Abstract
Aim
Hospital discharges with a diagnosis of cardiomyopathy have more than doubled in Sweden since 1987. We validated the cardiomyopathy diagnoses over this time period to investigate that the increase was real and not a result of improved recognition of the diagnosis and better diagnostic methods.
Methods and results
Every fifth year from 1989 to 2009, records for all patients with a cardiomyopathy diagnosis were identified by searching the local registers in three hospitals in Västra Götaland, Sweden. The diagnoses were validated according to criteria defined by the European Society of Cardiology from 2008. The population comprised 611 cases with cardiomyopathy diagnoses [mean age 58.9 (SD 15.5) years, 68.2% male], divided into three major groups: dilated, hypertrophic, and other cardiomyopathies. Hypertrophic cardiomyopathy and hypertrophic obstructive cardiomyopathy were analysed as a group. Cardiomyopathies for which there were few cases, such as restrictive, arrhythmogenic right ventricular, left ventricular non‐compaction, takotsubo, and peripartum cardiomyopathies, were analysed together and defined as ‘other cardiomyopathies’. Relevant co‐morbidities were registered. The use of echocardiography was 99.7%, of which 94.6% was complete echocardiography reports. The accuracy rates of the diagnoses dilated cardiomyopathy, hypertrophic cardiomyopathy, and other cardiomyopathies were 85.5%, 87.5%, and 100%, respectively, with no differences between the three hospitals or years studied; nor did the prevalence of co‐morbidities differ.
Conclusions
The accuracy rate of the cardiomyopathy diagnoses from in‐hospital records from >600 patients in western Sweden during a 20 year period was 86.6%, with no significant trend over time, strengthening epidemiological findings that this is likely due to an actual increase in cardiomyopathy diagnoses rather than changes in coding practices. The use of echocardiography was high, and there was no significant difference in co‐morbidities during the study period. The accuracy rate of the cardiomyopathy diagnoses during the 20 year period was high. The use of diagnostic tools did not increase under the study period, and once cardiomyopathy diagnoses were suspected, echocardiography was performed in almost all cases. In this study, the occurrence of cardiomyopathy was increasing over time without significant increase of co‐morbidity, supporting that an actual increase of cardiomyopathy has occurred.
Keywords: Cardiomyopathy, Diagnosis, Validation, Co‐morbidity
Introduction
Heart failure (HF) is a serious condition, with an overall 5 year mortality of ~50%,1 mostly affecting older people; but it may also occur in the young.2 There is always an underlying cause to HF.3, 4, 5, 6 Cardiomyopathies form a comparatively minor but important aetiological group, especially among young patients, in whom it is seen in about 20% of a hospitalized population with HF.7
Cardiomyopathy includes a heterogeneous group of diseases.8 More detailed and specific definitions have evolved over the years.8, 9, 10, 11 In the 1980s, cardiomyopathies were defined as heart muscle disease of unknown cause,9, 10, 12 primary or secondary to other systemic diseases.13, 14 This often resulted in difficulties in differentiating between the two groups. With increasing knowledge of pathogenesis, aetiology, and genetic defects, cardiomyopathy was defined in 1995 as ‘heart muscle disease associated with cardiac dysfunction’.14 A new classification by the European Society of Cardiology (ESC)8 was introduced in 2008: ‘A myocardial disease in which the heart muscle is structurally and functionally abnormal, in the absence of coronary artery disease, hypertension, valvular disease and congenital heart disease sufficient to cause the observed myocardial abnormality.’ This includes hereditary forms as well as those induced by viral infection, inflammatory disease, medication, alcohol and/or drug abuse, tachycardia, and dilated cardiomyopathy (DCM) in pregnancies.8 A recent update of the ESC guidelines from 2014 of the diagnosis of hypertrophic cardiomyopathy (HCM) is not taken into account in this study.
Data from the Swedish National Inpatient Registry (IPR) have shown that HF associated with cardiomyopathies more than doubled in all age groups between 1987 and 2006 with no tendency to flatten out.7 If this is a real increase, we can look forward to an HF epidemic, not just due to an aging population, but also due to an increase in the young, still working population. However, potentially, this increase may not be a result of a real increase but, rather, of improved recognition of the diagnosis and better diagnostic methods. Accordingly, validation of the cardiomyopathy diagnoses over an extended time period is important. To our knowledge, no such validation exists. The present study aims to investigate not only the accuracy of the diagnosis but also whether there has been a change in validity over time, while taking into account changes in the use of diagnostic methods and the prevalence of co‐morbidities.
