Abstract
Aims
The effect of extravascular lung water (EVLW) and relationship to functional status as a result of acute decompensated heart failure (ADHF) are not well understood. We sought to quantify changes in clinical variables, EVLW, airway anatomy, spirometry, and diffusing capacity for carbon monoxide before and after treatment for ADHF.
Methods and results
Fifteen patients were recruited within 24 h of hospital admission. Spirometry, diffusing capacity for carbon monoxide, and surrogates of EVLW by computed tomography were measured and were then repeated within 24 h of discharge. From the computed tomography (CT) scan, surrogates of EVLW were calculated from the distribution of CT attenuation of the lung tissue. Airways were segmented using the vida apollo software. Patients were hospitalized for 4.6 ± 2.1 days, had 10 ± 4.8 L of fluid removed (7.0 ± 4.2 L between study visits), and lost 7.1 ± 4.9 kg. Patients had significant clearance of fluid from the lungs (per cent change: mean, 4.2 ± 6.1%; skew, 17.5 ± 27.0%; kurtosis, 37.6 ± 56.7%; full‐width half‐maximum, 10.2 ± 13.5%). Static lung volumes and maximal flows improved significantly (per cent change: forced vital capacity, 14.5 ± 13.6%; forced expiratory volume in 1 s, 15.9 ± 14.0%; forced expiratory flow at 25–75% of forced vital capacity, 27.2 ± 42.9%). The ratio of membrane conductance to capillary blood volume improved significantly (per cent change: alveolar–capillary membrane conductance/capillary blood volume, 23.4 ± 22.8%). Weight loss during hospitalization was significantly correlated with improved spirometry and diffusing capacity.
Conclusions
Extravascular lung water contributes to the pulmonary congestive syndrome in ADHF patients, and its clearance is an important component of the improvement in pulmonary function as a result of inpatient treatment.
Keywords: Computed tomography, Acutely decompensated heart failure, Extravascular lung water, Pulmonary function
Introduction
Hospital admission and readmission for heart failure (HF) is a growing clinical problem in the USA with worsening clinical outcomes after hospitalization.1 In left HF, decompensation is precipitated by increased left ventricular filling pressures as a result of volume overload or redistribution.2 As a result, elevated pulmonary capillary wedge pressure promotes increasing fluid transudation into the interstitial tissue with formation of oedema.
Patients are generally admitted with symptoms of dyspnoea as well as pulmonary and peripheral oedema.3 Primary treatment consists of diuresis until patients have reduced signs of pulmonary and systemic venous congestion and respiratory symptoms have abated. Grading systems based on the presence of peripheral and lung congestion and adequate perfusion have been shown to be predictive of mortality.4, 5 These systems include common clinical metrics including body weight and blood pressure that can assess patient status during hospitalization. While a change in body weight generally occurs, whether this fluid is removed from the interstitial or intravascular volumes depends on a number of factors including HF aetiology.6, 7 Additionally, there is uncertainty regarding the amount of fluid removed from the periphery versus the pulmonary compartments. The relationship between the clinical metrics, fluid volumes, and functional outcomes such as lung function and pulmonary gas exchange has not been clearly established.
We have previously demonstrated increased levels of extravascular lung water (EVLW) associated with decreased pulmonary function in chronic, stable HF patients.8 In these patients, there is decreased pulmonary function, including forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC), and forced expiratory flow at 25–75% of FVC (FEF25–75); decreased diffusing capacity for carbon monoxide (DLCO); and altered gas exchange at both rest and exercise.9, 10, 11 However, the degree to which these functional variables may be modified by therapeutic intervention during an acute decompensation and recovery during a course of inpatient treatment has not been well characterized. One study followed spirometry and DLCO beginning with hospitalization for acute decompensation and found significant improvement in spirometry but minimal changes in DLCO with treatment,12 suggesting persistent extravascular fluid in the alveolar–capillary membrane. This study did not evaluate other clinical measurements such as weight or blood pressure, leaving the relationship between clinical and functional measures unclear.
In the study herein, we sought to evaluate pulmonary congestion during inpatient treatment for acutely decompensated HF by investigating the relationship between clinical and functional variables and response to treatment. We were specifically interested in the changes in EVLW, airway anatomy, lung function, and their relationship to changes in body weight, blood pressure, and other clinical measures. We hypothesized that EVLW would fall with diuresis; that there would be subsequent increases in airway luminal area, improvements in maximal lung volumes and flows, and improvements in DLCO; and that these changes would correlate with clinical measures of body weight and blood pressure from hospital admission to discharge.
Methods
Participants
Fifteen participants were recruited within 24 h of hospital admission for acute HF decompensation and diuresis. Patients were recruited with evidence of volume overload and a previous diagnosis of HF regardless of aetiology or the presence of other co‐morbidities. The protocol was approved by the Mayo Clinic Institutional Review Board, and written informed consent was obtained from all patients prior to testing.
