Abstract
Background & Aims
Cirrhosis results from accumulation of myofibroblasts derived from quiescent hepatic stellate cells (Q-HSCs); it regresses when myofibroblastic HSCs are depleted. Hedgehog signaling promotes transdifferentiation of HSCs by activating Yes-associated protein 1 (YAP1 or YAP) and inducing aerobic glycolysis. However, increased aerobic glycolysis alone cannot meet the high metabolic demands of myofibroblastic HSCs. Determining the metabolic processes of these cells could lead to strategies to prevent progressive liver fibrosis, so we investigated whether glutaminolysis (conversion of glutamine to alpha-ketoglutarate) sustains energy metabolism and permits anabolism when Q-HSCs become myofibroblastic, and whether this is controlled by hedgehog signaling to YAP.
Methods
Primary HSCs were isolated from C57BL/6 or Smoflox/flox mice; we also performed studies with rat and human myofibroblastic HSCs. We measured changes of glutaminolytic genes during culture-induced primary HSC transdifferentiation. Glutaminolysis was disrupted in cells by glutamine deprivation or pathway inhibitors (BPTES, CB-839, EGCG and AOA), and effects on mitochondrial respiration, cell growth and migration, and fibrogenesis were measured. Hedgehog signaling to YAP was disrupted in cells by adenovirus expression of Cre-recombinase or by small hairpin RNA knockdown of YAP. Hedgehog and YAP activity were inhibited by incubation cells with cyclopamine or verteporfin, and effects on glutaminolysis were measured. Acute and chronic liver fibrosis were induced in mice by intraperitoneal injection of CCl4 or methionine choline-deficient diet. Some mice were then given injections of BPTES to inhibit glutaminolysis, and myofibroblast accumulation was measured. We also performed mRNA and immunohistochemical analyses of percutaneous liver biopsies from healthy human and 4 patients with no fibrosis, 6 patients with mild fibrosis, and 3 patients with severe fibrosis.
Results
Expression of genes that regulate glutaminolysis increased during transdifferentiation of primary Q-HSCs into myofibroblastic HSCs, and inhibition of glutaminolysis disrupted transdifferentitation. Blocking glutaminolysis in myofibroblastic HSCs suppressed mitochondrial respiration, cell growth and migration, and fibrogenesis; replenishing glutaminolysis metabolites to these cells restored these activities. Knockout of the hedgehog signaling intermediate SMO or knockdown of YAP inhibited expression of glutaminase, the rate limiting enzyme in glutaminolysis. Hedgehog and YAP inhibitors blocked glutaminolysis and suppressed myofibroblastic activities in HSCs. In livers of patients and of mice with acute or chronic fibrosis, glutaminolysis was induced in myofibroblastic HSCs. In mice with liver fibrosis, inhibition of glutaminase blocked accumulation of myofibroblasts and fibrosis progression.
Conclusions
Glutaminolysis controls accumulation of myofibroblast HSCs in mice and might be a therapeutic target for cirrhosis.
Keywords: Liver Diseases, Metabolic Reprogramming, Fibrogenesis, Hippo Pathway
Introduction
Liver fibrosis develops when myofibroblasts (MFs) excessively accumulate in the injured liver. Progressive liver fibrosis increases the risk for cirrhosis and liver-related morbidities and mortality.1 Quiescent hepatic stellate cells (Q-HSCs) are the dominant source of fibrogenic myofibroblasts in chronic liver diseases. HSCs are mesenchymal stem-like cells and thus, have inherent plasticity that permits their reprogramming in response to microenvironmental cues. Liver injury stimulates HSCs to transdifferentiate from a quiescent state to become proliferative, migratory and fibrogenic myofibroblasts.2 Transient accumulation of myofibroblastic HSCs is necessary for effective liver regeneration, but prolonged excessive accumulation of such cells causes progressive fibrosis, defective repair and ultimately, cirrhosis. Because the outcome of liver injury is dictated by factors that control HSC activation, the mechanisms that orchestrate HSC reprogramming are attractive therapeutic targets. In theory, reprogramming mechanisms might be manipulated to optimize repair of liver damage, providing novel strategies to prevent cirrhosis. Herein, we investigate the role of metabolism in HSC reprogramming and focus on glutaminolysis. We postulate that glutaminolysis is a particularly tractable mechanism for regulating HSC activation because glutaminolytic activity can fuel anapleurosis and thus, might enable HSC to meet the high bioenergetic and biosynthetic demands of the myofibroblastic phenotype.
Myofibroblastic HSCs are similar to highly proliferative cancer cells with regards to their bioenergetics and biosynthetic requirements. There is growing evidence that induction of glutaminolysis is a key component of the metabolic reprogramming that is necessary to fuel cancer cell growth.3 Briefly, it has long been known that one of the key metabolic hallmarks of cancer cells is the Warburg effect, i.e., cancer cells take up glucose at much higher rates than nonmalignant cells but oxidize glucose incompletely, even in the presence of oxygen, presumably so that the residual glucose-derived carbon molecules can be used to synthesize new biomass. However, newer data indicate that amino acids, rather than glucose, account for the majority of cell mass in proliferating mammalian cells.4–6 Glutamine is the most abundant amino acid in mammalian plasma, and increased glutamine metabolism (glutaminolysis) is another key metabolic characteristic of cancer cells.5 The glutaminolytic pathway enzymes, glutaminase (GLS), glutamate dehydrogenase (GDH), and transaminases convert glutamine to glutamate and glutamate to alpha-ketoglutarate (α-KG). Glutamine-derived α-KG can supplement α-KG that is regenerated during the TCA cycle to enhance TCA cycle activity, increasing both ATP production and key metabolic intermediates for the biosynthesis of nucleic acids, amino acids, and lipids. Indeed, cancer cells are glutamine addicted, and inhibition of glutaminolysis has been proposed recently as an attractive therapeutic option for cancers.5 However, the importance of glutaminolysis in HSC transdifferentiation and growth has not been elucidated.
On the other hand, the transdifferentiation of HSC into myofibroblastic HSC and maintenance of the myofibroblastic HSC phenotype are known to require activation of the hedgehog pathway. Hedgehog signaling plays a critical role in both embryonic tissue development and adult tissue maintenance and regeneration.7 It affects a variety of cellular functions including cell proliferation, migration and linage commitment.7 Hedgehog signaling is negligible in healthy adult liver in which most HSCs are quiescent. Liver injury induces accumulation of factors that activate the hedgehog pathway and this promotes transdifferentiation of Q-HSCs into myofibroblastic HSCs. While transient hedgehog pathway activation is required for effective liver regeneration, dysregulated excessive hedgehog signaling promotes liver pathology, including liver fibrosis and cancer.1 Like hedgehog, Yes-associated protein 1 (YAP) is a morphogenic signaling protein that is relatively inactive in healthy liver, but dramatically activated in HSCs during liver injury.8 The hedgehog pathway was recently shown to control the activity of YAP in HSCs, and blocking hedgehog pathway and YAP activation prevented Q-HSCs from transdifferentiating into myofibroblastic HSCs.9 Hedgehog signaling regulates aerobic glycolysis during adipocyte differentiation and HSC transdifferentiatiion.10, 11 Interestingly, glycolysis is necessary for YAP activation in some cells,12, 13 suggesting that morphogens may modulate metabolism to induce cell fate changes that are required for adult tissues to survive and recover from injury. Consistent with this concept, YAP-signaling dependent increases in glutaminolysis are necessary for vascular remodeling during pulmonary hypertension.14 YAP also reprograms hepatocyte glutamine metabolism to increase nucleotide biosynthesis and enable liver regeneration in zebrafish.15 High YAP activity is a characteristic of hepatocellular carcinoma (HCC) and targeted inhibition of glutaminase was recently shown to reduce HCC growth without apparent toxicity.16 Considering the critical role of glutaminolysis in fate-directive metabolic reprogramming, and the regulatory functions of hedgehog-YAP in metabolic reprogramming, we hypothesized that hedgehog-mediated YAP activation directs Q-HSCs transdifferentiation and myofibroblastic HSCs proliferation by stimulating glutaminolysis.
Methods and Materials
Detailed methods are available in Supplementary Material and Methods.
Cell culture studies
Primary HSCs were isolated from 12–16 week-old C57BL/6J mice or Smoflox/flox mice. Cells were cultured to induce activation for up to 7 days in Dulbecco’s Modified Eagle Medium containing 10% fetal bovine serum, and 1% penicillin-streptomycin (Life Technologies, Grand Island, NY). To genetically ablate hedgehog signaling, HSCs from Smoflox/flox mice were transduced with adenovirus harboring either GFP or Cre-recombinase on culture day 4. Primary human HSCs (obtained from Life Technology) and rat myofibroblastic HSC (8B cells from Marcos Rojkind, George Washington University, Washington, DC) 17 were maintained in DMEM medium supplemented with 10% fetal bovine serum and 1% penicillin streptomycin. To genetically ablate YAP signaling, 8B cells were treated with YAP (YAPΔ1, YAPΔ2) or non-targeting control shRNA lentiviruses as described.9 Hedgehog signaling and Yap activity were inhibited pharmacologically by treating cells with either cyclopamine or verteporfin. To test the role of glucose and glutamine, cells were cultured in complete medium (4500 mg/L glucose and 4 mM glutamine) with or without glutaminolytic inhibitors (e.g., CB-839, BPTES, EGCG or AOA), or in medium deprived of glucose and/or glutamine. Cell migration was monitored using scratch assays 18 and cell growth was monitored with Cell Counting Kit-8 (CCK8, Dojindo Molecular Technologies, Inc, Rockville, MD).
