Abstract
Aims
N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) is the most frequently used biomarker in heart failure (HF), but its prognostic utility across ethnicities is unclear.
Methods and results
This study included 546 Caucasians with HF from the Coordinating Study Evaluating Outcomes of Advising and Counseling in Heart Failure and 578 Asians with HF from the Singapore Heart Failure Outcomes and Phenotypes study. NT‐proBNP was measured at discharge after HF hospitalization. The studied outcome was a composite of all‐cause mortality and HF hospitalization at 18 months. Compared with Caucasian patients, Asian patients were younger (63 ± 12 vs. 71 ± 11 years); less often female (26% vs. 39%); and had lower body mass index (26 vs. 27 kg/m2), better renal function (61 ± 37 vs. 54 ± 20 mL/min/1.73 m2), lower rates of atrial fibrillation (25% vs. 46%), strikingly higher rates of diabetes (59% vs. 30%), and higher rates of hypertension (76% vs. 44%). Despite these clear inter‐group differences in individual drivers of NT‐proBNP, average levels were similar in Asians [2709 (1350, 6302) pg/mL] and Caucasians [2545 (1308, 5484) pg/mL] (P = 0.514). NT‐proBNP was strongly associated with outcome [hazard ratio 1.28 (per doubling), 95% confidence interval 1.18–1.39, P < 0.001], regardless of ethnicity (P interaction = 0.719). NT‐proBNP was similarly associated with outcome in HF with reduced and preserved ejection fraction in Asian (P interaction = 0.776) and Caucasian patients (P interaction = 0.558).
Conclusions
NT‐proBNP has similar prognostic performance in Asians and Caucasians with HF despite ethnic differences in known clinical determinants of plasma NT‐proBNP.
Keywords: Ethnicity, Heart failure, Prognosis, NT‐proBNP, HFpEF
Introduction
Inter‐ethnic differences in the incidence, symptoms, underlying pathophysiology, and treatment of heart failure (HF) have been described.1, 2, 3, 4 However, most epidemiological studies and therapeutic trials have focused exclusively on Caucasian populations, and available data on HF in Asian populations are scarce.5
Previously, Asian HF patients have been reported to have lower mortality rates than did their Caucasian peers.6 Higher rates of HF with a preserved ejection fraction (HFpEF) within Asian patients together with higher rates of co‐morbidities such as hypertension, with lower rates of documented myocardial infarction, have also been claimed.7
N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) is the best‐validated biomarker in risk stratification and management of HF.8, 9, 10 B‐type cardiac natriuretic peptide (BNP) and NT‐proBNP have proven their prognostic power in patients with HF across the entire spectrum of ejection fraction.11 A recent study showed that Asian‐American and African‐American HF patients have higher BNP levels than did their Caucasian peers and that predictive value for in‐hospital mortality was similar between ethnicities in an acute HF population.12 Furthermore, NT‐proBNP had higher discriminatory power for identifying HF in Asian dyspnoeic individuals in the emergency department than in Caucasian dyspnoeic individuals.13 However, no previous reports compare results from multi‐ethnic populations with those from stable HF in Asian and Western settings. Therefore, we report the clinical associations and prognostic performance of NT‐proBNP in Asian and Caucasian patients with chronic HF.
Methods
Study design and population
Data were combined from the studies of Coordinating Study Evaluating Outcomes of Advising and Counseling in Heart Failure (COACH) and the Singapore Heart Failure Outcomes and Phenotypes (SHOP).14, 15, 16
In brief, the COACH trial studied the effects of additional intensive nurse‐led support on clinical outcomes in 1023 HF patients.15 Overall, the results of the trial were neutral.14 NT‐proBNP measurements were available in a sub‐cohort of 546 Caucasian patients measured at discharge (see Supporting Information, Figure S1 ). The SHOP study prospectively enrolled 1099 HF patients from six different centres in Singapore. The main objective of the SHOP study is to document the prevalence, characteristics, and outcomes of Asian HF patients in Singapore and to determine the relative proportion of HFpEF patients in this population.16 The Asian population used in this study refers to a SHOP sub‐cohort of 578 patients with NT‐proBNP measurements available at discharge following hospitalization (see Supporting Information, Figure S1 ). Inclusion and exclusion criteria were similar for both studies, which both included patients >18 years of age, either presenting as new onset HF or having previous HF hospitalizations.15, 16 In both studies, blood sampling for NT‐proBNP assay was performed at or around discharge after hospitalization for acute decompensated HF.15, 16 Only participants recruited as inpatients were included in this report. This study complies with the Declaration of Helsinki, local medical ethics committees approved the study, and all patients provided written informed consent.
Outcome
The endpoint analysed was a combined outcome of all‐cause mortality or HF hospitalizations within 18 months. In both studies, endpoints were adjudicated by an independent committee.
Study and laboratory measurements
Blood samples, echocardiography, and all other data were obtained at discharge from index hospitalization for both COACH and SHOP. HFpEF was defined by a left ventricular ejection fraction (LVEF) of ≥50%, and heart failure with a reduced ejection fraction (HFrEF) by an LVEF < 40%. Recently, patients with an intermediate LVEF (HF with a mid‐range ejection fraction) have been considered to be a specific patient group and were, therefore, left out of the definition of HFrEF and HFpEF but were included in the analyses on the total population in this study.17 Assessment of LVEF was performed at admission or within 6 months prior to admission. Measurements of NT‐proBNP were performed on the Elecsys proBNP ECLIA platform (Roche Diagnostics, Mannheim, Germany) in both the SHOP and COACH cohorts. This assay has intra‐assay and inter‐assay coefficients of variation of 4% and 5%, respectively. The estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease formula.18
Statistical analysis
Continuous variables are presented as medians with interquartile range or means ± SD, where appropriate. Categorical variables are presented as numbers with percentages. Inter‐group differences were tested using Student's t‐test or Mann–Whitney U‐test for continuous variables or χ2 test for categorical variables.