Study design
The study was performed on two visits, one within 24 h of hospital admission and one within 24 h of discharge from the hospital. On both visits, patients were brought to the laboratory to perform, in the sitting position, an FVC manoeuvre, DLCO and diffusing capacity for nitric oxide (DLNO) via rebreathe, and 10 min of resting gas exchange. A thoracic computed tomography (CT) scan was then obtained. All measurements were repeated on the second visit.
Diffusing capacity for carbon monoxide and diffusing capacity for nitric oxide rebreathe
Patients performed a DLCO/DLNO rebreathe manoeuvre in the sitting positions using a mouthpiece attached to a pneumotachometer. This was attached to a switching valve with one port to room air and the other to a 6 L anaesthesia bag filled with a mixture of 35% O2, 9% He, 0.6% C2H2, 0.23% C18O, 40 ppm NO, and balance N2. In four patients, the multiple O2 tensions of 35% and 70% were used instead of DLNO for the calculation of capillary blood volume (Vc) and alveolar–capillary membrane conductance (Dm) owing to technical difficulties. Patients synchronized their breathing with a metronome set to 32 breaths per minute before being switched onto the gas mixture for 8–10 breaths. DLCO and DLNO values were calculated using custom software. The Vc and Dm were calculated using established methods.13
Computed tomography scanning
All CT scans were performed on the same scanner (GE Litespeed 16‐slice CT scanner, GE Healthcare). Scans were obtained with 2.5‐mm‐thick slices and 1.2 mm overlap and reconstructed to 1.25‐mm‐thick slices with a 0.6 mm overlap; the total radiation dose for all scans combined was 25.2 mSv. An initial scout scan was performed to ensure capture of the entire lung volume. The location of the scanner table, field of view, and number of images were recorded, and a mark was made on the subject to ensure alignment between admission and discharge scans. Patients were instructed to hold their breath at total lung capacity during all scans.
Computed tomography quantitative analysis was carried out using the MATLAB (MathWorks, Inc., Natick, MA) software. The lung tissue was automatically segmented out from the surrounding tissue, and pixels outside the range of −1000 to 0 Hounsfield unit were excluded to remove airways from the analysis. Values for the mean, skewness, kurtosis, and full‐width half‐maximum (FWHM) of the distributions were calculated from the segmented areas as indicators of EVLW status.
Statistical analysis
All statistical analysis was carried out using SPSS (SPSS, Chicago, IL). The Wilcoxon matched‐pairs signed rank test was used to compare admission vs. discharge measurements. Linear regression and Kendall's tau coefficient were used to assess the relationship between functional and clinical data. All results are expressed as mean ± standard deviation unless otherwise stated. The acceptable level of Type I error was set at P < 0.05.
Results
Demographics
Fifteen patients who were admitted for decompensated HF associated with volume overload were recruited for the study (Table 1). All patients were treated with 580 mg of intravenous diuretics on average during hospital treatment. Patients had left and right ventricular HF. Additionally, both HF with reduced ejection fraction (HFrEF, n = 7) and HF with preserved ejection fraction (HFpEF, n = 8) were represented. Patients spent between 2 and 8 days in hospital and lost between 3.3 and 20.5 L of fluid during hospitalization (−0.2 to 16.3 L between study visits). Changes in clinical values are shown in Table 2. Patients lost approximately 7 kg of body weight during hospitalization, had significant haemoconcentration, and had reduction in blood pressure. No differences were observed in heart rate, respiratory rate, or oxygen saturation. Pulmonary oedema was observed by chest X‐ray in nine patients at hospital admission. Finally, peripheral oedema was observed in all patients at admission. Peripheral oedema improved in all patients but was only fully resolved in three patients at discharge.
Table 1.
Patient demographics at hospital admission
| Characteristic | Value |
|---|---|
| N (female) | 15 (4) |
| Age, years | 70.9 ± 12.2 |
| Height, cm | 168.7 ± 13.2 |
| BMI, kg m−2 | 34.5 ± 9.7 |
| LVEF, % | 43 ± 17 |
| NT‐pro‐BNP, pg mL−1 | 4810 (790, 22 040) |
| eGFR, mL min−1 1.73 m−2 | 40.4 (17.6, 79.0) |
| Days in hospital | 4 (2, 8) |
| Total fluid loss, L | 8.9 (3.3, 20.5) |
| Fluid loss between study visits, L | 8.0 (−0.2, 16.3) |
| Intravenous Lasix administered, mg | 460 (140, 1500) |
| Heart failure classification | |
| HFrEF (LVEF ≤ 40%) | 7 |
| HFpEF (LVEF > 40%) | 8 |
| Co‐morbidities | |
| Diabetes | 5 |
| Chronic kidney disease | 4 |
| COPD | 3 |
| Coronary artery disease | 2 |
| Pulmonary arterial hypertension | 2 |
| Peripheral artery disease | 1 |
| Pacemaker | 3 |
BMI, body mass index; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; LVEF, left ventricular ejection fraction; NT‐pro‐BNP, N‐terminal pro‐BNP. Patients were a mix of both HFrEF and HFpEF and had evidence of volume overload. Patients were treated with intravenous Lasix during hospitalization for volume overload and approximately 70% of fluid loss was captured between study visits. Age, height, weight, BMI, and LVEF are mean ± standard deviation. NT‐pro‐BNP, eGFR, days in hospital, total fluid loss, fluid loss between study visits, and intravenous Lasix administered are median (range).