Animal studies
To acutely induce liver fibrosis, C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME) were injected intraperitoneally with corn oil or CCl4 (1200 mg/kg). To determine if inhibiting glutaminolysis altered MF accumulation, BPTES (12.5 mg/kg) or its vehicle (10% DMSO in PBS) was intraperitoneally administered at 6h and 30h post-CCl4. Mice were sacrificed 48h post-CCl4 treatment (n=4–6/group). To chronically induce liver fibrosis, C57BL/6 mice (Hyochang, Dae-gu, Korea) received 0.6 ml/kg CCl4 or corn oil by i.p. injection, twice a week for 10 weeks (n= 6).19 Liver tissues were harvested at 48 h after the last injection of CCl4 or corn oil. To induce dietary liver fibrosis, mice were fed methionine choline-deficient diet (MCD) (MP Biomedicals, #960439; n = 6) or chow diet (Picolab Rodent diet 20, #5053; n=6) for 8 weeks.20 Liver tissue was fixed in phosphate-buffered formalin for histology, or flash-frozen in liquid nitrogen and stored at −80 °C. All studies were approved by the Duke University (acute CCl4 model and MCD model) or Pusan National University (chronic CCl4 model) Institutional Animal Care and Use Committee as set forth in the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health.
Human Subjects
De-identified frozen tissues from percutaneous liver biopsies from healthy human and 13 NAFLD patients with histologically staged fibrosis (no fibrosis (F0) (n = 4), mild fibrosis (F1–2) (n = 6), severe fibrosis (F3–4) (n = 3)) were obtained from the Duke University School of Medicine Tissue Bank Shared Resource and used in accordance with NIH and institutional guidelines for human subject research.
Statistics
Data are expressed as mean ± SEM. Statistical significance between two groups was evaluated using student’s t test, while comparisons of multiple groups were assessed by one-way analysis of variance (ANOVA), followed by Student–Newman–Keul’s test. p ≤ 0.05 was considered to be statistically significant.
Results
Glutaminolysis is induced during the transdifferentiation of HSCs into myofibroblastic HSCs
Metabolic reprogramming, particularly increased conversion of the amino acid glutamine into α-KG (i.e., glutaminolysis), is a hallmark of neoplasia. α-KG is a critical intermediate of the TCA cycle and extra α-KG generated by glutaminolysis enhances TCA cycle activity. This promotes cancer cell proliferation and growth because increased TCA cycle activity enables ATP production while providing precursors for synthesis of new biomass.21, 22 Given that bioenergetic/biosynthetic demands increase dramatically when nonproliferative Q-HSC become proliferative and myofibroblastic, we postulated that HSC transdifferentiation must involve cancer-like metabolic reprogramming so that myofibroblastic HSC can satisfy these needs. Expression of genes involved in metabolism was profiled in mouse primary HSCs when they were freshly-isolated (i.e., quiescent) and after culture for up to 7 days under conditions that stimulate their transdifferentiation into myofibroblastic HSCs. Consistent with evidence that metabolism of amino acids (rather than glucose) accounts for the majority of cell mass in proliferating mammalian cells,4, 5 our microarray analysis revealed that the most pronounced changes in metabolic gene profiles during HSC transdifferentiation involve protein metabolism. More specifically, genome set enrichment analysis of quiescent and culture-activated primary mouse HSC showed that ~38% of the differentially expressed metabolic genes regulate protein metabolism while only ~6% are involved in carbohydrate metabolism (Supplementary Figure 1A). Similar results were found in human HSC (Supplementary Figure 1B).
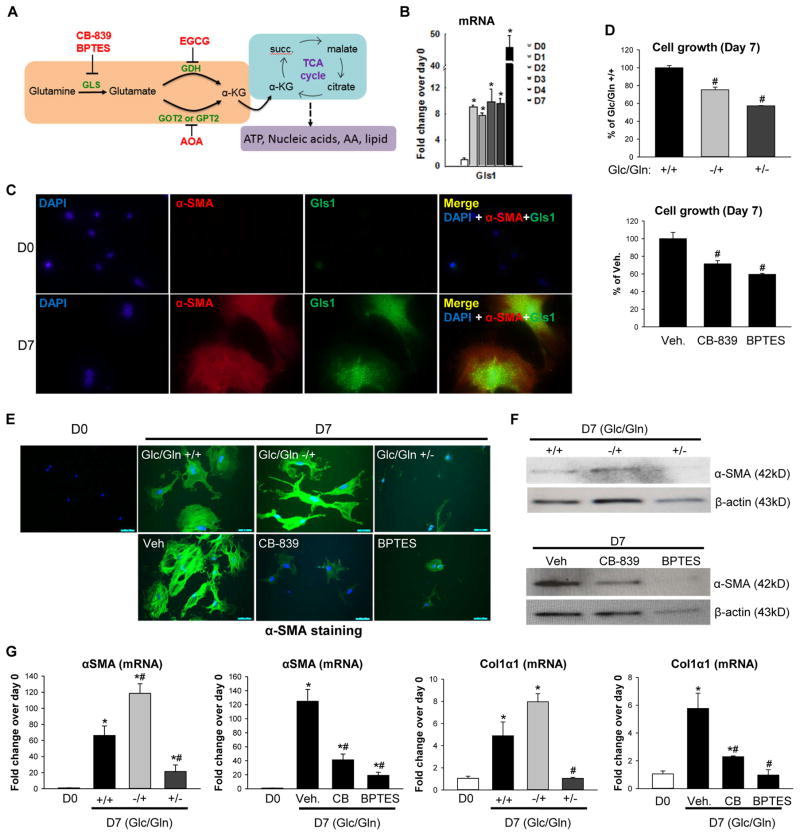
Glutamine is taken into cells by transporters (SNAT1, SNAT2, etc.). Glutaminase, the first enzyme in the catabolic pathway, converts glutamine to glutamate and comes in two distinct forms: the “kidney”-type Gls (here called Gls1 for clarity), and the “liver”-type Gls2. Gls1 is the rate limiting enzyme for glutamine utilization by cancer cells (Figure 2A). Our microarray analysis revealed that these transporters and Gls1 were up-regulated in myofibroblastic HSCs (Supplementary Figure 1C, p < 0.05 with a false discovery rate threshold of 5%). qRT-PCR analysis (Figure 1A) and immunocytochemistry (Figure 1B) confirmed that HSC transdifferentiation increased expression of Gls1 mRNA and protein together with the increased MF-HSC marker, αSMA. Bis-2-[5-phenylacetamido-1,2,4-thiadiazol-2-yl] ethyl sulfide (BPTES) is currently the best characterized Gls1 inhibitor. It allosterically inhibits the dimer-to-tetramer transition of Gls1 and displays an antiproliferative effect in numerous cancer cell lines.23 Its derivative CB-839, a highly potent and selective Gls1 inhibitor, is in several clinical trials.24 To determine if glutamine’s effects on HSC activation require glutamine catabolism, we deprived glucose and/or glutamine from the culture medium, and used pharmacologic inhibitors of Gls1. Glutaminolytic activity increased during HSC transdifferentiation, because removing glutamine from the culture medium on day 4 decreased subsequent cell growth. Indeed, glutamine deprivation had a more profound growth-inhibitory effect than glucose deprivation (Figure 1C). Furthermore, treatment of myofibroblastic HSCs with pharmacologic inhibitors of Gls1 (CB-839, BPTES) also suppressed HSC growth (Figure 1D). Interestingly, either deprivation of the glutamine from the culture medium or treatment with inhibitors of Gls1 blocked HSC from acquiring the MF phenotype, as indicated by a less myofibroblastic appearance (Supplementary Figure 2A) and suppression of MF marker αSMA (Figure 1E, F, G) and Col1α1 (Figure 1G). Because glutamine deprivation and BPTES had only minor effects on HSC viability (Supplementary Figure 2B, C), glutaminolysis inhibition reduces myofibroblastic HSC accumulation mainly by suppressing HSC activation, rather than by inducing HSC death.
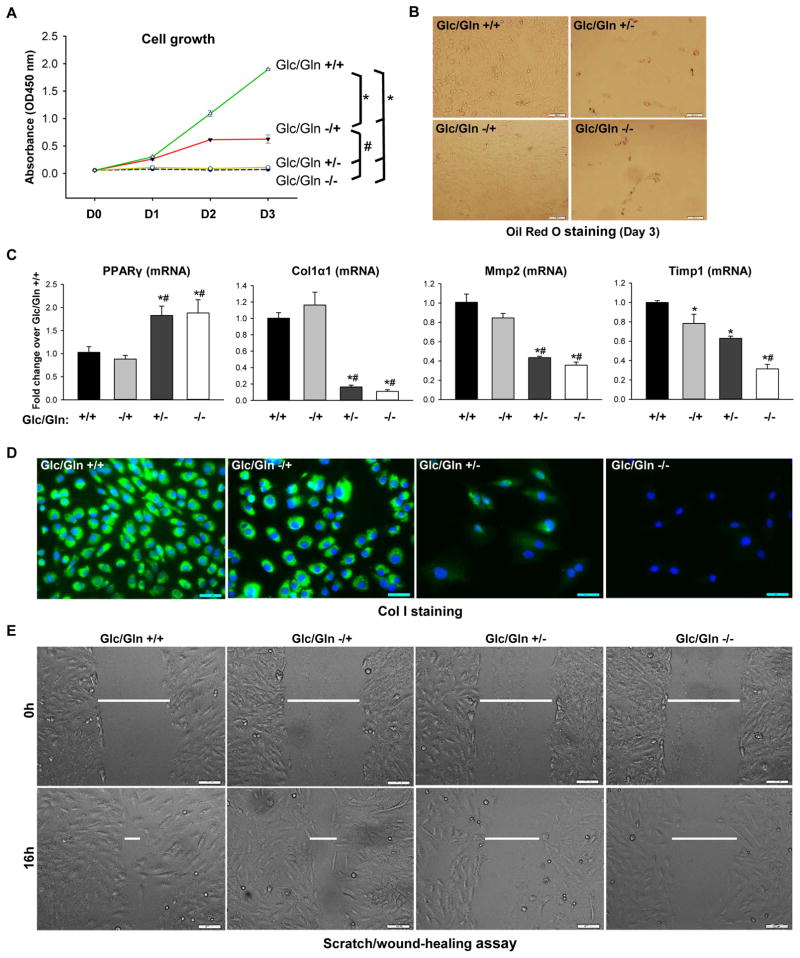
Figure 2. MF/HSCs are highly dependent on glutamine.
