS relied on presence of LGE; in case of early relative gadolinium enhancement and pathological T2‐weighted imaging, cardiac sarcoid involvement was suspected. The diagnostic value of myocardial T2 mapping to detect CS has been subject to prior studies. Crouser and colleagues conducted a retrospective study of 50 patients with histologically proven sarcoidosis that underwent CMR for suspected CS.25 CMR results were compared with those obtained in 14 healthy control subjects. They observed a significantly elevated T2 in sarcoidosis patients as compared with controls. Moreover, LGE and T2 abnormalities did not always go hand in hand, suggesting complementary diagnostic information from both techniques to identify CS. They concluded that a comprehensive CMR approach to detect CS should include both T2‐weighted imaging and LGE.
As to early relative gadolinium enhancement, Schulz‐Menger et al. identified early relative contrast enhancement in patients with suspected cardiac sarcoidal involvement that normalized after glucocorticoid therapy.18 In a population of 16 patients presenting systemic sarcoidosis, Shimada et al. detected early enhancing lesions that were likewise remitting under steroid treatment.26 Both studies illustrate the value of early local and global contrast enhancement in viable myocardium that may mainly occur in early CS stages. In equal manner to the above depicted courses, we observed remission of early relative enhancement under immunosuppressive treatment in five cases, implying remission of early stage CS.
Additional complexity emerges from the value of standard clinical testing. Despite being enlisted in the current agreement papers,12, 13 electrical recording and conventional echocardiography failed to be indicative of CS: application of the HRS consensus recommendations on ECG, Holter monitoring, and echocardiography would have enabled us to solely diagnose three out of 14 CS cases. In addition to established CS diagnostic criteria, we examined heart rate variability and T‐wave alternans as general risk stratifiers for sudden cardiac death by influencing the onset and sustainment of malignant ventricular arrhythmias.11, 27, 28 Neither comparison of persistently CS‐positive with CS‐negative patients nor comparison of PET‐positive with PET‐negative patients revealed significantly different values of the aforementioned risk stratifiers. This finding is consistent with the presently observed lack in sustained ventricular tachycardia and points at a more favourable CS course and prognosis.
A more sophisticated imaging tool for evaluating patients with suspected or confirmed CS arises from radionuclide approaches. Among these, gallium scintigraphy and 18F‐FDG PET/CT represent the best‐studied techniques, with the latter offering a higher sensitivity than single photon‐emitting procedures.29 SSTR‐directed PET/CT has been demonstrated to be feasible for imaging atherosclerotic and myocardial inflammatory processes.10, 30 The potential of SSTR‐directed PET/CT in the field of granulomatous diseases has foremost been described for 111In‐labelled octreotide.31
We performed 68Ga‐DOTATOC PET/CT instead of 18F‐FDG PET/CT in consequence of the limitations that FDG entails: specificity of FDG PET/CT is hampered by the need for dedicated patient preparation that otherwise may limit its diagnostic value.32 By contrast, 68Ga‐DOTATOC PET/CT overcomes this disadvantage: as the fasting preparation protocol is less rigorous, the rate of adequately prepared patients and consequently the procedure's validity are higher. Moreover, a shorter examination time and lower radiation exposure favour SSTR imaging.
However, the use of the SSTR ligand 68Ga‐DOTATOC for the evaluation of CS is limited to a study by Lapa et al.33 and a case report by Reiter et al.34 Lapa and colleagues compared SSTR‐PET/CT and CMR imaging findings in 15 patients with biopsy‐proven CS or extra‐CS and clinical suspicion on cardiac involvement. SSTR‐PET/CT and CMR imaging were concordantly in 79.3% of all CMR positive segments (23/29 segments), and an overall concordance of 96.1% of all segments (245/255) was described. In three cases, PET/CT and CMR results differed (negative PET/CT results vs. positive CMR results); two of these three patients were under immunosuppressants, and the remaining one was missed owing to minor wall involvement in CMR (<25% of wall thickness). None of the PET/CT positive patients were under immunosuppressive treatment at PET/CT conduction.
Reiter et al. also described concordance of CMR‐stated and 68Ga‐DOTATOC PET/CT‐stated acute myocardial damage in a case report with histologically proven extra‐CS and a response of cardiac involvement after systemic glucocorticoid therapy.34
We presently identified one patient with pathologically increased radiotracer uptake; the hereby visualized myocardial localization corresponded to cardiac areas of acute inflammation. This observation is consistent with the tracer's biodistribution that can be deduced from its binding profile: as the SSTR is overexpressed on activated macrophages, lymphocytes, and epithelioid cells, specific SSTR‐targeted radiotracers like 68Ga‐DOTATOC may directly display their distribution and thereby identify sites of inflammation.10, 35 By contrast, LGE primarily depicts myocardial scar; given the aforesaid biodistribution of 68Ga‐DOTATOC, PET/CT presently failed at imaging CMR‐detected LGE.
