Abstract
Aims
Iron deficiency is highly prevalent in Southeast Asians with heart failure (HF) and associated with worse outcomes. This trial aimed to assess the effect of intravenous iron in Southeast Asians hospitalized with decompensated HF.
Methods and results
Fifty patients hospitalized for acute decompensated HF, regardless of ejection fraction, with iron deficiency (defined as serum ferritin <300 ng/mL if transferrin saturation is <20%) were randomized to receive either one dose of intravenous ferric carboxymaltose (FCM) 1000 mg or placebo (0.9% saline) following HF stabilization and before discharge in two Singapore tertiary centres. The primary endpoint was difference in 6‐min walk test (6MWT) distance over 12 weeks, while secondary endpoints were quality of life assessed using validated Kansas City Cardiomyopathy Questionnaire (KCCQ) and Visual Analogue Scale (VAS). Improvement in 6MWT distance at Week 12 was observed in both FCM and placebo groups (from 252 ± 123 to 334 ± 128 m and from 243 ± 67 to 301 ± 83 m, respectively). Unadjusted analysis showed 6MWT distance for FCM exceeded that for placebo, but adjustment for baseline covariates and time attenuated this effect {adjusted mean difference between groups: 0.88 m [95% confidence interval (CI) −30.2 to 32.0, P = 0.956]}. KCCQ overall summary and VAS were similar in both groups [adjusted mean difference: KCCQ −1.48 (95% CI −8.27 to 5.31, P = 0.670) and VAS 0.26 (95% CI −0.33 to 0.86, P = 0.386)]. FCM was well tolerated with no serious treatment‐related adverse events.
Conclusions
Intravenous FCM administered pre‐discharge in Southeast Asians hospitalized with decompensated HF is clinically feasible. Changes in 6MWT distance should be measured beyond Week 12 to account for background therapy effects.
Keywords: Iron deficiency, Southeast Asian heart failure, Ferric carboxymaltose
Introduction
Asian patients with heart failure (HF) manifest an aggressive clinical phenotype with younger age at presentation and increased mortality rates compared with those in America or Europe based on registry data.1, 2 Prevalence rates of iron deficiency (ID) in Southeast Asian HF patients also exceed those in European patients, averaging 61% and peaking at 80% in patients of Indian ethnicity.3 Regardless of ethnicity, geographical location, and anaemia status, concurrent HF and ID has consistently been associated with poor outcomes such as reduced exercise capacity, reduced quality of life (QoL), and elevated risks of HF hospitalization and mortality.4, 5, 6, 7
The landmark Ferric Carboxymaltose in Patients with Heart Failure and Iron Deficiency (FAIR‐HF) trial has shown success, demonstrating beneficial effects of intravenous (i.v.) ferric carboxymaltose (FCM) on exercise capacity and QoL. It was followed by the Beneficial Effects of Long‐term Intravenous Iron Therapy with Ferric Carboxymaltose in Patients with Symptomatic Heart Failure and Iron Deficiency (CONFIRM‐HF) trial, consolidating these findings as well as proving the sustainability of these outcomes over 1 year.8, 9 Results from these trials have established i.v. FCM as a feasible option in the management of HF in patients with concurrent ID irrespective of anaemia status, receiving a Class IIa recommendation in the 2016 European Society of Cardiology guidelines for the treatment of HF.10, 11 Most recently, the Effect of Ferric Carboxymaltose on Exercise Capacity in Patients with Iron Deficiency and Chronic Heart Failure (EFFECT‐HF) trial reinforced the results of these landmark trials by demonstrating significant benefit with i.v. FCM vs. standard of care for improving peak oxygen uptake along with QoL.12 These trials have been carried out within ambulatory settings in patients with HF with reduced ejection fraction (HFrEF), involving predominantly European populations.
Less is known about patients with acute decompensated HF and concurrent ID. Cohen‐Solal et al. published the first descriptive study of ID prevalence in this cohort, showing that ID was more common in acute settings compared with ambulatory cohorts with HF in Europe, as 69% of men and 75% of women admitted with decompensated HF were found to have ID.13 In a more recent study, Núñez et al. showed that amongst inpatients with decompensated HF, absolute ID was associated with higher risk of readmission within 30 days [hazard ratio 1.72; 95% confidence interval (CI) 1.13–2.60, P = 0.011].14
Our study aimed to bridge knowledge gaps with respect to the efficacy and tolerability of iron repletion in higher‐risk HF patients of Southeast Asian ethnicity receiving FCM pre‐discharge following hospitalization for acute decompensated HF. Collection of preliminary data in this population would also form the basis of sample size calculation for a larger‐scale multicentre therapeutic trial.
Methods
Study design and oversight
Between September 2013 and June 2015, 50 eligible participants were enrolled in two tertiary centres in Singapore. The study design has been published.15 The study protocol was approved by the local ethics institutional regulatory board and conducted in accordance with the principles of the World Medical Association Declaration of Helsinki on ethics in medical research and International Conference on Harmonization guidelines for Good Clinical Practice. Written informed consent was obtained from all participants.
The trial was designed by the authors. Implementation and clinical management were the responsibility of the two study sites. An independent body, the Singapore Clinical Research Institute, was responsible for on‐site monitoring, data management, and reviewing safety data including deaths throughout the trial period. All analyses were performed by an independent statistician (B. C. T.) in accordance with a pre‐defined statistical analysis plan. The manuscript was prepared and submitted for publication by the authors. All authors have full access to study data and vouch for the accuracy and completeness of the reported analyses. This trial is registered at https://ClinicalTrials.gov/ (NCT01922479).
Recruitment and follow‐up of participants
Eligible participants included patients hospitalized for acute decompensated HF regardless of left ventricular ejection fraction (LVEF), with concurrent ID (defined as serum ferritin <300 ng/mL if transferrin saturation is <20%) and haemoglobin (Hb) ≤14 g/dL. This cut‐off for ID was based on our prior experience in an Asian HF population where transferrin saturation independently correlated with functional status and event‐free survival.3 All subjects had to be able to complete the 6‐min walk test (6MWT). Those with serious medical conditions deemed prohibitive to study participation or completion were excluded. Full inclusion and exclusion criteria are presented in Table 1.
Table 1.
