Abstract
The present update is dedicated to the evolution of the interaction between heart failure (HF) and exercise and how the scientific community has handled it. Indeed, on the one hand, HF is a leading cause of morbidity and mortality with a stable prevalence from 1998 onward varying between 6.3% and 13.3%. On the other hand, exercise is seen as a diagnostic and prognostic tool as well as a therapeutic intervention in chronic HF. More precisely, the knowledge, the clinical application, and the research interest on the mutual interactions between exercise and HF have different phases in disease progression:
Before HF onset (past): exercise provides protective benefit in preventing HF (primary prevention).
With HF present: exercise improvement with training provides benefits in HF (secondary prevention).
The prediction of future in HF patients: exercise impairment, as a leading characteristic of HF, is used as a prognostic factor.
Keywords: Exercise, Heart failure, Prevention, Training, Prognosis
Introduction
The present update is dedicated to the evolution of the interaction between heart failure (HF) and exercise and how the scientific community has handled it. Indeed, on the one hand, HF is the leading cause of morbidity and mortality with a stable prevalence from 1998 onward varying between 6.3% and 13.3%,1 and 50% of HF patients with reduced ejection fraction (HFrEF) die within 5 years from diagnosis.2 On the other hand, exercise is seen as a diagnostic and prognostic tool as well as a therapeutic intervention in chronic HF.
In the scientific community, exercise in HF is a hot topic as shown in Figure 1 , which reports the number of PubMed‐included papers by searching for ‘exercise and HF’.
Figure 1.
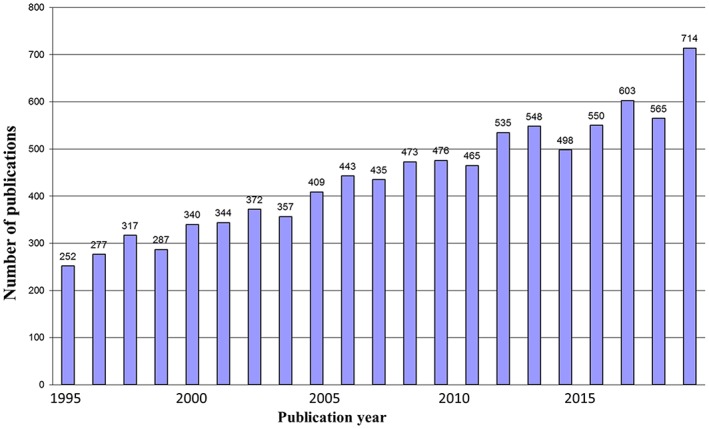
PubMed results searching for ‘exercise and heart failure’.
More precisely, the knowledge, the clinical application, and the research interest on the mutual interactions between exercise and HF have different phases in HF progression (Figure 2 ):
Before HF onset (past): exercise provides protective benefit in preventing HF (primary prevention).
With HF present: exercise improvement with training provides benefits in HF (secondary prevention).
The prediction of future in HF patients: exercise impairment, as a leading characteristic of HF, is used as a prognostic factor.
Figure 2.
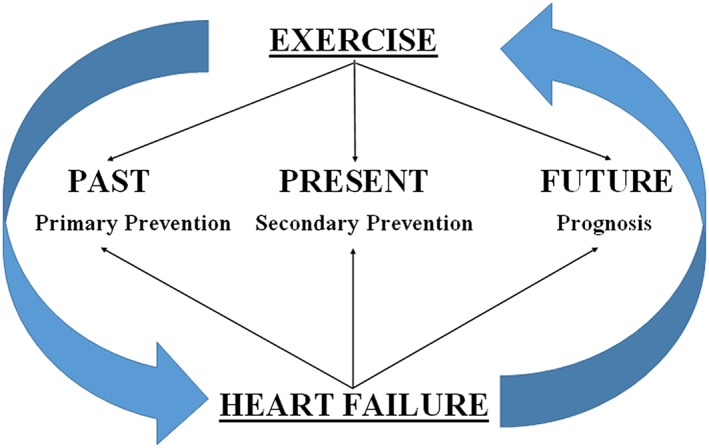
Scheme of interactions between exercise and heart failure in different phases of the disease.
Before heart failure onset (past): exercise as a tool for primary prevention in heart failure
Several epidemiological studies consistently reported inverse associations between cardiorespiratory fitness and risk of cardiovascular disease3 and mortality.4 A 41% reduction in mortality was reported in 786 Tour de France cyclists compared with the general French male population.5 Wilson et al.6 recently reviewed the basic science behind the cardiovascular benefit of exercise, suggesting functional, structural, cellular, and molecular adaptations in the heart in response to exercise. Considering how much and how intense exercise should be, the authors concluded that there is no lower exercise threshold for cardiovascular benefits to be seen, signifying that ‘some exercise is better than none’.
Data on the association of physical activity and HF are sparse but can be summarized in the following important findings.
Protective association between physical activity/fitness and heart failure risk
Seminal works from Kenchaiah et al., Hu et al., NHANES I Epidemiologic Follow‐up Study and FINMONICA Study7, 8, 9, 10 provided the first data about the association between physical activity and HF. Kraigher‐Krainer et al.11 in 2013 evaluated 1142 elderly participants from the Framingham Study in a longitudinal study on follow‐up of 10 years. They observed that lower physical activity, assessed with a standardized validated questionnaire, is associated with higher incidence of HF. More recently, Khan et al.12 in 2014 evaluated, in a first population‐based prospective study, 1873 men (aged 42–61) from eastern Finland to assess the prospective associations (follow‐up of 20.4 years) of cardiorespiratory fitness and the risk of HF. This study demonstrated a strong, inverse, and independent association between long‐term cardiorespiratory fitness and risk of HF, with men in the top quartile of long‐term fitness levels having 53% lower risk of developing HF. A 1 unit higher cardiorespiratory fitness was associated with 6% lower risk of incident HF events corresponding to a 21% lower risk of HF per 1 metabolic equivalent (MET) greater of cardiorespiratory fitness. Moreover, Kenchaiah et al.,7 Hu et al.,8 and the more recent work from Cooper Center for Longitudinal Study13, 14 in 2017 demonstrated the protective association of physical activity against HF risk across all body mass index range and independent of the presence of cardiac and non‐cardiac co‐morbidities. Finally, Berry et al.15 studied 20 642 participants with fitness measured at the mean age of 49 years and who survived to receive Medicare coverage from 1999 and 2009. A 1 unit greater fitness level in MET achieved in midlife was associated with 20% lower risk for HF hospitalization after the age of 65 years but only 10% lower risk for acute myocardial infarction in men and not association in women, suggesting that a higher fitness is more protective against risk of HF than of myocardial infarction.
Specific effects of exercise on cardiac structure and function important for long‐term heart failure risk
Recent data from the Cooper Center Longitudinal Study14 evaluated the association between fitness and non‐fatal cardiovascular events, such as HF and coronary artery disease, hypothesizing that the specific effects of exercise on cardiac structure and function might be particularly important for long‐term HF risk. Accordingly, recent works from large cohorts demonstrated significant associations between higher levels of fitness or physical activity and favourable left ventricular structure and function:
Florido et al.16 demonstrated that physical activity is inversely associated with chronic subclinical myocardial damage, which could represent a mechanism of the described HF risk reduction by physical activity.
The CARDIA Study17 observed that lower cardiorespiratory fitness in young adults was associated with abnormal left ventricle remodelling and a higher prevalence of subclinical abnormalities in left ventricle systolic and diastolic function in middle age.
Kamimura et al.18 demonstrated that physical activity is associated with reduced left ventricular mass in obese and hypertensive African Americans, consistent with beneficial effect of physical activity on cardiac structure and function.