Rat myofibroblastic HSCs (8B cells) were grown in conditional medium Glc/Gln ±/± for 3 days. (A) Cell growth determined by CCK8 assay at 450nm. (B) Lipid content assessed by Oil Red O staining on day 3. (C) Gene expression quantified by RT-PCR on day 3. (D) Collagen I expression assessed by ICC on day 3. (E) Cell migration determined by scratch/wound-healing assay. Bars represent mean ± SEM of n = 4–5 assays. *p < 0.05 vs Glc/Gln +/+ group; #p < 0.05 vs Glc/Gln −/+ group.
Figure 1. Glutaminolysis is induced during primary HSC activation.
(A) Glutamine is converted into glutamate by glutaminase (Gls), and then into α-KG by GDH or transaminases (GOT2, GPT2) to fuel the TCA cycle and sustain ATP production and anabolism by supplying biosynthetic precursors for nucleic acids, amino acids (AA) and lipids. Glutaminolysis inhibitors include the Gls1 inhibitor CB-839 and BPTES, the GDH inhibitor EGCG, and the GOT2 and GPT2 inhibitor AOA. (B) Primary HSCs were isolated from adult mice (n=4 mice/experiment). A portion of the pooled isolate was harvested for Q-HSCs (Day 0; D0) and remaining cells were cultured for up to 7 days. At the indicated times, mRNA was analyzed using qRT-PCR. Results are expressed as mean ± SEM of 3–5 independent experiments. *p<0.05 vs D0. (C) Representative immunocytochemistry of α-SMA, Gls1 and DAPI staining. (D–G) Primary HSCs were cultured in complete medium containing both glucose (Glc) and glutamine (Gln) (Glc/Gln +/+) until day 4. In some cultures, medium was then replaced with Glc/Gln −/+ or Glc/Gln +/− medium, or supplemented with glutaminolysis inhibitors (CB-839, 0.3 μM or BPTES, 10 μM) or vehicle (0.1% DMSO). On day 7, cell growth was assessed by CCK8 assay and compared to Glc/Gln +/+ group or vehicle group (100%) (D), changes in α-SMA protein were assessed by ICC (E) and western blotting (F), and changes in mRNA expression were assessed by qRT-PCR (G). Bars represent mean ± SEM of n= 3–4 experiments. *p < 0.05 vs D0. #p < 0.05 vs Glc/Gln +/+ group or vehicle group on day 7.
Myofibroblastic HSCs are highly dependent on glutamine
To further assess the role of glutamine in regulating myofibroblastic HSCs, rat myofibroblastic HSCs (8B cells) were cultured in complete medium or medium with varying concentrations of glutamine/glucose. Glutamine deprivation completely inhibited myofibroblastic HSC growth (Figure 2A; Supplementary Figure 3A, C). In contrast, glucose deprivation merely slowed the growth of myofibroblastic HSCs in glutamine-containing medium (Figure 2A; Supplementary Figure 3B, C), demonstrating that myofibroblastic HSC growth is mainly dependent on glutamine. Interestingly, as observed in primary mouse HSCs, glutamine deprivation also caused fat accumulation (Figure 2B) and increased expression of PPARγ (Figure 2C), but decreased expression of Col1α1 in the rat myofibroblastic HSC line (Figures 2C, D). In contrast, neither fat accumulation/PPARγ expression (Figure 2B, C), nor expression of Col1α1 (Figure 2C, D), changed when glucose was removed from the medium, suggesting that HSC require glutamine rather than glucose to maintain the fibrogenic myofibroblastic phenotype. Since glutamine dependence has been linked to cancer cell invasiveness,25 we next sought to determine if glutamine controls the migratory capacity of myofibroblastic HSCs. Deprivation of glutamine (but not glucose) reduced migration of myofibroblastic HSCs (Figure 2E) and decreased expression of genes that encode the matrix remodeling enzymes Mmp2 and Timp-1 (Figure 2C). All these data suggest that the proliferative myofibroblastic phenotype of HSCs is critically dependent upon glutamine.
Inhibiting glutaminolysis in myofibroblastic HSC reduces their proliferative, myofibroblastic state
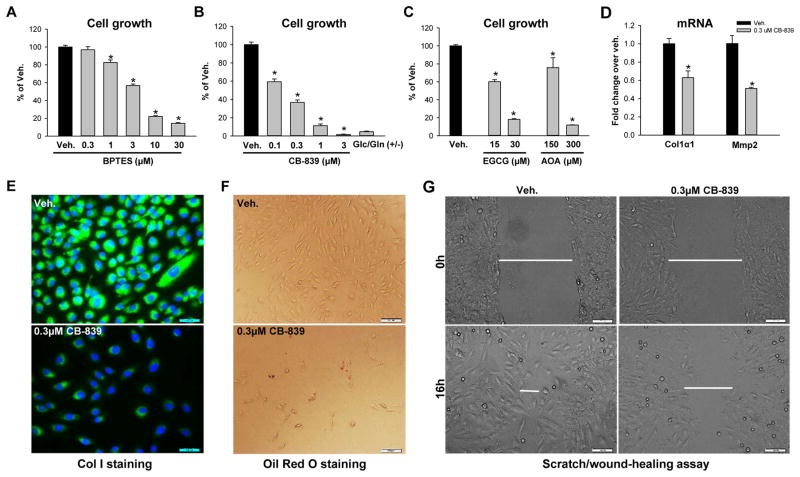
To determine if glutamine’s effects on myofibroblastic HSCs require glutamine catabolism, we treated myofibroblastic HSCs with pharmacologic inhibitors of enzymes in the glutaminolytic pathway (Figure 1A). BPTES dose-dependently inhibited the growth of myofibroblastic HSCs (Figure 3A; Supplementary Figure 3D). Its derivative CB-839, also strongly inhibited the growth of myofibroblastic HSCs in a dose-dependent manner (Figure 3B; Supplementary Figure 3D). Glutamate is converted into α-KG by glutamate dehydrogenase (GDH) or transaminases, such as glutamate oxaloacetate transaminases (GOTs, aspartate aminotransferase) and glutamate pyruvate transaminases (GPTs, alanine aminotransferases) (Figure 1A). Both the GDH inhibitor EGCG and the transaminase inhibitor AOA caused dose-dependent inhibition of myofibroblastic HSC growth (Figure 3C; Supplementary Figure 3D). The glutaminase inhibitor CB-839 also decreased Col1α1 (Figure 3D, E) and Mmp2 (Figure 3D) expression, caused lipid accumulation (Figure 3F), and impaired the migratory capacity of myofibroblastic HSCs (Figure 3G). As in primary mouse HSCs (Supplementary Figure 2B, C), in rat myofibroblastic HSCs the suppression of myofibroblastic characteristics caused by culture in glutamine-depleted medium or with Gls1 inhibitor was not associated with significant loss of cell viability (Supplementary Figure 3A). Thus, the aggregate data provide novel evidence that myofibroblastic HSCs require glutaminolysis to retain a proliferative myofibroblastic phenotype.
Figure 3. Inhibiting glutaminolysis suppresses myofibroblastic HSCs proliferative-myofibroblastic state.
(A–C) Rat myofibroblastic HSCs (8B cells) were grown in complete medium treated with glutaminolysis inhibitors or vehicle (0.1% DMSO) for 3 days. Cells growing in Glc/Gln +/− medium were used as positive controls. Cell growth was determined by CCK8 assay. (D) Gene expression was quantified by qRT-PCR. (E) Collagen I expression was assessed by ICC. (F) Lipid accumulation was assessed by Oil Red O staining. (G) Cell migration was assessed by scratch/wound-healing assay. Bars represent mean ± SEM of n = 4–5 assays. *p < 0.05 vs vehicle group (0.1% DMSO).
Glutaminolysis is critical for energy production and anabolism of myofibroblastic HSCs
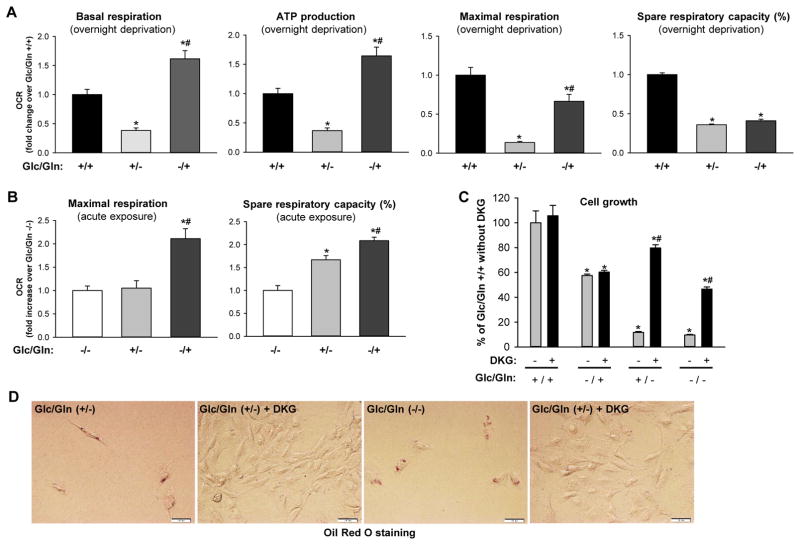
We hypothesized that glutaminolysis sustains the proliferative myofibroblastic phenotype of HSCs by replenishing the TCA cycle to assure that requirements for energy and anabolism are satisfied. To test this, we compared the mitochondrial respiratory capacity of myofibroblastic HSCs cultured overnight in glutamine- or glucose-deprived medium versus complete medium. Glutamine deprivation dramatically reduced basal respiration, ATP production, maximal respiration and spare respiratory capacity (Figure 4A). In contrast, glucose deprivation only moderately reduced maximal respiration and actually increased basal mitochondrial respiration and ATP content (Figure 4A). These data suggested that myofibroblastic HSCs are highly dependent on glutamine for energy metabolism. To assess the acute effects of glucose and glutamine on myofibroblastic HSCs, cells were deprived of both glucose and glutamine overnight and these substrates were then supplemented by acute injection during the measurement of oxygen consumption rate (OCR). While acute exposure of the cells to glucose did not affect maximal mitochondrial respiration, acute exposure to glutamine immediately promoted maximal respiration and increased mitochondrial respiratory capacity (Figure 4B), suggesting that glutamine is the preferred energy substrate of myofibroblastic HSCs in urgent need of nutrients. Since glutamate is converted into α-KG to replenish the TCA cycle for energy production and anabolism, we tested whether adding cell-permeable dimethyl α-ketoglutarate (DKG) to the medium could rescue the growth inhibition caused by glutamine deprivation. As predicted, DKG did not affect growth in glutamine containing medium (Glc/Gln +/+ or −/+) but respectively rescued 80% and 50% of the growth inhibition in glutamine free medium (Glc/Gln +/− and Glc/Gln −/−) (Figure 4C; Supplementary Figure 5). Interestingly, DKG also prevented fat accumulation in glutamine-deprived conditions (Figure 4D), suggesting that supplementing α-KG promoted recovery of a more myofibroblastic phenotype. Inhibiting glutaminolysis with Gls1 inhibitor CB-839 or GDH inhibitor EGCG also disrupted mitochondrial energy metabolism of myofibroblastic HSCs (Supplementary Figure 6A, B) and DKG was able to partially rescue this growth inhibition (Supplementary Figure 6C, E). Furthermore, DKG reversed the reduction of Col1α1 gene expression (Supplementary Figure 6D) and prevented the fat accumulation caused by glutaminolysis inhibitors (Supplementary Figure 6F).