We compared absolute values of NT‐proBNP between ethnicities. For all subsequent (Cox) regression analysis, NT‐proBNP was log2 transformed. The following results for NT‐proBNP should be interpreted as per doubling (e.g. 2–4 and 4–8). An interaction analysis was performed to assess if the relationship between individual clinical variables and NT‐proBNP levels differed between ethnicities. Univariable linear regression analysis was performed separately for Caucasians and Asians with NT‐proBNP as the dependent variable. Multivariable adjustment was then performed for factors known to influence NT‐proBNP levels including age, sex, body mass index (BMI), LVEF, New York Heart Association class, previous myocardial infarction, systolic blood pressure, a history of atrial fibrillation, and eGFR.19, 20, 21, 22, 23, 24
Cox regression analysis (including interaction analysis in univariable and multivariable models) was performed to further investigate the possible differential association of NT‐proBNP with outcome between different ethnicities. Multivariable models were produced based on significant differences at baseline between ethnicities as well as incorporating known clinically meaningful variables. Model fit was tested using the Hosmer–Lemeshow goodness‐of‐fit test. Additionally, to graphically depict the relationship between ethnicity and the primary outcome, Kaplan–Meier curves stratified by ethnicity were produced. Differences were tested using the log‐rank test. For multivariable Cox regression analysis, the proportional hazards assumption was tested using Schoenfeld residuals and found to be valid. We have provided results of continuous net reclassification analysis to study whether NT‐proBNP significantly improves a model of clinical variables equally in Asian and Caucasian HF patients. Furthermore, we studied the ability of NT‐proBNP to predict outcome on top of a clinical model using the area under the receiver operating characteristic curve. Tests were performed two sided, and a P‐value of <0.05 was considered significant. All statistical analyses were performed using STATA version 13.0 (StataCorp LP, College Station, Texas, USA).
Results
Baseline characteristics
Baseline demographic and clinical characteristics of Asian and Caucasian patients are presented in Table 1. Asian patients were younger and less often female than were their Caucasian peers. Additionally, Asian patients had a lower BMI and a better renal function and an overall lower burden of chronic obstructive pulmonary disease, atrial fibrillation, and peripheral vascular disease, while having higher rates of diabetes mellitus and hypertension (Table 1). Furthermore, Asian HF patients were more often treated with beta‐blockers (Table 1). Despite these differences, absolute plasma concentrations of NT‐proBNP in Asian and Caucasian patients were similar (P = 0.514; Figure 1). After correcting for clinical characteristics influencing NT‐proBNP levels including age, sex, BMI, eGFR, systolic blood pressure, history of atrial fibrillation, and usage of angiotensin‐converting enzyme inhibitors, diuretics, and beta‐blockers, NT‐proBNP levels were equal between Caucasians and Asians (β = 0.045, P = 0.161, for Asian compared with Caucasian HF patients).
Table 1.
Baseline characteristics
| Total cohort (n = 1124) | Caucasian (n = 546) | Asian (n = 578) | P value | ||
|---|---|---|---|---|---|
| Demographics | |||||
| Age (years) | 66.9 (12.3) | 70.7 (11.2) | 63.2 (12.3) | <0.001 | |
| Female sex, n (%) | 362 (32.1%) | 210 (38.5%) | 152 (26.3%) | <0.001 | |
| NYHA class, n (%) | I/II | 689 (62.2%) | 257 (47.4%) | 432 (76.3%) | <0.001 |
| III | 391 (35.3%) | 271 (50.0%) | 120 (21.2%) | ||
| IV | 28 (2.5%) | 14 (2.6%) | 14 (2.5%) | ||
| BMI (kg/m2) | 26.6 (5.5) | 27.1 (5.5) | 26.1 (5.5) | 0.004 | |
| Systolic blood pressure (mmHg) | 119.3 (20.2) | 118.1 (21.0) | 120.3 (19.3) | 0.066 | |
| Diastolic blood pressure (mmHg) | 68.9 (12.1) | 68.6 (12.2) | 69.2 (12.1) | 0.43 | |
| Heart rate (b.p.m.) | 76.0 (13.9) | 74.6 (13.3) | 77.4 (14.3) | <0.001 | |
| LVEF (%) | 29.0 (20.0, 42.0) | 30.0 (22.0, 40.0) | 28.0 (20.0, 45.0) | 0.990 | |
| HFpEF, n (%) | 169 (18.5%) | 49 (12.7%) | 118 (22.5%) | <0.001 | |
| Medical history, n (%) | |||||
| Myocardial infarction | 388 (39.7%) | 215 (39.4%) | 173 (40.0%) | 0.830 | |
| Hypertension | 676 (60.4%) | 240 (44.0%) | 436 (76.1%) | <0.001 | |
| COPD | 194 (17.3%) | 147 (26.9%) | 47 (8.2%) | <0.001 | |
| Atrial fibrillation | 394 (35.2%) | 251 (46.0%) | 143 (24.9%) | <0.001 | |
| Diabetes mellitus | 503 (44.8%) | 162 (29.7%) | 341 (59.2%) | <0.001 | |
| Peripheral vascular disease | 130 (11.6%) | 91 (16.7%) | 39 (6.8%) | <0.001 | |
| Stroke | 147 (13.1%) | 83 (15.2%) | 64 (11.1%) | 0.040 | |
| Prior medication, n (%) | |||||
| ACE‐inhibitors | 723 (65.0%) | 394 (72.2%) | 329 (58.1%) | <0.001 | |
| ARB | 203 (18.3%) | 62 (11.4%) | 141 (24.9%) | <0.001 | |
| ACE‐inhibitors and/or ARB | 911 (81.9%) | 449 (82.2%) | 462 (81.6%) | 0.790 | |
| Beta‐blocker | 861 (77.4%) | 372 (68.1%) | 489 (86.4%) | <0.001 | |
| Aldosterone antagonists | 535 (48.1%) | 296 (54.2%) | 239 (42.2%) | <0.001 | |
| Diuretics | 1039 (93.4%) | 522 (95.6%) | 517 (91.3%) | 0.004 | |
| Digoxin | 325 (29.2%) | 177 (32.4%) | 148 (26.1%) | 0.022 | |
| Laboratory | |||||
| eGFR (mL/min/1.73 m2) | 57.8 (30.2) | 54.1 (19.6) | 61.3 (37.1) | <0.001 | |
| Potassium (mEq/L) | 4.1 (3.8, 4.5) | 4.2 (3.9, 4.6) | 4.0 (3.7, 4.4) | <0.001 | |
| Sodium (mEq/L) | 138.0 (136.0, 141.0) | 139.0 (136.0, 142.0) | 138.0 (136.0, 140.0) | <0.001 | |
ACE, angiotensin‐converting enzyme; ARB, angiotensin‐II receptor blocker; BMI, body mass index; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; HFpEF, heart failure with a preserved ejection fraction; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association. Data in bold are P‐values <0.05.