Heart failure phenotype associated with physical inactivity and low fitness: heart failure with preserved ejection fraction vs. heart failure with reduced ejection fraction
Brinker et al.19 examined a subset of patients at the Cooper Center who underwent fitness testing for screening purposes and received echocardiographic testing. They observed that lower fitness was associated with a higher prevalence of diastolic dysfunction and abnormal left ventricle remodelling, which represent important intermediate phenotypes in the natural history of heart failure with preserved ejection fraction (HFpEF). These data suggested that low fitness associated risk of HF is more likely to be in HFpEF than in HFrEF. Accordingly, a recent work20 in 2017 from an individual‐level pooled analysis of three large cohort studies confirmed a strong and dose‐dependent association of physical activity with HFpEF but not with HFrEF. Adequately designed randomized prevention trials are needed to further evaluate these evidences.
Association between changes in physical activity and heart failure risk
Pandey et al.,14 evaluating Cooper Center Longitudinal Study and Centers for Medicare & Medicaid Services data, demonstrated that individuals who increased their fitness levels had a lower rate of HF hospitalization. Furthermore, longitudinal changes in cardiorespiratory fitness, but not body mass index, were found to be associated with HF risk.13 Moreover, in young adults evaluated in the CARDIA Study,17 the decline in cardiorespiratory fitness over follow‐up was a significant predictor of subclinical systolic dysfunction and elevated diastolic filling pressure in middle age independent of baseline cardiorespiratory fitness levels. These data were confirmed in HFpEF: low cardiorespiratory fitness could be not only considered as an early stage marker but also a target for prevention, owing to the impact of exercise training on risk factor profile, on left ventricle structure and function, on myocardial strain pattern, and on vascular stiffness.19 It was calculated that 1 MET improvement in physical activity was associated with 17% reduction of HFpEF risk at a later age21 and that daily dynamic exercise for >30 min four or five times weekly over the course of a lifetime preserves left ventricle diastolic function.22
More activity is better for heart failure prevention
Pandey et al.23 confirmed a dose–response relationship between physical activity and risk of HF, performing the largest meta‐analysis from 12 prospective cohort studies (papers published between 1995 and 2014; 370 460 subjects included; 20 203 HF events; 13 years of median follow‐up). The pooled results confirmed a consistent, linear, inverse, and dose–response association between physical activity and HF risk. A linear dose response was observed across a wide dose range, without upper or lower threshold effect, again suggesting that ‘more activity is better than some activity for HF prevention’. Subjects with a 500 METs‐min/work of physical activity, that is, the minimum guidelines recommended, had a 10% lower risk of HF compared with those with no physical activity, while practising 1000 or 2000 METs‐min/work results in a 19% and 35% lower risk of HF, respectively, suggesting a re‐evaluation of the actual guidelines.24
All these observations are all in line with the idea of exercise as a tool of primary prevention in HF patients.
With heart failure present: exercise improvement with training provides benefits in heart failure (secondary prevention)
Exercise in HF patients was historically proscribed and the concept of reducing physical activity to avoid exercise‐induced symptoms and haemodynamic overload for the diseased ventricle dominated the textbooks.25 Indeed, there was a consensus in the 1970s that patients with all stages of HF should be advised to refrain from physical activity with bed rest prescription.26 In 1988, the comprehensive seminal paper of Sullivan et al.27 about exercise training in 12 HF patients demonstrated for the first time that ambulatory HF patients can significantly improve their exercise tolerance. This study is the cornerstone for three decades of subsequent research, changing deeply the scientific approach to the relationship between exercise and HF. Recently, Doukky et al.28 demonstrated that in patients with symptomatic chronic HF, physical inactivity (i.e. failure to exercise or television screen time) is associated with nearly twice all‐cause and cardiac mortality, and even modest exercise was associated with survival benefit. The authors calculated the propensity scores of spending >4 h/day watching television, showing that increasing sedentary time was associated with a stepwise increase in the risk of all‐cause death in HF patients.
Exercise training is now a well‐establish therapy for HF patients,29 and in the last European Society of Cardiology (ESC) HF guideline 2016, ‘patients with HF are recommended to perform properly designed exercise training’.30 In a recent review titled ‘Exercise as medicine—evidence for prescribing exercise as therapy in 26 different chronic diseases’, Pedersen and Saltin wrote a chapter about HF patients.31 Moreover, Arena recently wrote: ‘we are only now coming to the realization that functional capacity is a vital sign and exercise is a medicine’ in the preface of an issue in Heart Failure Clinics written as ‘a journey from bench to bedside to clinic to community with respect to all things regarding functional assessment and exercise training’.32 Along this line, HF‐ACTION33 was designed as a large multicentre prospective randomized controlled trial to test the long‐term safety and efficacy of aerobic exercise training in stable HFrEF for patients and New York Heart Association class II to IV. Patients randomized to exercise participated in 3 months of supervised exercise training with moderate intensity 3 days/week. In the end, between 2003 and 2007, HF‐ACTION Investigators randomized 2331 patients showing that exercise training was associated with modest significant reductions for both all‐cause mortality or hospitalization and cardiovascular mortality or HF hospitalization only after adjustment for highly prognostic predictors of the primary endpoint. These results were confirmed by a recent meta‐analysis34, 35 that failed to clearly demonstrate a mortality benefit from exercise training. However, results from the HF‐ACTION trial confirmed the safety and clinical benefit of exercise training in HF, and they were helpful in persuading the US Centers for Medicare & Medicaid Services to approve coverage for cardiac rehabilitation for selected HFrEF that matched the HF‐ACTION trial inclusion criteria.36, 37
However, despite the existing evidence and guidelines in favour of exercise training programmes in HF patients, only 10% of eligible HF patients received cardiac rehabilitation referral at discharge after hospitalization for HF.38 This is due to physician (low degree of perceived benefit, lack of strong demonstrated mortality benefit, and safety concerns) and patient‐level factors (older age, lower socio‐economic status, logistic problems, insurance status, and co‐morbidity burden). A recent ExtraHF Survey confirmed the lack of utilization of exercise training and cardiac rehabilitation in HF patients,39 suggesting the need for constant activities of education in this field. A somewhat encouraging finding is a recently reported increasing trend in the proportion of HF patients referred for cardiac rehabilitation by ~40% in relative terms over the past decade.38 Moreover, 60% of the cardiac centres in ESC‐related countries have developed a programme of exercise training for their HF patients, showing an improvement vs. previous survey.39
It became progressively clear that different organ systems, such as the heart,40, 41 skeletal muscle,27, 42 vascular function,27, 42 respiratory function, and neuro‐hormonal systems are involved in HF disease progression and modulation by exercise training.43 Measuring simultaneously peak oxygen consumption (VO2) and peak cardiac output before and after training in 70 HF patients, our group demonstrated that exercise training improves physical performance by changing peak exercise cardiac output without changing artero‐venous O2 difference. Moreover, a reduction after training of arteriovenous O2 difference with an increase in cardiac output at peak exercise is frequent and it is suggestive of blood flow redistribution after workout.44 In systolic HF patients, the effects of exercise training on neurovascular control and skeletal myopathy,45 on the mechanisms of improving skeletal muscle O2 transport and utilization,46 and on the prevention of the deterioration in the arterial baroreflex control and sympathetic nerve47 were recently reviewed. Esposito et al.48 demonstrated in HF muscles not only perfusive but also diffusive oxygen impairment as a new possible target of exercise training.