Figure 4. Glutaminolysis sustains energy metabolism and anabolism of myofibroblastic HSCs.
(A) Rat myofibroblastic HSCs (8B cells) were cultured in Glc/Gln +/+ or +/− or −/+ medium overnight. Oxygen consumption rate (OCR) was measured using a Seahorse XFp Analyzer. Bars represent mean ± SEM of triplicate assays. *p < 0.05 vs Glc/Gln +/+ group, #p < 0.05 vs Glc/Gln +/− group. (B) To measure the acute response of cells to glucose or glutamine, cells were cultured in Glc/Gln −/− medium overnight. Glucose or glutamine was then added back acutely and OCR was measured. Bars represent mean ± SEM of triplicate assays. *p < 0.05 vs Glc/Gln −/− group, #p < 0.05 vs Glc/Gln +/− group. (C) Cell growth assessed by CCK8 assay after 3 days culture in various medium conditions ± 4 mM dimethyl α-ketoglutarate (DKG). Bars represent mean ± SEM of n=4–5 assays. *p < 0.05 vs Glc/Gln +/+ group, #p < 0.05 vs DKG (−) group. (D) Lipid accumulation assessed by Oil Red O staining of cells described in (C).
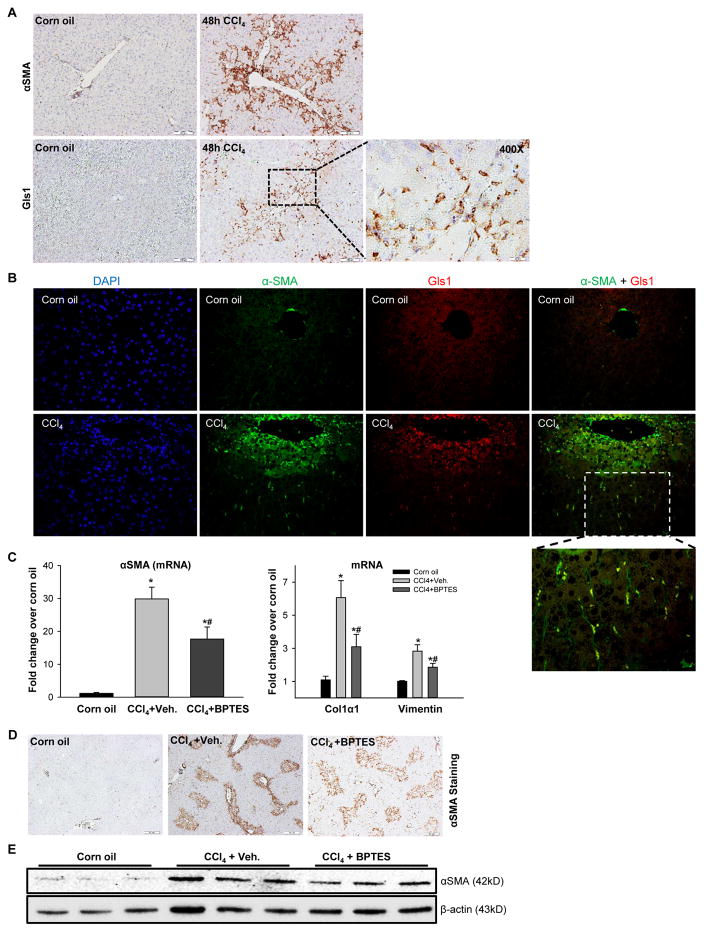
To determine if glutaminolysis increases when HSCs are stimulated to become myofibroblasts during liver repair in vivo, an animal model of liver injury that rapidly induces accumulation of fibrogenic myofibroblastic HSC was examined.10, 26 Mice were injected with a single dose of CCl4 and pilot studies were done to define the kinetics of MF accumulation following acute exposure to this hepatotoxin. Consistent with published literature, we found that CCl4 treatment kills zone 3 hepatocytes within 2 days and activates repair responses that completely replace dead hepatocytes by 7 days post-exposure.27, 28 In this model, zone 3 sinusoidal accumulation of cells expressing the myofibroblastic HSC marker, alpha smooth muscle (α-SMA), as well as expression of Gls1, peak during the period when hepatocyte death is maximal (i.e., at 2 days post-CCl4 injection) (Figure 5A; Supplementary Figure 7A, B). Notably, co-immunoflorescence microscopy revealed co-localization of these markers, indicating that activated HSCs upregulate Gls1 (Figure 5B). Interestingly, we also observed that while expression of glutamine transporter Slc38a1 and both the KGA and GAC isoforms of Gls1 were induced, liver-type Gls2 was downregulated in injured liver (Supplementary Figure 7A, B). Differential regulation of Gls1 and 2 was also reported recently in fibrotic liver and HCC,16, 29 suggesting that there might be a metabolic switch from Gls2 to Gls1 during liver damage. Therefore, we investigated the effects of the specific Gls1 inhibitor BPTES on this early myofibroblastic response in the CCl4 acute liver injury mouse model. BPTES treatment did not affect liver injury as assessed by serum AST and liver histology (Supplementary Figure 7C, D), but significantly inhibited the early fibrogenic response induced by CCl4 treatment, as shown by ~50% decrease in α-SMA gene expression at 48 h post-CCl4 injection (Figure 5C). The reduction in α-SMA mRNA was paralleled by a corresponding decrease in protein expression as assessed by immunohistostaining (Figure 5D; Supplementary Figure 7E) and western blot (Figure 5E). BPTES also suppressed typical injury-related induction of myofibroblastic associated genes that encode Col1α1 and Vimentin (Figure 5C), and reduced hepatic hydroxyproline accumulation (Supplementary Figure 7F). These data confirm that induction of glutaminolysis is important for myofibroblastic HSCs to accumulate in injured livers and validate the utility of the in vitro systems for interrogating the mechanisms that regulate this metabolic reprogramming.
Figure 5. Glutaminolysis activates myofibroblastic HSCs in acute liver injury.
Adult mice were injected intraperitoneally with corn oil vehicle or CCl4 (1200 mg/kg), and BPTES or its vehicle were injected at 6h and 30h post-CCl4 injection. (A) Representative Gls1 and α-SMA IHC stained liver sections. (B) Representative Gls1 and α-SMA co-immunofluorescence stained liver sections. (C) mRNA levels of α-SMA, Col1α1 and Vimentin determined by qRT-PCR. (D) Representative α-SMA stained liver sections. (E) Western blot for α-SMA using β-actin as the loading control. Bars represent mean ± SEM of n=4–6 mice/group. *p < 0.05 vs corn oil, #p < 0.05 vs CCl4+vehicle group.
Glutaminolysis is induced in chronically-injured fibrotic livers in both mouse and human
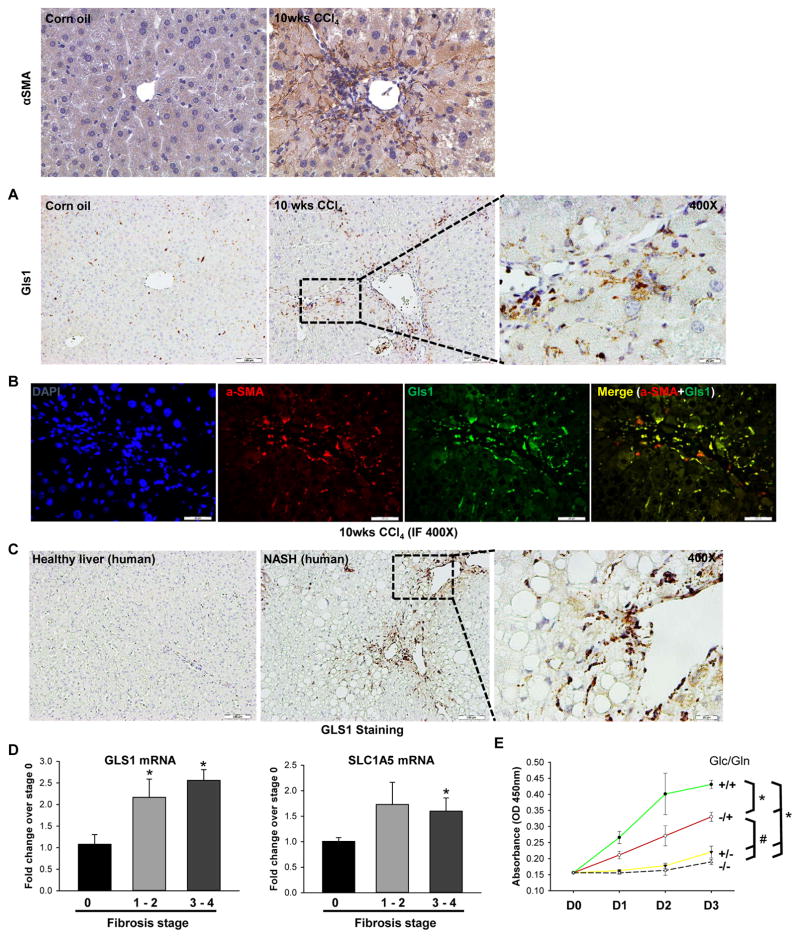
To determine if outcomes in the acute CCl4 model also occur in chronically fibrotic liver, we compared expression of Gls1 in mice injected intraperitoneally with CCl4 or vehicle (corn oil) twice per week for 10 weeks. Chronic CCl4 treatment caused massive liver fibrosis,19 as indicated by the substantial accumulation of αSMA-positive HSCs around zone 3 hepatocytes (Figure 6A). Importantly, Gls1 expression was also remarkably upregulated (Figure 6A), and coimmunofluorescence microscopy revealed that αSMA and Gls1 strictly co-localized in these areas, indicating that the activated HSCs upregulate Gls1 (Figure 6B). Consistent with this, in the methionine choline-deficient (MCD) diet-induced liver fibrosis murine model,20 Gls1 expression was also significantly higher than in the chow-fed control group (Supplementary Figure 8). Interestingly, we also observed that Gls2 (the isoform down-regulated in proliferative cancer cells) decreased in the dietary fibrotic liver (Supplementary Figure 8).