Overall, among the 17 patients of our study population for whom PET/CT data were available, CMR‐based T2 imaging and early relative gadolinium enhancement detected acute inflammatory injury in five patients; out of these five patients, only one patient with abnormal T2 finding presented PET/CT pathologies. This discrepancy may be ascribed to the medication use at the time of radionuclide imaging: whereas the aforementioned PET/CT‐positive patient did not receive immunosuppressants at the time of PET/CT conduction, the remaining four patients were under immunosuppressive medication. This finding is consistent with the observation made by Lapa et al.33: 68Ga‐DOTATOC PET/CT detected acute inflammatory processes in patients not receiving immunosuppressants. Therefore, 68Ga‐DOTATOC PET/CT may be a useful tool to monitor diseases' activity under immunosuppressive therapy especially in patients with contraindications to CMR like device therapy or renal impairment. Nonetheless, given that only one patient presented PET/CT pathologies, future studies are warranted to definitely determine the value of SSTR scintigraphy as imaging modality for evaluation of CS activity and for following response to treatment.
There are several limitations that should be addressed. First, the non‐randomized and single‐centred study design may have affected our data through procedure bias. As we performed follow‐up of previously diagnosed CS patients, the study population size was modest, and the chosen time frame of 2.6 years may have been too short to properly assess patients' outcome, as the number of events resulted to be low. In consequence of the limited sample size, adjustment for pre‐existing clinical symptomatology, medical treatment, and size of CMR‐detected lesions was not feasible and might mitigate the study's conclusion on prognosis in CS. In keeping with this, longer‐termed follow‐up would have enabled us to additionally address the predictive value of PET/CT findings. Finally, as PET/CT data were retrospectively extracted, a prospective approach examining the diagnostic impact of 68Ga‐DOTATOC PET/CT, especially in comparison with 18F‐FDG PET/CT, would be of additional value.
In conclusion, in our CS‐positive study population, cardiac sarcoid affection persisted in barely half the patients after a period of 2 years, and the patients were not afflicted with cardiac death. It points at minor diseases' perseverance and at a more favourable disease's course. 68Ga‐DOTATOC PET/CT imaging allowed for visualization of acute myocardial inflammation. However, to definitively determine its value in CS imaging, future studies are warranted.
Conflict of interest
None declared.
Acknowledgements
The support provided by Bernd and Hildegard Stachetzki who chair ‘Sarkoidose‐Netzwerk e.V.’, a German sarcoidosis self‐help association, is greatly appreciated and acknowledged.
Pizarro, C. , Kluenker, F. , Dabir, D. , Thomas, D. , Gaertner, F. C. , Essler, M. , Grohé, C. , Nickenig, G. , and Skowasch, D. (2018) Cardiovascular magnetic resonance imaging and clinical performance of somatostatin receptor positron emission tomography in cardiac sarcoidosis. ESC Heart Failure, 5: 249–261. doi: 10.1002/ehf2.12243.
References
- 1. Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med 1999; 160: 736–755. [DOI] [PubMed] [Google Scholar]
- 2. Thomas KW, Hunninghake GW. Sarcoidosis. JAMA 2003; 289: 3300–3303. [DOI] [PubMed] [Google Scholar]
- 3. Porterfield CP, Ferguson JD. Arrhythmia in cardiac sarcoidosis. J Cardiovasc Electrophysiol 2014; 25: 177–178. [DOI] [PubMed] [Google Scholar]
- 4. Gideon NM, Mannino DM. Sarcoidosis mortality in the United States 1979–1991: an analysis of multiple‐cause mortality data. Am J Med 1996; 100: 423–427. [DOI] [PubMed] [Google Scholar]
- 5. Iwai K, Tachibana T, Takemura T, Matsui Y, Kitaichi M, Kawabata Y. Pathological studies on sarcoidosis autopsy. I. Epidemiological features of 320 cases in Japan. Acta Pathol Jpn 1993; 43: 372–376. [DOI] [PubMed] [Google Scholar]