A meeting in 2012 in Bethesda of the National Heart, Lung, and Blood Institute49 aimed to evaluate, to optimize, and to translate the potential role of exercise training in HF. They identified many possible knowledge gaps and six final recommendations as the highest priority in advancing exercise training as a therapy in HF: (i) better understanding of the basic mechanisms of exercise intolerance, (ii) better knowledge of different phenotypes, (iii) better measurement of the results after training, (iv) improvement in adherence, (v) optimization of training regimens, and (vi) combination of training with other lifestyle interventions. Of interest, the role of sex differences in HF and training was emphasized: women with HF generally have a lower functional capacity than men have, but they show a greater benefit of training. Moreover, the authors underlined the effects of aging, multiple co‐morbidities, and frailty on the use of exercise training, leading to the necessity of a personalized exercise‐based rehabilitation. This argument was recently reinforced by Rod Taylor during ‘Late Breaking Trial III: focus on trial updates, registries and meta‐analyses’ session during the Heart Failure 2016 Congress in Florence, presenting the results from ExTraMATCH II. More recently, Luo et al. evaluated exercise training in patients with chronic HF and atrial fibrillation.50 They demonstrated that, despite having more severe HF, stable outpatients with HF and atrial fibrillation were able to receive similar benefits with exercise training when using a structured and monitored intervention. Importantly, exercise training did not lead to an increase in atrial fibrillation events. Finally, Reeves et al. performed the REHAB‐HF Pilot study,51 which suggested the safety and the efficacy of a novel multidomain physical rehabilitation intervention to improve physical function and reduce hospitalization in older and frail patients with acute decompensated HF with multiple co‐morbidities.
Exercise training modalities
Moderate continuous training is efficient, safe, and well tolerated by HF patients, and it is recommended by the Heart Failure Association Guidelines.30 Improvement in exercise capacity of HF patients undergoing continuous aerobic exercise training is primarily determined by the total energy expenditure, such as the product of training intensity, session duration, session frequency, and programme duration of the training program.52 In addition to continuous moderate‐intensity aerobic training, and also high‐intensity and low‐intensity interval training models, respiratory training and strength training demonstrated efficacy in this setting. The key components of the ESC position paper were written in 2011 to strongly advise standardization of exercise prescription.53 In a recent issue in Heart Failure Clinics,54, 55, 56, 57 the rehabilitation practice patterns performed all over the world are widely reported. More recently, in 2016, Cornelis et al.58 published a paper about the first systematic review and meta‐analysis to provide a complete overview of randomized clinical trials in order to assess the effectiveness of different exercise training modalities on prognostic cardiopulmonary exercise test (CPET) parameters, quality of life (QoL), and left ventricle remodelling. Five studies compared interval training with combined interval‐strength training; 3 studies, continuous training with combined continuous‐strength training; 11 studies, continuous training with interval training; and finally only 1 study, continuous training with strength training. Finally, an increase of prognostic CPET parameters was not significantly favoured by specific training modalities, while left ventricular remodelling revealed significant improvement after interval training vs. other modalities. In 2017, Ellingsen et al.59 published the first randomized multicentre trial evaluating high‐intensity interval training in chronic HFrEF demonstrating that 12 weeks of high‐intensity interval training was not superior to moderate continuous training with respect to left ventricular reverse remodelling or improving secondary endpoints. Considering that adherence to the prescribed exercise intensity based on heart rate may be difficult to achieve, moderate continuous training remains the standard exercise modality for patients with chronic HF. More standardized, high qualitative, rigorous multicentre clinical trials should be planned in the near future.
Special populations
Heart failure with preserved ejection fraction
While the majority of trials about exercise training in HF included patients with unspecified EF or HFrEF, recent trials have recruited those with HFpEF. Kitzman et al.,60 with the largest data on exercise in HFpEF in randomized controlled settings, demonstrated a significant improvement in fitness with training among HFpEF patients. More recently, Pandey et al.61 performed an updated and comprehensive evaluation of the effect of training in patients with HFpEF in a meta‐analysis of randomized trials including 276 patients enrolled in six randomized controlled trial; taken together, the available literature suggests that exercise training may improve exercise tolerance in these patients through peripheral mechanisms leading to an improved oxygen extraction in the active skeletal muscles without a significant change in left ventricle diastolic function. More recently, in 2017, Kitzman et al.62 recently demonstrated significant and additive positive effects of the combination of caloric restriction and aerobic exercise training among obese older patients with clinically stable HFpEF.
Left ventricular assist device patients
Jung and Gustafsson63 provided a systematic description of the different components of exercise physiology in adult left ventricular assist device patients, pointing out potential solutions and future researches; of note, exercise training in left ventricular assist device patients improves peak VO2 and should be encouraged.
Chemotherapy‐related cardiomyopathy
Nair and Gongora64 recently evaluated the role of exercise training in chemotherapy‐related cardiomyopathy, suggesting a scheme for exercise prescription based on the baseline evaluation of left ventricle ejection fraction and global longitudinal strain or tissue Doppler imaging. The authors recommend the identification of subclinical disease prior to appearance of symptoms as a key factor to prevent cardiovascular injury and enhance survival as well as QoL.
Alternative approaches to exercise in heart failure patients
Tai Chi training
Tai Chi is a form of low‐intensity physical activity originating in China, with positive effects on balance control, flexibility, cardiovascular fitness, pain, fatigue, insomnia, and psychological well‐being; Pan et al.65 performed a meta‐analysis to evaluate the effects of Tai Chi training on exercise capacity, QoL, and other clinical outcomes in patients with HF including 242 patients enrolled in four randomized clinical trials; the meta‐analysis results suggest Tai Chi training as a valuable and suggestive tool for improving QoL in patients with HF; however, more data are needed to demonstrate improvement of other clinical and exercise parameters.
Muscle electrical stimulation
Exercise training is not recommended in unstable HF patients; Groehs et al.66 investigated the effects of muscle functional electrical stimulation on muscle sympathetic nerve activity, muscle blood flow, and exercise tolerance in hospitalized patients for stabilization of HF; functional electrical stimulation improves muscle sympathetic nerve activity, vasoconstriction, exercise tolerance, muscle strength, and QoL, suggesting this approach during hospitalization to treat decompensated HF.
Wii gaming
The Nintendo Wii platform is the most tested exergame in adults; the HF‐Wii study is a randomized study to evaluate the effect of exergaming in patients with HF; recruitment was expected to be completed by December 2016, and the study should close at the beginning of 2018.67
Robot‐assisted training
Schoenrath et al. tested in five HF patients with New York Heart Association functional class 2 and 3 a robot‐assisted gait therapy with the Lokomat® system, obtaining promising data for further HF trials in more impaired and hospitalized patients.68
Holidays
Shah et al.69 evaluated the impact of winter season and holidays in 22 727 HF patients; the authors demonstrated that Christmas and Independence Day were associated with increased HF admissions immediately after the holidays, suggesting overeating, emotional stressors, lesser exercise, and postponing medicals around holidays as possible causes.
The prediction of the future in heart failure patients
Directly measured oxygen consumption (VO2) is the objective and quantitative measure of cardiorespiratory fitness, and it represents cardiac, circulatory, and respiratory functions and muscle oxygen use. VO2 at peak exercise is a well‐established prognostic factor in HF, but other exercise parameters are proved as powerful predictors of mortality such as ventilation (VE)/carbonic monoxide production (VCO2) slope, VO2 at anaerobic threshold, O2 uptake efficiency slope, VO2/work rate relationship, mean response time, and haemodynamic measurement during exercise. Recently, Alba et al.70 confirmed the role of peak VO2, O2 uptake efficiency slope, and VE/VCO2 as independent predictors with similar discriminatory capacity over recognized clinical variables. Again recently, Malhotra et al. in a state‐of‐the‐art paper71 and Keteyan et al.72 underlined the known role of CPET for careful measurement of ventilatory and O2 uptake patterns in HF to quantify disease severity, prognosis, and relative contributions of organ system to exercise intolerance.