Methods
Procedures
Sweden has a publicly funded national health system providing a comprehensive set of facilities and hospital care for all citizens. Sahlgrenska University Hospital/Östra is a university hospital with a catchment area of 260 000 inhabitants, while Kungälv Hospital and Lidköping Hospital are local hospitals with catchment areas of 122 000 and 38 000 inhabitants, respectively. Patient records with a cardiomyopathy diagnosis from the departments of internal medicine, including the cardiology wards from the three hospitals, were identified from the local hospital discharge registries. Diagnostic codes for all hospital discharges are entered into the IPR with complete coverage from 1987 onwards. A validation of other major diagnoses in the IPR was performed by the National Board of Health and Welfare in 2011, confirming the high validity (up to 85–95%) of major cardiovascular diagnoses.22 The International Classification of Diseases (ICD) is used in all Swedish hospitals. From 1987 to 1996, the ICD‐9 was in use, and thereafter the ICD‐10. ICD codes used in this study are presented in Table S1 .
We obtained all records from all patients who were hospitalized or visited any of the outpatient clinics at the three hospitals and diagnosed with any form of cardiomyopathy over five selected periods between 1989 and 2009 (see Table S2 for details). Information was collected from the records in a structured manner and included gender, age at time of diagnosis, co‐morbidities, family history, height and weight, alcohol and/or drug abuse, medication potentially affecting the left ventricle (e.g. chemotherapy), and radiation therapy to the chest. In addition, type of cardiomyopathy and diagnostic criteria for the specific type of cardiomyopathy according to the diagnostic criteria and definition of the ESC from 20088 were recorded. Details from echocardiography, coronary angiography, and cardiac magnetic resonance imaging, when performed, were collected.
For each time period, the most recent and comprehensive set of diagnostic criteria according to the ESC guidelines from 2008 was used.8 Specific types of cardiomyopathy such as restrictive, arrhythmogenic right ventricular, left ventricular non‐compaction, and takotsubo cardiomyopathies were also defined according to the ESC classification from 2008.8 Validation of the cardiomyopathy diagnoses was categorized as definite, uncertain, or miscoded. The validation was made independently by two physicians, and in questionable cases, two other highly experienced cardiologists studied the records separately, after which a final decision was made.
A diagnosis was defined as definite if the ESC diagnostic criteria from 20088 were fulfilled. If not all criteria were fulfilled, such as in patients with HCM and underlying untreated or poorly controlled hypertension or DCM and a prior myocardial infarction with secondary HF, the diagnoses were defined as miscoded. In some cases, medical records had insufficient documentation, for example, where an echocardiography had been performed but the complete report was missing. In these cases, the diagnoses were defined as uncertain.
Hypertension was defined as a recorded systolic blood pressure ≥ 140 mmHg, a diastolic blood pressure ≥ 90 mmHg, or both, on at least three occasions, or if blood pressure‐lowering drugs had been prescribed prior to the cardiomyopathy diagnosis. Medical records were also scrutinized for potential impact of insufficiently treated high blood pressure. A concurrent or prior diagnosis of diabetes mellitus or atrial fibrillation was documented. Valvular disease was defined as present if recorded as a primary or contributory diagnosis and if the echocardiography report indicated moderate to severe valvular disease (e.g. in the case of mitral or aortic valve regurgitation, a leakage of at least grade 3 or 4). Coronary artery disease was defined as present if medical records revealed information of previous myocardial infarction or revascularization and if the patient's symptomology, coronary angiography, echocardiography, electrocardiogram, or laboratory reports contained supporting data in accordance with ESC guidelines.15, 16, 17 Data about alcohol and/or drug abuse found in the records were recorded.
In addition, 20 medical records were randomly selected, and two experienced cardiologists validated the cardiomyopathy diagnoses separately.
Finally, 20 medical records with a diagnostic code of HF (I50) and no obvious cause (for details see Supporting Information Procedure 1) of cardiomyopathies; ischaemic heart disease; hypertension; aortic, mitral, and tricuspid valve disease; myocarditis; tachycardia, congenital heart disease; thyrotoxicosis and thyroiditis; and diabetes were identified and reviewed for the possible presence of cardiomyopathy, five for each year in 1997, 1999, 2004, and 2009.
Statistical analysis
Descriptive statistics are presented as percentages or mean values with standard deviation (SD). Accuracy rates of diagnosis between different periods and hospitals including the frequency of co‐morbidities were examined using the Pearson χ2 test for categorical variables and Student's t‐test for continuous variables. All P values are two sided, and values <0.05 were considered statistically significant. All statistical analyses were performed using SPSS for Windows version 18.0 (SPSS Inc., Chicago, IL, USA).
Results
A total of 640 patient records with a cardiomyopathy diagnosis were identified for analysis. Twenty‐five records were excluded because of lack of background information, for example, patients with a single short‐term admission living in another part of Sweden and with no further documentation available. Besides, four cases with a working diagnosis of ‘unspecified cardiomyopathy’ were excluded after a diagnostic workup had revealed a different aetiological cause of cardiac dysfunction, and cardiomyopathy as a diagnosis was therefore rejected. Thus, the final population included 611 cases with a recorded diagnosis of cardiomyopathy.