Table 2.
Clinical measures associated with disease state and extravascular lung water status
| Admission | Discharge | |
|---|---|---|
| Weight, kg | 99.5 ± 30.6 | 92.4 ± 27.1* |
| Hgb, mg dL−1 | 11.3 ± 2.3 | 12.6 ± 2.1* |
| MAP, mmHg | 95.1 ± 27.8 | 78.5 ± 11.9* |
| HR, b.p.m. | 86.3 ± 19.1 | 82.8 ± 18.5 |
| RR, b.p.m. | 17.3 ± 3.5 | 18.2 ± 3.7 |
| SpO2, % | 95.5 ± 2.8 | 96.1 ± 1.5 |
| Drugs (HFrEF, HFpEF) | ||
| ACE inhibitor | 3 (1, 2) | 4 (3, 1) |
| Angiotensin II blocker | 2 (1, 1) | 2 (1, 1) |
| Beta‐blocker | 8 (5, 3) | 12 (12, 0) |
| Digitalis | 1 (1, 0) | 3 (2, 1) |
| Diuretic | 12 (5, 7) | 15 (7, 8) |
| CT indices | ||
| Mean, HU | −813 ± 40.4 | −847 ± 50.5** |
| Skew | 2.35 ± 0.67 | 2.64 ± 0.56* |
| Kurtosis | 10.5 ± 4.40 | 12.9 ± 3.90** |
| FWHM | 125 ± 36.8 | 110 ± 28.4** |
ACE, angiotensin‐converting enzyme; CT, computed tomography; FWHM, full‐width half‐maximum; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; Hgb, haemoglobin concentration; HR, heart rate; HU, Hounsfield unit; MAP, mean arterial pressure; RR, respiratory rate; SpO2, oxygen saturation.
Patients had significant weight loss, increase in Hgb, and decrease in MAP. No change was observed between study visits in HR, RR, or SpO2. Patient drug regimens were optimized during inpatient treatment. Quantitative CT indices are representative of extravascular lung water status. Decreased mean, increased skew, increased kurtosis, and FWHM are indicative of fluid clearance. Data are mean ± standard deviation.
P < 0.01,
P < 0.05.
Lung function
Spirometric measurements were greatly reduced at admission, expressed as a per cent predicted (Figure 1 ). After treatment, FVC, FEV1, and FEF25–75 improved significantly but were still lower than predicted [admission vs. discharge: FVC, 71.0 ± 23.6% vs. 78.7 ± 22.4%; FEV1, 68.7 ± 26.8% vs. 78.0 ± 27.9%; FEV1/FVC, 96.8 ± 11.0% vs. 98.0 ± 12.7%; FEF25–75, 69.4 ± 51.0% vs. 86.8 ± 64.2%; peak expiratory flow (PEF), 81.8 ± 29.8% vs. 82.1 ± 34.0%]. DLCO, Dm, Vc, and Vc/Dm ratio are shown in Figure 1 . DLCO and Vc did not change, but Dm and the ratio of Dm/Vc improved significantly at discharge visit (admission vs. discharge: DLCO, 11.1 ± 5.6 vs. 11.3 ± 5.9 mL min−1 mmHg−1; Dm, 23.5 ± 12.8 vs. 27.1 ± 13.6 mL min−1 mmHg−1; Vc, 46.6 ± 20.9 vs. 39.4 ± 19.7 mL; Dm/Vc, 0.58 ± 0.45 vs. 0.90 ± 0.82 min−1 mmHg−1).
Figure 1.