Figure 1.

Boxplots showing N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) levels in Asian and Caucasian heart failure patients.
N‐terminal pro‐B‐type natriuretic peptide and clinical variables
Univariable and multivariable associations between levels of NT‐proBNP and clinical variables are shown in Table 2. In univariable analysis, NT‐proBNP levels were more strongly associated with the male sex in Asians than in Caucasians (β = −0.033, P = 0.442, in Caucasians; β = −0.137, P = 0.001, in Asians; P interaction = 0.046); however, no difference in levels of NT‐proBNP between sexes were observed after correcting for the presence of HFpEF in both Asians and Caucasians. Equal associations were found between levels of NT‐proBNP and clinical covariates such as a history of atrial fibrillation, hypertension, BMI, LVEF, and eGFR in both Asians and Caucasians (P interaction all >0.05). The R 2 for the multivariable model in Caucasian HF patients (0.17) was lower than in Asian HF patients (0.29).
Table 2.
Clinical associations of N‐terminal pro‐B‐type natriuretic peptide
| Caucasian | Asian | P‐value (interaction) | |||
|---|---|---|---|---|---|
| Univariable | Multivariablea | Univariable | Multivariablea | ||
| β (P‐value) | β (P‐value) | β (P‐value) | β (P‐value) | ||
| Demographics | |||||
| Age | 0.114 (0.008) | 0.055 (0.275) | 0.108 (0.009) | 0.155 (0.001) | 0.890 |
| Female sex | −0.033 (0.442) | 0.014 (0.756) | −0.137 (0.001) | −0.013 (0.752) | 0.046 |
| NYHA class | 0.127 (0.003) | 0.081 (0.085) | 0.140 (0.001) | 0.108 (0.004) | 0.542 |
| BMI | −0.262 (<0.001) | −0.188 (<0.001) | −0.272 (<0.001) | −0.193 (<0.001) | 0.588 |
| Heart rate | 0.073 (0.091) | 0.064 (0.169) | 0.045 (0.280) | 0.016 (0.678) | 0.642 |
| LVEF | −0.253 (<0.001) | −0.259 (<0.001) | −0.345 (<0.001) | −0.456 (<0.001) | 0.258 |
| HFpEF | −0.232 (<0.001) | −0.064 (0.457) | −0.376 (<0.001) | −0.238 (0.007) | 0.076 |
| Medical history | |||||
| Myocardial infarction | 0.063 (0.140) | −0.029 (0.547) | 0.116 (0.015) | 0.031 (0.475) | 0.361 |
| Peripheral vascular disease | 0.055 (0.199) | 0.019 (0.685) | 0.095 (0.022) | 0.090 (0.015) | 0.216 |
| Hypertension | 0.041 (0.335) | 0.073 (0.128) | −0.059 (0.158) | −0.043 (0.266) | 0.087 |
| Atrial fibrillation | 0.051 (0.231) | 0.033 (0.491) | 0.094 (0.023) | 0.124 (0.001) | 0.323 |
| Diabetes | 0.000 (0.991) | 0.051 (0.278) | 0.020 (0.625) | 0.037 (0.327) | 0.728 |
| Laboratory | |||||
| eGFR | −0.219 (<0.001) | −0.223 (<0.001) | −0.223 (<0.001) | −0.185 (<0.001) | 0.061 |
BMI, body mass index; eGFR, estimated glomerular filtration rate; HFpEF, heart failure with a preserved ejection fraction; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association.
Explanatory note: univariable and multivariable associations for between clinical variables and N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) levels are shown. The P‐value for interaction is the interaction between ethnicity (Asian and Caucasian) and the clinical variable for the association with NT‐proBNP. Data in bold are P‐values <0.05.
Corrected for age, sex, BMI, LVEF, systolic blood pressure, eGFR, and a history of atrial fibrillation.
Outcome in Asian and Caucasian heart failure patients
In the entire cohort, 507 (45%) patients [including 273 (47%) Asians] incurred the primary composite outcome. Asian patients reached the primary combined outcome of all‐cause mortality or HF hospitalizations at 18 months more often than did their Caucasian counterparts [hazard ratio (HR) 1.35; 95% confidence interval (CI) 1.13–1.60; P = 0.001]. Also after multivariable correction, Asian patients were more often subject to all‐cause mortality or HF rehospitalizations over 18 months (HR 1.36; 95% CI 1.08–1.72; P = 0.009, see Supporting Information, Table S1 and Figure S2 ). For all‐cause mortality alone, 154 (28%) Caucasian HF patients died within 18 months compared with 102 (18%) Asian patients (P < 0.001).