Patient characteristics are presented in Table 1. The study population comprised 611 patients of mean age 58.9 (SD 15.5) years, of whom 417 (68.2%) were men. In all, 72.0% of the patients were treated at Sahlgrenska University Hospital/Östra, 17.7% at Kungälv Hospital, and 10.3% at Lidköping Hospital. A total of 407 records with a DCM diagnosis, 100 records with an HCM diagnosis, and 84 records with a diagnosis of obstructive HCM (OHCM) diagnosis were identified as well as 20 records with other cardiomyopathy diagnoses.
Table 1.
Patient characteristics by hospital discharge or outpatient visit diagnosis
|
DCM n = 407 |
HCM and OHCM n = 184 |
Other n = 20 |
Total n = 611 |
|
|---|---|---|---|---|
| Age, mean (SD) | 59.6 (13.7) | 58.3 (18.6) | 53.1 (15.2) | 58.9 (15.5) |
| ≤54 years | 145 (35.6) | 75 (40.8) | 11 (55.0) | 231 (37.8) |
| 55 to 64 years | 107 (26.3) | 32 (17.4) | 4 (20.0) | 143 (23.4) |
| ≥65 years | 155 (38.1) | 77 (41.8) | 5 (25.0) | 237 (38.8) |
| Gender | ||||
| Male | 309 (75.9) | 103 (55.97) | 5 (25.0) | 417 (68.2) |
| Female | 98 (24.1) | 81 (44.0) | 15 (75.0) | 194 (31.8) |
| Hypertension | 94 (23.1) | 11 (5.97) | 8 (40.0) | 113 (18.5) |
| Coronary artery disease | 70 (17.2) | 2 (1.09) | 3 (15.0) | 75 (12.3) |
| Valve disease | 64 (15.7) | 2 (1.09) | 5 (25.0) | 71 (11.6) |
| Diabetes mellitus | 71 (17.4) | 3 (1.63) | 3 (15.0) | 77 (12.6) |
| Atrial fibrillation | 134 (32.9) | 6 (3.26) | 12 (60.0) | 152 (24.9) |
| Alcohol or drug abuse | 98 (24.1) | 1 (0.54) | 4 (20.0) | 103 (16.9) |
| Hospital | ||||
| SU/Östra | 277 (68.1) | 149 (80.97) | 14 (70.0) | 440 (72.0) |
| Kungälv | 81 (19.9) | 22 (11.95) | 5 (25.0) | 108 (17.7) |
| Lidköping | 49 (12.0) | 13 (7.07) | 1 (5.00) | 63 (10.3) |
All values are presented as n (%) if not otherwise stated.
DCM, dilated cardiomyopathy; HCM, hypertrophic cardiomyopathy; OHCM, obstructive hypertrophic cardiomyopathy; Other, peripartum, restrictive, takotsubo, arrhythmogenic right ventricular or left ventricular non‐compaction cardiomyopathy.
Of 407 medical records diagnosed as DCM, 85.5% were classified as definite and 13.3% as miscoded, while 1.23% were classified as uncertain (Table 2). The accuracy rates of the DCM diagnosis at Sahlgrenska University Hospital/Östra, Kungälv Hospital, and Lidköping Hospital were 84.8%, 86.4%, and 87.8%, respectively. The 184 HCM and OHCM cases were analysed together, and in 87.5%, the diagnosis was classified as definite, in 8.69% as miscoded, and in 3.8% as uncertain (Table 2). The accuracy rates of the HCM and OHCM diagnoses at Sahlgrenska University Hospital/Östra, Kungälv Hospital, and Lidköping Hospital were 88.2%, 86.4%, and 84.6%, respectively. As other cardiomyopathies only comprised 20 cases (3.3%), they were analysed together. All of these cases were classified as definite (100%).
Table 2.