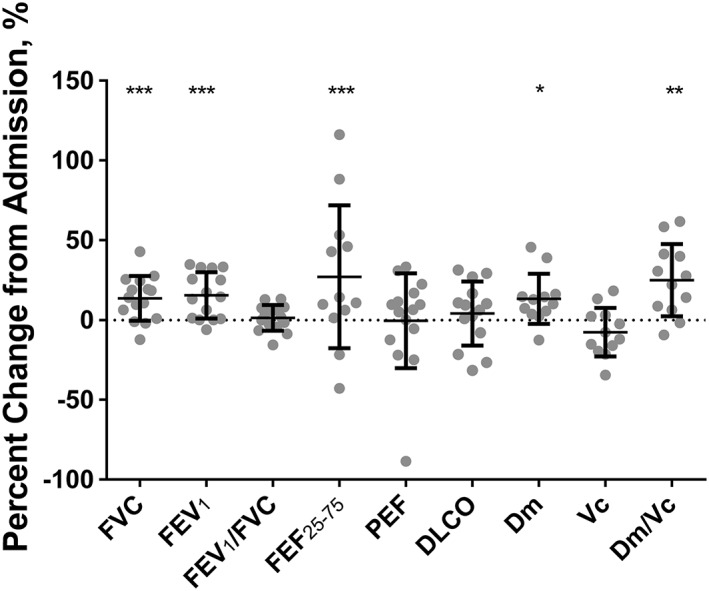
Change in pulmonary function measurements from hospital admission. Grey dots are individual subject response. Error bars are standard deviation. *P < 0.05, **P < 0.01, ***P < 0.001. DLCO, diffusing capacity for carbon monoxide; Dm, alveolar–capillary membrane conductance; FEF, forced expiratory flow at 25–75% of forced vital capacity; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; PEF, peak expiratory flow; Vc, capillary blood volume.
Figure 2 shows the average attenuation of the lung tissue at the admission and discharge study visits, and Table 2 shows the CT quantitative indices at admission and discharge. Qualitatively, the histogram moves to the left, becomes narrower, and becomes taller, suggesting the clearance of fluid. Quantitatively, the mean was more negative, skew increased, kurtosis increased, and FWHM decreased, all suggestive of decreased EVLW (per cent change: mean, 4.2 ± 6.1%; skew, 17.5 ± 27.0%; kurtosis, 37.6 ± 56.7%; FWHM, 10.2 ± 13.5%). Thirteen of the 15 patients had clear evidence of reduced EVLW based on CT indices.
Figure 2.
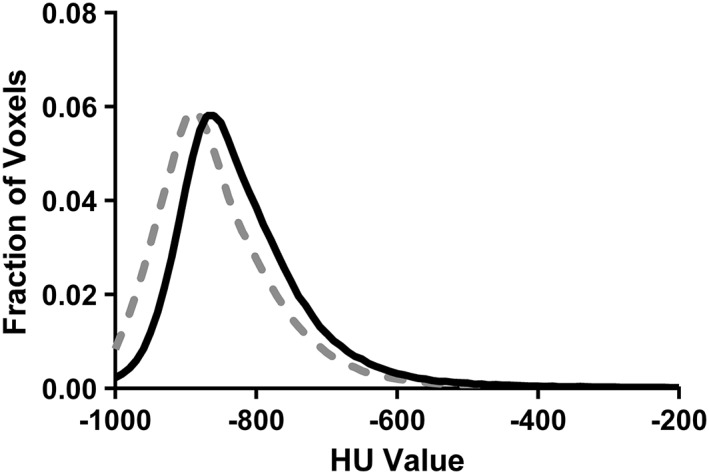
Group mean histogram of computed tomography attenuation of lung tissue for admission (black) and discharge (grey). Leftward shift and higher peak suggest clearance of fluid from admission to discharge. Standard deviation is not shown for clarity.
Airway anatomy
Airway wall thickness is shown in Figure 3 A, and luminal area is shown in Figure 3 B. No changes were observed in airway wall thickness or airway luminal area through six generations where the trachea is Generation 1.
Figure 3.
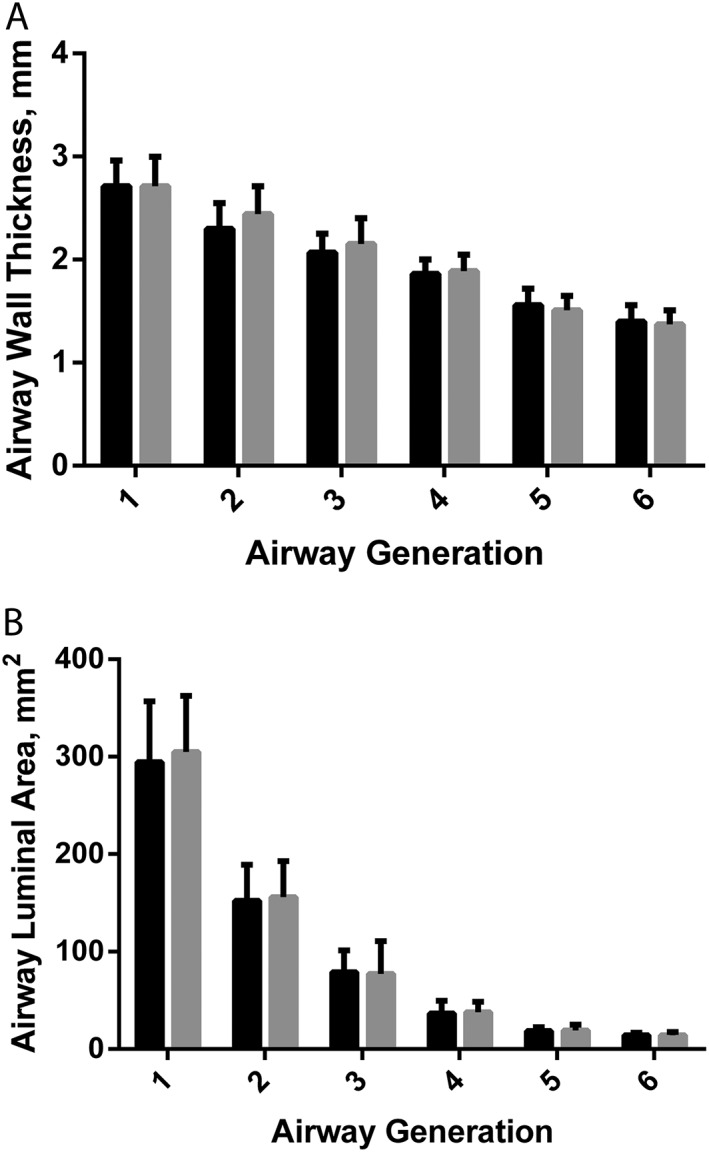
Measurements of airway wall thickness (A) and luminal area (B). Black is admission and grey is discharge. Generation 1 is the trachea, and generation number represents the number of branches from the trachea. Error bars are standard deviation.