Trial inclusion and exclusion criteria
| Inclusion criteria |
| Clinical diagnosis of acute decompensated heart failure (based on the European Society of Cardiology guidelines), regardless of left ventricular ejection fraction |
| Iron deficiency defined as serum ferritin <300 ng/mL if transferrin saturation is <20% |
| Able to complete the 6‐min walk test |
| Age 21 years and above |
| Exclusion criteria |
| Haemoglobin level above 14 g/dL |
| Known sensitivity to ferric carboxymaltose |
| Intravenous iron therapy or blood transfusion within 4 weeks prior to randomization |
| Body weight ≤35 kg |
| Iron storage disorders (e.g. haemochromatosis) |
| Serious medical conditions deemed prohibitive to study participation or completion by the investigators |
Randomization
Participants were randomized to receive either i.v. FCM (study drug) or 0.9% saline (placebo) in a 1:1 ratio via random permuted blocks of size 4 with stratification by centre using a central phone‐based system. Prior to administration of treatment, clinical history was obtained, and baseline physical examination, electrocardiogram, blood sampling, 6MWT, and QoL assessment were performed.
Therapy and blinding
There was no restriction on guideline‐based pharmacotherapy for HF in both FCM and placebo groups. Prior to discharge from hospital and upon clinical stabilization, the study drug, i.v. FCM solution (Ferinject®, Vifor Pharma), was administered as a single dose of 1000 mg (50 mg of iron per millilitre), as an undiluted i.v. bolus injection of 20 mL over 15 min to all patients randomized to the FCM group. The equivalent volume (20 mL) of i.v. 0.9% saline was used as a placebo.
This single‐dose regimen was chosen as it forms the minimum cumulative iron dose, according to Ganzoni's formula, in the majority of Asian patients fulfilling the assumptions of baseline Hb ≥10 g/dL and body weight between 35 and 70 kg.16 In addition, administration of study drug while participants are hospitalized allows effective utilization of resources that may be scarce in the outpatient setting, in particular i.v. administration of medication.
Intravenous FCM is a dark brown solution and clearly distinguishable from 0.9% saline. Consequently, study personnel involved in the preparation and administration of the study drug were not involved in any of the study assessments for efficacy or safety. Preparation of all administered medication was carried out away from participants. Apart from the transparent tubing of the i.v. cannula and line that enabled the colour of administered solution to be visible, all other identifiers differentiating FCM and placebo were removed (i.e. labels and containers), and the solutions were placed in identical bags. Study patients did not have visual contact with one another throughout their hospitalization.
Primary and secondary endpoints
The primary endpoint of our study is the change in 6MWT distance over the 12‐week study period. Conduct of the 6MWT was carried out in accordance with the American Thoracic Society 2002 guidelines.17 The total distance walked in 6 min was recorded, to the nearest metre. All tests were carried out by study personnel blinded to participants' treatment allocation, and every effort was made to have the same person supervise all 6MWTs for a specific participant. The 6MWT assessments were performed at baseline and Weeks 4 and 12 of follow‐up.
Secondary endpoints include the change in QoL assessed using the Kansas City Cardiomyopathy Questionnaire (KCCQ) overall summaries and Visual Analogue Scale (VAS) scores and change in New York Heart Association (NYHA) functional class, as well as any HF readmissions. Safety endpoints included serious and non‐serious adverse events assessed up to Week 12. These assessments were performed at baseline and Weeks 1, 4, and 12 of follow‐up.
Statistical analysis
Sample size calculations were based on the predicted 6MWT distance between FCM and placebo groups, using data from a study of 6MWT distance in Asian patients with HF.18 A mean difference of 40 m in 6MWT between groups and a standard deviation (SD) of 60 m with an autocorrelation coefficient of 0.6 were assumed for a repeated‐measures study design with one pre‐intervention and two post‐intervention measurements. Therefore, a sample size of 50 (25 per group) would have a 90% power to detect the hypothesized difference at a two‐sided alpha of 0.05.
For the primary outcome of 6MWT and secondary outcomes of QoL measures, mixed model analyses were utilized to evaluate the effect of FCM compared with placebo over time, in order to account for intra‐subject correlation arising from repeated measures, with adjustment made for baseline covariates and the effect of time. STATA statistical software Version 13 (StataCorp LP, Texas) was used for all statistical analyses, assuming a two‐sided test at the 5% level of significance. All outcomes were analysed on the basis of intention to treat.
Results
A total of 50 patients were randomized from the two study sites—25 to i.v. FCM and 25 to placebo. The Consolidated Standards of Reporting Trials trial flowchart is presented in Figure 1.
Figure 1.
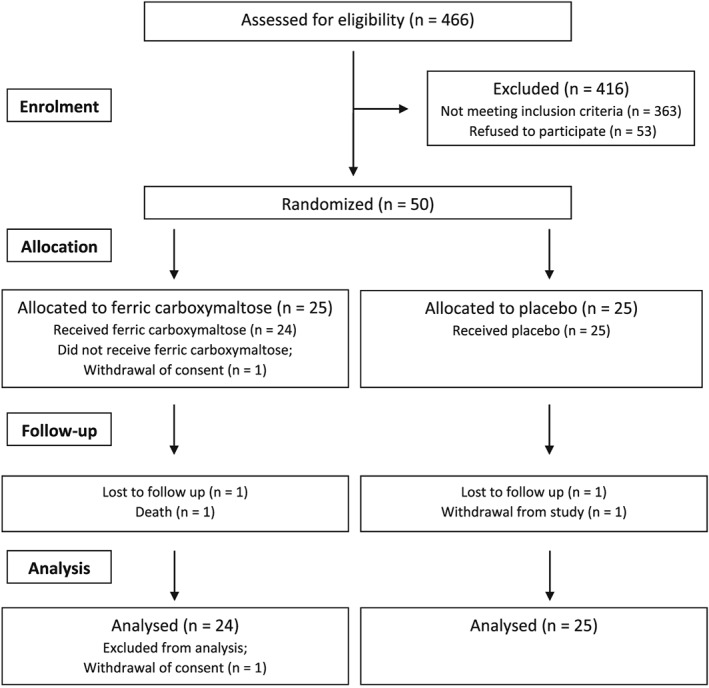
Trial flow.
Baseline characteristics
Both groups were comparable in terms of gender and ethnicity breakdown, as well as baseline cardiovascular medication and co‐morbidities. The FCM group was younger, heavier, and had an increased baseline 6MWT distance compared with the placebo group (Table 2).
Table 2.