Accuracy of the specific cardiomyopathy diagnoses identified from the hospital registries by year
| Validity of diagnosis | 1989–90 | 1994–96 | 1997–99 | 2004 | 2009 | Total | |
|---|---|---|---|---|---|---|---|
| n = 20 | n = 56 | n = 76 | n = 105 | n = 150 | n = 407 | ||
| DCM | Definite | 17 (85.0) | 47 (83.9) | 60 (78.9) | 92 (87.6) | 132 (88.0) | 348 (85.5) |
| Miscoded | 3 (15.0) | 8 (14.3) | 15 (19.7) | 12 (11.4) | 16 (10.7) | 54 (13.3) | |
| Uncertain | 0 | 1 (1.78) | 1 (1.32) | 1 (0.95) | 2 (1.33) | 5 (1.23) | |
| n = 2 | n = 8 | n = 34 | n = 32 | n = 108 | n = 184 | ||
| HCM and OHCM | Definite | 2 (100) | 6 (75.0) | 29 (85.3) | 28 (88.5) | 96 (88.9) | 161 (87.5) |
| Miscoded | 0 | 1 (12.5) | 3 (8.82) | 2 (6.25) | 10 (9.26) | 16 (8.69) | |
| Uncertain | 0 | 1 (12.5) | 2 (5.88) | 2 (6.25) | 2 (1.85) | 7 (3.80) |
All values are presented as n (%). Owing to few cases, all cases from 1990 were included in 1989; all cases from 1996 were included in 1994; and all cases from 1997 were included in 1999. Records older than 1997 from Kungälv Hospital and 1999 from Lidköping Hospital were not identified. A diagnosis was defined as definite if the ESC diagnostic criteria from 20088 were fulfilled. Besides, in cases with present coronary artery disease and DCM with underlying causes such as tachycardia, alcohol and drug abuse, and myocarditis, or where coronary angiography revealed minimal signs of coronary artery disease not sufficient to cause a global reduction of systolic cardiac function and, finally, in cases with present hypertension but well treated and insufficient to cause global impact on the cardiac function, the diagnosis was defined as definite. If the ESC diagnostic criteria from 2008 were not fulfilled, the diagnosis was defined as miscoded. The diagnosis was defined as uncertain in cases with insufficient documentation.
DCM, dilated cardiomyopathy; ESC, European Society of Cardiology; HCM, hypertrophic cardiomyopathy; OHCM, obstructive hypertrophic cardiomyopathy.
In cases of DCM that were miscoded, underlying coronary artery disease was present in 44/54 (81.5%), with only very few cases of primary valvular disease (5/54, 9.26%) or hypertension (3/54, 5.56%). The most common reason for miscoded HCM and OHCM diagnoses was hypertension (12/16, 75.0%).
A complete echocardiography report was found in 77.3% in 1989–90, thereafter varying between 91.2% and 98.2% (Table 3). Of 22 cases of possible cardiomyopathy in 1989 and 1990, a complete echocardiography report was missing in four cases, and one case lacked information on whether echocardiography had been performed. In two cases from 1994–96 and 1997–99, the echocardiography report was incomplete. In 2004, no echocardiography report was found in one case, or the echocardiography report was incomplete (n = 11), as was the situation in 12 cases in 2009. The use of coronary angiography increased from 68.2% in 1989–90 to 84.9% in 2009 (Figure 1).
Table 3.
Use of ultrasonic cardiography and coronary angiography by year in absolute numbers (percentages) in the whole population
| 1989–90 | 1994–96 | 1997–99 | 2004 | 2009 | Total | |
|---|---|---|---|---|---|---|
| UCG | n = 22 | n = 64 | n = 110 | n = 137 | n = 278 | n = 611 |
| Yes | 17 (77.3) | 62 (96.9) | 108 (98.2) | 125 (91.2) | 266 (95.7) | 578 (94.6) |
| No | 1 (4.55) | 0 | 0 | 1 (0.72) | 0 | 2 (0.33) |
| Incomplete reports | 4 (18.2) | 2 (3.12) | 2 (1.81) | 11 (8.03) | 12 (4.31) | 31 (5.07) |
| Coronary angiography | n = 22 | n = 64 | n = 110 | n = 137 | n = 278 | n = 611 |
| Yes | 5 (22.7) | 14 (21.9) | 35 (31.9) | 51 (37.2) | 88 (31.7) | 193 (31.6) |
| No | 7 (31.8) | 30 (46.9) | 27 (24.5) | 25 (18.2) | 42 (15.1) | 131 (21.4) |
| Incomplete reports | 10 (45.5) | 20 (31.2) | 48 (43.6) | 61 (44.5) | 148 (53.2) | 287 (46.9) |
Owing to few cases, all cases from 1990 were included in 1989; all cases from 1996 were included in 1994; and all cases from 1997 were included in 1999. Records older than 1997 from Kungälv Hospital and 1999 from Lidköping Hospital were not identified.
UCG, ultrasonic cardiography.
Figure 1.
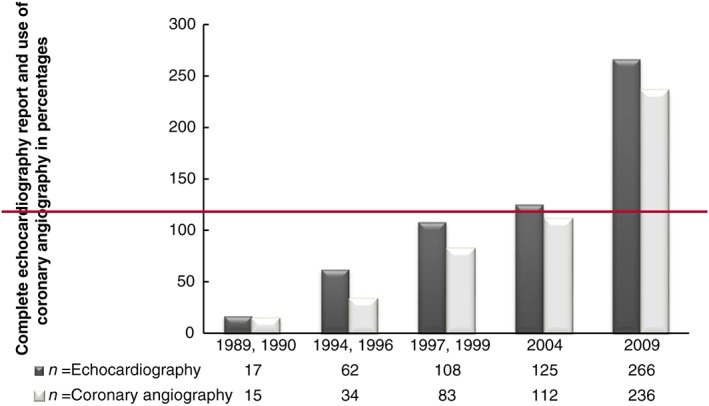
Complete echocardiography report and use of coronary angiography by year in the whole population studied.