Nonetheless, the prognostic power of CPET‐derived parameters is deeply supported by literature, but they were poorly considered for prognostic scores. Indeed, only HF survival score73 and HF‐ACTION predictive risk score model74 included only peak VO2 and exercise duration at CPET among other clinical parameters, totally excluding ventilatory parameters.
In 2011, 13 leading and experienced Italian HF Units joined together with the purpose of building a new risk score for systolic HF patients, integrating measures with potential prognostic value from CPET with established clinical, laboratory, and echocardiographic risk factors. From a database of 2716 systolic HF patients, six variables, which resulted independently in relation to prognosis (haemoglobin, sodium, kidney function, left ventricle ejection fraction, peak VO2, and VE/VCO2 slope), were included in a new score called MECKI (metabolic exercise test data combined with cardiac and kidney indexes). The MECKI score is CPET centred, and it is a long‐term prognostic score with the highest area under the curve.75
The MECKI Score Research Group proceeded with the aim to prove the central role of CPET in HF patients. Table 1 reports all the papers published by MECKI Score Research Group. They recently performed a validation study to legitimate MECKI score employment in daily HF routine as a prognostic tool, together with the simplicity of the model with easy available measurements.76 Moreover, the MECKI Score Research Group promoted further evaluations to deepen the role of CPET for risk stratification in specific population:
Indeterminable anaerobic threshold (AT): First‐time observation of the absence of an identified AT as an independent prognostic factor in HF patients; patients with HF with an unidentifiable AT should be considered at high risk.77
Severe HF: Optimal medical therapy with beta‐blockers in specialized HF Units offers a long‐term survival benefit not different from HF patients who underwent heart transplant; moreover, in severe HF patients with optimal medical therapy with beta‐blockers, peak VO2 maintains its crucial role as prognostic factor and as a criterion for HF transplant but with lower cut‐off than conventionally considered.78
Atrial fibrillation (AF): AF is a marker of advanced disease in chronic HF patients, but it is not independently associated with adverse prognosis79; a higher cut‐off value of VO2 at AT is suggested to identify low‐risk HF patients in case of AF.80
Low peak respiratory exchange ratio (pRER): The measurement of pRER is a way to ensure that patients are tested at their maximal effort level and routine methods exclude CPET results with low pRER from the risk stratification process; in contrast, the MECKI Score Research Group demonstrated that incorporating both clinical and CPET variables in a score appears appropriate for risk assessment even when CPET is symptom limited and low pRER occurs.81
Renal dysfunction: In HF patients with poor renal function, peak VO2 offers limited prognostic information; the pathophysiological link between renal dysfunction and decreased exercise capacity in HF is poorly understood.82
Elderly HF patients: Older patients with HF are a high‐risk population with lower exercise performance, and the MECKI score maintains its prognostic value also in older patients.83
Women with HF: Women with HF live longer than do men even though their peak VO2 is lower; however, the female prognostic advantage is lost when sex‐specific differences are correctly taken into account with propensity score matching, suggesting adjustments for sex‐related characteristics in HF model.84
Obese with HF: The prognostic contribution of peak VO2 overwhelms the prognostic capacity of body mass index, so that the so‐called obesity paradox in systolic HF does not exist, and it is due to a patient selection bias.85
Idiopathic dilated cardiomyopathy: Peak VO2 (% of predicted) and VE/VCO2 slope emerged as the strongest prognostic CPET variables, while peak VO2 conventionally measured in millilitre per kilogram was not predictive.86
Anaemic HF patients: Anaemic HF patients have a worse prognosis; CPET can be safely performed in HF patients with anaemia; peak VO2 and left ventricle ejection fraction, but not VE/VCO2 slope, maintain their prognostic power also in HF patients with anaemia, suggesting the use of CPET and prognostic scores also in HF patients with low haemoglobin.87
Table 1.
Papers published by MECKI Score Research Group
| First author | Paper | Year | No. patients | Issue |
|---|---|---|---|---|
| Agostoni PG | Int J Cardiol | 2013 | 2716 | General HF population |
| Cattadori G | Int J Cardiol | 2013 | 715 | Severe HF patients |
| Agostoni PG | Circ Heart Fail | 2013 | 3058 | Indeterminable anaerobic threshold |
| Corrà U | Int J Cardiol | 2015 | 969 | Low peak respiratory exchange ratio |
| Carubelli V | Circ J | 2015 | 3794 | Elderly HF patients |
| Paolillo S | Eur J Intern Med | 2015 | 3447 | Atrial fibrillation |
| Scrutinio D | Circ J | 2015 | 2938 | Renal dysfunction |
| Corrà U | Int J Cardiol | 2016 | 2009/992 | General HF population (validation study) |
| Corrà U | Can J Cardiol | 2016 | 2985 | Sex profile |
| Magrì D | Eur J Prev Cardiol | 2016 | 4221 | Anaerobic threshold and atrial fibrillation |
| Piepoli MF | Eur J Heart Fail | 2016 | 4623 | Obese HF patients |
| Cattadori G | Eur J Intern Med | 2016 | 3913 | Anaemic HF patients |
| Sinagra G | Int J Cardiol | 2016 | 381 | Idiopathic dilated cardiomyopathy |
Conclusions
We provided a comprehensive review of the impact of fitness and physical activity on the risk, management, and prognosis associated with HF development.
We divided the paper into three main chapters—the past, present, and future—showing a continuum in the mutual connection between physical activity and HF.
In summary:
Exercise is a tool of primary prevention in HF patients (past).
Exercise training is a therapy for HF patients (present).
Exercise capacity is a strong prognostic parameter in HF patients (future).
Conflict of interest
None declared.
Cattadori, G. , Segurini, C. , Picozzi, A. , Padeletti, L. , and Anzà, C. (2018) Exercise and heart failure: an update. ESC Heart Failure, 5: 222–232. doi: 10.1002/ehf2.12225.