Demographic and clinical characteristics of trial participants
| FCM (n = 24) | Saline (n = 25) | ||
|---|---|---|---|
| Age, years (SD) | 61.1 (10.8) | 64 (10) | |
| Gender (n, %) | Male | 18 (75) | 20 (80) |
| Ethnicity (n, %) | Chinese | 10 (41.7) | 15 (60) |
| Indian | 3 (12.5) | 4 (16) | |
| Malay | 10 (41.7) | 6 (24) | |
| Other | 1 (4.2) | 0 (0) | |
| Weight, kg (SD) | 73.1 (13.5) | 64.9 (12.6) | |
| Height, cm (SD) | 161.0 (8.4) | 162.8 (9.7) | |
| Medication (n, %) | Diuretics | 21 (87.5) | 23 (92) |
| ACEI | 11 (45.8) | 8 (32) | |
| ARB | 8 (33.3) | 5 (20) | |
| Beta‐blocker | 24 (100) | 20 (80) | |
| Antiplatelet | 14 (58.3) | 20 (80) | |
| MRA | 7 (29.2) | 10 (40) | |
| Lipid lowering | 22 (91.7) | 21 (84) | |
| Iron supplement | 15 (62.5) | 12 (48) | |
| Co‐morbidities (n, %) | Type 2 DM | 15 (62.5) | 15 (60) |
| Hypertension | 21 (87.5) | 18 (72) | |
| Hyperlipidaemia | 20 (83.3) | 20 (80) | |
| Previous AMI | 12 (50) | 13 (52) | |
| Current smoking | 5 (20.8) | 5 (20) | |
| LVEF, % (SD) | 38.8 (17.5) | 33.2 (14.8) | |
| HFpEF (n, %) | 9 (39.1) | 4 (16) | |
| 6MWT distance, m (SD) | 252.4 (122.7) | 242.6 (66.8) | |
| Quality of life measures | KCCQ Overall | 50.0 (17.7) | 51.2 (14.5) |
| VAS score | 6.4 (1.6) | 5.7 (1.0) | |
| Laboratory measurements | Hb, g/dL (SD) | 11.6 (1.9) | 13.1 (1.3) |
| Ferritin, ng/mL (SD) | 91.4 (80.4) | 84.1 (63.7) | |
| TSAT, % (SD) | 15.7 (10.1) | 13.9 (6.8) |
ACEI, angiotensin‐converting enzyme inhibitor; AMI, acute myocardial infarction; ARB, angiotensin receptor blocker; DM, diabetes mellitus; FCM, ferric carboxymaltose; Hb, haemoglobin; HFpEF, heart failure with preserved ejection fraction; KCCQ, Kansas City Cardiomyopathy Questionnaire; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association; SD, standard deviation; 6MWT, 6‐minute walk test; TSAT, transferrin saturation; VAS, Visual Analogue Score.
Follow‐up
Over the 12 week duration of the study, there were one withdrawal after recruitment, one loss to follow‐up after Week 1, and one death after Week 4 in the FCM group. There were one withdrawal and one loss to follow‐up in the placebo group, both after Week 1. Apart from the FCM patient who withdrew participation after recruitment, data from all other patients (n = 49) contributed to the outcome analysis of the trial.
Primary endpoint
From baseline to Week 4, the 6MWT distance increased from 252 ± 123 to 322 ± 122 m for the FCM group and from 243 ± 67 to 300 ± 70 m for the placebo group. Between Weeks 4 and 12, the 6MWT distance for the FCM group continued to increase to a final value of 334 ± 128 m, whereas a plateau was observed in the placebo group (final 6MWT distance 301 ± 83 m; Figure 2). Unadjusted analysis for the change in 6MWT distance over 12 weeks showed a mean difference between groups of 15.6 m in favour of FCM (95% CI −43.9 to 75.0, P = 0.607). Following adjustment for baseline 6MWT distance and effect of time, taking into account possible intra‐subject correlation in repeated measurements, analysis based on the mixed effect model with random intercept showed a non‐significant mean difference in 6MWT distance between groups of 0.9 m (95% CI −30.2 to 32.0, P = 0.607) (Table 3).
Figure 2.
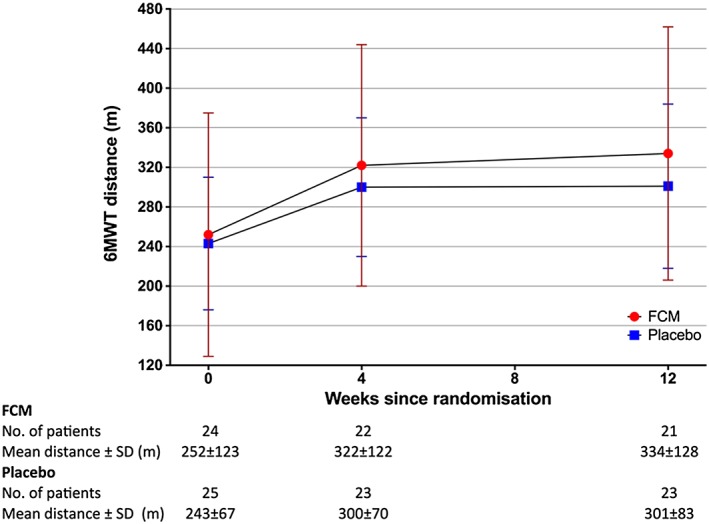
Absolute 6‐min walk test (6MWT) distances for both groups over time.
Table 3.
Estimate of treatment effect on 6‐min walk test distance (mixed model)
| Model | Mean difference | 95% confidence interval | P‐value |
|---|---|---|---|
| Unadjusted | 15.59 | −43.87 to 75.04 | 0.607 |
| Adjusteda | 0.88 | −30.22 to 31.97 | 0.956 |
Adjusted for baseline 6‐min walk test distance and effect of time.
Of our study cohort, 10 had HF with preserved ejection fraction (HFpEF) (six from the FCM arm and four from the placebo arm). Formal subgroup analysis was not pre‐specified a priori in the trial protocol and was not performed based on the Consolidated Standards of Reporting Trials statement as well as because of the small numbers in the HFpEF cohort.
Secondary endpoints
Similar patterns of change in the overall KCCQ score were observed in both FCM and placebo groups, increasing from a baseline of 50.0 ± 17.7 and 51.2 ± 14.5 (FCM and placebo, respectively) to 75.1 ± 20.3 and 76.3 ± 12.2 (FCM and placebo, respectively) by Week 1. At Week 12, the overall KCCQ score was 82.4 ± 15.2 for FCM and 87.1 ± 6.4 for placebo (Figure 3). Mixed model analysis revealed no significant differences between the two study groups both with and without adjustment (Table 4).