Co‐morbidities including hypertension, coronary artery disease, valvular disease, diabetes, and atrial fibrillation as well as risk factors such as alcohol and drug abuse in all patients with a recorded diagnosis of cardiomyopathy are presented in Table 4. A total of 326 patients (53.4%) had up to five co‐morbidities whereas 285 patients had none, with no systematic variation over time for any specific co‐morbidity. In the group where the cardiomyopathy diagnoses were correct, there were 230 patients with DCM (66.1%) and 15 patients with HCM or OHCM (9.3%) who had co‐morbidities and/or risk factors (Table 5), again with no systematic changes over time.
Table 4.
Co‐morbidities by year among patients with any cardiomyopathy diagnosis
|
1989–90 n = 22 |
1994–96 n = 64 |
1997–99 n = 110 |
2004 n = 137 |
2009 n = 278 |
Total n = 611 |
|
|---|---|---|---|---|---|---|
| Hypertension | 3 (13.6) | 14 (21.9) | 18 (16.4) | 33 (24.1) | 45 (16.2) | 113 (18.5) |
| Coronary artery disease | 2 (9.1) | 14 (21.9) | 23 (20.9) | 21 (15.3) | 15 (5.39) | 75 (12.3) |
| Valve disease | 4 (18.2) | 9 (14.1) | 28 (25.5) | 13 (9.49) | 17 (6.12) | 71 (11.6) |
| Diabetes mellitus | 5 (22.7) | 9 (14.1) | 16 (14.5) | 22 (16.1) | 25 (8.99) | 77 (12.6) |
| Atrial fibrillation | 12 (54.5) | 18 (28.1) | 28 (25.5) | 37 (27.0) | 57 (20.5) | 152 (24.9) |
| Alcohol or drug abuse | 6 (27.3) | 13 (20.3) | 16 (14.5) | 29 (21.2) | 39 (14.0) | 103 (16.9) |
| Any co‐morbidity | 15 (68.2) | 41 (64.1) | 63 (57.3) | 86 (62.8) | 121 (43.5) | 326 (53.4) |
All values are presented as n (%). Owing to few cases, all cases from 1990 were included in 1989; cases from 1996 were included in 1994; and cases from 1997 were included in 1999. Records older than 1997 from Kungälv Hospital and 1999 from Lidköping Hospital were not identified.
Table 5.
Co‐morbidities by year among patients with definite dilated cardiomyopathy (DCM), hypertrophic cardiomyopathy, and obstructive hypertrophic cardiomyopathy diagnoses
| DCM |
1989–90 n = 17 |
1994–96 n = 47 |
1997–99 n = 60 |
2004 n = 92 |
2009 n = 132 |
Total n = 348 |
|---|---|---|---|---|---|---|
| Hypertension | 3 (17.6) | 13 (27.7) | 7 (11.7) | 23 (25.0) | 28 (21.2) | 74 (21.3) |
| Coronary artery disease | 0 | 9 (19.1) | 4 (6.67) | 11 (11.95) | 2 (1.51) | 26 (7.47) |
| Valve disease | 4 (23.5) | 7 (14.9) | 17 (28.3) | 12 (13.0) | 14 (10.6) | 54 (15.5) |
| Diabetes mellitus | 4 (23.5) | 8 (17.0) | 7 (11.7) | 18 (19.6) | 19 (14.4) | 56 (16.1) |
| Atrial fibrillation | 9 (52.9) | 14 (29.8) | 19 (31.7) | 27 (29.3) | 42 (31.8) | 111 (31.9) |
| Alcohol or drug abuse | 5 (29.4) | 11 (23.4) | 11 (18.3) | 23 (25.0) | 24 (18.2) | 74 (21.3) |
| Any co‐morbidity | 12 (70.6) | 33 (70.2) | 37 (61.7) | 64 (69.6) | 84 (63.6) | 230 (66.1) |
All values are presented as n (%). Owing to few cases, all cases from 1990 were included in 1989; all cases from 1996 were included in 1994; and all cases from 1997 were included in 1999. Records older than 1997 from Kungälv Hospital and 1999 from Lidköping Hospital were not identified. The same patients could have one to five co‐morbidities.
Two experienced cardiologists analysed separately 20 of the medical records and were in complete agreement. Finally, among 20 medical records with an HF code in the absence of any complementary diagnostic code for ischaemic heart disease, hypertension, tachycardia, atrial fibrillation, valvular disease, myocarditis, congenital heart disease, diabetes, and hyperthyreosis, three cases with an underlying cardiomyopathy were identified (one in 1997 and one in 1999 with DCM, and one in 2009 with peripartum cardiomyopathy). In all three records, the cardiomyopathy diagnosis was recorded in the text, but first on a later occasion registered as discharge diagnosis.