References
- 1. Van Riet EES, Hoes AW, Wagenaar KP, Limburg A, Landman MA, Rutten FH. Epidemiology of heart failure: the prevalence of heart failure and ventricular dysfunction in older adults over time. A systematic review. Eur J Heart Fail 2016; 18: 242–252. [DOI] [PubMed] [Google Scholar]
- 2. Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Executive summary: heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation 2014; 129: 399–410. [DOI] [PubMed] [Google Scholar]
- 3. Laukkanen KS, Salonen R, Rauramaa R, Salonen JT. The predictive value of cardiorespiratory fitness for cardiovascular events in men with various risk profiles: a prospective population‐based cohort study. Eur Heart J 2004; 25: 1428–1437. [DOI] [PubMed] [Google Scholar]
- 4. Ekelung LG, Haskell WL, Johnson JL, Whaley FS, Criqui MH, Sheps DS. Physical fitness as a predictor of cardiovascular mortality in asymptomatic North American men. The Lipid Research Clinics Mortality Follow‐up Study. N Engl J Med 1988; 319: 1379–1384. [DOI] [PubMed] [Google Scholar]
- 5. Marijon E, Tafflet M, Antero‐Jacquemin J, El Helou N, Berthelot G, Celermajer DS, Bougouin W, Combes N, Hermine O, Empana JP, Rey G, Toussaint JF, Jouven X. Mortality of French participants in the Tour de France (1947–2012). Eur Heart J 2013; 343: 3145–3150. [DOI] [PubMed] [Google Scholar]
- 6. Wilson MG, Ellison GM, Cable NT. Basic science behind the cardiovascular benefits of exercise. Heart 2015; 101: 758–765. [DOI] [PubMed] [Google Scholar]
- 7. Kenchaiah S, Sesso HD, Gaziano JM. Body mass index and vigorous physical activity and the risk of heart failure. Circulation 2009; 119: 44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hu G, Jousilahti P, Antikainen R, Katzmarzyk PT, Tuomilehto J. Joint effects of physical activity, body mass index, waist circumference and waist‐to‐hip ratio on the risk of heart failure. Circulation 2010; 121: 237–244. [DOI] [PubMed] [Google Scholar]
- 9. He J, Ogden LG, Bazzano LA, Vupputuri S, Loria C, Whelton PK. Risk factor for congestive heart failure in US men and women: NHANES I Epidemiologic Follow‐up study. Arch Intern Med 2001; 161: 996–1002. [DOI] [PubMed] [Google Scholar]
- 10. McMurray JJ, Stewart S. Epidemiology, aetiology, and prognosis of heart failure. Heart 2000; 83: 596–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kraigher‐Krainer E, Lyass A, Massaro JM, Lee DS, Ho JE, Levy D, Kannel WB, Vasan RS. Association of physical activity and heart failure with preserved vs. reduced ejection fraction in the elderly: the Framingham Heart Study. Eur J Heart Fail 2013; 15: 742–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Khan H, Kunutsor S, Rauramaa R, Savonen K, Kalogeropoulos AP, Georgiopoulou VV, Butler J, Laukkanen JA. Cardio‐respiratory fitness and risk of heart failure: a population‐based follow‐up study. Eur J Heart Fail 2014; 16: 180–188. [DOI] [PubMed] [Google Scholar]
- 13. Pandey A, Cornwell WK 3rd, Willis B, Neeland IJ, Gao A, Leonard D, DeFina L, Berry JD. Body mass index and cardiorespiratory fitness in mid‐life and risk of heart failure hospitalization in older age. JACC Heart Fail 2017; 5: 367–374. [DOI] [PubMed] [Google Scholar]
- 14. Pandey A, Patel M, Gao A, Willis BL, Das SR, Leonard D, Drazner MH, de Lemos JA, DeFina L, Berry JD. Changes in mid‐life fitness predicts heart failure risk at a later age independent of interval development of cardiac and noncardiac risk factors: the Cooper Center Longitudinal Study. Am Heart J 2015; 169: 290–297 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Berry JD, Pandey A, Gao A, Leonard D, Farzaneh‐Far R, Ayers C, DeFina L, Willis B. Physical fitness and risk for heart failure and coronary artery disease. Circ Heart Fail 2013; 6: 627–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Florido, Ndumele CE, Kwak L, Pang Y, Matsushita K, Schrack JA, Lazo M, Nambi V, Blumenthal RS, Folsom AR, Coresh J, Ballantyne CM, Selvin E. Physical activity, obesity an subclinical myocardial damage. JACC Heart Fail 2017; 5: 377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pandey A, Allen NB, Ayers C, Reis JP, Moreira HT, Sidney S, Rana JS, Jacobs DR Jr, Chow LS, de Lemos JA, Carnethon M, Berry JD. Fitness in young adulthood and long‐term cardiac structure and function. The CARDIA Study. JACC Heart Fail 2017; 5: 347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kamimura D, Loprinzi PD, Wang W, Suzuki T, Butler KR, Mosley TH, Hall ME. Physical activity is associated with reduced left ventricular mass in obese and hypertensive African Americans. Am J Hypertens 2017; 30: 617–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brinker SK, Pandey A, Ayers CR, Barlow CE, DeFina LF, Willis BL, Radford NB, Farzaneh‐Far R, de Lemos JA, Drazner MH, Berry JD. Association of cardiorespiratory fitness with left ventricular remodeling and diastolic function: the Cooper Center Longitudinal Study. JACC Heart Fail 2014; 2: 238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pandey A, LaMonte M, Klein L, Ayers C, Psaty BM, Eaton CB, Allen NB, de Lemos JA, Carnethon M, Greenland P, Berry JD. Relationship between physical activity, body mass index and risk of heart failure. JACC 2017; 69: 1129–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pandey A, Darden D, Berry JD. Low fitness in midlife: a novel therapeutic target for heart failure with preserved ejection fraction prevention. Prog Cardiovasc Dis 2015; 58: 87–93. [DOI] [PubMed] [Google Scholar]
- 22. Bhella PS, Hastings JL, Fujimoto N, Shibata S, Carrick‐Ranson G, Palmer MD, Boyd KN, Adams‐Huet B, Levine BD. Impact of lifelong exercise “dose” on left ventricular compliance and distensibility. J Am Coll Cardiol 2014; 64: 1257–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pandey A, Garg S, Khunger M, Darden D, Ayers C, Kumbhani DJ, Mayo HG, de Lemos JA, Berry JD. Dose–response relationship between physical activity and risk of heart failure. A meta‐analysis. Circulation 2015; 132: 1786–1794. [DOI] [PubMed] [Google Scholar]
- 24. Intwala S, Balady GJ. Physical activity in the prevention of heart failure. Another step forward. Circulation 2015; 132: 1777–1779. [DOI] [PubMed] [Google Scholar]
- 25. Zipes DB, Libby P, Bonow RO, Braunwald E. Branuwald's Heart disease. A Textbook of Cardiovascular Medicine, 1st ed. Elsevier: Philadelphia, Pennsylvania 19106: 1980. [Google Scholar]
- 26. McDonald CD, Burch GE, Walsh JJ. Prolonged bed rest in the treatment of idiopathic cardiomyopathy. Am J Med 1972; 52: 41–50. [DOI] [PubMed] [Google Scholar]
- 27. Sullivan MJ, Higginbotham MB, Cobb FR. Exercise training in patients with severe left ventricular dysfunction. Heamodynamic and metabolic effects. Circulation 1988; 78: 506–515. [DOI] [PubMed] [Google Scholar]
- 28. Doukky R, Mangla A, Ibrahim Z, Poulin MF, Avery E, Collado FM, Kaplan J, Richardson D, Powell LH. Impact of physical inactivity on mortality in patients with heart failure. Am J Cardiol 2016; 117: 1135–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fleg JL, Cooper LS, Borlaug BA, Haykowsky MJ, Kraus WE, Levine BD, Pfeffer MA, Piña IL, Poole DC, Reeves GR, Whellan DJ, Kitzman DW, National Heart, Lung, and Blood Institute Working Group . Exercise training as therapy for heart failure: current status and future directions. Circ Heart Fail 2015; 8: 209–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ponikowsky P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 31. Pedersen BK, Saltin B. Exercise as medicine—evidence for prescribing exercise as therapy in 26 different chronic disease. Scand J Med Sci Sports 2015; 25: 1–72. [DOI] [PubMed] [Google Scholar]
- 32. Arena RA. Preface. Functional capacity and exercise training have earned a primary role in the assessment and treatment of patients with heart failure. Heart Failure Clin 2015; 11: XV–XVII. [DOI] [PubMed] [Google Scholar]
- 33. O'Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA, Rendall DS, Miller NH, Fleg JL, Schulman KA, McKelvie RS, Zannad F, Piña IL, HF‐ACTION Investigators . Efficacy and safety of exercise training in patients with chronic heart failure: HF Action randomized control trial. JAMA 2009; 301: 1451–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sagar VA, Davies EJ, Briscoe S, Coats AJ, Dalal HM, Lough F, Rees K, Singh S, Taylor RS. Exercise‐based rehabilitation for heart failure: systematic review and meta‐analysis. Open Heart 2015; 2: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Taylor RS, Sagar VA, Davies EJ, Briscoe S, Coats AJ, Dalal H, Lough F, Rees K, Singh S. Exercise‐based rehabilitation for heart failure (review). Cochrane Database Syst Rev 2014; CD003331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Forman DE, Sanderson BK, Josephson RA, Raikhelkar J, Bittner V, American College of Cardiology's Prevention of Cardiovascular Disease Section . Heart failure as a newly approved diagnosis for cardiac rehabilitation. Challenges and opportunities. J Am Coll Cardiol 2015; 65: 2652–2659. [DOI] [PubMed] [Google Scholar]
- 37. Jacques L. Decision memorandum for coverage of cardiac rehabilitation programs for chronic heart failure. February 18, 2014. Centers for Medicare & Medicaid Services. Available at: http://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=270. Accessed April 26, 2015.
