Abstract
Cumulative evidence suggests that cocaine use could alter the structure and function of different brain systems. However, the extent to which the altered brain structure and function possibly recover over time after cocaine abstinence remains less clear. The present study examines 39 male military veterans with different stages of cocaine addiction and long-term abstinence (from 1 year up to 30 years) and evaluates plausible changes in brain structure and function of specific brain regions that sub-serve addictions. These include the striatum that is involved in cocaine reward; the lateral prefrontal cortex (especially the dorsolateral PFC) that plays a major role in inhibitory control; the insula, which has been implicated in craving; and the medial orbitofrontal (OFC) and ventromedial prefrontal cortex (VMPFC) shown to play key roles in foresight and decision-making. The results suggest that there are differences in both brain structure (gray matter volume, GMV) and function between cocaine USERS and CONTROLS, with USERS showing plausible relative strengthening in neural systems for processing reward and craving, and relative weakening in neural systems involved in inhibitory control and decision-making. Examination of possible neural changes after abstinence suggests that presumed recovery occurs mostly in neural systems related to reward, craving, and inhibitory control, but to a lesser extent in neural systems related to decision-making. Given the limitations of the data in terms of a small sample size, as well as the lack of certainty about occasional use in the abstinent group, these results may be considered as preliminary. However, they are compelling in that they suggest that male military veterans cocaine USERS are indefinitely at a higher risk for making lapses in judgment and decision-making leading to possible relapse, if reward salience and craving become more intense. Understanding the neurobiology of long-term cocaine abstinence in vulnerable populations and beyond could help devising better therapeutic strategies that prevent relapse.
Keywords: Cocaine Addiction, Functional Connectivity, fMRI, VBM, Abstinence
Introduction
Cocaine is a powerful addictive drug that leads to numerous short-term and long-term health consequences. In the United States, the National Survey on Drug Use and Health of the National Institute on Drug Abuse (NIDA) has estimated that the prevalence of lifetime cocaine use in 2013 was 11.6% between the age of 18 and 25, and 16.5% over the age of 26 (http://www.drugabuse.gov/national-survey-drug-use-health). This high prevalence rate has major implications for individuals and societies. For example, in the United States, cocaine use along with other illicit drugs pose estimated societal costs in terms of healthcare, law enforcement and lost productivity of $181 billion per year (NIDA, 2008).
Cumulative evidence suggests that cocaine use could be associated with altered structure and function of brain regions involved in impulsion, cravings, inhibitory control and decision making, all of which are essential for addiction development and maintenance (Noel et al., 2013). Specifically, it has been shown that cocaine use can be associated with structural and functional abnormalities in the striatum, which is known to play a key role in cocaine reward; the lateral prefrontal cortex (especially the dorsolateral PFC), which is known to play a major role in inhibitory control; the insula, which is implicated in craving; and the medial orbitofrontal (OFC) and ventromedial prefrontal cortex (VMPFC), which are involved in integrative and reflective thought leading to decision-making.
One explanation for such associations is that cocaine blocks the recycling process of dopamine in synapses within the reward system, causing an excessive amount of dopamine to float between neurons (Kuhar et al., 1991). With repeated use, it can cause long-term molecular biological changes in the reward system, which in turn will increase cocaine use and promote addiction. Moreover, compared with healthy controls, cocaine dependent individuals showed reduced glutamate metabolism in both reward system and dorsal lateral prefrontal cortex, a critical brain region for inhibitory control (Baker et al., 2003; Schmaal et al., 2012; Sun and Rebec, 2006; Yang et al., 2009). Also, structural brain imaging demonstrated that repeated cocaine exposure changes regional gray matter and white matter in these brain systems (Romero et al., 2010). For example, Franklin et al. (2002) reported that cocaine users had less GMV in the ventromedial orbitofrontal, anterior cingulate, anteroventral insular, and superior temporal cortices. Sim et al. (2007a) reported similar results, and showed that cocaine users had less GMV in the premotor cortex bilaterally, right orbitofrontal cortex, bilateral temporal cortex, left thalamus, and bilateral cerebellum, as well as lower right cerebellar white matter volume relative to a control group. They also observed an inverse correlation between duration of cocaine use, and the right and left cerebellar gray matter volumes. Lastly, using functional brain imaging, researchers have found altered brain activity and connectivity in these systems for cocaine users compared to controls (Adinoff et al., 2015; Hu et al., 2015; Tomasi et al., 2007). For example, Hu et al. (2015) found that cocaine use was associated with increased resting state functional connectivity in striatal-frontal circuits; and with decreased resting state functional connectivity between the striatum and cingulate, striatal, temporal, hippocampal/amygdala, and insular regions.
Although these studies have reported that cocaine use was associated with changes in brain structure and function in key neural regions linked to addictive behaviors, little is known about possible corrective functional and structural alterations that take place in the brain during recovery, especially over long abstinence periods. Specifically, only few studies tried to explore the possibility that the altered brain structure and function could recover after cocaine abstinence (Bell et al., 2011; Hanlon et al., 2011). These studies, however, have focused mostly on short-term abstinence. They found no difference in brain activity and response inhibition behavior (Bell et al., 2014a; Bell et al., 2014b; Morie et al., 2014). Nevertheless, possible effects of long-term abstinence are yet to be examined (Bell et al., 2011). This is especially true given the neurotoxicity of substances, the presence of which can slow down neuroplasticity (Kanai and Rees, 2011). Moreover, examining the neurobiology of long-term cocaine abstinence is important, because it could help understanding mechanisms leading to abstinence success and/or to lapses; this knowledge can serve as a basis for developing relapse prevention programs.