Relationship between clinical and functional measures
Weight loss during hospitalization was correlated with improved spirometric function (τ; FEV1: −0.51; FEF25–75: −0.64; PEF: −0.52; P < 0.005). Improved DLCO and Vc were also correlated with greater weight loss (τ; DLCO: −0.38; Vc: −0.43; P < 0.05). These correlations are shown in Figure 4 A–D. Additionally, improvement in spirometry was correlated with improvement in DLCO (τ: FVC: 0.62; FEV1: 0.58; PEF: 0.51; P < 0.01). Number of days in hospital and decrease in mean arterial pressure were not correlated with any functional measure. Finally, no differences were observed between patients with HFrEF and HFpEF for any measure of EVLW, airway anatomy, lung function, or clinical measurement.
Figure 4.
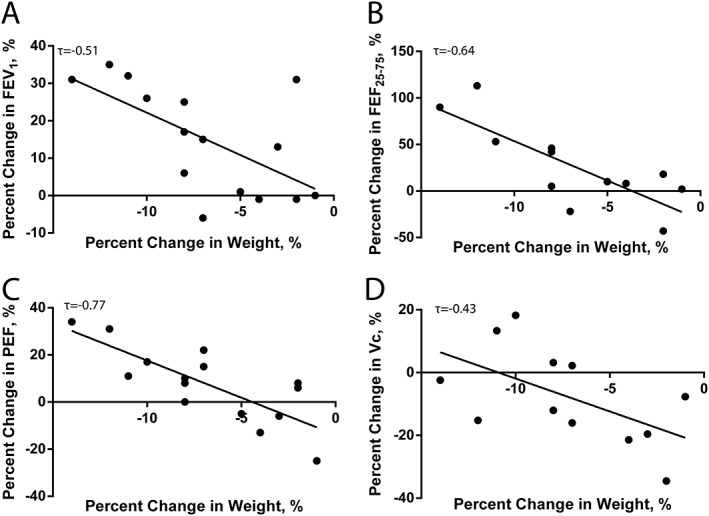
Correlations between change in weight during hospitalization and lung function measures. Greater weight loss was associated with increases in forced expiratory volume in 1 s (FEV1) (A), forced expiratory flow at 25–75% of forced vital capacity (FEF25–75) (B), peak expiratory flow (PEF) (C), and capillary blood volume (Vc) (D).
Discussion
In the present study, we sought to better understand the pulmonary congestive syndrome during hospitalization for acute HF decompensation and its effect on functional measurements of the respiratory system and response to therapy as these measures have previously not been simultaneously followed during treatment for acute HF decompensation. We found that our patients were admitted to the hospital with symptoms of dyspnoea and a history of HF for extensive diuresis and subsequently had significant improvement in clinical measures. Patients had significantly reduced FVC, FEV1, and FEF25–75 as a per cent predicted but, at baseline, had better spirometric measures than those observed in previous studies.12, 14 Following treatment, spirometric measures were greatly improved but remained lower than those of chronic, stable patients observed in our laboratory.8, 14 Additionally, DLCO and Vc did not change, but Dm and Dm/Vc ratio improved. These changes were paralleled by clearance of EVLW, suggesting the pulmonary congestion present at admission was at least partially cleared by hospital discharge. However, there was no change in the anatomy of the large airways through the sixth generation. Additionally, no differences were observed in changes in clinical measures, lung function measurements, or EVLW clearance between patients with HFrEF and HFpEF. Weight loss during hospitalization was strongly associated with improvement in pulmonary function.