- 38. Piepoli MF, Binno S, Corrà U, Seferovic P, Conraads V, Jaarsma T, Schmid JP, Filippatos G, Ponikowski PP, Committee on Exercise Physiology & Training of the Heart Failure Association of the ESC . ExtraHF survey: the first European survey on implementation of exercise training in heart failure patients. Eur J Heart Fail 2015; 17: 631–638. [DOI] [PubMed] [Google Scholar]
- 39. Bjarnason‐Wehrens B, McGee H, Zwisler AD, Piepoli MF, Benzer W, Schmid JP, Dendale P, Pogosova NG, Zdrenghea D, Niebauer J, Mendes M, Cardiac Rehabilitation Section European Association of Cardiovascular Prevention and Rehabilitation . Cardiac rehabilitation in Europe: results from the European cardiac rehabilitation Inventory survey. Eur J Cardiovasc Prev Rehabil 2010; 17: 410–418. [DOI] [PubMed] [Google Scholar]
- 40. Haykowsky MJ, Liang Y, Pechter D, Jones LW, McAlister FA, Clark AM. A meta‐analysis of the effect of exercise training on left ventricular remodeling in heart failure: the benefit depends on the type of training performed. J Am Coll Cardiol 2007; 49: 2329–2336. [DOI] [PubMed] [Google Scholar]
- 41. Hambrecht R, Gielen S, Linke A, Fiehn E, Yu J, Walther C, Schoene N, Schuler G. Effects of exercise training on left ventricular function and peripheral resistance in patients with chronic heart failure: a randomized trial. JAMA 2000; 283: 3095–3101. [DOI] [PubMed] [Google Scholar]
- 42. Jettè M, Heller R, Landry F, Blümchen G. Randomized 4‐week exercise program in patients with impaired left ventricular function. Circulation 1991; 84: 1561–1567. [DOI] [PubMed] [Google Scholar]
- 43. Phillips SA, Vuckovic K, Cahalin LP, Baynard T. Defining the system: contributors to exercise limitations in heart failure. Heart Fail Clin 2015; 11: 1–16. [DOI] [PubMed] [Google Scholar]
- 44. Cattadori G, Schmid JP, Brugger N, Gondoni E, Palermo P, Agostoni P. Hemodynamic effects of exercise training in heart failure. J Cardiac Fail 2011; 17: 916–922. [DOI] [PubMed] [Google Scholar]
- 45. Negrao CE, Middlekauff HR, Gomes‐Santos IL, Antunes‐Correa LM. Effects of exercise training on neurovascular control and skeletal myopathy in systolic heart failure. Am J Physiol Heart Circ Physiol 2015; 308: H792–H802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hirai DM, Musch TI, Poole DC. Exercise training in chronic heart failure: improving skeletal muscle O2 transport and utilization. Am J Physiol Heart Circ Physiol 2015; 309: H1419–H1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Groehs RV, Toschi‐Dias E, Antunes‐Correa LM, Trevizan PF, Rondon MU, Oliveira P, Alves MJ, Almeida DR, Middlekauff HR, Negrão CE. Exercise training prevents the deterioration in the arterial baroreflex control of sympathetic nerve activity in chronic heart failure patients. Am J Physiol Heart Circ Physiol 2015; 308: H1096–H1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Esposito F, Reese V, Shabetai R, Wagner PD, Richardson RS. Isolated quadriceps training increases maximal exercise capacity in chronic heart failure: the role of skeletal muscle convective and diffusive oxygen transport. J Am Coll Cardiol 2011; 58: 1353–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meeting in 2012 in Bethesda of the National Heart, Lung, and Blood Institute in Fleg JL et al. Exercise training as therapy for heart failure: current status and future directions. Circ Heart Fail 2015; 8: 209–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Luo N, Merrill P, Parikh KS, Whellan DJ, Pina IL, Fiuzat M, Kraus WE, Kitzman DW, Keteyan SJ, O'Connor CM, Mentz RJ. Exercise training in patients with chronic heart failure and atrial fibrillation. J Am Coll Cardiol 2017; 69: 1683–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Reeves GR, Whellan DJ, O'Connor CM, Duncan P, Eggebeen JD, Morgan TM, Hewston LA, Pastva A, Patel MJ, Kitzman DW. A novel rehabilitation intervention for older patients with acute decompensated heart failure. The REHAB Pilot Study. JACC Heart Fail 2017; 5: 337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Vromen T, Kraal JJ, Kuiper J, Spee RF, Peek N, Kemps HM. The influence of training characteristics on the effect of aerobic exercise training in patients with chronic heart failure: a meta‐regression analysis. Int J Cardiol 2016; 208: 120–127. [DOI] [PubMed] [Google Scholar]
- 53. Piepoli MF. Exercise training in heart failure: from theory to practise. A consensus document of the Heart Failure Association and the European Association for Cardiovascular Prevention and Rehabilitation. Eur J Heart Fail 2011; 13: 347–357. [DOI] [PubMed] [Google Scholar]
- 54. Labate V, Guazzi M. Past, present, and future rehabilitation practice patterns for patients with heart failure. The European Perpective. Heart Fail Clin 2015; 11: 105–115. [DOI] [PubMed] [Google Scholar]
- 55. Forman DE. Rehabilitation practise patterns for patients with heart failure. The United States Perspective. Heart Fail Clin 2015; 11: 89–94. [DOI] [PubMed] [Google Scholar]
- 56. Borghi‐Silva A, Trimer R, Mendes RG, Arena RA, Schwartzmann PV. Rehabilitation practise patterns for patients with heart failure. The South American Perspective. Heart Fail Clin 2015; 11: 73–82. [DOI] [PubMed] [Google Scholar]
- 57. Sun X. Rehabilitation practise patterns for patients with heart failure. The Asian perspective. Heart Fail Clin 2015; 11: 95–104. [DOI] [PubMed] [Google Scholar]
- 58. Cornelis J, Beckers P, Taeymans J, Vrints C, Vissers D. Comparing exercise training modalities in heart failure: a systematic review and meta‐analysis. Int J Cardiol 2016; 221: 867–876. [DOI] [PubMed] [Google Scholar]