N‐terminal pro‐B‐type natriuretic peptide levels and outcome
The association of NT‐proBNP with the primary combined outcome was similar (P interaction = 0.631) in Asians (HR 1.56; 95% CI 1.29–1.89; P < 0.001) and Caucasians in both unadjusted and adjusted analyses (HR 1.28; 95% CI 1.21–1.35, Table 3 and Figure 2; and HR 1.33; 95% CI 1.16–1.54; P < 0.001, Table 3 and Figure 3, respectively). The predictive value of NT‐proBNP in patients with HFrEF and HFpEF was similar in Asian and Caucasian patients specifically; NT‐proBNP had equal predictive power in both HFrEF and HFpEF subgroups (P interaction > 0.05 across univariable and multivariable models for both Asian and Caucasians) for the combined outcome of death and HF hospitalization (Table 3). In multivariable analysis, NT‐proBNP remained equally predictive for the combined endpoint in both Asian and Caucasian HFrEF and HFpEF patients (see Supporting Information, Figure S3 ). Results were similar for all‐cause mortality alone (see Supporting Information, Table S2 ). When exploring subgroup associations with outcome in both Asian and Caucasian HF patients, no significant differences were found (P‐value for interaction all >0.05; see Supporting Information, Figures S4 and S5 ). The receiver operating characteristic curve of the clinical model (Model 3 from Table 3) increased from 0.65 to 0.69 when NT‐proBNP was added in Asian patients (P < 0.001) and from 0.70 to 0.72 in Caucasian patients (P = 0.037). Net‐reclassification analysis showed that both Asian [net reclassification index (NRI) 0.377, P < 0.001] and Caucasian (NRI 0.325, P < 0.001) patients with HF were significantly reclassified after adding NT‐proBNP to a model of clinical variables (Model 3, Table 3).
Table 3.
Cox regression analysis of N‐terminal pro‐B‐type natriuretic peptide corrected for ethnicity
| Adjustments | Cox HRa (95% CI) | P‐value | Total | Caucasian | Asian |
|---|---|---|---|---|---|
| P‐value interaction (ethnicity) | P‐value interaction (HF status) | P‐value interaction (HF status) | |||
| Univariable | 1.28 (1.21–1.35) | <0.001 | NA | 0.338 | 0.077 |
| Ethnicity | 1.28 (1.21–1.35) | <0.001 | 0.631 | NA | NA |
| Model 1 | 1.26 (1.19–1.33) | <0.001 | 0.520 | 0.865 | 0.092 |
| Model 2 | 1.23 (1.15–1.31) | <0.001 | 0.825 | 0.411 | 0.583 |
| Model 3 | 1.28 (1.18–1.39) | <0.001 | 0.719 | 0.558 | 0.776 |
CI, confidence interval; HF, heart failure; HR, hazard ratio.
Model 1: Ethnicity, Age; Sex.
Model 2: Model 1; body mass index; estimated glomerular filtration rate; systolic blood pressure; history of peripheral vascular disease; chronic obstructive pulmonary disease; diabetes; atrial fibrillation; myocardial infarction, and New York Heart Association class.
Model 3: Model 2; left ventricular ejection fraction; usage of aldosterone antagonists; diuretics; digoxin; beta‐blockers; angiotensin‐converting enzyme inhibitors; angiotensin‐II receptor blockers.
Hazard ratios are per doubling of levels of N‐terminal pro‐B‐type natriuretic peptide.
Figure 2.
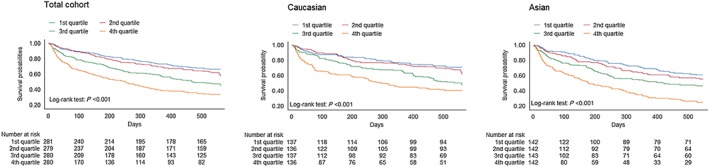
Kaplan–Meier curves showing the relationship of N‐terminal pro‐B‐type natriuretic peptide levels with outcome for the total cohort, Caucasian patients, and Asian patients.
Figure 3.
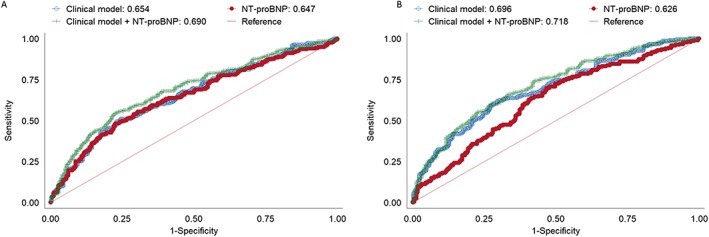
Receiver operating characteristic curves for a clinical model, N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP), and a clinical model + NT‐proBNP for predicting the primary combined outcome in Asian (A) and Caucasian (B) patients.
Discussion
This analysis shows that NT‐proBNP is a strong and independent predictor of adverse outcome in both Asian and Caucasian HF patients. In a similar clinical setting of stable HF, Asian HF patients have NT‐proBNP similar to that of their Caucasian peers. For a given relative increase of NT‐proBNP, outcome is equally poor in both Asian and Caucasian patients. This holds true in both HFpEF and HFrEF, regardless of ethnicity. Additionally, the associations of NT‐proBNP with its prime well‐recognized drivers are similar across ethnicities. These findings have clinical implications, namely, that a relative NT‐proBNP increase in both Asian and Caucasian patients can be interpreted similarly. Furthermore, it is likely that NT‐proBNP can be used for selection of patients at high risk for adverse outcomes amongst both Asian and Caucasian ethnicities.