In this study, we address this objective and examine possible altered brain structure and function associated with different stages of cocaine addiction and long-term abstinence (from 1 year to 30 years) using structural Magnetic Resonance Imaging (sMRI) and functional Magnetic Resonance Imaging (fMRI). Our analysis focuses on the abovementioned four neural regions, as these are important for addiction development and maintenance, and alterations in them are often associated with cocaine use. Prior research specifically showed that the striatum tends to be more efficient (often manifested through reduced gray matter volume) in cases of addiction (He et al., 2017); the insular cortex tends to be hypo-active in cases of addiction (often manifested through reduced gray matter volume), but the findings regarding the insula are mixed, as lesions to it actually disturb addiction (Droutman et al., 2015); and that the frontal regions we focus on (dorsolateral PFC, medial OFC and VMPFC) tend to be hypo-active in cases of addiction (often manifested through reduced gray matter volume). Assuming that neural recovery involves corrective actions related to these regions, and given their proven flexible morphology, we hypothesize that abstinence will be associated with opposite changes, both in brain function and structure (Thomas et al., 2008). We further conjecture that these changes will likely be more pronounced in subcortical regions, as these are more prone to morphological adaptations compared with prefrontal regions (Kanai and Rees, 2011). We hence suggest that while recovery can help with desired changes in the striatum and insula, it may be less effective (though still possible) in terms of alterations in prefrontal regions.
Materials and Methods
Participants
Thirty-nine males (32 cases and 7 controls) from the greater Los Angeles area were recruited to participant in this study. These participants were a subsample of a long-term cohort study investigating the longitudinal patterns and consequences of cocaine use and addiction (Hser et al., 2006). Cocaine-dependent patients were screened and recruited from the original 321 male military veterans who met dependence criteria for cocaine and were admitted to cocaine treatment in 1988–1989. Follow-up interviews were conducted in 1990–1991, 1991–1992, and 2002–2003. The normal control subjects were of similar ages and ethnicity/race; they responded to flyers posted in local Veterans Affairs facilities. A screening process was conducted to exclude candidates who : 1) are suffering from Axis 1 psychiatric conditions including PTSD and alcohol dependence; 2) have medical conditions that might interfere with safe study participation; and/or 3) are currently taking medications for psychiatric or medical conditions. A urine testing was also conducted to verify recent self-reported cocaine use or abstinence.
The mean age for these participants was 56.5 ± 5.4 years. They were in different stages of cocaine abstinence without treatment. While twelve were found to use cocaine in the last year (USERS), 20 were at different stages of addiction abstinence (ABSTINENCE) [five with 1-5 years of abstinence (ABS1), five with 6-10 years of abstinence (ABS2), and 10 with over 10 years of abstinence (ABS3)]. The remaining seven subjects were controls (CONTROLS); they had matched age, sex, years of education, and race, to the case subjects. The controls never tried cocaine but lived in the same neighborhood. They were carefully screened for any neurological and psychiatric disorders (e.g., current psychoses, anxiety, or bipolar disorders) and use of medications that impact the central nervous system; no exclusions based on these criteria were made. All participants had normal or corrected-to-normal vision. All participants gave informed consent to the study procedures, which were approved by Institutional Review Boards of the University of Southern California and the University of California, Los Angeles.
Procedures
Participants were asked to come to a Cognitive Neuroscience Imaging Center to sign a consent form, complete a behavioral interview and go through an MRI scan. Prior to the MRI scan, they provided a urine sample. The MRI scan took about 30 minutes. During the scan, images for one high-resolution structural scan and one session of cue task were acquired, with a short resting period in between.
Behavior Interviews
Measures included natural history of drug use, alcohol and tobacco use, mental health (e.g., Beck Depression, SCL-58), quality of health (e.g., SF36), sensation-seeking, and self-efficacy. It captured up-to-date drug use and abstinence (e.g., years of cocaine abstinence) and other measures (e.g., current health and mental health). Urine samples were used to reasonably confirm participants’ abstinence.
fMRI Protocol
MRI images were acquired using a 3T Siemens MAGNETOM Tim/Trio scanner at the Dana and David Dornsife Cognitive Neuroscience Imaging Center at the University of Southern California. Participants lay in the supine position on the scanner bed. Foam pads were used to minimize head motion. They were instructed to rest and keep their head very still during the structural scan. During the fMRI scan, the task was back-projected onto a screen through a mirror attached to the head coil. Stimulus presentation and timing of all stimuli and response events were operationalized with Matlab (Mathworks) and Psychtoolbox (www.psychtoolbox.org) on an IBM-compatible PC. Participants’ responses were collected online using an MRI-compatible button box. The structural image was obtained with T1-weighted 3D-Magnetization Prepared Rapid Gradient Echo (MPRAGE) sequence, covering the whole brain with the following scanning parameters: TR/TE = 2530/3.39 ms, flip angel = 7°, matrix = 256 × 256, number of slices = 128, and slice thickness = 1.33 mm.
Functional MRI images were acquired with blood oxygen level dependent (BOLD) scan using a z-shim gradient echo EPI (echo planer imaging) sequence with PACE (prospective acquisition correction). This specific sequence is designed to reduce signal loss in the prefrontal and orbitofrontal areas. The PACE option can help reducing the impact of head motion during data acquisition. The scanning parameters were: TR/TE = 2000/25 ms; flip angle = 90°; 64 × 64 matrix size with resolution 3 × 3 mm2. Thirty-one 3.5-mm axial slices were used to cover the whole cerebral cortex and most of the cerebellum with no gaps. The slices were tilted about 30° clockwise along the AC-PC plane to obtain better signals in the orbitofrontal cortex.
fMRI Cue Task
Participants performed a cue task in the scanner to measure their brain activity when watching two categories of stimuli: cocaine-related pictures and natural scene pictures (see example stimuli in Figure 1). The fMRI task used a block design and participants were asked to passively view the images. MRI-compatible eye-tracking system was used to monitor participants’ pupils to ensure they are awake during the fMRI task. No exclusions based on this were made. Within each 24 second block, 8 pictures were presented for 2.5 second each with an inter-trial interval of 0.5 seconds. There was a 12 second resting period between blocks of stimuli. There were 16 cocaine-related pictures and 16 natural scene pictures. These pictures were selected based on a pilot study. Each participant viewed 8 blocks of each stimuli type and the order of different stimuli presentation was counterbalanced across participants using either ABBABAAB or BAABABBA sequence. There was a 12 second resting period both at the beginning and the end of the task. The total scanning time for the cue task was about 10 minutes.