Figure 3.
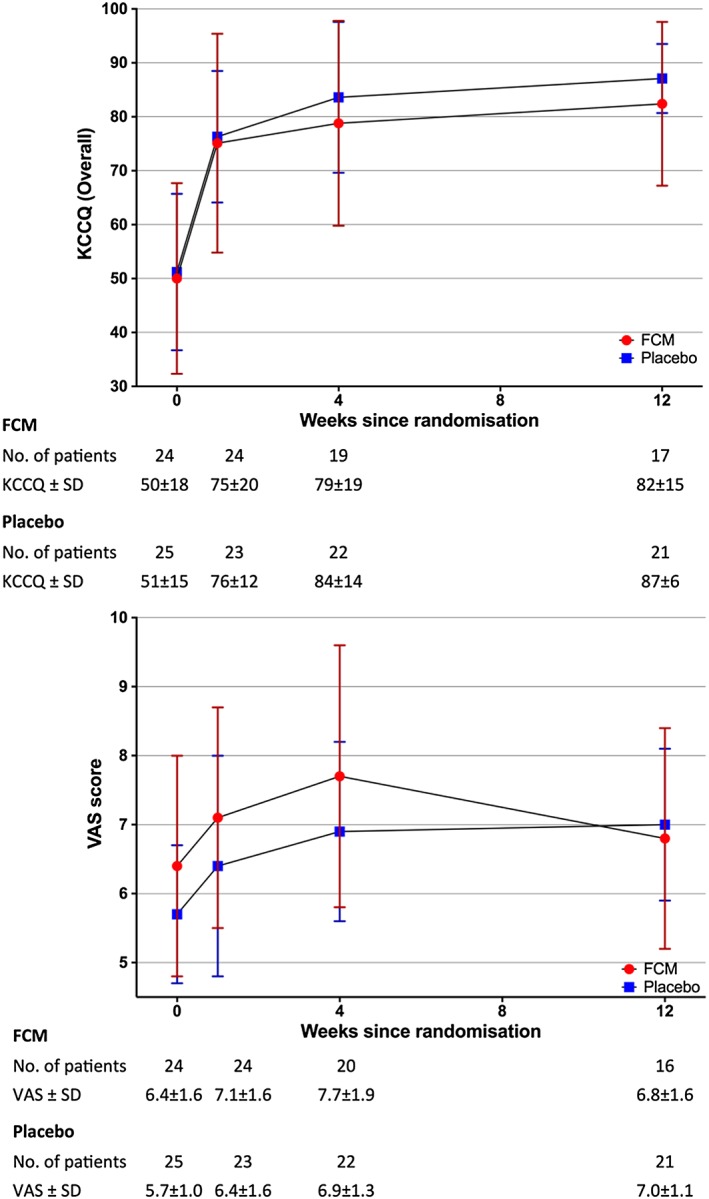
Kansas City Cardiomyopathy Questionnaire (KCCQ) and Visual Analogue Scale (VAS) scores for both groups over time.
Table 4.
Estimate of treatment effect on quality of life indicators
| Quality of life indicators | Mean difference | 95% confidence interval | P‐value |
|---|---|---|---|
| Kansas City Cardiomyopathy Questionnaire | |||
| Unadjusted | −3.00 | −11.72 to 5.73 | 0.501 |
| Adjusteda | −1.48 | −8.27 to 5.31 | 0.670 |
| Visual Analogue Scale | |||
| Unadjusted | 0.47 | −0.18 to 1.13 | 0.152 |
| Adjusteda | 0.26 | −0.33 to 0.86 | 0.386 |
Adjusted for baseline overall Kansas City Cardiomyopathy Questionnaire score and Visual Analogue Scale, respectively, and effect of time.
The VAS score results for both groups were comparable with the overall KCCQ score, with a sharp increase from baseline to Week 4 before stabilizing at Week 12 (Figure 3). An unadjusted mean difference of 0.47 was observed between groups in favour of FCM but did not reach statistical significance (95% CI −0.18 to 1.13, P = 0.152). After adjusting for baseline VAS score and the effect of time, the magnitude of difference was further reduced (mean difference 0.26, 95% CI −0.33 to 0.86, P = 0.386) (Table 4).
Haematological and iron indices
The screening iron indices for all recruited patients fulfilled both the PRACTICE‐ASIA‐HF criteria for ID and the definition for ID in the FAIR‐HF trial (ferritin <100 ng/mL or between 100 and 299 ng/mL, if transferrin saturation is <20%). Improvements in haematological and iron indices were observed over the study period in the FCM group. In particular, 95.0% of patients in the FCM group achieved ferritin levels above 100 ng/mL compared with 39.1% of patients in the placebo group at Week 12 (P < 0.001). In absolute terms, mean ferritin levels were 406 ± 57 vs. 98 ± 17 ng/mL (P < 0.001) in the FCM and placebo arms, respectively, at the end of the study. This is comparable with levels achieved in the FAIR‐HF trial. Likewise, 80.0% of patients in the FCM group no longer had ID at Week 12 compared with 47.8% of patients in the placebo group (P < 0.001). At Week 4, almost twice the number of patients in the FCM group achieved transferrin saturation ≥20% compared with the placebo group (72.7% vs. 39.1%, P = 0.04) although this difference was attenuated by Week 12 (70.0% vs. 47.8%, P = 0.22) (Table 5). No significant differences in mean corpuscular volume, mean corpuscular Hb, haematocrit, and red cell distribution width were detected between study groups.
Table 5.
Change in haematological and iron biomarkers
| FCM | Saline | P‐value | ||
|---|---|---|---|---|
| Ferritin ≥ 100 ng/mL (%) | Baseline | 10 (41.7) | 8 (32.0) | 0.56 |
| Weeks 4–6 | 20 (90.9) | 9 (39.1) | <0.0001 | |
| Week 12 | 19 (95.0) | 9 (39.1) | <0.0001 | |
| TSAT ≥ 20% (%) | Baseline | 8 (34.8) | 6 (24) | 0.53 |
| Weeks 4–6 | 16 (72.7) | 9 (39.1) | 0.04 | |
| Week 12 | 14 (70.0) | 11 (47.8) | 0.22 | |
| IDa (%) | Baseline | 15 (62.5) | 19 (76.0) | 0.48 |
| Weeks 4–6 | 3 (13.6) | 12 (52.2) | <0.0001 | |
| Week 12 | 4 (20.0) | 12 (52.2) | <0.0001 | |
| MCV (fL) | Baseline | 82 ± 7 | 84 ± 8 | 0.9 |
| Weeks 4–6 | 84 ± 6 | 84 ± 9 | 0.66 | |
| Week 12 | 85 ± 5 | 84 ± 9 | 0.13 |
Hb, haemoglobin; FCM, ferric carboxymaltose; ID, iron deficiency; MCV, mean corpuscular volume; TSAT, transferrin saturation.