Figure 6. Glutaminolysis is induced in chronically-injured fibrotic livers.
(A–B) Adult mice were injected intraperitoneally with corn oil or CCl4 (0.6 ml/kg) twice per week for 10 weeks; livers were harvested at 48h post last injection. Representative α-SMA and Gls1 IHC stained liver sections (A). Representative α-SMA and Gls1 co-immunofluorescence (B). (C–D) GLS1 IHC stained liver sections (C) and GLS1 and SLC1A5 mRNA expression (D) from healthy liver and NAFLD livers with different stages of liver fibrosis. Bars represent mean ± SEM of n=3–6 patients/group. *p < 0.05 vs fibrosis stage 0. (E) Human HSCs were grown in conditional Glc/Gln ±/± medium for 3 days. Cell growth was determined by CCK8 assay. Bars represent mean ± SEM of n = 4 assays. *p < 0.05 vs Glc/Gln +/+ group; #p < 0.05 vs Glc/Gln −/+ group.
To determine if findings in mouse models reflected responses in fibrotic human livers, we re-examined our transcriptomic data derived from microarray analysis of over 70 NASH patients with varying degrees of liver fibrosis,30 and our data revealed that GLS1 expression was 1.6 fold higher in the group of patients with advanced (F3–4) liver fibrosis (n = 32) than in the group with mild (F0–1) liver fibrosis (n = 40), and this difference remained statistically significant even after correcting for multiple comparisons. In addition, we compared expression of GLS1 in liver biopsies from healthy human liver and patients with histologically-staged fibrosis. Compared to healthy liver, fibrotic livers showed accumulation of GLS1-positive HSCs (Figure 6C), with highest expression of GLS1 and glutamine transporter SLC1A5 occurring in severely fibrotic livers (Figure 6D). Furthermore, we evaluated the growth of human HSCs cultured in complete medium or medium deprived of glutamine and/or glucose for up to 3 days. As noted in the in vivo and in vitro murine HSC systems, human HSCs were also highly dependent on glutamine for their growth (Figure 6E). The aggregate data indicate that glutaminolysis is a conserved driver of myofibroblastic HSC accumulation during liver fibrosis.
Hedgehog-YAP signaling regulates glutaminolysis to direct metabolic reprogramming and transdifferentiation of HSCs
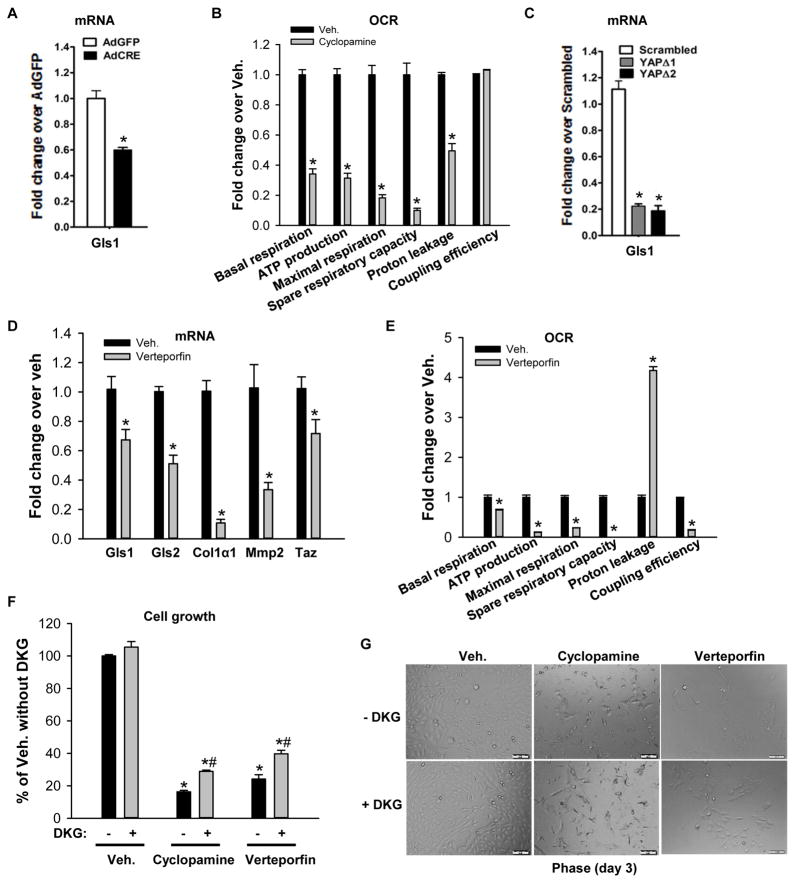
Previously, we reported that hedgehog signaling directs a global reprogramming of HSC gene expression to activate metabolic processes that promote glycolysis and lactate accumulation, while inhibiting lipogenesis and gluconeogenesis.10 During this process, mitochondrial number also increases dramatically,10 suggesting an urgent demand for mitochondrial processes that promote energy production and anabolism. Metabolic reprogramming activates glutaminolysis to fuel the TCA cycle in mitochondria of rapidly proliferating cancer cells.31 Therefore, we hypothesized that hedgehog-regulated signaling networks also induce glutaminolysis to satisfy the increased demands for energy and anabolic substrates when HSC transdifferentiate to become myofibroblastic. To examine this, we treated 4-day cultured HSCs from Smoflox/flox mice with Cre recombinase to conditionally delete the hedgehog signaling intermediate Smo, inhibit hedgehog signaling, and suppress the myofibroblastic HSCs phenotype.9, 32 Conditional deletion of Smo in myofibroblastic HSCs significantly reduced mRNA of glutaminolytic enzyme Gls1 (Figure 7A), indicating that hedgehog signaling is involved in directing the increase in glutaminolysis in myofibroblastic HSCs. In support of this, the hedgehog pathway inhibitor cyclopamine also dramatically decreased mitochondrial respiration (Figure 6B) and cell growth of myofibroblastic HSCs (Figure 7F, G). Since YAP is a downstream effector of the hedgehog pathway during liver repair,9 we next assessed whether YAP is a mediator of hedgehog-directed changes in glutaminolytic enzymes during HSC transdifferentiation. To accomplish this, rat myofibroblastic HSCs were treated with short hairpin RNA (shRNA) lentiviral constructs to knock down YAP expression.9 Reducing YAP suppressed Gls1 gene expression in myofibroblastic HSCs, suggesting that hedgehog activates YAP to regulate Gls1 expression in myofibroblastic HSCs (Figure 7C). In support of this, treating myofibroblastic HSCs with the Yap inhibitor verteporfin also decreased gene expression of Gls (both kidney type Gls1 and liver type Gls 2), Col1α1, and MMP2 (Figure 7D). Interestingly, YAP-inhibitor treated cells demonstrated a dramatic decrease in mitochondrial respiration (Figure 7E) and cell growth (Figure 7F, G) compared to vehicle treated cells. Supplementing the culture medium with the cell permeable analog of α-KG (DKG) partially rescued cells from growth inhibition (Figure 7F, G). Together, the aggregate data demonstrate that hedgehog-YAP signaling controls HSC transdifferentiation at least in part by regulating glutaminolysis, providing a mechanism to explain why inhibiting glutaminolysis acutely blocked myofibroblastic HSC accumulation during liver injury (Figure 5), similar to genetic approaches that directly disrupt hedgehog-YAP signaling.9, 18 YAP collaborates with a related transcriptional regulator, TAZ, to regulate expression of genes with TEAD binding sites. Sequence analysis predicts the presence of TEAD binding sites in the promotor regions of Gls1 and this has been confirmed by ChIP-qPCR assay14. Thus, evidence that verteporfin lowers TAZ expression in myofibroblastic HSCs (Figure 7D) suggests that YAP may work with TAZ through TEAD binding sites to regulate Gls1 expression in HSCs.
Figure 7. Hedgehog-YAP signaling regulates glutaminolysis in MF/HSCs.