In this study, Asian HF patients differed significantly from their Caucasian counterparts. They were younger yet had more adverse outcomes. With regard to co‐morbidities, Asian patients had significantly higher rates of diabetes and hypertension, while having lower rates of renal impairment and atrial fibrillation. Additionally, they had lower BMIs than did their Caucasian peers. Similar differences with regard to diabetes and atrial fibrillation have been previously observed in an earlier study involving both Asian and Caucasian subjects.12 Despite greater age and burden of co‐morbidities, we found that Caucasian HF patients performed better on the primary combined outcome than did Asian HF patients. Nevertheless, Caucasian HF patients had higher mortality rates than did their Asian peers, suggesting that the difference between ethnicities for the combined outcome is mainly driven by higher HF rehospitalization rates. Higher mortality rates in Caucasian patients are potentially explained by the lower rates of beta‐blocker usage in the Caucasian patients in this study. The difference in HF rehospitalizations can be explained by differences in health‐care‐seeking behaviours as well as differences in dosages and effectiveness of guideline‐directed treatment in Asian HF patients, which deserves further study.25, 26, 27 Additionally, ethnic differences can potentially be explained by genetic differences rather than race per se. Yet in the absence of genetic data, race provides a good surrogate parameter.28
Median NT‐proBNP levels were similar in Asian and Caucasian HF patients. Also, clinical characteristics known for influencing NT‐proBNP levels had similar effects. Clinical determinants of BNP levels have been previously found to be relatively similar across ethnicities.12 Nevertheless, discriminatory power of NT‐proBNP for acute HF is superior in Asian acute HF patients than in Caucasian acute HF patients in the emergency department.13 NT‐proBNP levels were similar in our study between Asian and Caucasian patients despite differences in distribution of key drivers of NT‐proBNP levels between Asians such as atrial fibrillation and BMI. This suggests that, overall, the drivers of NT‐proBNP levels were balanced between Asian and Caucasian HF patients and that NT‐proBNP is similarly prognostic irrespective of the exact combination and proportional magnitude of drivers for production and clearance of plasma NT‐proBNP concentrations. Nevertheless, uncaptured confounders such as nutritional status and differences in health‐care systems and nutritional status might influence NT‐proBNP levels.29
NT‐proBNP was similarly associated with both HF rehospitalizations and all‐cause mortality across ethnicities. This finding is in line with an earlier report in acute HF patients, in which levels of BNP measured at admission were found to hold equal predictive value for in‐hospital mortality and length of stay.12 Additionally, several key studies have evaluated the predictive value of BNP in Asian populations and showed that BNP is a key predictor of outcomes in these patients.30, 31, 32 Also, NT‐proBNP levels had equal predictive value in both HFpEF and HFrEF. This has been previously reported in a Caucasian population.11 This suggests that clinical applications of NT‐proBNP are similar in both Asian and Caucasian populations. Interestingly, values of NT‐proBNP have been shown to be higher in patients with HFrEF compared with HFpEF, yet the predictive value of NT‐proBNP is similar.11 Outcome of patients with HFpEF was previously found to be better than that of patients with HFrEF in the MAGGIC meta‐analysis study.33 This might in part be explained by lower NT‐proBNP levels; nevertheless, there might be different factors currently unknown that are driving the mortality in HFpEF.34, 35 Of note, sensitivity and specificity for a given level of NT‐proBNP were comparable between Asian and Caucasian HF patients.
NT‐proBNP is equally prognostic in both Asian and Caucasian populations. This is despite striking inter‐ethnic differences in the mean level (e.g. younger age in Asians) or prevalence (e.g. far more frequent atrial fibrillation in Caucasians) of key background drivers for plasma NT‐proBNP. Notably, each driver exhibits similar strength and slope of association with NT‐proBNP between ethnicities. The findings suggest that clinical applications of NT‐proBNP measurements in diagnosis and management of HF will be similarly effective across ethnicities. Secondly, application of NT‐proBNP as an entry criterion for enrichment of event rates in future trials is equally applicable to Caucasians and Asians. With ethnic differences with regard to treatment response and outcome becoming ever more apparent, future HF trials will most probably be more inclusive of non‐western ethnicities. The findings of this study suggest that NT‐proBNP is equally valuable as an epidemiologic and clinical tool in both Asian and Caucasian HF patients.
Limitations
This study is a post hoc analysis with all limitations coming with that, including potential selection bias. Since no data are available on treatment during admission for HF prior to discharge, this might confound some of the reported findings. No information was available on difference in nutritional status, although differences in health‐care systems between Singapore and the Netherlands might have influenced NT‐proBNP levels. In this context, patients in this study cover a grey area between acute decompensated and chronic HF patients. Corroboration of our findings will require further studies in independent Asian and Caucasian HF cohorts.
Conclusions
NT‐proBNP has equal predictive power in both Caucasian and Asian HF populations at discharge after admission for acute HF. Clinical associations with NT‐proBNP do not differ between ethnicities, suggesting that a given value can be similarly interpreted in both Asian and Caucasian HF patients.
Conflict of interest
C.S.P.L. is supported by a Clinician Scientist Award from the National Medical Research Council of Singapore; has received research support from Boston Scientific, Bayer, Thermo Fisher, Medtronic, and Vifor Pharma; and has consulted for Bayer, Novartis, Takeda, Merck, AstraZeneca, Janssen Research & Development, LLC, Menarini, Boehringer Ingelheim, and Abbott Diagnostics. A.M.R. is supported by in‐kind research support and/or speaker's honoraria and/or sit on advisory boards from/for Roche Diagnostics, Abbott Laboratories, Thermo Fisher, Critical Diagnostics, AstraZeneca, and Novartis. R.d.B. is supported by the Netherlands Heart Foundation (CVON‐DOSIS, grant 2014‐40), the Innovational Research Incentives Scheme programme of the Netherlands Organization for Scientific Research (NWO VIDI, grant 917.13.350), AstraZeneca, Bristol‐Myers Squibb, and Trevena; and has received speaker fees and is on the advisory boards of Novartis and Roche Diagnostics. A.A.V. has received speaker fees from Roche Diagnostics. All other authors have nothing to disclose with regard to this manuscript.