Serum ferritin <300 ng/mL if transferrin saturation is <20%.
Adverse events
A total of 10 patients experienced 14 HF readmissions over the course of the study: five in the FCM group and five in the placebo group. There were no statistically significant differences in HF readmissions between the two groups analysed using either the total number of affected patients (χ2 statistic 0.005, P = 0.94) or total number of episodes (χ2 statistic 1.5, P = 0.23) (Table 6).
Table 6.
List of adverse events
| Adverse events | FCM | Placebo |
|---|---|---|
| Heart failure recurrence (episodes) | 9 | 5 |
| Drug‐related | ||
| Serious/severe | 0 | 0 |
| General disorders and administration site conditions including fever | 3 | 0 |
| Skin and subcutaneous tissue disorders including rash and pruritus | 2 | 1 |
| Nervous system disorders including headache | 1 | 1 |
| Gastrointestinal disorders | 0 | 0 |
| Vascular disorders | 0 | 0 |
| Investigations | 0 | 0 |
| Ear and labyrinth disorders including dizziness | 1 | 1 |
| Injury, poisoning, and procedural complications | 0 | 0 |
| Cardiac disorders | 0 | 0 |
| Musculoskeletal disorders including myalgia/arthralgia/gout | 2 | 1 |
| Respiratory disorders | 1 | 0 |
| Total | 10 | 4 |
| Unrelated to drug | ||
| Death (from colon carcinoma and complications) | 1 | 0 |
| Moderate (NSTEMI with AF) | 1 | 0 |
| Mild (elevated creatinine, hyperglycaemia/hypoglycaemia, rib fracture, fall, haematuria, gynecomastia, UTI) | 3 | 3 |
| Total | 5 | 3 |
AF, atrial fibrillation; FCM, ferric carboxymaltose; NSTEMI, non‐ST segment elevation myocardial infarction; UTI, urinary tract infection.
Safety analyses
A death occurred in the FCM group that was deemed unrelated to the study drug. The patient was a 61‐year‐old Malay male with ischaemic cardiomyopathy who fulfilled all inclusion criteria and had no contraindications to trial participation at the time of recruitment. He was subsequently diagnosed with metastatic colon carcinoma complicated by active gastrointestinal bleeding and died 2 months after enrolment.
Consequently, no serious treatment‐related adverse events were encountered over the study duration in either group. A total of 15 patients in the FCM group were found to have non‐serious adverse events, of which five (inclusive of the death) were unrelated to the study drug. In the placebo group, seven patients experienced non‐serious adverse events, of which three were unrelated to the study drug (Table 6).
Discussion
The PRACTICE‐ASIA‐HF pilot study is the first prospective randomized controlled trial to compare the effect of a single dose of i.v. FCM with placebo administered pre‐discharge in an inpatient cohort of Southeast Asian patients recovering from an acute episode of decompensated HF regardless of LVEF and with concurrent ID. Over a period of 12 weeks, FCM corrected ID in over 75% of treated patients and improved functional capacity, as measured using the 6MWT, compared with placebo, but this effect was attenuated following adjustment for baseline covariates and effect of time. QoL measures did not differ significantly between FCM and placebo groups. FCM was well tolerated with no serious treatment‐related adverse events.
The beneficial effects of i.v. iron in ambulatory patients with concurrent HFrEF and ID have been well described ever since Toblli et al. and Okonko et al. first demonstrated the efficacy of i.v. iron sucrose in two separate trials.19, 20 These include reduced risk of cardiovascular death or hospitalization for worsening HF, improved 6MWT distance, improved QoL, and improved NYHA class with no increased risk of adverse events as supported by two separate meta‐analyses of available evidence.21, 22 Of the trials included for these meta‐analyses, FAIR‐HF and CONFIRM‐HF accounted for the majority of patients owing to their sample sizes and were largely responsible for the findings. PRACTICE‐ASIA‐HF adds to the existing pool of evidence from a different perspective given our trial design and study population.
Compared with the landmark iron replacement trials, our study adopts a unique timing for FCM administration. The rationale behind this takes into consideration the feasibility of ambulatory i.v. medication administration, where additional time and manpower required for this service may not be available in low‐income to medium‐income countries. Furthermore, knowledge deficiencies pertaining to their heart condition are not uncommon in Southeast Asian patients, leading to hesitancy in consumption and even omission of medication, especially in ambulatory settings.23 Inpatient administration of i.v. FCM prior to discharge utilizes minimal additional resources for hospitals and, given their inpatient status, ensures patients are more receptive to therapy. Our trial demonstrates that administration of i.v. FCM prior to discharge is feasible and can be potentially implemented in clinical practice.
Apart from inpatient i.v. FCM administration, our trial also utilized a simplified single dose of 1000 mg FCM for all subjects, considering the smaller overall size of an average Southeast Asian HF patient compared with a Caucasian. In our study, the mean weight of participants (70 kg) differed from those involving Caucasian patients (77 to 80 kg) by up to 10 kg, and the majority of patients in the FCM arm no longer had ID at Week 12. Despite the lower mean weight of the enrolled patients, 50% of those in the FCM arm had body weight exceeding 70 kg, and 25% had baseline Hb of <10g/dL. Of the four patients in the FCM arm who continued to have ID at the end of the study, three had a body weight >70 kg (mean body weight 91.5 kg with mean body mass index 34 kg/m2), and the remainder had baseline Hb of 9.6 g/dL. While a one‐off dosing regimen may benefit most Southeast Asian patients, body‐weight‐guided and Hb‐guided FCM dosing as proposed by Ponikowski et al. remains relevant and should be considered in patients ≥70 kg and with Hb <10 g/dL.24 Alternatively, if a dosing‐guided initial estimate is not used, iron levels should be checked at a follow‐up visit, ideally between 1 and 3 months, with repeat dosing administered if ID has not been corrected.