(A) Primary HSCs isolated from Smoflox/flox mice were transduced with adenovirus harboring either GFP or Cre recombinase on culture day 4. Total RNA was harvested on day 7 and analyzed for Gls1 mRNAs by qRT-PCR. (B, E) Rat myofibroblastic HSCs (8B cells) were treated with cyclopamine or verteporfin or vehicle (0.1% DMSO) overnight. OCR was measured using a Seahorse XFp Analyzer. (C) Rat myofibroblastic HSCs were treated with YAP (YAPΔ1, YAPΔ2) or non-targeting control shRNA lentiviruses and analyzed for Gls1 mRNAs by qRT-PCR. (D) Rat myofibroblastic HSCs were treated with verteporfin or its vehicle (0.1% DMSO) for 72h. Gene expression was quantified by qRT-PCR. (F) Cell growth was assessed by CCK8 assay after 3 days culture in cyclopamine- or verteporfin- or vehicle-treated medium ± 4 mM dimethyl α-ketoglutarate (DKG). (E) Representative phase contrast images to illustrate growth differences ± DKG. Bars represent mean ± SEM of n = 4–5 assays. *p < 0.05 vs the GFP (A), scrambled (C), vehicle (0.1% DMSO) control (B, D, E, F). #p < 0.05 vs DKG (−) group (F).
Discussion
Treatments to prevent and reverse cirrhosis in individuals with ongoing liver injury are needed to reduce liver disease-related morbidity and mortality, but efforts to develop such agents have proven to be unsuccessful. Myofibroblasts derived from liver-resident hepatic stellate cells (myofibroblastic HSCs) are the major producers of the fibrous matrix that accumulates as cirrhosis evolves and thus, limiting accumulation of myofibroblastic HSCs in injured livers should prevent cirrhosis. Theoretically, this could be accomplished by inhibiting injury-related mechanisms that stimulate the myofibroblastic transdifferentiation of HSC that are quiescent in healthy liver, blocking the proliferation of myofibroblastic HSCs, promoting the reversion of myofibroblastic HSCs back to a more quiescent phenotype, or killing myofibroblastic HSCs. Large numbers of quiescent HSCs reside in healthy livers suggesting that these cells have important homeostatic roles. During liver injury, HSC-derived myofibroblasts orchestrate matrix remodeling and several other wound healing responses that are necessary for the liver to regenerate effectively. Thus, the pools of Q-HSC and myofibroblastic HSC vary reciprocally during injury: the population of Q-HSCs shrinks initially and then recovers, while the myofibroblastic HSC population initially expands and then dissipates as injured livers regenerate. The mechanisms that naturally orchestrate these changes in HSC fate are poorly understood but would seem to be ideal therapeutic targets to limit accumulation of myofibroblastic HSCs and prevent excessively fibrogenic repair.
Metabolic pathways that enable HSCs to satisfy the increased bioenergetic and biosynthetic demands of the myofibroblastic phenotype are attractive therapeutic candidates for cirrhosis prevention. Inhibiting such pathways should have little impact on quiescent HSC viability but would be expected to restrict myofibroblastic transdifferentiation and limit the proliferative activity of myofibroblastic HSCs that have already emerged. Further, the dose and duration of exposure to agents that inhibit the metabolic reprogramming required to fuel highly proliferative myofibroblastic cells could be titrated and “personalized” to optimize wound healing responses during liver injury with little adverse consequence to healthy adult tissues. Identifying the relevant metabolic targets is essential for the success of this approach.
Our studies show that, like highly proliferative cancer cells, myofibroblastic HSC are highly dependent on glutamine in vitro. Further, we demonstrate that glutamine is not merely required for myofibroblastic HSC growth, but necessary for HSC to acquire and maintain a myofibroblastic phenotype. Our work proves that HSCs must catabolize glutamine to achieve these effects, and identifies α-KG, the end-product of glutaminolysis, as the ultimate effector. Our data support the concept that glutamine-derived α-KG helps to satisfy the high bioenergetic and biosynthetic demands of myofibroblastic HSCs by enhancing the activity of the TCA cycle, a key source of substrates that are converted to biomass and ATP in highly proliferative cells. In addition, we discovered that hedgehog signaling and activated Yap are key endogenous regulators of glutaminolytic activity in HSCs. This finding is important because earlier research proved that these nascent pleiotropic mediators of morphogenesis are rapidly co-activated by injury-related microenvironmental factors, and showed that they interact to promote efficient myofibroblastic transdifferentiation of HSCs both during liver injury and during culture.9 Evidence that hedgehog signaling and Yap collaborate to orchestrate tissue repair is not surprising because effects of the hedgehog pathway and activated Yap somewhat overlap. Both not only direct cell proliferation, differentiation, and viability during tissue growth, but seem to be master regulators of cellular metabolism. For example, activation of the hedgehog pathway has been shown to trigger glucose uptake and aerobic glycolysis in multiple organs, including liver,33 and Yap promotes liver growth at least in part by stimulating glutaminolysis.15 The hedgehog pathway controls Yap activity during liver regeneration9 and as mentioned earlier, transient expansion of myofibroblastic HSC populations is necessary for injured livers to regenerate. Bertero et al recently reported that YAP/TAZ regulates glutaminolysis to drive pulmonary hypertension through a mechanoactive feedback loop that augments fibrosis.14, 34 Due to the increasing recognition of the critical role of mechanical factors in liver fibrosis,35, 36 it will be intriguing to determine whether such a mechanoactive feedback loop can also exist via the Hedgehog-YAP axis in stellate cells. Our new results show that accumulation of the glutaminolytic end-product, α-KG, is necessary for HSCs to become and remain proliferative myofibroblasts. This suggests that limiting α-KG production might be a novel “targeted” strategy to safely block one key downstream consequence of hedgehog-Yap signaling. Our studies in a mouse model of acute toxin-mediated liver injury support the efficacy of this approach by demonstrating that pharmacologic inhibition of glutaminolysis is sufficient to abort the accumulation of myofibroblastic HSCs that immediately follows liver injury. In this model, reducing MF accumulation safely attenuated the fibrogenic response to liver injury and acutely reduced collagen accumulation. Because the systemically-administered pharmacologic inhibitor was not designed to target any specific cell type, we emphasize that we cannot exclude the possibility that cells other than stellate cells might also have been impacted by this approach. HSC glutaminolysis is also increased in chronically fibrotic murine and human livers, and HSC growth is highly glutamine-dependent in both species. Intriguingly, during the reviewing process of our study, another research group also reported similar findings demonstrating that HSCs are highly dependent on glutamine metabolism.37 Therefore, further research to validate the utility of glutaminolysis inhibitors in models of chronic liver injury and fibrosis is justified.
Future studies might also investigate the relative merits of combining inhibitors of glutaminolysis (a process that is strongly regulated by Yap) and inhibitors of glycolysis (a particular target of the hedgehog pathway) as an anti-fibrotic strategy. Previously, we reported that HSC transdifferentiation depends upon glucose uptake and induction of aerobic glycolysis (the Warburg effect).10 Glutamine-dependent anapleurosis dictates glucose uptake and utilization in transformed cells,38 suggesting that both glycolysis and glutaminolysis may be necessary to fulfill inherent metabolic requirements of the myofibroblastic state. Indeed, our present studies reveal that both glucose and glutamine are necessary for the optimal growth of HCS in vitro. Aerobic glycolysis and glutaminolysis are probably coordinately regulated because both processes are necessary to safely satisfy the bioenergetic demands of highly proliferative cells.39, 40 For example, glycolytic metabolism of glucose to generate lactate promotes acidification of the cellular environment that could threaten cell survival. Ammonia, a by-product of glutaminolysis, neutralizes glycolysis-associated acidification and thus maintains cellular acid-base homeostasis.41 Based on this information, restricting both metabolic adaptations might be the most physiologic (and safest) approach to limit accumulation of myofibroblastic HSCs during liver injury.
In summary, the results of the present study complement and extend other evidence that effective liver repair demands metabolic adaptations in resident liver cells that are involved in wound healing responses. Injury-induced metabolic reprogramming is essential but dynamic in order to assure transient accumulation of cell types that are relatively unabundant in healthy liver, such as myofibroblastic HSCs. Since the accumulation of myofibroblastic HSCs demands maintenance of their metabolically-adapted state, metabolically-disruptive interventions are expected to reduce myofibroblastic HSC accumulation and thus, improve associated liver fibrosis. This concept is supported by work in cultured HSC and research in a mouse model wherein myofibroblastic HSC rapidly accumulate after acute liver injury, justifying further research to explore the safety and efficacy of this novel anti-fibrotic strategy in models of chronic liver injury.
Supplementary Material
Primer used for qRT-PCR
Q-HSCs were freshly isolated from mouse or human tissue; some of the isolates were cultured to induce transdifferentiation into myofibroblastic HSCs; Q-HSC and myofibroblastic HSC RNA was isolated and subjected to microarray analysis as described.1 Gene ontogeny (GO) analysis was performed with the Gene Annotation Tool (http://gather.genome.duke.edu). Charts show distribution of the differentially-expressed genes according to GO pathway descriptors for either mouse HSCs (A) or human HSCs (B) in metabolic process. Fold-change in glutamine metabolism genes in mouse myofibroblastic HSCs versus Q-HSCs (C).
Primary HSCs were cultured in complete medium (Glc/Gln +/+) until day 4. In some cultures, medium was then replaced with glucose-(Glc/Gln −/+) or glutamine-free (Glc/Gln +/−) medium; in others complete medium was supplemented with glutaminolysis inhibitors (CB-839, 0.3 μM or BPTES, 10 μM) or vehicle (0.1% DMSO). (A) Representative phase pictures day 0 and 7. (B) Representative trypan blue exclusion staining for cytotoxicity at day 7. Arrows indicate typical nonviable cells stained blue. (C) Cell viability quantified by trypan blue staining and presented as percentage of living cells compared to total cells. Bars represent mean ± SEM of n = 4 assays. *p < 0.05 vs Glc/Gln +/+ group.
Rat myofibroblastic HSCs (8B cells) were grown in medium containing various concentrations of glucose (Glc) and/or glutamine (Gln) for 3 days, or in complete medium treated with glutaminolysis inhibitors (CB-839; BPTES; EGCG; AOA) or vehicle (0.1% DMSO) for 3 days. (A, B) Cell growth determined by CCK8 assay at 450nm (OD450nm) according to manufacturer’s instructions. (C, D) Representative phase contrast images to illustrate growth differences under each condition on day 3. Bars represent mean ± SEM of n = 4–5 assays.