- 6. Yigla M, Badarna‐Abu‐Ria N, Tov N, Ravell‐Weiller D, Rubin AH. Sarcoidosis in northern Israel; clinical characteristics of 120 patients. Sarcoidosis Vasc Diffuse Lung Dis 2002; 19: 220–226. [PubMed] [Google Scholar]
- 7. Pizarro C, Goebel A, Dabir D, Hammerstingl C, Pabst S, Grohé C, Fimmers R, Stoffel‐Wagner B, Nickenig G, Schild H, Skowasch D, Thomas D. Cardiovascular magnetic resonance‐guided diagnosis of cardiac affection in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2016; 32: 325–335. [PubMed] [Google Scholar]
- 8. Vanhagen PM, Krenning EP, Reubi JC, Kwekkeboom DJ, Bakker WH, Mulder AH, Laissue I, Hoogstede HC, Lamberts SW. Somatostatin analogue scintigraphy in granulomatous diseases. Eur J Nucl Med 1994; 21: 497–502. [DOI] [PubMed] [Google Scholar]
- 9. ten Bokum AM, Hofland LJ, de Jong G, Bouma J, Melief MJ, Kwekkeboom DJ, Schonbrunn A, Mooy CM, Laman JD, Lamberts SW, van Hagen PM. Immunohistochemical localization of somatostatin receptor sst2A in sarcoid granulomas. Eur J Clin Invest 1999; 29: 630–636. [DOI] [PubMed] [Google Scholar]
- 10. Lapa C, Reiter T, Li X, Werner RA, Samnick S, Jahns R, Buck AK, Ertl G, Bauer WR. Imaging of myocardial inflammation with somatostatin receptor based PET/CT—a comparison to cardiac MRI. Int J Cardiol 2015; 194: 44–49. [DOI] [PubMed] [Google Scholar]
- 11. Wellens HJ, Schwartz PJ, Lindemans FW, Buxton AE, Goldberger JJ, Hohnloser SH, Huikuri HV, Kääb S, La Rovere MT, Malik M, Myerburg RJ, Simoons ML, Swedberg K, Tijssen J, Voors AA, Wilde AA. Risk stratification for sudden cardiac death: current status and challenges for the future. Eur Heart J 2014; 35: 1642–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Birnie DH, Sauer WH, Bogun F, Cooper JM, Culver DA, Duvernoy CS, Judson MA, Kron J, Mehta D, Cosedis Nielsen J, Patel AR, Ohe T, Raatikainen P, Soejima K. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm 2014; 11: 1305–1323. [DOI] [PubMed] [Google Scholar]
- 13. Soejima K, Yada H. The work‐up and management of patients with apparent or subclinical cardiac sarcoidosis: with emphasis on the associated heart rhythm abnormalities. J Cardiovasc Electrophysiol 2009; 20: 578–583. [DOI] [PubMed] [Google Scholar]
- 14. Shah SA, Kambur T, Chan C, Herrington DM, Liu K, Shah SJ. Relation of short‐term heart rate variability to incident heart failure (from the Multi‐Ethnic Study of Atherosclerosis). Am J Cardiol 2013; 112: 533–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Narayan SM. T‐wave alternans and the susceptibility to ventricular arrhythmias. J Am Coll Cardiol 2006; 47: 269–281. [DOI] [PubMed] [Google Scholar]
- 16. Douglas PS, Garcia MJ, Haines DE, Lai WW, Manning WJ, Patel AR, Picard MH, Polk DM, Ragosta M, Parker Ward R, Weiner RB. ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 appropriate use criteria for echocardiography. J Am Soc Echocardiogr 2011; 24: 229–267.21338862 [Google Scholar]
- 17. Standardized lung function testing. Official statement of the European Respiratory Society. Eur Respir J Suppl 1993; 16: 1–100. [PubMed] [Google Scholar]
- 18. Schulz‐Menger J, Wassmuth R, Abdel‐Aty H, Siegel I, Franke A, Dietz R, Friedrich MG. Patterns of myocardial inflammation and scarring in sarcoidosis as assessed by cardiovascular magnetic resonance. Heart 2006; 92: 399–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS. American Heart Association Writing Group on Myocardial Segmentation and Registration for Cardiac Imaging. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 2002; 105: 539–542. [DOI] [PubMed] [Google Scholar]
- 20. Kramer CM, Barkhausen J, Flamm SD, Kim RJ, Nagel E. Society for Cardiovascular Magnetic Resonance Board of Trustees Task Force on Standardized Protocols. Standardized cardiovascular magnetic resonance (CMR) protocols 2013 update. J Cardiovasc Magn Reson 2013; 15: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nagai T, Kohsaka S, Okuda S, Anzai T, Asano K, Fukuda K. Incidence and prognostic significance of myocardial late gadolinium enhancement in patients with sarcoidosis without cardiac manifestation. Chest 2014; 146: 1064–1072. [DOI] [PubMed] [Google Scholar]