- 59. Ellingsen O, Halle M, Conraads V, Støylen A, Dalen H, Delagardelle C, Larsen AI, Hole T, Mezzani A, Van Craenenbroeck EM, Videm V, Beckers P, Christle JW, Winzer E, Mangner N, Woitek F, Höllriegel R, Pressler A, Monk‐Hansen T, Snoer M, Feiereisen P, Valborgland T, Kjekshus J, Hambrecht R, Gielen S, Karlsen T, Prescott E, Linke A; SMARTEX Heart Failure Study (Study of Myocardial Recovery After Exercise Training in Heart Failure) Group . High‐intensity interval training in patients with heart failure with reduced ejection fraction. Circulation 2017; 135: 839–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kitzman DW, Brubaker PH, Morgan TM, Stewart KP, Little WC. Exercise training in older patients with heart failure and preserved ejection fraction: a randomized, controlled, single‐blind trial. Circ Heart Fail 2010; 3: 659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pandey A, Parashar A, Kumbhani DJ, Agarwal S, Garg J, Kitzman D, Levine BD, Drazner M, Berry JD. Exercise training in patients with heart failure and preserved ejection fraction. Meta‐analysis of randomized control trials. Circ Heart Fail 2015; 8: 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kitzman DW, Brubaker P, Morgan T, Haykowsky M, Hundley G, Kraus WE, Eggebeen J, Nicklas BJ. Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction. A randomized clinical trial. JAMA 2016; 315: 36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Jung MH, Gustafsson F. Exercise in heart failure patients supported with a left ventricular assist device. J Heart Lung Transplant 2015; 34: 489–496. [DOI] [PubMed] [Google Scholar]
- 64. Nair N, Gongora E. Heart failure in chemotherapy‐related cardiomyopathy: can exercise make a difference? BBA Clin 2016; 6: 69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Pan L, Yan J, Guo Y, Yan J. Effects of Tai Chi training on exercise capacity and quality of life in patients with chronic heart failure: a meta‐analysis. Eur J Heart Fail 2013; 15: 316–323. [DOI] [PubMed] [Google Scholar]
- 66. Groehs RV, Antunes‐Correa LM, Nobre TS, Alves MJ, Rondon MU, Barreto AC, Negrão CE. Muscle electrical stimulation improves neurovascular control and exercise tolerance in hospitalised advanced heart failure patients. Eur J Prev Cardiol 2016; 23: 1599–1608. [DOI] [PubMed] [Google Scholar]
- 67. Jaarsma T, Klompstra L, Ben Gal T, Boyne J, Vellone E, Bäck M, Dickstein K, Fridlund B, Hoes A, Piepoli MF, Chialà O, Mårtensson J, Strömberg A. Increasing exercise capacity and quality of life of patients with heart failure through Wii gaming: the rationale, design and methodology of the HF‐Wii study; a multicentre randomized controlled trial. Eur J Heart Fail 2015; 17: 743–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Schoenrath F, Markendorf S, Brauchlin AE, Frank M, Wilhelm MJ, Saleh L, Riener R, Schmied CM, Falk V. Robot‐assisted training for heart failure patients—a small pilot study. Acta Cardiol 2015; 70: 665–671. [DOI] [PubMed] [Google Scholar]
- 69. Shah M, Bhalla V, Patnaik S, Maludum O, Lu M, Figueredo VM. Heart failure and the holidays. Clin Res Cardiol 2016; 105: 865–872. [DOI] [PubMed] [Google Scholar]
- 70. Alba AC, Adamson MW, MacIsaac J, Lalonde SD, Chan WS, Delgado DH, Ross HJ. The added value of exercise variables in heart failure prognosis. J Cardiac Fail 2016; 22: 492–497. [DOI] [PubMed] [Google Scholar]
- 71. Malhotra R, Bakken K, D'Elia E, Lewis GD. Cardiopulmonary exercise testing in heart failure. JACC Heart Fail 2016; 4: 607–616. [DOI] [PubMed] [Google Scholar]
- 72. Keteyian SJ, Patel M, Kraus WE, Brawner CA, McConnell TR, Piña IL, Leifer ES, Fleg JL, Blackburn G, Fonarow GC, Chase PJ, Piner L, Vest M, O'Connor CM, Ehrman JK, Walsh MN, Ewald G, Bensimhon D, Russell SD, HF‐ACTION Investigators . Variables measured during cardiopulmonary exercise testing as predictors of mortality in chronic heart failure. J Am Coll Cardiol 2016; 67: 780–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Aaronson KD, Schwartz JS, Chen TM, Wong KL, Goin JE, Mancini DM. Development and prospective validation of a clinical index to predict survival in ambulatory patients referred for cardiac transplant evaluation. Circulation 1997; 95: 2660–2667. [DOI] [PubMed] [Google Scholar]
- 74. O'Connor CM, Whellan DJ, Wojdyla D, Leifer E, Clare RM, Ellis SJ, Fine LJ, Fleg JL, Zannad F, Keteyian SJ, Kitzman DW, Kraus WE, Rendall D, Piña IL, Cooper LS, Fiuzat M, Lee KL. Factors related to morbidity and mortality in patients with chronic heart failure with systolic dysfunction: the HF‐ACTION predictive risk score model. Circ Heart Fail 2011; 5: 63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Agostoni P, Corrà U, Cattadori G, Veglia F, La Gioia R, Scardovi AB, Emdin M, Metra M, Sinagra G, Limongelli G, Raimondo R, Re F, Guazzi M, Belardinelli R, Parati G, Magrì D, Fiorentini C, Mezzani A, Salvioni E, Scrutinio D, Ricci R, Bettari L, Di Lenarda A, Pastormerlo LE, Pacileo G, Vaninetti R, Apostolo A, Iorio A, Paolillo S, Palermo P, Contini M, Confalonieri M, Giannuzzi P, Passantino A, Cas LD, Piepoli MF, Passino C, MECKI Score Research Group . Metabolic exercise test data combined with cardiac and kidney indexes, the MECKI score: a multiparametric approach to heart failure prognosis. Int J Cardiol 2013; 167: 2710–2718. [DOI] [PubMed] [Google Scholar]
- 76. Corrà U, Agostoni P, Giordano A, Cattadori G, Battaia E, La Gioia R, Scardovi AB, Emdin M, Metra M, Sinagra G, Limongelli G, Raimondo R, Re F, Guazzi M, Belardinelli R, Parati G, Magrì D, Fiorentini C, Cicoira M, Salvioni E, Giovannardi M, Veglia F, Mezzani A, Scrutinio D, Di Lenarda A, Ricci R, Apostolo A, Iorio AM, Paolillo S, Palermo P, Contini M, Vassanelli C, Passino C, Giannuzzi P, Piepoli MF, MECKI Score Research Group , Other Members of the MECKI Score Research Group , Antonioli L, Segurini C, Bertella E, Farina S, Bovis F, Pietrucci F, Malfatto G, Roselli T, Buono A, Calabrò R, De Maria R, Santoro D, Campanale S, Caputo D, Bertipaglia D, Berton E. The metabolic exercise test data combined with cardiac and kidney indexes (MECKI) score and prognosis in heart failure. Int J Cardiol 2016; 203: 1067–1072. [DOI] [PubMed] [Google Scholar]
- 77. Agostoni PG, Corrà U, Cattadori G, Veglia F, Battaia E, La Gioia R, Scardovi AB, Emdin M, Metra M, Sinagra G, Limongelli G, Raimondo R, Re F, Guazzi M, Belardinelli R, Parati G, Magrì D, Fiorentini C, Cicoira M, Salvioni E, Giovannardi M, Mezzani A, Scrutinio D, Di Lenarda A, Mantegazza V, Ricci R, Apostolo A, Iorio A, Paolillo S, Palermo P, Contini M, Vassanelli C, Passino C, Piepoli MF, MECKI Score Research Group . Prognostic value of indeterminable anaerobic threshold in heart failure. Circ Heart Fail 2013; 6: 977–987. [DOI] [PubMed] [Google Scholar]