Myofibroblastic HSCs were cultured for 3 days in complete medium (Glc/Gln +/+) or medium deprived either glucose (Glc/Gln −/+) or glutamine (Glc/Gln +/−); in other cultures, complete medium was supplemented with glutaminolysis inhibitors (CB-839, 0.3 μM or BPTES, 10 μM) or vehicle (0.1% DMSO). (A) Representative pictures of phase, PI staining and trypan blue exclusion staining for cytotoxicity detection at day 3. Arrows indicated typical nonviable cells stained blue. (B) Cell viability assessed by PI staining and trypan blue staining and presented as percentage of living cells compared to total cells. Bars represent mean ± SEM of n = 4 assays. *p < 0.05 vs Glc/Gln +/+ group.
Rat myofibroblastic HSCs (8B cells) were cultured in complete medium (Glc/Gln +/+) or medium depleted of glucose (Glc/Gln −/+) or glutamine (Glc/Gln +/−) or both (Glc/Gln −/−) without (−) or with (+) addition of 4 mM dimethyl α-ketoglutarate (DKG). Representative phase contrast images were acquired to demonstrate growth differences in the presence or absence of DKG.
(A, B)Myofibroblastic HSCs were cultured overnight in complete medium with glutaminolysis inhibitors (CB-839 or EGCG). Oxygen consumption rate (OCR) was measured with a Seahorse XFp Analyzer to determine basal respiration, ATP production, maximal respiration, spare respiratory capacity and proton leakage. Bars represent mean ± SEM of triplicate assays. *p < 0.05 vs vehicle (0.1% DMSO) group. (C) Cell growth determined by CCK8 assay in cultures treated with vehicle or glutaminolysis inhibitors (CB-839, EGCG or AOA) with (+) or without (−) addition of dimethyl α-ketoglutarate (DKG) for 3 days. Data expressed as percentage of the vehicle group (100%). (D) Col1α1 gene expression quantified by RT-PCR. Bars represent mean ± SEM of n = 4–5 assays. *p < 0.05 vs Glc/Gln +/+ group, #p < 0.05 vs DKG (−) group. (E) Representative phase contrast images were acquired to demonstrate growth differences in the presence or absence of DKG. (F) Lipid accumulation assessed by Oil Red O staining.
Adult mice were injected intraperitoneally with corn oil or CCl4 (1200 mg/kg). At 6h and 30h post-CCl4 injection, mice were intraperitoneally injected with Gls1 inhibitor BPTES or its vehicle (10% DMSO in PBS) and liver tissues were harvested at 48h post-CCl4. (A) Liver mRNA levels quantified by qRT-PCR. (B) Protein expression of both isoforms of Gls1 by western blotting. (C) Serum aspartate aminotransferase (AST) activity. (D) Representative H&E-stained liver sections with injured areas outlined. Injured areas were identified by lack of nuclear staining or pyknotic nuclei and lines were marked along the boundaries of injured areas of parenchyma. (E) Morphometric analysis of αSMA expression in the respective groups. (F) Quantification of hepatic hydroxyproline content in the respective groups. Bars represent mean ± SEM of n=4 mice/group. *p < 0.05 vs corn oil, #p < 0.05 vs CCl4+vehicle group.
C57Bl/6 wild-type male mice were fed either chow diet (n=6) or MCD diet (n=6) for 8 weeks. Gls1 and Gls2 expression in total liver were quantified by qRT-PCR. *p < 0.05 vs chow diet fed group.
Acknowledgments
Grant support: This work was supported by National Institutes of Health grants R37 AA010154, R01 DK077794, R56 DK106633 awarded to Anna Mae Diehl.
We thank our former colleague Dr. Yuping Chen for her contributions in Figure 1B and Figure 7A, C.
Abbreviations used in this paper
- AOA
aminooxyacetic acid
- α-KG
alpha-ketoglutarate
- α-SMA
alpha-smooth muscle actin
- BPTES
bis-2-[5-phenylacetamido-1,2,4-thiadiazol-2-yl] ethyl sulfide
- DKG
dimethyl α-ketoglutarate
- EGCG
epigallocatechin gallate
- GDH
glutamate dehydrogenase
- GLS
glutaminase
- GOTs
aspartate aminotransferase
- GPTs
alanine aminotransferases
- HCC
hepatocellular carcinoma
- HSC
hepatic stellate cell
- ICC
immunocytochemistry
- IF
immunofluorescence
- IHC
immunohistochemistry
- MF
myofibroblast
- OCR
oxygen consumption rate
- PI
propidium iodide
- Q-HSC
quiescent hepatic stellate cell
- qRT-PCR
quantitative reverse-transcription polymerase chain reaction
- SMO
smoothened
- TCA
tricarboxylic acid
- YAP
Yes-associated protein
Footnotes
Disclosures: The authors declare no conflicts of interest.
Author Contributions: K.D. and A.M.D. conceived of the experiments. K.D., J.H., R.T.P., S.C., G.A.M. and M.S.S. performed experiments. K.D., J.H., S.C., G.D.D. and A.M.D. analyzed data. Y.J. provided samples. E.T. and B.S.R. provided technical support. K.D., G.D.D., R.T.P. and A.M.D. wrote and edited the manuscript. A.M.D. provided funding.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Authors names in bold designate shared co-first authorship.
- 1.Angulo P, Machado MV, Diehl AM. Fibrosis in nonalcoholic Fatty liver disease: mechanisms and clinical implications. Semin Liver Dis. 2015;35:132–45. doi: 10.1055/s-0035-1550065. [DOI] [PubMed] [Google Scholar]
- 2.Iwaisako K, Brenner DA, Kisseleva T. What’s new in liver fibrosis? The origin of myofibroblasts in liver fibrosis. J Gastroenterol Hepatol. 2012;27(Suppl 2):65–8. doi: 10.1111/j.1440-1746.2011.07002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 4.Le A, Lane AN, Hamaker M, et al. Glucose-independent glutamine metabolism via TCA cycling for proliferation and survival in B cells. Cell Metab. 2012;15:110–21. doi: 10.1016/j.cmet.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu W, Pelicano H, Huang P. Cancer metabolism: is glutamine sweeter than glucose? Cancer Cell. 2010;18:199–200. doi: 10.1016/j.ccr.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hosios AM, Hecht VC, Danai LV, et al. Amino Acids Rather than Glucose Account for the Majority of Cell Mass in Proliferating Mammalian Cells. Dev Cell. 2016;36:540–9. doi: 10.1016/j.devcel.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ingham PW, Nakano Y, Seger C. Mechanisms and functions of Hedgehog signalling across the metazoa. Nat Rev Genet. 2011;12:393–406. doi: 10.1038/nrg2984. [DOI] [PubMed] [Google Scholar]
- 8.Machado MV, Diehl AM. Liver renewal: detecting misrepair and optimizing regeneration. Mayo Clin Proc. 2014;89:120–30. doi: 10.1016/j.mayocp.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 9.Swiderska-Syn M, Xie G, Michelotti GA, et al. Hedgehog regulates yes-associated protein 1 in regenerating mouse liver. Hepatology. 2016;64:232–44. doi: 10.1002/hep.28542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Y, Choi SS, Michelotti GA, et al. Hedgehog controls hepatic stellate cell fate by regulating metabolism. Gastroenterology. 2012;143:1319–29. e1–11. doi: 10.1053/j.gastro.2012.07.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teperino R, Amann S, Bayer M, et al. Hedgehog partial agonism drives Warburg-like metabolism in muscle and brown fat. Cell. 2012;151:414–26. doi: 10.1016/j.cell.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 12.Enzo E, Santinon G, Pocaterra A, et al. Aerobic glycolysis tunes YAP/TAZ transcriptional activity. EMBO J. 2015;34:1349–70. doi: 10.15252/embj.201490379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang W, Xiao ZD, Li X, et al. AMPK modulates Hippo pathway activity to regulate energy homeostasis. Nat Cell Biol. 2015;17:490–9. doi: 10.1038/ncb3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bertero T, Oldham WM, Cottrill KA, et al. Vascular stiffness mechanoactivates YAP/TAZ-dependent glutaminolysis to drive pulmonary hypertension. J Clin Invest. 2016;126:3313–35. doi: 10.1172/JCI86387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cox AG, Hwang KL, Brown KK, et al. Yap reprograms glutamine metabolism to increase nucleotide biosynthesis and enable liver growth. Nat Cell Biol. 2016;18:886–896. doi: 10.1038/ncb3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiang Y, Stine ZE, Xia J, et al. Targeted inhibition of tumor-specific glutaminase diminishes cell-autonomous tumorigenesis. J Clin Invest. 2015;125:2293–306. doi: 10.1172/JCI75836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greenwel P, Schwartz M, Rosas M, et al. Characterization of fat-storing cell lines derived from normal and CCl4-cirrhotic livers. Differences in the production of interleukin-6. Lab Invest. 1991;65:644–53. [PubMed] [Google Scholar]
- 18.Michelotti GA, Tucker A, Swiderska-Syn M, et al. Pleiotrophin regulates the ductular reaction by controlling the migration of cells in liver progenitor niches. Gut. 2016;65:683–692. doi: 10.1136/gutjnl-2014-308176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hyun J, Wang S, Kim J, et al. MicroRNA-378 limits activation of hepatic stellate cells and liver fibrosis by suppressing Gli3 expression. Nat Commun. 2016;7:10993. doi: 10.1038/ncomms10993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Machado MV, Michelotti GA, Xie G, et al. Mouse models of diet-induced nonalcoholic steatohepatitis reproduce the heterogeneity of the human disease. PLoS One. 2015;10:e0127991. doi: 10.1371/journal.pone.0127991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bode BP, Fuchs BC, Hurley BP, et al. Molecular and functional analysis of glutamine uptake in human hepatoma and liver-derived cells. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1062–73. doi: 10.1152/ajpgi.00031.2002. [DOI] [PubMed] [Google Scholar]
- 22.Linder-Horowitz M, Knox WE, Morris HP. Glutaminase activities and growth rates of rat hepatomas. Cancer Res. 1969;29:1195–9. [PubMed] [Google Scholar]