Figure 1.

Example stimuli of A) Cocaine related pictures, and B) natural scenes control pictures used in the present study.
Statistical Analysis
Similar analysis procedures were applied to the structural and functional scans. First, participants were divided into three major groups: USERS (those who have used cocaine within the last year, n = 12), ABSTINENCE (those who were at different stages of cocaine abstinence, n = 20) and CONTROLS (matched controls that have never used cocaine, n = 7). The first analysis focused on group differences between 1) USERS and CONTROLS, 2) USERS and ABSTINENCE, 3) ABSTINENCE and CONTROLS. Since the number of participants in the CONTROL group was relatively small, we first examined group difference between three groups as a means to preliminarily search for brain regions that may present differences. These differences were further refined by dividing the ABSTINENCE group into three subgroups: ABS1 (1–5 years of abstinence, n = 5), ABS2 (6–10 years of abstinence, n = 5), and ABS3 (abstinent for over 10 years, n = 10). This division was chosen based on how prior research has treated short meaningful abstinence, medium duration abstinence, and long-term abstinence. A second analysis was performed to shed more light on possible differences associated with abstinence. It included two inter-group comparisons between: 1) USERS and ABS1 (examining the differences between current users and recently abstinent); and 2) CONTROL and ABS3 (examining the differences between controls and long term abstinent). A third analysis included the calculation of years of abstinence for the ABSTINENCE group (N = 20). These data and their log10 transformation were then correlated with whole-brain imaging data. This was done to reveal association between abstinence duration and brain function and structure. The last (fourth) analysis supplemented the previous one by extracting region of interest (ROI) volumes and activations. These data were used for subgroup comparisons. For all correlational analysis, robust regression was used to minimize the impact of outliers in the behavioral data, using iteratively reweighted least squares implemented in the robustfit command in the MATLAB Statistics Toolbox. Reported r-values reflect (non-robust) Pearson product-moment correlation values, whereas the reported p-values and regression lines are based on the robust regression results. Because the sample size was relatively small, the effect sizes as well as their 95% confidence intervals were reported where available, following methods by Steiger and Fouladi (1997).
Voxel-Based Morphometry (VBM) Analysis
Structural MRI data were analyzed with FSL-VBM, an optimized voxel-based morphometry analysis toolbox implemented in FSL. This approach requires no prior information about the location of possible differences in gray matter, and has been proven to be operator-independent. First, structural images were extracted using BET. Next, tissue-type segmentation was performed using FAST4. The resulting gray-matter partial volume images were then aligned to the gray-matter template in the MNI152 standard space using the affine registration tool FLIRT, followed by nonlinear registration using FNIRT, which used a b-spline representation of the registration warp field. The spatially normalized images were then averaged to create a study-specific template, to which the native gray matter images were registered again using both linear and nonlinear algorithms as described above. The registered partial volume images were then modulated by dividing them with the Jacobian of the warp field to correct for local expansion or contraction. The modulated segmented images, which represent the GMV, were then smoothed with an isotropic Gaussian kernel with a 3 mm standard deviation. Finally, voxel-wise general linear models were used to examine correlations between brain data, group affiliation and years of abstinence. Non-parametric permutation methods (Randomise v2.1 in FSL) were used for inference on statistic maps. The null distribution at each voxel was constructed using 10,000 random permutations of the data. Threshold-free cluster enhancement (TFCE) was used to correct for multiple comparisons across the whole brain. The mean GMV in each significant cluster was then extracted for each individual.
fMRI Analysis
Functional MRI image preprocessing and statistical analyses were carried out using the FSL package (www.fmrib.ox.ac.uk/fsl). fMRI images were realigned to compensate for small residual head movements that were not captured by the PACE sequence. Translational movement parameters never exceeded 1 voxel in any direction for any participant. Data were spatially smoothed using a 5-mm full-width-half-maximum (FWHM) Gaussian kernel. The data were filtered using a nonlinear high pass filter with a 100-second cut-off.
A two-step registration procedure was used whereby EPI images were first registered to the MPRAGE structural image, and then into standard MNI space, using affine transformations. Registration from MPRAGE structural image to standard space was further refined using FNIRT nonlinear registration. Statistical analyses were performed in the native image space, with the statistical maps normalized to the standard space prior to higher-level analyses. The data were modeled at the first level using a general linear model (GLM) within FSL’s FILM module. Brain activations for both cocaine-related pictures and natural scene pictures were modeled in the single subject level. The boxcar response was convolved with the canonical hemodynamic response function (HRF, double-gamma) to generate regressors used in the GLM. Temporal derivatives were included as covariates of no interest to improve statistical sensitivity. Resting periods were not explicitly modeled, and therefore constituted an implicit baseline. The six movement parameters were also included as covariates in the model. Higher level random-effects models were tested for group analyses using FMRIB’s Local Analysis of Mixed Effect stage 1 only with automatic outlier detection. Group images were evaluated with a height threshold of Z > 3 and a cluster probability of p < 0.05, corrected for whole-brain multiple comparisons based on Gaussian random field theory.
Regions of interests (ROI) were created from clusters of voxels with significant activation in the voxel-wise analyses. Analyses were performed by extracting parameter estimates (betas) of each type of stimuli from the fitted model and averaging across all voxels in the cluster for each participant. Percent signal changes were calculated using a method suggested by Mumford (http://mumford.fmripower.org/perchange_guide.pdf).