Lung function and extravascular lung water changes following diuresis
Clearance of excess fluid during hospitalization is a primary objective during hospitalization for HF decompensation. This fluid clearance can be measured globally through changes in weight loss and peripherally by observing reductions in oedema, with fluid cleared from several different compartments with respect to intravascular and interstitial spaces.7 We investigated the pulmonary interstitial space using a histogram‐based approach to semi‐quantitatively determine EVLW in HF patients.15 This method has shown a relationship between CT lung density and EVLW as measured gravimetrically, suggesting the validity of this approach to quantifying changes in EVLW in patients.16 , 17 In the present study, consistent reductions in EVLW in patients during hospitalization were observed. Despite approximately 25% of total fluid removal occurring before the first CT scan, significant fluid clearance was still observed in the lung between study visits. There is likely a time delay between the clearance of vascular fluid and the subsequent clearance of interstitial fluid, especially in the lung. While the initial fluid clearance is important for the improvement of dyspnoea and other symptoms, subclinical levels of congestion likely remain, which may have an effect on pulmonary function.
Spirometric measurements have been used to follow improvement in acute HF patients following hospitalization. Previous studies have shown initiation of treatment with intravenous diuretics, which has been shown to result in immediate improvements in spirometry, with a maximal value reached after approximately 2 weeks, while DLCO does not improve significantly.12 , 18 However, these studies do not explain the relationship between the clearance of fluid and improved functional measures or the time delay in functional improvement. This delay is likely multifactorial but may be linked to continued clearance of the congested state, effects on airway reactivity, or changes in pharmacological interventions.
Compared with a previous study in our laboratory using the same histogram‐based technique, the HF patients had greater levels of EVLW compared with chronic, stable patients.14 EVLW is linked to decreased lung compliance and subsequent reduced FVC and FEV1. Engorgement of the small airways may limit FEF25–75, and this excess fluid likely contributes to the reduced Dm and Dm/Vc ratio. This remaining congestive state and the associated pulmonary oedema can increase airway reactivity and may contribute to the reduced function.19 If some degree of excess EVLW remains, this reactive airway state likely remains at hospital discharge, may persist after complete fluid clearance, and may contribute to the reduced function. Finally, recommended pharmacological interventions for HF may contribute to pulmonary function improvements. Angiotensin‐converting enzyme (ACE) inhibitors, angiotensin 2 receptor blockers, and diuretics improve fluid balance. As excess fluid is cleared, lung compliance increases, improving FVC and FEV1, and Dm increases as the alveolar–capillary membrane conductance improves.20, 21 Additionally, ACE inhibitors and spironolactone have been linked to improvements in the alveolar–capillary membrane function, independent of lung fluid changes.22, 23 Down‐regulation of the renin–angiotensin system and aldosterone activity may act to reduce factors that lead to thickening of the alveolar–capillary membrane and lung fibrosis.24, 25 Preventing these pathological changes would be expected to improve both spirometric measures and gas diffusion, and introduction of these medications to some patients may contribute to the functional improvement observed here, and patients for whom these medications are contraindicated due to reduced kidney function may be at risk for more rapid deterioration of pulmonary function.
Differences between patients with heart failure with reduced ejection fraction and those with heart failure with preserved ejection fraction
It is not fully understood where excess fluid is cleared from during inpatient diuresis. It has been shown that fluid is removed differently in patients with HFrEF, where greater blood volume is removed, versus HFpEF, where greater interstitial fluid is removed.7 However, it is not well understood how fluid accumulates in and is removed peripherally versus centrally. In the present study, we found similar levels of EVLW initially and similar levels of clearance from both HFrEF and HFpEF patients and similar subsequent improvements in pulmonary function. While in this study changes in pulmonary congestion seem similar between HFrEF and HFpEF patients, investigations into the pulmonary vascular blood volumes are necessary to fully understand differences in pulmonary congestion in these different HF populations.
Clinical relevance
Hospital readmission for HF decompensation is a growing clinical problem that is difficult to predict.26 The amount of EVLW has been suggested as a prognostically important factor towards understanding decompensated HF.27, 28 Additionally, the time course for changes in both EVLW and functional values may be important. While subject‐perceived symptoms can resolve rapidly, EVLW and spirometry take longer to fully resolve and may continue to improve after discharge from the hospital. Our results suggest that clearance of both peripheral congestion and pulmonary congestion occur and contribute to the improvements in clinical and functional measurements during hospitalization; however, their relative contributions are not clear. The present study, taken in concert with previous work, suggests that some degree of pulmonary congestion remains at hospital discharge. Residual congestion may allow for small perturbations to cause a recurrent bout of decompensation and hospitalization and contribute to the high rates of 30 day hospital readmission. Congestion is difficult to monitor in both the inpatient and outpatient settings. Spirometry may provide a method of understanding changes in a patient's congestive state to monitor both continued improvement immediately following hospitalization and long‐term functional status.