- 23.Thangavelu K, Pan CQ, Karlberg T, et al. Structural basis for the allosteric inhibitory mechanism of human kidney-type glutaminase (KGA) and its regulation by Raf-Mek-Erk signaling in cancer cell metabolism. Proc Natl Acad Sci U S A. 2012;109:7705–10. doi: 10.1073/pnas.1116573109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin L, Alesi GN, Kang S. Glutaminolysis as a target for cancer therapy. Oncogene. 2016;35:3619–25. doi: 10.1038/onc.2015.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang L, Moss T, Mangala LS, et al. Metabolic shifts toward glutamine regulate tumor growth, invasion and bioenergetics in ovarian cancer. Mol Syst Biol. 2014;10:728. doi: 10.1002/msb.20134892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ozturk Akcora B, Storm G, Prakash J, et al. Tyrosine kinase inhibitor BIBF1120 ameliorates inflammation, angiogenesis and fibrosis in CCl4-induced liver fibrogenesis mouse model. Sci Rep. 2017;7:44545. doi: 10.1038/srep44545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mehendale HM. Tissue repair: an important determinant of final outcome of toxicant-induced injury. Toxicol Pathol. 2005;33:41–51. doi: 10.1080/01926230590881808. [DOI] [PubMed] [Google Scholar]
- 28.Bezerra JA, Bugge TH, Melin-Aldana H, et al. Plasminogen deficiency leads to impaired remodeling after a toxic injury to the liver. Proc Natl Acad Sci U S A. 1999;96:15143–8. doi: 10.1073/pnas.96.26.15143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu D, Shi X, Meng G, et al. Kidney-type glutaminase (GLS1) is a biomarker for pathologic diagnosis and prognosis of hepatocellular carcinoma. Oncotarget. 2015;6:7619–31. doi: 10.18632/oncotarget.3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moylan CA, Pang H, Dellinger A, et al. Hepatic gene expression profiles differentiate presymptomatic patients with mild versus severe nonalcoholic fatty liver disease. Hepatology. 2014;59:471–82. doi: 10.1002/hep.26661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amelio I, Cutruzzola F, Antonov A, et al. Serine and glycine metabolism in cancer. Trends Biochem Sci. 2014;39:191–8. doi: 10.1016/j.tibs.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Michelotti GA, Xie G, Swiderska M, et al. Smoothened is a master regulator of adult liver repair. J Clin Invest. 2013;123:2380–94. doi: 10.1172/JCI66904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teperino R, Aberger F, Esterbauer H, et al. Canonical and non-canonical Hedgehog signalling and the control of metabolism. Semin Cell Dev Biol. 2014;33:81–92. doi: 10.1016/j.semcdb.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bertero T, Cottrill KA, Lu Y, et al. Matrix Remodeling Promotes Pulmonary Hypertension through Feedback Mechanoactivation of the YAP/TAZ-miR-130/301 Circuit. Cell Rep. 2015;13:1016–32. doi: 10.1016/j.celrep.2015.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olsen AL, Bloomer SA, Chan EP, et al. Hepatic stellate cells require a stiff environment for myofibroblastic differentiation. Am J Physiol Gastrointest Liver Physiol. 2011;301:G110–8. doi: 10.1152/ajpgi.00412.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhubanchaliyev A, Temirbekuly A, Kongrtay K, et al. Targeting Mechanotransduction at the Transcriptional Level: YAP and BRD4 Are Novel Therapeutic Targets for the Reversal of Liver Fibrosis. Front Pharmacol. 2016;7:462. doi: 10.3389/fphar.2016.00462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li J, Ghazwani M, Liu K, et al. Regulation of hepatic stellate cell proliferation and activation by glutamine metabolism. PLoS One. 2017;12:e0182679. doi: 10.1371/journal.pone.0182679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaadige MR, Looper RE, Kamalanaadhan S, et al. Glutamine-dependent anapleurosis dictates glucose uptake and cell growth by regulating MondoA transcriptional activity. Proc Natl Acad Sci U S A. 2009;106:14878–83. doi: 10.1073/pnas.0901221106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Medina MA, Nunez de Castro I. Glutaminolysis and glycolysis interactions in proliferant cells. Int J Biochem. 1990;22:681–3. doi: 10.1016/0020-711x(90)90001-j. [DOI] [PubMed] [Google Scholar]
- 40.Araujo L, Khim P, Mkhikian H, et al. Glycolysis and glutaminolysis cooperatively control T cell function by limiting metabolite supply to N-glycosylation. Elife. 2017:6. doi: 10.7554/eLife.21330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang L, Zhou H, Wang Y, et al. CtBP maintains cancer cell growth and metabolic homeostasis via regulating SIRT4. Cell Death Dis. 2015;6:e1620. doi: 10.1038/cddis.2014.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primer used for qRT-PCR
Q-HSCs were freshly isolated from mouse or human tissue; some of the isolates were cultured to induce transdifferentiation into myofibroblastic HSCs; Q-HSC and myofibroblastic HSC RNA was isolated and subjected to microarray analysis as described.1 Gene ontogeny (GO) analysis was performed with the Gene Annotation Tool (http://gather.genome.duke.edu). Charts show distribution of the differentially-expressed genes according to GO pathway descriptors for either mouse HSCs (A) or human HSCs (B) in metabolic process. Fold-change in glutamine metabolism genes in mouse myofibroblastic HSCs versus Q-HSCs (C).
Primary HSCs were cultured in complete medium (Glc/Gln +/+) until day 4. In some cultures, medium was then replaced with glucose-(Glc/Gln −/+) or glutamine-free (Glc/Gln +/−) medium; in others complete medium was supplemented with glutaminolysis inhibitors (CB-839, 0.3 μM or BPTES, 10 μM) or vehicle (0.1% DMSO). (A) Representative phase pictures day 0 and 7. (B) Representative trypan blue exclusion staining for cytotoxicity at day 7. Arrows indicate typical nonviable cells stained blue. (C) Cell viability quantified by trypan blue staining and presented as percentage of living cells compared to total cells. Bars represent mean ± SEM of n = 4 assays. *p < 0.05 vs Glc/Gln +/+ group.
Rat myofibroblastic HSCs (8B cells) were grown in medium containing various concentrations of glucose (Glc) and/or glutamine (Gln) for 3 days, or in complete medium treated with glutaminolysis inhibitors (CB-839; BPTES; EGCG; AOA) or vehicle (0.1% DMSO) for 3 days. (A, B) Cell growth determined by CCK8 assay at 450nm (OD450nm) according to manufacturer’s instructions. (C, D) Representative phase contrast images to illustrate growth differences under each condition on day 3. Bars represent mean ± SEM of n = 4–5 assays.
Myofibroblastic HSCs were cultured for 3 days in complete medium (Glc/Gln +/+) or medium deprived either glucose (Glc/Gln −/+) or glutamine (Glc/Gln +/−); in other cultures, complete medium was supplemented with glutaminolysis inhibitors (CB-839, 0.3 μM or BPTES, 10 μM) or vehicle (0.1% DMSO). (A) Representative pictures of phase, PI staining and trypan blue exclusion staining for cytotoxicity detection at day 3. Arrows indicated typical nonviable cells stained blue. (B) Cell viability assessed by PI staining and trypan blue staining and presented as percentage of living cells compared to total cells. Bars represent mean ± SEM of n = 4 assays. *p < 0.05 vs Glc/Gln +/+ group.
Rat myofibroblastic HSCs (8B cells) were cultured in complete medium (Glc/Gln +/+) or medium depleted of glucose (Glc/Gln −/+) or glutamine (Glc/Gln +/−) or both (Glc/Gln −/−) without (−) or with (+) addition of 4 mM dimethyl α-ketoglutarate (DKG). Representative phase contrast images were acquired to demonstrate growth differences in the presence or absence of DKG.
(A, B)Myofibroblastic HSCs were cultured overnight in complete medium with glutaminolysis inhibitors (CB-839 or EGCG). Oxygen consumption rate (OCR) was measured with a Seahorse XFp Analyzer to determine basal respiration, ATP production, maximal respiration, spare respiratory capacity and proton leakage. Bars represent mean ± SEM of triplicate assays. *p < 0.05 vs vehicle (0.1% DMSO) group. (C) Cell growth determined by CCK8 assay in cultures treated with vehicle or glutaminolysis inhibitors (CB-839, EGCG or AOA) with (+) or without (−) addition of dimethyl α-ketoglutarate (DKG) for 3 days. Data expressed as percentage of the vehicle group (100%). (D) Col1α1 gene expression quantified by RT-PCR. Bars represent mean ± SEM of n = 4–5 assays. *p < 0.05 vs Glc/Gln +/+ group, #p < 0.05 vs DKG (−) group. (E) Representative phase contrast images were acquired to demonstrate growth differences in the presence or absence of DKG. (F) Lipid accumulation assessed by Oil Red O staining.
Adult mice were injected intraperitoneally with corn oil or CCl4 (1200 mg/kg). At 6h and 30h post-CCl4 injection, mice were intraperitoneally injected with Gls1 inhibitor BPTES or its vehicle (10% DMSO in PBS) and liver tissues were harvested at 48h post-CCl4. (A) Liver mRNA levels quantified by qRT-PCR. (B) Protein expression of both isoforms of Gls1 by western blotting. (C) Serum aspartate aminotransferase (AST) activity. (D) Representative H&E-stained liver sections with injured areas outlined. Injured areas were identified by lack of nuclear staining or pyknotic nuclei and lines were marked along the boundaries of injured areas of parenchyma. (E) Morphometric analysis of αSMA expression in the respective groups. (F) Quantification of hepatic hydroxyproline content in the respective groups. Bars represent mean ± SEM of n=4 mice/group. *p < 0.05 vs corn oil, #p < 0.05 vs CCl4+vehicle group.
C57Bl/6 wild-type male mice were fed either chow diet (n=6) or MCD diet (n=6) for 8 weeks. Gls1 and Gls2 expression in total liver were quantified by qRT-PCR. *p < 0.05 vs chow diet fed group.