Functional Connectivity Analysis
To better understand key brain networks associated with long-term cocaine abstinence, the time courses of the following four seed regions revealed in the fMRI results were extracted: (1) the right striatum (MNI coordinates: 10, 14, 0); (2) the left insula (MNI coordinates: -40, 6, -10); (3) the right Lateral Frontal Pole (LFP; MNI coordinates: 24, 44, 42); (4) the left VMPFC (MNI coordinates: -14, 56, 14). These ROIs were defined as 6 mm spheres centered on the MNI coordinates mentioned above. Pearson product-moment correlational analysis was performed between the time courses of each pair of ROIs for each subject separately. Between-group differences and correlations with years of abstinence were then assessed.
Results
Gray Matter Volume and Cocaine Abstinence
The results suggest that there are significant differences in brain structure (GMV) between cocaine USERS and CONTROLS (Table 1), with USERS showing higher volumes in the neural systems for processing reward and craving (striatum and insula), and lower volumes in neural systems involved in both inhibitory control (lateral aspects of prefrontal cortex) and decision-making (medial aspects of OFC/VMPFC region). These differences can reflect the imbalance USERS typically have (Noel et al., 2013), as hypo-activity in prefrontal regions is often linked to reduced GMV and insular abnormality can be associated with increased GMV (Wood and Bechara, 2014).
Table 1.
Summary of VBM results
| L/R | Brain region | Voxels | MNI x |
MNI y |
MNI z |
TFCE corrected p |
|---|---|---|---|---|---|---|
| CONTROLS > USERS | ||||||
| R | LFP | 1018 | 34 | 48 | 6 | <.001 |
| L/R | DMPFC | 789 | 0 | 50 | 18 | <.001 |
| L | OFC | 638 | −16 | 10 | −20 | <.001 |
| R | OFC | 111 | 14 | 8 | −22 | .003 |
| L | DLPFC | 104 | 22 | 48 | 42 | .002 |
| USERS > CONTROLS | ||||||
| R | Striatum | 287 | 14 | −4 | 20 | <.001 |
| L | Insula | 133 | −40 | −2 | −2 | .001 |
| L | Striatum | 114 | −16 | 2 | 24 | .002 |
| ABSTINENCE > USERS | ||||||
| L/R | Lateral and Medial Frontal Pole | 2025 | 18 | 64 | 30 | <.001 |
| R | DLPFC | 282 | 58 | 14 | 28 | .001 |
| L | VMPFC | 208 | −22 | 68 | −12 | .003 |
| L | DLPFC | 180 | −48 | 12 | 12 | .002 |
| USERS > ABSTINENCE | ||||||
| R | Striatum | 230 | 18 | −4 | 24 | .001 |
| ABSTINENCE > CONTROLS | ||||||
| NONE | ||||||
| CONTROLS > ABSTINENCE | ||||||
| L | OFC | 176 | −12 | 8 | −22 | .002 |
| USER > ABS1 | ||||||
| R | Striatum | 144 | 18 | −2 | 24 | .003 |
| ABS1 > USER | ||||||
| L/R | Lateral and Medial Frontal Pole | 2372 | 34 | 66 | −10 | <.001 |
| L/R | DMPFC | 831 | −16 | 70 | 16 | <.001 |
| R | DLPFC | 400 | 54 | 14 | 32 | <.001 |
| ABS3 > CONTROL | ||||||
| NONE | ||||||
| CONTROL > ABS3 | ||||||
| L | OFC | 129 | −12 | 8 | −22 | .002 |
| R | OFC | 103 | 12 | 8 | −20 | .003 |
| POSITIVE CORRELATION WITH YEARS OF ABSTINENCE | ||||||
| R | LFP | 269 | 28 | 48 | 10 | <.001 |
| NEGATIVE CORRELATION WITH YEARS OF ABSTINENCE | ||||||
| L | Insula | 133 | −36 | −10 | −4 | <.001 |
| R | Striatum | 71 | 18 | −10 | 26 | .003 |
| POSITIVE CORRELATION WITH LOG-TRANSFORMED YEARS OF ABSTINENCE | ||||||
| R | LFP | 195 | 28 | 48 | 10 | .002 |
| NEGATIVE CORRELATION WITH LOG-TRANSFORMED YEARS OF ABSTINENCE | ||||||
| L | Insula | 133 | −36 | −8 | −4 | .001 |
| R | Striatum | 67 | 18 | −8 | 26 | .002 |
L: Left; R: Right; LFP: Lateral Frontal Pole; DMPFC: Dorsomedial Prefrontal Cortex; OFC: Orbital Frontal Cortex; DLPFC: Dorsolateral Prefrontal Cortex; VMPFC: Ventromedial Prefrontal Cortex.
Note: The voxel size in VBM analysis is 2 mm × 2 mm × 2 mm = 8 mm3.
These results further show that after a period of cocaine abstinence, there are observed differences in GMV that may reflect neural recovery compared with the USERS group. These possibly recovered regions stretch over three neural systems (reward, craving, and inhibitory control), but do not include the neural system linked to decision-making. Specifically, the ABSTINENCE group showed higher GMV (relative to USERS) in the lateral frontal pole bilaterally, and the DLPFC bilaterally, whereas USERS showed higher GMV (relative to the ABSTINENCE group) in the right striatum. This pattern held when comparing USERS to the most recent individuals in the ABSTINENCE group (ABS1): the ABS1 group showed more GMV (relative to USERS) in the lateral frontal pole bilaterally, the medial aspects of the dorsolateral prefrontal cortex (i.e., the medial aspects of the superior frontal gyrus) bilaterally, and the right DLPFC. Moreover, USERS had higher GMV (relative to ABS1) in the right striatum.
However, no structural differences in the medial OFC/VMPFC, that is brain regions critical for decision-making, were observed. These results may therefore imply no recovery in this area. Specifically, CONTROLS showed higher GMV in the left OFC relative to ABSTIENENCE. No other brain region showed differences in GMV in the ABSTINENCE group relative to CONTROLS. This was also true when comparing CONTROLS to the longest abstinent individuals of the ABSTINENCE group (ABS3). CONTROLS still showed higher GMV in the OFC, bilaterally, compared with the ABS3 group. No other brain region showed differences in GMV between the ABS3 and CONTROLS groups. Overall, this analysis revealed a possible tendency towards “normalization” in most key neural systems sub-serving cocaine addiction, except for prefrontal decision-making regions.