- 22. Greulich S, Deluigi CC, Gloekler S, Wahl A, Zürn C, Kramer U, Nothnagel D, Bültel H, Schumm J, Grün S, Ong P, Wagner A, Schneider S, Nassenstein K, Gawaz M, Sechtem U, Bruder O, Mahrholdt H. CMR imaging predicts death and other adverse events in suspected cardiac sarcoidosis. JACC Cardiovasc Imaging 2013; 6: 501–511. [DOI] [PubMed] [Google Scholar]
- 23. Blankstein R, Osborne M, Naya M, Waller A, Kim CK, Murthy VL, Kazemian P, Kwong RY, Tokuda M, Skali H, Padera R, Hainer J, Stevenson WG, Dorbala S, Di Carli MF. Cardiac positron emission tomography enhances prognostic assessments of patients with suspected cardiac sarcoidosis. J Am Coll Cardiol 2014; 63: 329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Birnie DH, Nery PB, Ha AC, Beanlands RS. Cardiac sarcoidosis. J Am Coll Cardiol 2016; 68: 411–421. [DOI] [PubMed] [Google Scholar]
- 25. Crouser ED, Ono C, Tran T, He X, Raman SV. Improved detection of cardiac sarcoidosis using magnetic resonance with myocardial T2 mapping. Am J Respir Crit Care Med 2014; 189: 109–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shimada T, Shimada K, Sakane T, Ochiai K, Tsukihashi H, Fukui M, Inoue S, Katoh H, Murakami Y, Ishibashi Y, Maruyama R. Diagnosis of cardiac sarcoidosis and evaluation of the effects of steroid therapy by gadolinium‐DTPA‐enhanced magnetic resonance imaging. Am J Med 2001; 110: 520–527. [DOI] [PubMed] [Google Scholar]
- 27. La Rovere MT, Pinna GD, Maestri R, Mortara A, Capomolla S, Febo O, Ferrari R, Franchini M, Gnemmi M, Opasich C, Riccardi PG, Traversi E, Cobelli F. Short‐term heart rate variability strongly predicts sudden cardiac death in chronic heart failure patients. Circulation 2003; 107: 565–570. [DOI] [PubMed] [Google Scholar]
- 28. Merchant FM, Ikeda T, Pedretti RF, Salerno‐Uriarte JA, Chow T, Chan PS, Bartone C, Hohnloser SH, Cohen RJ, Armoundas AA. Clinical utility of microvolt T‐wave alternans testing in identifying patients at high or low risk of sudden cardiac death. Heart Rhythm 2012; 9: 1256–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Okumura W, Iwasaki T, Toyama T, Iso T, Arai M, Oriuchi N, Endo K, Yokoyama T, Suzuki T, Kurabayashi M. Usefulness of fasting 18F‐FDG PET in identification of cardiac sarcoidosis. J Nucl Med 2004; 45: 1989–1998. [PubMed] [Google Scholar]
- 30. Schatka I, Wollenweber T, Haense C, Brunz F, Gratz KF, Bengel FM. Peptide receptor‐targeted radionuclide therapy alters inflammation in atherosclerotic plaques. J Am Coll Cardiol 2013; 62: 2344–2345. [DOI] [PubMed] [Google Scholar]
- 31. Cascini GL, Cuccurullo V, Mansi L. The non tumour uptake of (111)In‐octreotide creates new clinical indications in benign diseases, but also in oncology. Q J Nucl Med Mol Imaging 2010; 54: 24–36. [PubMed] [Google Scholar]
- 32. Harisankar CN, Mittal BR, Agrawal KL, Abrar ML, Bhattacharya A. Utility of high fat and low carbohydrate diet in suppressing myocardial FDG uptake. J Nucl Cardiol 2011; 18: 926–936. [DOI] [PubMed] [Google Scholar]
- 33. Lapa C, Reiter T, Kircher M, Schirbel A, Werner RA, Pelzer T, Pizarro C, Skowasch D, Thomas L, Schlesinger‐Irsch U, Thomas D, Bundschuh RA, Bauer WR, Gärtner FC. Somatostatin receptor based PET/CT in patients with the suspicion of cardiac sarcoidosis: an initial comparison to cardiac MRI. Oncotarget 2016; 7: 77807–77814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Reiter T, Werner RA, Bauer WR, Lapa C. Detection of cardiac sarcoidosis by macrophage‐directed somatostatin receptor 2‐based positron emission tomography/computed tomography. Eur Heart J 2015; 36: 2404. [DOI] [PubMed] [Google Scholar]
- 35. Ambrosini V, Nanni C, Fanti S. The use of gallium‐68 labeled somatostatin receptors in PET/CT imaging. PET Clin 2014; 9: 323–329. [DOI] [PubMed] [Google Scholar]