Discussion
The cardiomyopathy diagnoses were validated during the period 1989–2009 in three hospitals in western Sweden. The accuracy of the cardiomyopathy diagnoses during this 20 year period was >85%, with no significant differences between hospitals or time periods. To our knowledge, this is the first study to evaluate the validity of cardiomyopathy diagnoses.
In previous work, we found an unexpected rise in patients discharged with a diagnosis of cardiomyopathy.7 If this is the case, we can expect an increasing, heavy burden to the society of young patients with HF due to cardiomyopathy. Accordingly, we wished to investigate the accuracy of the diagnostic codes over time. To limit the impact of the change in definition over time, we used the 2008 classification8 because this was the most comprehensive, with an echocardiogram with a written report containing all relevant details as mandatory, and where a definite diagnosis of cardiomyopathy was not recorded if the echocardiography report was incomplete or missing.
Most of the miscoded DCM cases had underlying coronary artery disease of such magnitude that an appropriate diagnosis would have been coronary artery disease together with HF. According to the diagnostic criteria we used, diagnoses such as coronary artery disease or hypertension do not exclude the diagnosis of DCM. However, it is important to emphasize that these conditions are not allowed to be of sufficient magnitude as to cause global impairment of the systolic cardiac function.
The most common reason for miscoded HCM and OHCM diagnoses was hypertension. There were four cases whereby patients had definite HCM/OHCM and concomitant hypertension, but the condition was well treated and was not considered to have affected cardiac function. Other cardiomyopathies such as restrictive, takotsubo, and arrhythmogenic right and left ventricular non‐compaction cardiomyopathy, as well as peripartum cardiomyopathy, have very specific diagnostic criteria, which may explain the 100% validity for this mixed category.
A minor proportion of the cardiomyopathy diagnoses were classified as uncertain in this study, mostly because of a lack of relevant or divergent information. For example, cases with hypertension and possible HCM were defined as uncertain if the echocardiography report was incomplete.18 In addition, terms such as ‘unspecified cardiomyopathy’ were often used as a working diagnosis before all investigations had been performed. Once the diagnosis was upgraded from unspecified cardiomyopathy to cardiomyopathy, all details relevant for the diagnosis were assessed, and if missing or incomplete, the diagnosis was defined as miscoded or uncertain, respectively.
The use of echocardiography with complete reports in the medical records was high (on average 94.6%) for all periods of this study except the first time period, 1989–90. In a previous study that validated the diagnosis of HF, the validity declined over the years, although the use of echocardiography increased, and the authors argued that the validity did not depend on the availability of echocardiography.19 However, in that study, echocardiography was not required for a diagnosis of HF. In the present study, echocardiography was used in nearly all cases (in 99.6% of cases, of which 94.5% had complete reports). Thus, the availability of echocardiography was likely adequate throughout the study period. In addition, data from electrocardiograms, cardiac markers, and coronary angiography that could reveal the presence of ischaemic cardiovascular disease, for example, in questionable cases of DCM, were also taken into consideration. Importantly, coronary angiography was not a standard examination method for cardiomyopathy in Sweden during the time period studied, according to the recommendations of the National Board of Health (https://lakemedelsverket.se/upload/om-lakemedelsverket/publikationer/information-fran-lakemedelsverket/Info_fr_LV_2006-1.pdf).
When designing the study, we took into account that the cardiomyopathy diagnoses might have increased over time as a result of the widened definitions of cardiomyopathies during the study period. Accordingly, the frequency of co‐morbidities would be expected to increase as well. In an attempt to take this into consideration, we used the most recent as well as the most inclusive diagnostic criteria from 2008 in the whole population. We found no systematic increasing trends of co‐morbidities in the group as a whole or in the group with a definite diagnosis of cardiomyopathy. In addition, when analysing the 20 medical records with an HF diagnosis and without complementary diagnoses as described in Methods section, none represented a missed cardiomyopathy diagnosis at any time. However, in three cases, the ICD codes for cardiomyopathy were recorded in subsequent years, indicating that causation is necessary when interpreting results from the hospital discharge registry.20, 21, 22
Strengths and limitations
A strength of this study is the large number of medical records studied, at >600. Validation of the HF diagnosis has been provided in a few studies, including at most ~400 medical records. In addition, the present study included three hospitals in different settings, in three cities, in an unselected population covering a large part of western Sweden, without significant differences in diagnosis settings over an extended period of 20 years.