Limitations
Two limitations exist for the present study. First, the time delay between hospital admission and recruitment to the study was approximately 24 h. After this time, patients have had at least one additional dose of diuretics, significant fluid removal, and improvement in clinical symptoms. However, three‐quarters of the total fluid loss was still captured between the study visits, and many significant changes in physiology were observed. Second, the histogram technique measures contributions from thoracic air, tissue, blood, and EVLW. However, the longitudinal nature of the study minimizes the effect of differences related to the tissue and blood volumes as well as the presence of pacemakers or other objects that may create a CT artefact when comparing time points, and patients were instructed to hold their breath at total lung capacity to ensure similar air volumes.
Conclusions
The present study was designed to improve the understanding of pulmonary congestion during inpatient treatment for decompensated HF. Significant reductions in weight and blood pressure were observed as well as improvements in spirometry measures and Dm/Vc ratio. Additionally, significant clearance of EVLW was observed. Weight loss was strongly correlated with a number of functional outcomes including improved static lung volumes, improved maximal flows, and lower Vc. Finally, changes in EVLW, pulmonary function, and clinical measurements were similar between patients with HFrEF and HFpEF. Future studies should investigate the long‐term relationship between EVLW and clinical and functional measurements as well as the relationship between the clearance of peripheral oedema and EVLW in acutely decompensated HF patients.
Conflict of interest
None declared.
Funding
This work was supported by the National Heart, Lung, and Blood Institute, NIH (grant number HL71478), Mayo Clinic Graduate School of Biomedical Sciences, and ZOLL LifeVest.
Acknowledgements
The authors would like to thank Briana Ziegler, Alex Carlson, and the members of the CT and cardiac intensive care units.
Chase, S. C. , Fermoyle, C. C. , Wheatley, C. M. , Schaefer, J. J. , Olson, L. J. , and Johnson, B. D. (2018) The effect of diuresis on extravascular lung water and pulmonary function in acute decompensated heart failure. ESC Heart Failure, 5: 364–371. doi: 10.1002/ehf2.12253.
References
- 1. Solomon SD, Dobson J, Pocock S, Skali H, McMurray JJ, Granger CB, Yusuf S, Swedberg K, Young JB, Michelson EL, Pfeffer MA. Influence of nonfatal hospitalization for heart failure on subsequent mortality in patients with chronic heart failure. Circulation 2007; 116: 1482–1487. [DOI] [PubMed] [Google Scholar]
- 2. Cotter G, Metra M, Milo‐Cotter O, Dittrich HC, Gheorghiade M. Fluid overload in acute heart failure—re‐distribution and other mechanisms beyond fluid accumulation. Eur J Heart Fail 2008; 10: 165–169. [DOI] [PubMed] [Google Scholar]
- 3. O'Connor CM, Stough WG, Gallup DS, Hasselblad V, Gheorghiade M. Demographics, clinical characteristics, and outcomes of patients hospitalized for decompensated heart failure: observations from the IMPACT‐HF registry. J Card Fail 2005; 11: 200–205. [DOI] [PubMed] [Google Scholar]
- 4. Nohria A, Tsang SW, Fang JC, Lewis EF, Jarcho JA, Mudge GH, Stevenson LW. Clinical assessment identifies hemodynamic profiles that predict outcomes in patients admitted with heart failure. J Am Coll Cardiol 2003; 41: 1797–804. [DOI] [PubMed] [Google Scholar]
- 5. O'Connor CM, Abraham WT, Albert NM, Clare R, Stough WG, Gheorghiade M, Greenberg BH, Yancy CW, Young JB, Fonarow GC. Predictors of mortality after discharge in patients hospitalized with heart failure: an analysis from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE‐HF). Am Heart J 2008; 156: 662–673. [DOI] [PubMed] [Google Scholar]
- 6. Miller WL. Fluid volume overload and congestion in heart failure: time to reconsider pathophysiology and how volume is assessed. Circ Heart Fail 2016; 9: e002922. [DOI] [PubMed] [Google Scholar]
- 7. Miller WL, Mullan BP. Understanding the heterogeneity in volume overload and fluid distribution in decompensated heart failure is key to optimal volume management: role for blood volume quantitation. JACC Heart Fail 2014; 2:298–305. [DOI] [PubMed] [Google Scholar]
- 8. Chase SC, Wheatley CM, Olson LJ, Beck KC, Wentz RJ, Snyder EM, Taylor BJ, Johnson BD. Impact of chronic systolic heart failure on lung structure–function relationships in large airways. Phys Rep 2016; 4: e12867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Agostoni P, Bussotti M, Cattadori G, Margutti E, Contini M, Muratori M, Marenzi G, Fiorentini C. Gas diffusion and alveolar–capillary unit in chronic heart failure. Eur Heart J 2006; 27: 2538–2543. [DOI] [PubMed] [Google Scholar]