Next, correlations between GMV and both (1) years of abstinence, and (2) log-transformed years of abstinence (Table 1) were assessed. This analysis was restricted to those regions that showed differences between USERS and CONTROLS in an uncorrected (p < .05) statistical map. Results were consistent for years of abstinence and log-transformed years of abstinence. They showed that GMV in the right striatum and the left insula were negatively correlated with abstinence period, which reflects possible recovery. They also showed that GMV in the right LFP was positively correlated with abstinence duration, also possibly reflecting neural recovery (see Figure 2). Consistent with the between-group comparisons, no significant correlation between left OFC and abstinence period was observed.
Figure 2.
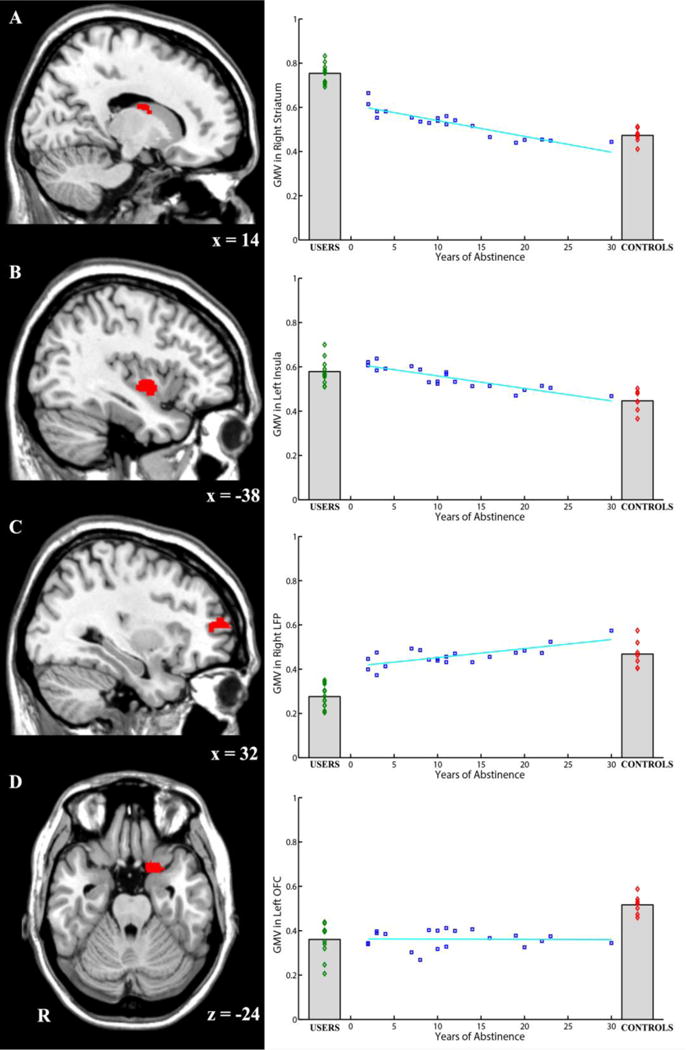
GMV in four key brain regions and its relationship with years of abstinence. A) The right striatum showed higher GMV in USERS than in CONTROLS and ABSTINENCE. There were also difference between USERS and ABS1 (i.e., those who recently quit using cocaine; less than 5 years ago). Years of abstinence inversely predicted the GMV in the right striatum. B) The left insula showed higher GMV in USERS than in CONTROLS. Years of abstinence also inversely predicted the GMV in the left insula. C) The right LFP showed lower GMV in USERS than in CONTROLS and ABSTINENCE. There was also observable difference between USERS and ABS1 (i.e., those who recently quit using cocaine; less than 5 years ago). Years of abstinence positively predicted the GMV in the right LFP. D) The left OFC showed higher GMV in CONTROLS than in USERS and ABSTINENCE. The difference was noticeable also between CONTROLS and ABS3 (i.e., those who quit using cocaine more than 11 years ago). The correlation suggested that there is no significant recovery in this region.
In the visualized brain slices, red color illustrates the corresponding brain region. R indicates the right hemisphere. Numbers below the slice indicate the MNI coordinates of the slice. ABC is displayed in sagittal view and D is displayed in axial view.
Brain Activation and Cocaine Abstinence
fMRI results (Table 2) showed that USERS (relative to CONTROLS) were more sensitive to cocaine pictures (as opposed to natural scenes pictures) in terms of eliciting higher neural activation responses in a large swath of subcortical regions that includes the striatum (caudate and putamen), the amygdala, hippocampus, thalamus, and insular cortex. Although the GMV results indicated possible weakening in the neural systems involved in inhibitory control in USERS (as reflected by relatively smaller GMV in the lateral prefrontal regions), we found an increase in activation responses to cocaine pictures (instead of a decrease) in the dorsal and ventral parts of the prefrontal cortex in USERS. One possible explanation is that USERS need to exert a stronger effort to inhibit response tendencies towards cocaine cues, as a result of their deteriorated inhibition faculties and abilities. As expected, cocaine pictures elicited increased neural activation responses compared to nature pictures in the more ventral part of the medial prefrontal cortex in CONTROLS (i.e., USERS showed weaker activation responses in this region) (Table 2), which is indicative of poorer decision-making capacity among the USERS group.
Table 2.