Once the cardiomyopathy diagnosis was suspected, echocardiography was performed in 99.6% of the cases; we thus presume that there has not been a change in access to diagnostic tools over the period studied. However, in a few cases, we were unable to obtain a complete echocardiography report. Another limitation is that there were very few records available to us from the first time period, that is, 1989–90, which might have influenced the results. The reason for this is unclear but could potentially reflect a real increase in cardiomyopathy diagnoses.7 A third limitation is that the diagnosis setting is dependent on the individual physician. Therefore, all records were examined and analysed by an experienced investigator, and upon having even the slightest doubt about the correct diagnosis, two cardiologists independently examined the medical records. In addition, 20 randomly selected medical records were examined twice by two cardiologists who arrived at the same results. Hence, we believe that the results are accurate. A fourth limitation is that the diagnostic criteria changed over time. When using the latest and most inclusive criteria for the whole time period studied, we accordingly tried to take this into account as much as possible. However, although this can only adjust for a correct diagnosis in the cases found, in the earlier time period studied with more limited diagnostic criteria, patients with cardiomyopathy according to the 2008 guidelines could have been excluded. We did not study potential diagnoses such as HF in conjunction with all potential co‐morbidities, although we did review 20 medical records with an HF diagnosis without any contributory ICD codes from 1997 to 2009 as described in Supporting Information Procedure 1. In the three cases where a cardiomyopathy was found, an ICD code for cardiomyopathy was recorded in the subsequent years. Hence, hospital discharge registers may underestimate the total burden of cardiomyopathy in the population23, 24 and false‐negative diagnoses in patients may be hidden in the population.23, 25, 26
Conclusions
During a period of 20 years, in hospital records from >600 patients in western Sweden, the overall accuracy of cardiomyopathy diagnoses was high, strengthening epidemiological findings that an actual increase in cardiomyopathy diagnoses has taken place. This indicates a need for targeted investigation of patients, but also structured monitoring, at both the individual level and group levels, to discover the common causes, if any, of the increase in this heterogeneous condition.
Conflict of interest
None declared.
Funding
Gothenburg Medical Society (GLS), Västra Götaland Region, Swedish Heart‐Lung Foundation, and the Cardiology Research Foundation of the Department of Medicine, Sahlgrenska University Hospital/Östra, Gothenburg, Sweden.
Supporting information
Table S1. ICD codes with descriptions used to detect medical records with cardiomyopathy diagnoses.
Table S2. Medical records identified by hospital and year.
Procedure S1
Basic, C. , Rosengren, A. , Lindström, S. , and Schaufelberger, M. (2018) High validity of cardiomyopathy diagnoses in western Sweden (1989–2009). ESC Heart Failure, 5: 233–240. doi: 10.1002/ehf2.12224.
References
- 1. Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart 2007; 93: 1137–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kannel WB. Incidence and epidemiology of heart failure. Heart Fail Rev 2000; 5: 167–173. [DOI] [PubMed] [Google Scholar]
- 3. Ponikowski P. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 4. McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez‐Sanchez MA, Jaarsma T, Køber L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Rønnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A; ESC Committee for Practice Guidelines . ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012; 33: 1787–1847. [DOI] [PubMed] [Google Scholar]
- 5. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P; Authors/Task Force Members . 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 6. Zarrinkoub R, Wettermark B, Wändell P, Mejhert M, Szulkin R, Ljunggren G, Kahan T. The epidemiology of heart failure, based on data for 2.1 million inhabitants in Sweden. Eur J Heart Fail 2013; 15: 995–1002. [DOI] [PubMed] [Google Scholar]
- 7. Barasa A, Schaufelberger M, Lappas G, Swedberg K, Dellborg M, Rosengren A. Heart failure in young adults: 20‐year trends in hospitalization, aetiology, and case fatality in Sweden. Eur Heart J 2013; 35: 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Elliott P, Andersson B, Arbustini E, Bilinska Z, Cecchi F, Charron P, Dubourg O, Kühl U, Maisch B, McKenna WJ, Monserrat L, Pankuweit S, Rapezzi C, Seferovic P, Tavazzi L, Keren A. Classification of the cardiomyopathies: a position statement from the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2008; 29: 270–276. [DOI] [PubMed] [Google Scholar]