The safety profile of i.v. FCM in the ambulatory Caucasian population has been well described with similar incidence of adverse events compared with placebo and no FCM‐related severe allergic reactions. Our study further demonstrates that i.v. FCM can be safely administered in a cohort of Southeast Asian patients with decompensated HF over a period of 12 weeks, with no serious treatment‐related adverse events. This is consistent with a recent meta‐analysis on the safety of i.v. iron preparations by Avni et al., showing that i.v. iron is not associated with an increase in severe adverse events.25 Given its efficacy, dosing flexibility, and safety profile compared with other iron preparations such as iron sucrose and ferric gluconate, i.v. FCM appears to be appropriate for iron replacement in patients with concurrent ID and HF.
We observed near‐parallel improvements in outcomes in both treatment groups up to Week 4, reflecting a common initial response to evidence‐based pharmacotherapy in this high‐risk cohort during hospitalization. The treatment effect reached a plateau for the placebo arm, whereas an incremental benefit of FCM was perceptible after Week 4, based on the divergence of 6MWT distance curves for both treatment arms. This delayed effect is consistent with findings from the FAIR‐HF and CONFIRM‐HF trials, where patients were administered i.v. FCM at least 4 weeks after receiving optimal medical therapy. Significant treatment effects in 6MWT and QoL scores became apparent at a later time point (24 weeks and beyond as observed in CONFIRM‐HF). This clinical effect may correspond to iron utilization by erythrocytes, which have a typical lifespan of around 120 days. In our trial, dosing of i.v. FCM was concurrent with optimization of medical therapy, and our results suggest the need for longer surveillance of patients after administration of i.v. FCM before differences in treatment effect can be appreciated. Additionally, it may be prudent to measure changes in efficacy from Week 4 onwards to minimize the impact of background standard therapy on patients' well‐being.
Study limitations
A systematic error was detected in the grading of NYHA status between study sites, due to miscoding in one of the two sites. This error was only detected at the close of the study, when the database was locked, thus not allowing post hoc correction. A decision was therefore made to exclude this variable from analysis based on invalid measurements. For the assessment of functional status, we focused on the objective measurement of 6MWT distance alone.
Our study was a single‐blind, open‐label trial where the colour of medication received was visible to subjects. Because of practical considerations and difficulty in achieving successful blinding in view of the distinctive dark‐coloured FCM, contemporary trials such as the European multicentre EFFECT‐HF trial and UK‐based IRONMAN trial have also adopted similar study designs.26, 27 Of note, the primary outcomes for the EFFECT‐HF and IRONMAN trials are peak oxygen consumption and mortality, respectively, which are less amenable to patient effort and bias compared with 6MWT.
There was an unexpected imbalance between study groups (mean 6MWT distance differing by >20 m between groups at baseline between the study sites) despite our best efforts to standardize randomization using a central phone‐based system for treatment allocation in addition to an independent study coordinator at each site. Centre‐based analysis confirmed one of the two centres was accountable for this discrepancy, where recruited patients in the FCM group (n = 12) had a baseline mean 6MWT distance of 245 ± 141 m compared with 222 ± 57 m in the placebo group (n = 12). In spite of this possible recruitment bias, results following unadjusted analyses favoured i.v. FCM, albeit non‐statistically significant (Figure S1).
Our original calculation estimated a sample size of 25 per group to have a 90% power to detect a mean difference of 40 m in 6MWT distance between FCM and placebo groups at a two‐sided alpha of 5%. Factors that led to under‐powering of our study were as follows:
The wide range of 6MWT distances resulted in inflation of SD from 60 to 120 m, with the study's 90% power insufficient in compensating for this large SD. This discrepancy between estimated and actual SDs could be explained by utilization of local data assessing the 6MWT in HF patients (SD for 6MWT distance ranged from 18 to 43 m) rather than contemporary data from CONFIRM‐HF (SD for baseline 6MWT distance 98 m).18
Unanticipated failure to complete the 6MWT in four patients following randomization coupled with withdrawal, loss to follow‐up, or death in a total of four further patients led to a substantial reduction in patient data at study completion.
A sample size recalculation was performed using data from this study in order to provide information on number of subjects required for subsequent studies. This yielded a total of 100 subjects (50 per arm) required for a repeated‐measures study design assuming 80% power with a similar magnitude of effect. Likewise, a total sample size of 200 would be necessary for a parallel study design as evaluated in the FAIR‐HF and CONFIRM‐HF trials. This number is in alignment with that from the EFFECT‐HF trial, which studied 174 European patients and was able to demonstrate statistically significant differences in favour of i.v. FCM improving peak oxygen consumption and QoL.12
New developments
The impressive efficacy of i.v. FCM has renewed interest in the possible role of oral iron preparations in concurrent HF and ID. The option of oral iron replacement as an alternative to i.v. FCM is of practical interest because of resource availability and logistical challenges being barriers to widespread adoption of i.v. administration despite multiple studies demonstrating its cost‐effectiveness.28, 29, 30, 31 While early trials of oral iron supplementation were negative, they comprised small sample sizes, involved single centres, were not the primary focus of investigation, and were often paired with erythropoiesis‐stimulating agents.32, 33 A multicentre randomized controlled trial has just been completed, investigating the effects of oral iron polysaccharide vs. oral placebo in improving functional capacity (measured by maximum oxygen uptake during cardiopulmonary exercise testing) in patients with HFrEF and ID (the IRONOUT‐HF trial).34 Despite being one of the largest multicentre trials investigating oral iron, the study presented negative results and did not support use of oral iron in patients with HFrEF.35
A dedicated randomized controlled trial focusing on the HFpEF population with concurrent ID is also being planned and will investigate the effect over 1 year of i.v. FCM on patients with LVEF ≥45% with diastolic dysfunction.36
Conclusions
Our results showed that in the majority of Southeast Asian patients with ID hospitalized for acute decompensated HF, a single dose of i.v. FCM 1000 mg administered pre‐discharge compared with placebo was safe and clinically feasible. Changes in 6MWT distance should be assessed beyond Week 12, and efficacy changes should be reviewed from Week 4 onwards to account for background therapy effects.
Conflict of interest
C. S. L. has received research support from Boston Scientific, Medtronic, and Vifor Pharma and has consulted for Bayer and Novartis. F. A. H. and T. C. were employees of Vifor Pharma at the time of writing.