Summary of fMRI results (Cocaine Pictures > Natural Scene Pictures)
| L/R | Brain region | Voxels | MNI x |
MNI y |
MNI z |
Z corrected p |
|---|---|---|---|---|---|---|
| USERS | ||||||
| L/R | Hippocampus/Putamen/Amygdala/Thalamus | 3223 | 6 | 0 | 2 | 4.64 |
| L | DLPFC Extending to Anterior Insula | 1740 | −42 | 12 | 26 | 4.51 |
| L/R | DMPFC/VMPFC | 1023 | 8 | 56 | −14 | 4.14 |
| R | DLPFC | 860 | 50 | 44 | −16 | 3.89 |
| L | Inferior Temporal Gyrus | 698 | −52 | −38 | −4 | 4.13 |
| PCC/Precuneus Extending to Right Superior Parietal | ||||||
| L/R | 696 | −14 | −50 | 30 | 3.90 | |
| Lobe | ||||||
| R | Lateral Occipital Cortex | 610 | 36 | −50 | 54 | 3.84 |
| R | DLPFC | 563 | 54 | 10 | 44 | 4.59 |
| R | LFP | 472 | 24 | 44 | 42 | 4.26 |
| CONTROLS | ||||||
| L/R | DMPFC/VMPFC/OFC (left lateralized) | 2567 | −8 | 62 | 12 | 4.38 |
| USERS > CONTROLS | ||||||
| Bilateral Caudate/Bilateral Thalamus/right | ||||||
| L/R | 1181 | 6 | 0 | 4 | 5.58 | |
| Insula/right Putamen/right Pallidum | ||||||
| L | Insula/DLPFC | 789 | −54 | 30 | 6 | 5.27 |
| R | DLPFC | 346 | 52 | 10 | 46 | 5.29 |
| R | LFP | 273 | 24 | 46 | 42 | 5.31 |
| L | Inferior Temporal Gyrus | 245 | −46 | −6 | −28 | 5.29 |
| CONTROLS > USERS | ||||||
| L | VMPFC/OFC | 405 | −14 | 34 | −14 | 4.84 |
| ABSTINENCE > USERS | ||||||
| NONE | ||||||
| USERS > ABSTINENCE | ||||||
| R | Caudate/Thalamus/Putamen/Pallidum | 439 | 12 | 0 | −8 | 5.39 |
| L | Caudate | 139 | −10 | 20 | 10 | 5.26 |
| L | DLPFC | 103 | −54 | 30 | 8 | 5.04 |
| ABSTINENCE > CONTROLS | ||||||
| R | LFP/DLPFC | 601 | 46 | 38 | 34 | 5.45 |
| CONTROLS > ABSTINENCE | ||||||
| L | VMPFC | 224 | −20 | 50 | 6 | 4.89 |
| USER > ABS1 | ||||||
| R | Striatum extending to ACC | 224 | 4 | 32 | 4 | 5.09 |
| ABS1 > USER | ||||||
| NONE | ||||||
| ABS3 > CONTROL | ||||||
| NONE | ||||||
| CONTROL > ABS3 | ||||||
| L | VMPFC | 164 | −14 | 56 | 14 | 5.43 |
| POSITIVE CORRELATION WITH YEARS OF ABSTINENCE | ||||||
| NONE | ||||||
| NEGATIVE CORRELATION WITH YEARS OF ABSTINENCE | ||||||
| R | LFP | 278 | 24 | 44 | 42 | 4.38 |
| L | Insula | 195 | −40 | 6 | −10 | 3.74 |
| R | Caudate | 189 | 10 | 14 | 0 | 4.25 |
| POSITIVE CORRELATION WITH LOG-TRANSFORMED YEARS OF ABSTINENCE | ||||||
| NONE | ||||||
| NEGATIVE CORRELATION WITH LOG-TRANSFORMED YEARS OF ABSTINENCE | ||||||
| L | Caudate | 194 | 10 | 14 | 0 | 4.19 |
| L | Insula | 165 | −40 | 8 | −10 | 4.05 |
| R | LFP | 137 | 24 | 46 | 44 | 3.89 |
L: Left; R: Right; DLPFC: Dorsolateral Prefrontal Cortex; DMPFC: Dorsomedial Prefrontal Cortex; VMPFC: Ventromedial Prefrontal Cortex; PCC: Posterior Cingulate Cortex; LFP: Lateral Frontal Pole; OFC: Orbital Frontal Cortex; ACC: Anterior Cingulate Cortex.
Note: The voxel size in fMRI analysis is 2 mm × 2 mm × 2 mm = 8 mm3.
We also compared different groups in relation to the contrast between cocaine pictures and natural scene pictures. The activation maps from the previous step were used as an inclusive mask to rule out the possibility that activation differences were due to a negative activation in one condition. Results suggested that USERS (relative to CONTROLS) showed increased activations in the left insula/DLPFC, right DLPFC, right LFP, left inferior temporal gyrus, and a large part of the subcortical areas, including the caudate/thalamus bilaterally, and the right putamen/pallidum. In contrast, CONTROLS (relative to USERS) showed increased activation in the left VMPFC/OFC (Table 2). USERS also showed, relative to the ABSTINENCE Group, higher activations in the right caudate/thalamus/putamen/pallidum, right caudate, and left DLPFC. The reverse comparison showed no difference (Table 2). Specifically, USERS showed higher activation in the left striatum (extending to the ACC region) than the most recent abstinent individuals of the ABSTINENCE group (ABS1). No other region in the ABS1 group showed higher activation relative to USERS (Table 2). The ABSTINENCE group showed more activation in the right LFP/DLPFC than the CONTROLS group did, while the CONTROLS group showed higher activation in the left VMPFC than the ABSTINENCE group did. CONTROLS also showed higher activation in the left VMPFC compared with the longest abstinent individuals of the ABSTINENCE group (ABS3). No other region in the ABS3 group showed higher activation relative to CONTROLS (Table 2).
Lastly, we analyzed the correlation between brain activation differences and (1) years of abstinence, and (2) log-transformed years of abstinence (Table 2). This analysis was restricted to those regions that showed differences between USERS and CONTROLS in a liberal (p < .05, uncorrected) statistical map. Results consistently suggested that no brain areas were positively correlated with abstinence time and its log transformation. However, activations in three brain regions (right caudate, left insula, and right LFP) showed negative correlations with abstinence time and its log transformation (Table 2).