- 9. Oakley CM. Report of the WHO/ISFC task force on the definition and classification of cardiomyopathies. Br Heart J 1980; 48: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hudson REB. The cardiomyopathies: order from chaos. Am J Cardiol 1970; 25: 70–77. [DOI] [PubMed] [Google Scholar]
- 11. Brandeburg RO, Chazov E, Cherian G et al. Report of the WHO/ISFC Task Force on the definition and classification of cardiomyopathies. Br Heart J 1980; 44: 672–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goodwin JF. Cardiac function in primary myocardial disorders I. Br Med J 1964; 1: 1527–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goodwin JF. Cardiac function in primary myocardial disorders II. Br Med J 1964; 1: 1595–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Richardson P, McKenna RW, Bristow M, Maisch B, Mautner B, O'Connell J. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the definition and classification of cardiomyopathies. Circulation 1996; 93: 841–842. [DOI] [PubMed] [Google Scholar]
- 15. Task Force Members , Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, Bugiardini R, Crea F, Cuisset T, Di Mario C, Ferreira JR, Gersh BJ, Gitt AK, Hulot JS, Marx N, Opie LH, Pfisterer M, Prescott E, Ruschitzka F, Sabaté M, Senior R, Taggart DP, van der Wall EE, Vrints CJ, ESC Committee for Practice Guidelines , Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S; Document Reviewers , Knuuti J, Valgimigli M, Bueno H, Claeys MJ, Donner‐Banzhoff N, Erol C, Frank H, Funck‐Brentano C, Gaemperli O, Gonzalez‐Juanatey JR, Hamilos M, Hasdai D, Husted S, James SK, Kervinen K, Kolh P, Kristensen SD, Lancellotti P, Maggioni AP, Piepoli MF, Pries AR, Romeo F, Rydén L, Simoons ML, Sirnes PA, Steg PG, Timmis A, Wijns W, Windecker S, Yildirir A, Zamorano JL. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J 2013; 34: 2949–3003. [DOI] [PubMed] [Google Scholar]
- 16. Hamm CW, Bassand JP, Agewall S, Bax J, Boersma E, Bueno H, Caso P, Dudek D, Gielen S, Huber K, Ohman M, Petrie MC, Sonntag F, Uva MS, Storey RF, Wijns W, Zahger D; ESC Committee for Practice Guidelines . ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation: the Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST‐segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2011; 32: 2999–3054. [DOI] [PubMed] [Google Scholar]
- 17. Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC) ; Steg PG, James SK, Atar D, Badano LP, Blömstrom‐Lundqvist C, Borger MA, Di Mario C, Dickstein K, Ducrocq G, Fernandez‐Aviles F, Gershlick AH, Giannuzzi P, Halvorsen S, Huber K, Juni P, Kastrati A, Knuuti J, Lenzen MJ, Mahaffey KW, Valgimigli M, van't Hof A, Widimsky P, Zahger D. ESC guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation. Eur Heart J 2012; 33: 2569–2619. [DOI] [PubMed] [Google Scholar]
- 18. Williams LK, Frenneaux MP, Steeds RP. Echocardiography in hypertrophic cardiomyopathy diagnosis, prognosis, and role in management. Eur J Echocardiogr 2009; 10: iii9–14. https://doi.org/10.1093/ejechocard/jep157 [DOI] [PubMed] [Google Scholar]
- 19. Ingelsson E, Arnlov J, Sundstrom J, Lind L. The validity of a diagnosis of heart failure in a hospital discharge register. Eur J Heart Fail 2005; 7: 787–791. [DOI] [PubMed] [Google Scholar]
- 20. Khand AU, Shaw M, Gemmel I, Cleland JG. Do discharge codes underestimate hospitalisation due to heart failure? Validation study of hospital discharge coding for heart failure. Eur J Heart Fail 2005; 7: 792–797. [DOI] [PubMed] [Google Scholar]
- 21. Fonseca C, Sarmento PM, Marques F, Ceia F. Validity of a discharge diagnosis of heart failure: implications of misdiagnosing. Congest Heart Fail 2008; 14: 187–191. [DOI] [PubMed] [Google Scholar]
- 22. Kümler T, Gislason GH, Kirk V, Bay M, Nielsen OW, Køber L, Torp‐Pedersen C. Accuracy of a heart failure diagnosis in administrative registers. Eur J Heart Fail 2008; 10: 658–660. [DOI] [PubMed] [Google Scholar]
- 23. Merry AH, Boer JM, Schouten LJ, Feskens EJ, Verschuren WM, Gorgels AP, van den Brandt PA. Validity of coronary heart diseases and heart failure based on hospital discharge and mortality data in the Netherlands using the cardiovascular registry Maastricht cohort study. Eur J Epidemiol 2009; 24: 237–247. [DOI] [PubMed] [Google Scholar]
- 24. Mähönen M, Jula A, Harald K, Antikainen R, Tuomilehto J, Zeller T, Blankenberg S, Salomaa V. The validity of heart failure diagnoses obtained from administrative registers. Eur J Prev Cardiol 2013; 20: 254–259. [DOI] [PubMed] [Google Scholar]
- 25. Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, Heurgren M, Olausson PO. External review and validation of the Swedish national inpatient register. BMC Public Health 2011; 11: 450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mard S, Nielsen FE. Positive predictive value and impact of misdiagnosis of a heart failure diagnosis in administrative registers among patients admitted to a University Hospital cardiac care unit. Clin Epidemiol 2010; 2: 235–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. ICD codes with descriptions used to detect medical records with cardiomyopathy diagnoses.
Table S2. Medical records identified by hospital and year.
Procedure S1