Funding
Coordinating Study Evaluating Outcomes of Advising and Counseling in Heart Failure was supported by grant 2000Z003 from the Netherlands Heart Foundation and by additional unrestricted grants from Roche Diagnostics Nederland BV, Venlo, The Netherlands (N‐terminal pro‐hormone brain natriuretic peptide), and Novartis Pharma BV, Arnhem, The Netherlands. The SHOP study is funded by the National Medical Research Council, Singapore (grant no. R‐172‐003‐219‐511), the A*STAR–NZ HRC (grant no. JGC 10_027), and the Clinician Scientist Award (C.S.P.L.).
Supporting information
Table S1. Difference with regard to all‐cause mortality and HF rehospitalization for Asian HF patients vs. Caucasian HF patients.
Table S2. Association of levels of NT‐proBNP with all‐cause mortality.
Figure S1. Flow chart study design.
Figure S2. Outcome in Caucasian vs Asian HF patients.
Figure S3. Association of quartiles of NT‐proBNP with the combined outcome for Asian patients with HFrEF (A) and HFpEF (B) as well as Caucasian patients with HFrEF (C) and HFpEF (D).
Figure S4. hazard ratios in subgroups of Asian HF patients for the primary outcome, p‐value for interaction for all is >0.05. Abbreviations: BMI, body mass index; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HT, hypertension.
Figure S5. hazard ratios for the primary outcome in subgroups of Caucasian HF patients, p‐value for interaction for all is >0.05. Abbreviations: BMI, body mass index; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HT, hypertension.
Tromp, J. , Richards, A. M. , Tay, W. T. , Teng, T.‐H. K. , Yeo, P. S. D. , Sim, D. , Jaufeerally, F. , Leong, G. , Ong, H. Y. , Ling, L. H. , van Veldhuisen, D. J. , Jaarsma, T. , Voors, A. A. , van der Meer, P. , de Boer, R. A. , and Lam, C. S. P. (2018) N‐terminal pro‐B‐type natriuretic peptide and prognosis in Caucasian vs. Asian patients with heart failure. ESC Heart Failure, 5: 279–287. doi: 10.1002/ehf2.12252.
Contributor Information
Rudolf A. de Boer, Email: r.a.de.boer@umcg.nl
Carolyn S.P. Lam, Email: carolyn.lam@duke-nus.edu.sg
References
- 1. Bibbins‐Domingo K, Pletcher MJ, Lin F, Vittinghoff E, Gardin JM, Arynchyn A, Lewis CE, Williams OD, Hulley SB. Racial differences in incident heart failure among young adults. N Engl J Med 2009; 360: 1179–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dickson VV, Knafl GJ, Wald J, Riegel B. Racial differences in clinical treatment and self‐care behaviors of adults with chronic heart failure. J Am Heart Assoc 2015; 4: e001561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vardeny O, Cavallari LH, Claggett B, Desai AS, Anand I, Rossignol P, Zannad F, Pitt B, Solomon SD. Race influences the safety and efficacy of spironolactone in severe heart failure. Circ Heart Fail 2013; 6: 970–976. [DOI] [PubMed] [Google Scholar]
- 4. Gotsman I, Avishai‐Eliner S, Jabara R, Zemora Z, Shauer A, Lotan C, Keren A. Ethnic disparity in the clinical characteristics of patients with heart failure. Eur J Heart Fail 2015; 17: 801–808. [DOI] [PubMed] [Google Scholar]
- 5. Sosin MD, Bhatia GS, Davis RC, Lip GYH. Heart failure—the importance of ethnicity. Eur J Heart Fail 2004; 6: 831–843. [DOI] [PubMed] [Google Scholar]
- 6. Kaul P, McAlister FA, Ezekowitz JA, Grover VK, Quan H. Ethnic differences in 1‐year mortality among patients hospitalised with heart failure. Heart 2011; 97: 1048–1053. [DOI] [PubMed] [Google Scholar]
- 7. Gurwitz JH, Magid DJ, Smith DH, Hsu G, Sung SH, Allen LA, McManus DD, Goldberg RJ, Go AS. The complex relationship of race to outcomes in heart failure with preserved ejection fraction. Am J Med 2015; 128: 591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lainchbury JG, Troughton RW, Strangman KM, Frampton CM, Pilbrow A, Yandle TG, Hamid AK, Nicholls MG, Richards AM. N‐terminal pro‐B‐type natriuretic peptide‐guided treatment for chronic heart failure: results from the BATTLESCARRED (NT‐proBNP‐Assisted Treatment To Lessen Serial Cardiac Readmissions and Death) trial. J Am Coll Cardiol 2009; 55: 53–60. [DOI] [PubMed] [Google Scholar]
- 9. Januzzi JL, van Kimmenade R, Lainchbury J, Bayes‐Genis A, Ordonez‐Llanos J, Santalo‐Bel M, Pinto YM, Richards M. NT‐proBNP testing for diagnosis and short‐term prognosis in acute destabilized heart failure: an international pooled analysis of 1256 patients: the International Collaborative of NT‐proBNP Study. Eur Heart J 2006; 27: 330–337. [DOI] [PubMed] [Google Scholar]
- 10. Maisel A, Mueller C, Nowak R, Peacock WF, Landsberg JW, Ponikowski P, Mockel M, Hogan C, Wu AHB, Richards M, Clopton P, Filippatos GS, Di Somma S, Anand I, Ng L, Daniels LB, Neath S‐X, Christenson R, Potocki M, McCord J, Terracciano G, Kremastinos D, Hartmann O, von Haehling S, Bergmann A, Morgenthaler NG, Anker SD. Mid‐region pro‐hormone markers for diagnosis and prognosis in acute dyspnea: results from the BACH (Biomarkers in Acute Heart Failure) trial. J Am Coll Cardiol 2010; 55: 2062–2076. [DOI] [PubMed] [Google Scholar]