- 10. Dimopoulou I, Daganou M, Tsintzas OK, Tzelepis GE. Effects of severity of long‐standing congestive heart failure on pulmonary function. Respir Med 1998; 92: 1321–1325. [DOI] [PubMed] [Google Scholar]
- 11. Guazzi M, Agostoni P, Matturri M, Pontone G, Guazzi MD. Pulmonary function, cardiac function, and exercise capacity in a follow‐up of patients with congestive heart failure treated with carvedilol. Am Heart J 1999; 138: 460–467. [DOI] [PubMed] [Google Scholar]
- 12. Light RW, George RB. Serial pulmonary function in patients with acute heart failure. Arch Intern Med 1983; 143: 429–433. [PubMed] [Google Scholar]
- 13. Ceridon ML, Beck KC, Olson TP, Bilezikian JA, Johnson BD. Calculating alveolar capillary conductance and pulmonary capillary blood volume: comparing the multiple‐ and single‐inspired oxygen tension methods. J Appl Physiol 2010; 109: 643–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chase SC, Taylor BJ, Cross TJ, Coffman KE, Olson LJ, Johnson BD. Influence of thoracic fluid compartments on pulmonary congestion in chronic heart failure. J Card Fail 2017; 23: 690–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kato S, Nakamoto T, Iizuka M. Early diagnosis and estimation of pulmonary congestion and edema in patients with left‐sided heart diseases from histogram of pulmonary CT number. Chest 1996; 109: 1439–1445. [DOI] [PubMed] [Google Scholar]
- 16. Forster BB, Muller NL, Mayo JR, Okazawa M, Wiggs BJ, Pare PD. High‐resolution computed tomography of experimental hydrostatic pulmonary edema. Chest 1992; 101: 1434–1437. [DOI] [PubMed] [Google Scholar]
- 17. Hedlund LW, Vock P, Effmann EL, Lischko MM, Putman CE. Hydrostatic pulmonary edema. An analysis of lung density changes by computed tomography. Invest Radiol 1984; 19: 254–262. [DOI] [PubMed] [Google Scholar]
- 18. McNicol MW, Kirby BJ, Bhoola KD, Everest ME, Price HV, Freedman SF. Pulmonary function in acute myocardial infarction. Br Med J 1965; 2: 1270–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brown RH, Zerhouni EA, Mitzner W. Airway edema potentiates airway reactivity. J Appl Physiol 1995; 79: 1242–1248. [DOI] [PubMed] [Google Scholar]
- 20. Cattadori G, Wasserman K, Meloni C, Mustaq S, Contini M, Apostolo A, Andreini D, Magrì D, Sciomer S, Veglia F, Berna G, Introcaso G, Palermo P, Fiorentini C, Agostoni P. Alveolar membrane conductance decreases as BNP increases during exercise in heart failure. Rationale for BNP in the evaluation of dyspnea. J Card Fail 2009; 15: 136–144. [DOI] [PubMed] [Google Scholar]
- 21. Frank NR, Lyons HA, Siebens AA, Nealon TF. Pulmonary compliance in patients with cardiac disease. Am J Med 1957; 22: 516–523. [DOI] [PubMed] [Google Scholar]
- 22. Agostoni P, Magini A, Andreini D, Contini M, Apostolo A, Bussotti M, Cattadori G, Palermo P. Spironolactone improves lung diffusion in chronic heart failure. Eur Heart J 2005; 26: 159–164. [DOI] [PubMed] [Google Scholar]
- 23. Guazzi M, Marenzi G, Alimento M, Contini M, Agostoni P. Improvement of alveolar–capillary membrane diffusing capacity with enalapril in chronic heart failure and counteracting effect of aspirin. Circulation 1997; 95: 1930–1936. [DOI] [PubMed] [Google Scholar]
- 24. Agostoni P, Guazzi M, Bussotti M, Grazi M, Palermo P, Marenzi G. Lack of improvement of lung diffusing capacity following fluid withdrawal by ultrafiltration in chronic heart failure. J Am Coll Cardiol 2000; 36: 1600–1604. [DOI] [PubMed] [Google Scholar]
- 25. Kay JM, Edwards FR. Ultrastructure of the alveolar–capillary wall in mitral stenosis. J Pathol 1973; 111: 239–245. [DOI] [PubMed] [Google Scholar]
- 26. Gheorghiade M, Vaduganathan M, Fonarow GC, Bonow RO. Rehospitalization for heart failure: problems and perspectives. J Am Coll Cardiol 2013; 61: 391–403. [DOI] [PubMed] [Google Scholar]
- 27. Volpicelli G, Caramello V, Cardinale L, Mussa A, Bar F, Frascisco MF. Bedside ultrasound of the lung for the monitoring of acute decompensated heart failure. Am J Emerg Med 2008; 26: 585–591. [DOI] [PubMed] [Google Scholar]
- 28. Frassi F, Gargani L, Tesorio P, Raciti M, Mottola G, Picano E. Prognostic value of extravascular lung water assessed with ultrasound lung comets by chest sonography in patients with dyspnea and/or chest pain. J Card Fail 2007; 13: 830–835. [DOI] [PubMed] [Google Scholar]