Funding
This work was supported by the National Medical Research Council, Singapore (Centre Grant: R‐172‐003‐219‐511 and Clinician Scientist Award to C. S. L) and an unrestricted research grant from Vifor Pharma.
Supporting information
Figure S1. Absolute 6MWT distances over time by centre.
Acknowledgements
We would like to acknowledge the research coordinators, nurses, and ward staff members who contributed to this paper, including Ms Marissa Lim, for their tireless efforts in patient recruitment for this study.
Yeo, T. J. , Yeo, P. S. D. , Hadi, F. A. , Cushway, T. , Lee, K. Y. , Yin, F. F. , Ching, A. , Li, R. , Loh, S. Y. , Lim, S. L. , Wong, R. C.‐C. , Tai, B. C. , Richards, A. M. , and Lam, C. S. P. (2018) Single‐dose intravenous iron in Southeast Asian heart failure patients: A pilot randomized placebo‐controlled study (PRACTICE‐ASIA‐HF). ESC Heart Failure, 5: 344–353. doi: 10.1002/ehf2.12250.
References
- 1. Atherton JJ, Hayward CS, Wan Ahmad WA, Kwok B, Jorge J, Hernandez AF, Liang L, Kociol RD, Krum H. Patient characteristics from a regional multicenter database of acute decompensated heart failure in Asia Pacific (ADHERE International‐Asia Pacific). J Card Fail 2012; 18: 82–88. [DOI] [PubMed] [Google Scholar]
- 2. Lam CS, Anand I, Zhang S, Shimizu W, Narasimhan C, Park SW, Yu CM, Ngarmukos T, Omar R, Reyes EB, Siswanto B, Ling LH, Richards AM. Asian Sudden Cardiac Death in Heart Failure (ASIAN‐HF) registry. Eur J Heart Fail 2013; 15: 928–936. [DOI] [PubMed] [Google Scholar]
- 3. Yeo TJ, Yeo PS, Ching‐Chiew Wong R, Ong HY, Leong KT, Jaufeerally F, Sim D, Santhanakrishnan R, Lim SL, M MYC, Chai P, Low AF, Ling LH, Ng TP, Richards AM, Lam CS. Iron deficiency in a multi‐ethnic Asian population with and without heart failure: prevalence, clinical correlates, functional significance and prognosis. Eur J Heart Fail 2014; 16: 1125–1132. [DOI] [PubMed] [Google Scholar]
- 4. Comin‐Colet J, Enjuanes C, Gonzalez G, Torrens A, Cladellas M, Merono O, Ribas N, Ruiz S, Gomez M, Verdu JM, Bruguera J. Iron deficiency is a key determinant of health‐related quality of life in patients with chronic heart failure regardless of anaemia status. Eur J Heart Fail 2013; 15: 1164–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jankowska EA, Rozentryt P, Witkowska A, Nowak J, Hartmann O, Ponikowska B, Borodulin‐Nadzieja L, von Haehling S, Doehner W, Banasiak W, Polonski L, Filippatos G, Anker SD, Ponikowski P. Iron deficiency predicts impaired exercise capacity in patients with systolic chronic heart failure. J Card Fail 2011; 17: 899–906. [DOI] [PubMed] [Google Scholar]
- 6. Klip IT, Comin‐Colet J, Voors AA, Ponikowski P, Enjuanes C, Banasiak W, Lok DJ, Rosentryt P, Torrens A, Polonski L, van Veldhuisen DJ, van der Meer P, Jankowska EA. Iron deficiency in chronic heart failure: an international pooled analysis. Am Heart J 2013; 165: 575–582.e3. [DOI] [PubMed] [Google Scholar]
- 7. Okonko DO, Mandal AK, Missouris CG, Poole‐Wilson PA. Disordered iron homeostasis in chronic heart failure: prevalence, predictors, and relation to anemia, exercise capacity, and survival. J Am Coll Cardiol 2011; 58: 1241–1251. [DOI] [PubMed] [Google Scholar]
- 8. Anker SD, Comin Colet J, Filippatos G, Willenheimer R, Dickstein K, Drexler H, Luscher TF, Bart B, Banasiak W, Niegowska J, Kirwan BA, Mori C, von Eisenhart RB, Pocock SJ, Poole‐Wilson PA, Ponikowski P. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med 2009; 361: 2436–2448. [DOI] [PubMed] [Google Scholar]
- 9. Ponikowski P, van Veldhuisen DJ, Comin‐Colet J, Ertl G, Komajda M, Mareev V, McDonagh T, Parkhomenko A, Tavazzi L, Levesque V, Mori C, Roubert B, Filippatos G, Ruschitzka F, Anker SD. Beneficial effects of long‐term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency. Eur Heart J 2015; 36: 657–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 11. Filippatos G, Farmakis D, Colet JC, Dickstein K, Luscher TF, Willenheimer R, Parissis J, Gaudesius G, Mori C, von Eisenhart RB, Greenlaw N, Ford I, Ponikowski P, Anker SD. Intravenous ferric carboxymaltose in iron‐deficient chronic heart failure patients with and without anaemia: a subanalysis of the FAIR‐HF trial. Eur J Heart Fail 2013; 15: 1267–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Veldhuisen DJv. Effect of Ferric Carboxymaltose on Exercise Capacity in Patients with Iron Deficiency and Chronic Heart Failure (EFFECT‐HF). 2016.