- 11. van Veldhuisen DJ, Linssen GCM, Jaarsma T, van Gilst WH, Hoes AW, Tijssen JGP, Paulus WJ, Voors AA, Hillege HL. B‐type natriuretic peptide and prognosis in heart failure patients with preserved and reduced ejection fraction. J Am Coll Cardiol 2013; 61: 1498–1506. [DOI] [PubMed] [Google Scholar]
- 12. Krim SR, Vivo RP, Krim NR, Qian F, Cox M, Ventura H, Hernandez AF, Bhatt DL, Fonarow GC. Racial/ethnic differences in B‐type natriuretic peptide levels and their association with care and outcomes among patients hospitalized with heart failure: findings from Get With The Guidelines‐Heart Failure. JACC Heart Fail 2013; 1: 345–352. [DOI] [PubMed] [Google Scholar]
- 13. Ibrahim I, Sen KW, Frampton C, Troughton R, Liew OW, Chong JPC, Chan SP, Tan LL, Lin WQ, Pemberton CJ, Ooi SBS, Richards AM. Superior performance of N‐terminal pro brain natriuretic peptide for diagnosis of acute decompensated heart failure in an Asian compared with a Western setting. Eur J Heart Fail 2017; 19: 209–217. [DOI] [PubMed] [Google Scholar]
- 14. Jaarsma T, van der Wal MHL, Lesman‐Leegte I, Luttik M‐L, Hogenhuis J, Veeger NJ, Sanderman R, Hoes AW, van Gilst WH, Lok DJA, Dunselman PHJM, Tijssen JGP, Hillege HL, van Veldhuisen DJ. Effect of moderate or intensive disease management program on outcome in patients with heart failure: Coordinating Study Evaluating Outcomes of Advising and Counseling in Heart Failure (COACH). Arch Intern Med 2008; 168: 316–324. [DOI] [PubMed] [Google Scholar]
- 15. Jaarsma T, Van Der Wal MHL, Hogenhuis J, Lesman I, Luttik M‐LA, Veeger NJGM, Van Veldhuisen DJ. Design and methodology of the COACH study: a multicenter randomised Coordinating study evaluating Outcomes of Advising and Counselling in Heart failure. Eur J Heart Fail 2004; 6: 227–233. [DOI] [PubMed] [Google Scholar]
- 16. Santhanakrishnan R, Ng TP, Cameron VA, Gamble GD, Ling LH, Sim D, Leong GKT, Yeo PSD, Ong HY, Jaufeerally F, Wong RC‐C, Chai P, Low AF, Lund M, Devlin G, Troughton R, Richards AM, Doughty RN, Lam CSP. The Singapore Heart Failure Outcomes and Phenotypes (SHOP) study and Prospective Evaluation of Outcome in Patients with Heart Failure with Preserved Left Ventricular Ejection Fraction (PEOPLE) study: rationale and design. J Card Fail 2013; 19: 156–162. [DOI] [PubMed] [Google Scholar]
- 17. Lam CSP, Solomon SD. The middle child in heart failure: heart failure with mid‐range ejection fraction (40–50%). Eur J Heart Fail 2014; 16: 1049–1055. [DOI] [PubMed] [Google Scholar]
- 18. Smilde TDJ, van Veldhuisen DJ, Navis G, Voors AA, Hillege HL. Drawbacks and prognostic value of formulas estimating renal function in patients with chronic heart failure and systolic dysfunction. Circulation 2006; 114: 1572–1580. [DOI] [PubMed] [Google Scholar]
- 19. McCullough PA, Duc P, Omland T, McCord J, Nowak RM, Hollander JE, Herrmann HC, Steg PG, Westheim A, Knudsen CW, Storrow AB, Abraham WT, Lamba S, Wu AHB, Perez A, Clopton P, Krishnaswamy P, Kazanegra R, Maisel AS. B‐type natriuretic peptide and renal function in the diagnosis of heart failure: an analysis from the Breathing Not Properly Multinational Study. Am J Kidney Dis 2003; 41: 571–579. [DOI] [PubMed] [Google Scholar]
- 20. DeFilippi C, van Kimmenade RRJ, Pinto YM. Amino‐terminal pro‐B‐type natriuretic peptide testing in renal disease. Am J Cardiol 2008; 101: 82–88. [DOI] [PubMed] [Google Scholar]
- 21. Morello A, Lloyd‐Jones DM, Chae CU, van Kimmenade RRJ, Chen AC, Baggish AL, O'Donoghue M, Lee‐Lewandrowski E, Januzzi JL. Association of atrial fibrillation and amino‐terminal pro‐brain natriuretic peptide concentrations in dyspneic subjects with and without acute heart failure: results from the ProBNP Investigation of Dyspnea in the Emergency Department (PRIDE) study. Am Heart J 2007; 153: 90–97. [DOI] [PubMed] [Google Scholar]
- 22. Richards M, Di Somma S, Mueller C, Nowak R, Peacock WF, Ponikowski P, Mockel M, Hogan C, Wu AHB, Clopton P, Filippatos GS, Anand I, Ng L, Daniels LB, Neath S‐X, Shah K, Christenson R, Hartmann O, Anker SD, Maisel A. Atrial fibrillation impairs the diagnostic performance of cardiac natriuretic peptides in dyspneic patients: results from the BACH Study (Biomarkers in ACute Heart Failure). JACC Heart Fail 2013; 1: 192–199. [DOI] [PubMed] [Google Scholar]
- 23. Daniels LB, Clopton P, Bhalla V, Krishnaswamy P, Nowak RM, McCord J, Hollander JE, Duc P, Omland T, Storrow AB, Abraham WT, Wu AHB, Steg PG, Westheim A, Knudsen CW, Perez A, Kazanegra R, Herrmann HC, McCullough PA, Maisel AS. How obesity affects the cut‐points for B‐type natriuretic peptide in the diagnosis of acute heart failure. Results from the Breathing Not Properly Multinational Study. Am Heart J 2006; 151: 999–1005. [DOI] [PubMed] [Google Scholar]