- 78. Cattadori G, Agostoni P, Corrà U, Di Lenarda A, Sinagra G, Veglia F, Salvioni E, La Gioia R, Scardovi AB, Emdin M, Metra M, Limongelli G, Raimondo R, Re F, Guazzi M, Belardinelli R, Parati G, Magrì D, Fiorentini C, Mezzani A, Scrutinio D, Pacileo G, Apostolo A, Iorio A, Paolillo S, Palermo P, Contini M, Giannuzzi P, Battaia E, Cicoira M, Passino C, Piepoli MF, MECKI Score Research Group . Severe heart failure prognosis evaluation for transplant selection in the era of beta‐blockers: role of peak oxygen consumption. Int J Cardiol 2013: 5078–5081. [DOI] [PubMed] [Google Scholar]
- 79. Paolillo S, Agostoni P, Masarone D, Corrà U, Passino C, Scrutinio D, Correale M, Cattadori G, Metra M, Girola D, Piepoli MF, Salvioni E, Giovannardi M, Iorio A, Emdin M, Raimondo R, Re F, Cicoira M, Belardinelli R, Guazzi M, Clemenza F, Parati G, Scardovi AB, Di Lenarda A, La Gioia R, Frigerio M, Lombardi C, Gargiulo P, Sinagra G, Pacileo G, Perrone‐Filardi P, Limongelli G, Metabolic Exercise test data combined with Cardiac and Kidney Indexes (MECKI) Score Research Group . Prognostic role of atrial fibrillation in patients affected by chronic heart failure. Data from the MECKI Score Research Group. Eur J Intern Med 2015; 26: 515–520. [DOI] [PubMed] [Google Scholar]
- 80. Magrì D, Agostoni P, Corrà U, Passino C, Scrutinio D, Perrone‐Filardi P, Correale M, Cattadori G, Metra M, Girola D, Piepoli MF, Iorio A, Emdin M, Raimondo R, Re F, Cicoira M, Belardinelli R, Guazzi M, Limongelli G, Clemenza F, Parati G, Frigerio M, Casenghi M, Scardovi AB, Ferraironi A, Di Lenarda A, Bussotti M, Apostolo A, Paolillo S, La Gioia R, Gargiulo P, Palermo P, Minà C, Farina S, Battaia E, Maruotti A, Pacileo G, Contini M, Oliva F, Ricci R, Sinagra G, Metabolic Exercise test data combined with Cardiac and Kidney Indexes (MECKI) Score Research Group . Deceptive meaning of oxygen uptake measured at the anaerobic threshold in patients with systolic heart failure and atrial fibrillation. Eur J Prev Cardiol 2015; 22: 1046–1055. [DOI] [PubMed] [Google Scholar]
- 81. Corrà U, Agostoni P, Piepoli MF, MECKI Score Research Group . Metabolic exercise data combined with cardiac and kidney indexes: MECKI score. Predictive role in cardiopulmonary exercise testing with low respiratory exchange ratio in heart failure. Int J Cardiol 2015; 184: 299–301. [DOI] [PubMed] [Google Scholar]
- 82. Scrutinio D, Agostoni P, Gesualdo L, Corrà U, Mezzani A, Piepoli M, Di Lenarda A, Iorio A, Passino C, Magrì D, Masarone D, Battaia E, Girola D, Re F, Cattadori G, Parati G, Sinagra G, Villani GQ, Limongelli G, Pacileo G, Guazzi M, Metra M, Frigerio M, Cicoira M, Minà C, Malfatto G, Caravita S, Bussotti M, Salvioni E, Veglia F, Correale M, Scardovi AB, Emdin M, Giannuzzi P, Gargiulo P, Giovannardi M, Perrone‐Filardi P, Raimondo R, Ricci R, Paolillo S, Farina S, Belardinelli R, Passantino A, La Gioia R, Metabolic Exercise test data combined with Cardiac and Kidney Indexes (MECKI) Score Research Group . Renal function and peak exercise oxygen consumption in chronic heart failure with reduced left ventricular ejection fraction. Circ J 2015; 79: 583–591. [DOI] [PubMed] [Google Scholar]
- 83. Carubelli V, Metra M, Corrà U, Magrì D, Passino C, Lombardi C, Scrutinio D, Correale M, Cattadori G, Piepoli MF, Salvioni E, Giovannardi M, Raimondo R, Cicoira M, Belardinelli R, Guazzi M, Limongelli G, Clemenza F, Parati G, Scardovi AB, Di Lenarda A, Bussotti M, La Gioia R, Agostoni P, MECKI Score Research Group . Exercise performance is a prognostic indicator in elderly patients with chronic heart failure. Circ J 2015; 79: 2608–2615. [DOI] [PubMed] [Google Scholar]
- 84. Corrà U, Agostoni P, Giordano A, Cattadori G, Battaia E, La Gioia R, Scardovi AB, Emdin M, Metra M, Sinagra G, Limongelli G, Raimondo R, Re F, Guazzi M, Belardinelli R, Parati G, Magrì D, Fiorentini C, Cicoira M, Salvioni E, Giovannardi M, Veglia F, Mezzani A, Scrutinio D, Di Lenarda A, Ricci R, Apostolo A, Iorio AM, Paolillo S, Palermo P, Contini M, Vassanelli C, Passino C, Giannuzzi P, Piepoli MF, MECKI Score Research Group . Sex profile and risk assessment with cardiopulmonary exercise testing in heart failure: propensity score matching for sex selection bias. Can J Cardiol 2016; 32: 754–759. [DOI] [PubMed] [Google Scholar]
- 85. Piepoli MF, Corrà U, Veglia F, Bonomi A, Salvioni E, Cattadori G, Metra M, Lombardi C, Sinagra G, Limongelli G, Raimondo R, Re F, Magrì D, Belardinelli R, Parati G, Minà C, Scardovi AB, Guazzi M, Cicoira M, Scrutinio D, Di Lenarda A, Bussotti M, Frigerio M, Correale M, Villani GQ, Paolillo S, Passino C, Agostoni P, MECKI Score Research Group . Exercise tolerance can explain the obesity paradox in patients with systolic heart failure: data from the MECKI Score Research Group. Eur J Heart Fail 2016; 18: 545–553. [DOI] [PubMed] [Google Scholar]
- 86. Sinagra G, Iorio A, Merlo M, Cannatà A, Stolfo D, Zambon E, Di Nora C, Paolillo S, Barbati G, Berton E, Carriere C, Magrì D, Cattadori G, Confalonieri M, Di Lenarda A, Agostoni P. Prognostic value of cardiopulmonary exercise testing in idiopathic dilated cardiomyopathy. Int J Cardiol 2016; 223: 596–603. [DOI] [PubMed] [Google Scholar]
- 87. Cattadori G, Agostoni P, Corrà U, Sinagra G, Veglia F, Salvioni E, Bonomi A, La Gioia R, Scardovi AB, Ferraironi A, Emdin M, Metra M, Di Lenarda A, Limongelli G, Raimondo R, Re F, Guazzi M, Belardinelli R, Parati G, Caravita S, Magrì D, Lombardi C, Frigerio M, Oliva F, Girola D, Mezzani A, Farina S, Mapelli M, Scrutinio D, Pacileo G, Apostolo A, Iorio A, Paolillo S, Filardi PP, Gargiulo P, Bussotti M, Marchese G, Correale M, Badagliacca R, Sciomer S, Palermo P, Contini M, Giannuzzi P, Battaia E, Cicoira M, Clemenza F, Minà C, Binno S, Passino C, Piepoli MF, MECKI Score Research Group . Heart failure and anemia: effects on prognostic variables (2017). Eur J Int Med 2016; 37: 56–63. [DOI] [PubMed] [Google Scholar]