- 13. Cohen‐Solal A, Damy T, Terbah M, Kerebel S, Baguet JP, Hanon O, Zannad F, Laperche T, Leclercq C, Concas V, Duvillie L, Darne B, Anker S, Mebazaa A. High prevalence of iron deficiency in patients with acute decompensated heart failure. Eur J Heart Fail 2014; 16: 984–991. [DOI] [PubMed] [Google Scholar]
- 14. Núñez J, Comín‐Colet J, Miñana G, Núñez E, Santas E, Mollar A, Valero E, García‐Blas S, Cardells I, Bodí V, Chorro FJ, Sanchis J. Iron deficiency and risk of early readmission following a hospitalization for acute heart failure. Eur J Heart Fail 2016; 18: 798–802. [DOI] [PubMed] [Google Scholar]
- 15. Yeo TJ, Yeo PSD, Hadi FA, Cushway T, Lee KY, Tai BC, Lam CSP. Rationale and design of a pilot randomized controlled trial to assess the role of intravenous ferric carboxymaltose in Asian patients with heart failure (PRACTICE‐ASIA‐HF). ESC Heart Failure 2016; 3: 71–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ganzoni AM. Intravenous iron‐dextran: therapeutic and experimental possibilities. Schweiz Med Wochenschr 1970; 100: 301–303. [PubMed] [Google Scholar]
- 17. ATS statement: guidelines for the six‐minute walk test. Am J Respir Crit Care Med 2002; 166: 111–117. [DOI] [PubMed] [Google Scholar]
- 18. Lee R, Chan YH, Wong J, Lau D, Ng K. The 6‐minute walk test predicts clinical outcome in Asian patients with chronic congestive heart failure on contemporary medical therapy: a study of the multiracial population in Singapore. Int J Cardiol 2007; 119: 168–175. [DOI] [PubMed] [Google Scholar]
- 19. Toblli JE, Lombrana A, Duarte P, Di Gennaro F. Intravenous iron reduces NT‐pro‐brain natriuretic peptide in anemic patients with chronic heart failure and renal insufficiency. J Am Coll Cardiol 2007; 50: 1657–1665. [DOI] [PubMed] [Google Scholar]
- 20. Okonko DO, Grzeslo A, Witkowski T, Mandal AK, Slater RM, Roughton M, Foldes G, Thum T, Majda J, Banasiak W, Missouris CG, Poole‐Wilson PA, Anker SD, Ponikowski P. Effect of intravenous iron sucrose on exercise tolerance in anemic and nonanemic patients with symptomatic chronic heart failure and iron deficiency FERRIC‐HF: a randomized, controlled, observer‐blinded trial. J Am Coll Cardiol 2008; 51: 103–112. [DOI] [PubMed] [Google Scholar]
- 21. Qian C, Wei B, Ding J, Wu H, Wang Y. The efficacy and safety of iron supplementation in patients with heart failure and iron deficiency: a systematic review and meta‐analysis. Can J Cardiol 2016; 32: 151–159. [DOI] [PubMed] [Google Scholar]
- 22. Jankowska EA, Tkaczyszyn M, Suchocki T, Drozd M, von Haehling S, Doehner W, Banasiak W, Filippatos G, Anker SD, Ponikowski P. Effects of intravenous iron therapy in iron‐deficient patients with systolic heart failure: a meta‐analysis of randomized controlled trials. Eur J Heart Fail 2016; 18: 786–795. [DOI] [PubMed] [Google Scholar]
- 23. Lip GY, Khan H, Bhatnagar A, Brahmabhatt N, Crook P, Davies MK. Ethnic differences in patient perceptions of heart failure and treatment: the West Birmingham heart failure project. Heart 2004; 90: 1016–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ponikowski P, van Veldhuisen DJ, Comin‐Colet J, Ertl G, Komajda M, Mareev V, McDonagh TA, Parkhomenko A, Tavazzi L, Levesque V, Mori C, Roubert B, Filippatos G, Ruschitzka F, Anker SD. Rationale and design of the CONFIRM‐HF study: a double‐blind, randomized, placebo‐controlled study to assess the effects of intravenous ferric carboxymaltose on functional capacity in patients with chronic heart failure and iron deficiency. ESC Heart Failure 2014; 1: 52–58. [DOI] [PubMed] [Google Scholar]
- 25. Avni T, Bieber A, Grossman A, Green H, Leibovici L, Gafter‐Gvili A. The safety of intravenous iron preparations: systematic review and meta‐analysis. Mayo Clin Proc 2015; 90: 12–23. [DOI] [PubMed] [Google Scholar]
- 26. Medicine USNLo . Intravenous Iron Treatment in Patients with Heart Failure and Iron Deficiency: IRONMAN. 2015.
- 27. Medicine USNLo . Effect of Ferric Carboxymaltose on Exercise Capacity in Patients with Iron Deficiency and Chronic Heart Failure (EFFECT‐HF). 2015.
- 28. Comin‐Colet J, Rubio‐Rodriguez D, Rubio‐Terres C, Enjuanes‐Grau C, Gutzwiller FS, Anker SD, Ponikowski P. A cost‐effectiveness analysis of ferric carboxymaltose in patients with iron deficiency and chronic heart failure in Spain. Rev Esp Cardiol (Engl Ed) 2015; 68: 846–851. [DOI] [PubMed] [Google Scholar]
- 29. Gutzwiller FS, Schwenkglenks M, Blank PR, Braunhofer PG, Mori C, Szucs TD, Ponikowski P, Anker SD. Health economic assessment of ferric carboxymaltose in patients with iron deficiency and chronic heart failure based on the FAIR‐HF trial: an analysis for the UK. Eur J Heart Fail 2012; 14: 782–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hofmarcher T, Borg S. Cost‐effectiveness analysis of ferric carboxymaltose in iron‐deficient patients with chronic heart failure in Sweden. J Med Econ 2015; 18: 492–501. [DOI] [PubMed] [Google Scholar]
- 31. Lim EA, Sohn HS, Lee H, Choi SE. Cost‐utility of ferric carboxymaltose (Ferinject(R)) for iron‐deficiency anemia patients with chronic heart failure in South Korea. Cost Eff Resour Alloc 2014; 12: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Maurer MS, Teruya S, Chakraborty B, Helmke S, Mancini D. Treating anemia in older adults with heart failure with a preserved ejection fraction with epoetin alfa: single‐blind randomized clinical trial of safety and efficacy. Circ Heart Fail 2013; 6: 254–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Parissis JT, Kourea K, Panou F, Farmakis D, Paraskevaidis I, Ikonomidis I, Filippatos G, Kremastinos DT et al Am Heart J 2008; 155 751.e1‐7. [DOI] [PubMed] [Google Scholar]
- 34. Lewis GD, Semigran MJ, Givertz MM, Malhotra R, Anstrom KJ, Hernandez AF, Shah MR, Braunwald E. Oral iron therapy for heart failure with reduced ejection fraction: design and rationale for oral iron repletion effects on oxygen uptake in heart failure. Circ Heart Fail 2016: 9(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lewis GD. Oral Iron Repletion Effects on Oxygen Uptake in Heart Failure (IRONOUT). 2016. [DOI] [PMC free article] [PubMed]
- 36. von Haehling S, Jankowska EA, van Veldhuisen DJ, Ponikowski P, Anker SD. Iron deficiency and cardiovascular disease. Nat Rev Cardiol 2015; 12: 659–669. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Absolute 6MWT distances over time by centre.