- 24. Hogenhuis J, Voors AA, Jaarsma T, Hoes AW, Hillege HL, Kragten JA, van Veldhuisen DJ. Anaemia and renal dysfunction are independently associated with BNP and NT‐proBNP levels in patients with heart failure. Eur J Heart Fail 2007; 9: 787–794. [DOI] [PubMed] [Google Scholar]
- 25. Lee R, Chan S‐P, Chan Y‐H, Wong J, Lau D, Ng K. Impact of race on morbidity and mortality in patients with congestive heart failure: a study of the multiracial population in Singapore. Int J Cardiol Elsevier 2009; 134: 422–425. [DOI] [PubMed] [Google Scholar]
- 26. Blair JEA, Zannad F, Konstam MA, Cook T, Traver B, Burnett JC, Grinfeld L, Krasa H, Maggioni AP, Orlandi C, Swedberg K, Udelson JE, Zimmer C, Gheorghiade M, Investigators EVEREST. Continental differences in clinical characteristics, management, and outcomes in patients hospitalized with worsening heart failure results from the EVEREST (Efficacy of Vasopressin Antagonism in Heart Failure: Outcome Study with Tolvaptan) program. J Am Coll Cardiol 2008; 52: 1640–1648. [DOI] [PubMed] [Google Scholar]
- 27. Mentz RJ, Cotter G, Cleland JGF, Stevens SR, Chiswell K, Davison BA, Teerlink JR, Metra M, Voors AA, Grinfeld L, Ruda M, Mareev V, Lotan C, Bloomfield DM, Fiuzat M, Givertz MM, Ponikowski P, Massie BM, O'Connor CM. International differences in clinical characteristics, management, and outcomes in acute heart failure patients: better short‐term outcomes in patients enrolled in Eastern Europe and Russia in the PROTECT trial. Eur J Heart Fail 2014; 16: 614–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jorde LB, Wooding SP. Genetic variation, classification and ‘race’. Nat Genet Nature Publishing Group 2004; 36: S28–S33. [DOI] [PubMed] [Google Scholar]
- 29. Ducros J, Larifla L, Merault H, Foucan L. NT‐proBNP, cardiometabolic risk factors, and nutritional status in hemodialysis patients. Int J Nephrol Hindawi 2017; 2017: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Choi D‐J, Han S, Jeon E‐S, Cho M‐C, Kim J‐J, Yoo B‐S, Shin M‐S, Seong I‐W, Ahn Y, Kang S‐M, Kim Y‐J, Kim HS, Chae SC, Oh B‐H, Lee M‐M, Ryu K‐H, Registry KHF. Characteristics, outcomes and predictors of long‐term mortality for patients hospitalized for acute heart failure: a report from the Korean heart failure registry. Korean Circ J 2011; 41: 363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Maeda K, Tsutamoto T, Wada A, Mabuchi N, Hayashi M, Tsutsui T, Ohnishi M, Sawaki M, Fujii M, Matsumoto T, Kinoshita M. High levels of plasma brain natriuretic peptide and interleukin‐6 after optimized treatment for heart failure are independent risk factors for morbidity and mortality in patients with congestive heart failure. J Am Coll Cardiol 2000; 36: 1587–1593. [DOI] [PubMed] [Google Scholar]
- 32. Yu CM, Sanderson JE. Plasma brain natriuretic peptide—an independent predictor of cardiovascular mortality in acute heart failure. Eur J Heart Fail 1999; 1: 59–65. [DOI] [PubMed] [Google Scholar]
- 33. Meta‐analysis Global Group in Chronic Heart Failure (MAGGIC) . The survival of patients with heart failure with preserved or reduced left ventricular ejection fraction: an individual patient data meta‐analysis. Eur Heart J 2012; 33: 1750–1757. [DOI] [PubMed] [Google Scholar]
- 34. Ter Maaten JM, Damman K, Verhaar MC, Paulus WJ, Duncker DJ, Cheng C, van Heerebeek L, Hillege HL, Lam CSP, Navis G, Voors AA. Connecting heart failure with preserved ejection fraction and renal dysfunction: the role of endothelial dysfunction and inflammation. Eur J Heart Fail 2016; 18: 588–598. [DOI] [PubMed] [Google Scholar]
- 35. Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 2013; 62: 263–271. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Difference with regard to all‐cause mortality and HF rehospitalization for Asian HF patients vs. Caucasian HF patients.
Table S2. Association of levels of NT‐proBNP with all‐cause mortality.
Figure S1. Flow chart study design.
Figure S2. Outcome in Caucasian vs Asian HF patients.
Figure S3. Association of quartiles of NT‐proBNP with the combined outcome for Asian patients with HFrEF (A) and HFpEF (B) as well as Caucasian patients with HFrEF (C) and HFpEF (D).
Figure S4. hazard ratios in subgroups of Asian HF patients for the primary outcome, p‐value for interaction for all is >0.05. Abbreviations: BMI, body mass index; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HT, hypertension.
Figure S5. hazard ratios for the primary outcome in subgroups of Caucasian HF patients, p‐value for interaction for all is >0.05. Abbreviations: BMI, body mass index; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HT, hypertension.