With the exception of the observed increased activation in the lateral prefrontal cortex in USERS relative to CONTROLS, the functional neuroimaging results are largely consistent with the structural (GMV) results in that USERS show (1) a relative strengthening in neural systems for processing reward and craving (striatum and insula), and (2) a relative weakening in neural systems involved in inhibitory control (lateral aspects of prefrontal cortex), and to some extent decision-making (medial aspects of OFC/VMPFC). Furthermore, after a period of cocaine abstinence, there are observed differences (possibly reflecting recovery) in activity in these regions. These may reflect a neural adaptation back to the levels of CONTROLS, in at least 3 key neural systems (reward, craving, and inhibitory control), but not to the same extent in the neural system serving decision-making (Figure 3).
Figure 3.
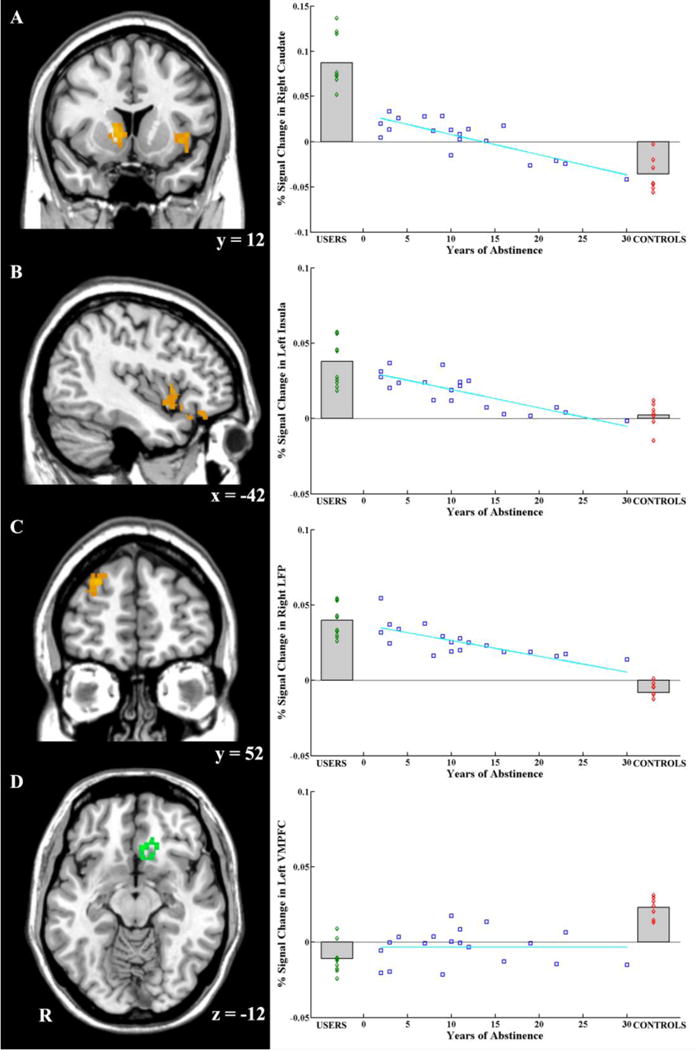
Brain activation in four key brain regions and relationships with years of abstinence. A) The right striatum showed higher activation in USERS when facing cocaine pictures than when facing control pictures. The activation in this area of USERS was higher than this of CONTROLS and ABSTINENCE, and even higher than this of ABS1 (i.e., those who recently quit using cocaine; less than 5 years ago). Years of abstinence could inversely predict brain activation in the right striatum. B) The left insula showed higher activation in USERS when facing cocaine pictures than when facing control pictures. The activation in this area for USERS is higher than this for CONTROLS. Years of abstinence could also inversely predict brain activation in the left insula. C) The right LFP in USERS showed higher activation when facing cocaine pictures than when facing control pictures. The activation in this area of USERS and ABSTINENCE was higher than in this area of CONTROLS. Years of abstinence could inversely predict brain activation in right LFP. D) The left VMPFC showed higher activation when facing cocaine pictures than when facing control pictures in CONTROLS. The activation in this area of CONTROLS was higher than this of USERS and ABSTINENCE, and even higher than this of ABS3 (i.e., those who quit using cocaine more than 11 years ago). The correlation suggested that there is no recovery for this region.
In the visualized brain slices, yellow or green colors illustrate the corresponding brain region. R indicates the right hemisphere. Numbers below the slice indicate the MNI coordinates of the slice. AC are displayed in coronal view, B is displayed in sagittal view, and D is displayed in axial view. R indicates the right hemisphere.
Functional Connectivity and Cocaine Abstinence
For functional connectivity analysis, we first used one sample t test to examine whether the correlation coefficients (rs) were significantly different from 0 in USERS and CONTROLS. Figure 4A demonstrated that except for the connectivity between the left VMPFC and the right LFP, and the connectivity between the left Insula and the right LFP, all other connections were significant for CONTROLS (all mean rs > 0.43, all ts > 18.66, all ps < 0.001). However, Figure 4B showed that in USERS, there is only significant connectivity between the left VMPFC and the left Insula (mean r = 0.39, SD = 0.085, t = 15.95, p < 0.001). Next, brain connectivity was compared between USERS and CONTROLS after Fisher-Z transformation of the correlation coefficients. These results are expressed in Figure 4C. It shows that there are three connections that differed between CONTROLS and USERS, including the left Insula and right Striatum connection (t(17) = 3.30, p = 0.004, Cohen’s d = 1.60, 95% CI = 0.97-1.78), left VMPFC and right striatum connection (t(17) = 2.98, p = 0.008, Cohen’s d = 1.45, 95% CI = 0.95-1.67), and right LFP and right Striatum connection (t(17) = 2.45, p = 0.025, Cohen’s d = 1.19, 95% CI = 1.03-1.45). Two connections (Figure 4D) also showed significant correlations with years of abstinence, including the connections between right LFP and right Stratum (r(19) = 0.48, p = 0.028, 95% CI = 0.43-0.56) and left Insula and right Striatum (r(19) = 0.57, p = 0.007, 95% CI = 0.49-0.61). However, the connection between the left VMPFC and the right striatum showed no correlation with years of abstinence (r(19) = 0.12, p = 0.60, 95% CI = -0.13-0.21).
Figure 4.
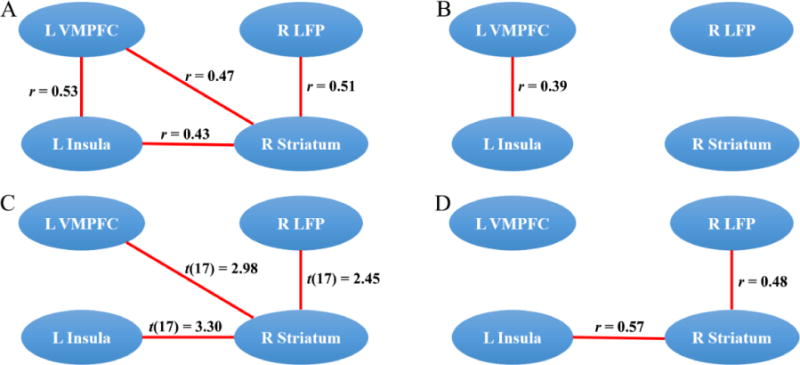
A) Significant connections for the CONTROLS group tested by one sample t tests. Except for the connectivity between the left VMPFC and the right LFP, and the connectivity between the left Insula and the right LFP, all other connections were significant (in red). The mean correlational coefficients are displayed for each significant connection. B) Significant connections for the USERS group tested by one sample t tests. Results suggested that only the connectivity between the left VMPFC and the left Insula is significant. The mean correlational coefficient is displayed for this connection. C) Three connections significantly differed between CONTROLS and USERS, including connections between the left Insula and the right Striatum, the left VMPFC and the right striatum, and the right LFP and right Striatum. T statistics for each significant connection are displayed. D) Two connections were also significantly positively correlated with years of abstinence, including the connections between the right LFP and the right Striatum and between the left Insula and the right Striatum. However, the connection between the left VMPFC and the right striatum did not correlate with years of abstinence. Correlational coefficients are displayed for each significant correlation.
Discussion
This study is unique in that it examined differences (possible changes) in brain structure, activation and connectivity as a function of long-term cocaine abstinence for a duration ranging from 1 year to 30 years, among military veterans. It specifically examined how cocaine use or abstinence duration may be associated with differences in four brain regions that are instrumental in addiction formation and maintenance. The findings point to both GMV and functional activation differences in USERS compared to CONTROLS in all of these target regions, namely the striatum; the lateral prefrontal cortex (especially the dorsolateral PFC); the insula; and the medial orbitofrontal cortex (OFC) and ventromedial prefrontal cortex (VMPFC). Specifically, USERS showed presumed unhealthy strengthening of neural systems for processing reward and craving (higher GMV and higher activation in the right striatum and left insula), and relative weakening of neural systems involved in (1) inhibitory control (the right lateral frontal pole [LFP] showed lower GMV, but interestingly higher functional activation, which may reflect more effortful engagement due to structural deficits), and (2) decision-making (the left OFC/VMPFC showed lower GMV and functional activation). Although compelling, the results are limited in that they include a relatively small sample size, as well as they lack methods that rule out with certainty any occasional use in the long-term cocaine abstinent group. Therefore, these results should be considered as preliminary. However, these findings reaffirm the centrality of these regions in addiction development and maintenance.
Examination of possible neural changes after abstinence suggests that presumed recovery can occur mostly in neural systems related to reward, craving, and inhibitory control, but to a lesser extent in neural systems related to decision-making. This is consistent with many observations that prefrontal deficit can be difficult to fix (Bechara et al., 2000) and that these brain regions are less prone to morphological changes compared to sub-cortical regions (He et al., 2017). Specifically, GMV and functional activation negatively correlated with years of abstinence from cocaine use in the ABSTINENCE group, in the right striatum and left insula. Moreover, in the ABSTINENCE group, GMV in the right LFP positively correlated with abstinence duration, while functional activation in the right LFP negatively correlated with years of abstinence. In contrast, both GMV and functional activation levels in the left OFC/VMPFC regions were not associated with years of abstinence. Additional analyses also suggested that USERS had reduced functional connectivity between the right striatum and the left insula, left VMPFC, and right LFP. While two of these connections (between the right LFP and the right striatum, and between the left insula and the right striatum) correlated with years of abstinence, the connection between left VMPFC and right striatum did not correlate with abstinence duration. This endorses the idea that after cocaine abstinence, it is possible that at least some neural systems related to reward, craving, and inhibitory control adapt; and their recovery is a function of time. The required recovery time, though, is long (several years). However, neural systems related to foresight and decision-making may not show similar recovery patterns. This implies that cocaine USERS may be indefinitely at a higher risk for making lapses in judgment and decision-making, and that these may be one reason for their ongoing relapses, especially if reward salience and craving become more intense. Hence, therapies that target decision making improvement, for example through mindfulness training, may be useful for improving abstinence rates; only under the assumption that USERS have the capacity to exert the needed inhibition. If the inhibition facilities do not recover, strategies to reduce cue salience (e.g., avoiding cues related to Cocaine) may be more efficacious. These propositions, though, merit further research, and their efficacy may depend on the possible plasticity of relevant brain regions.
The gray matter abnormalities identified in the current study in the striatum, insula, frontal pole, and OFC/VMPFC are consistent with numerous studies on abnormalities in gray matter morphology in people who use various substances, including cocaine (Ansell et al., 2012; Barrós-Loscertales et al., 2011; Franklin et al., 2002; Kaag et al., 2014; Konova et al., 2012; Moreno-López et al., 2012; Sim et al., 2007b), heroin (Liu et al., 2009; Wang et al., 2012; Yuan et al., 2010; Yuan et al., 2009), cannabis (Cousijn et al., 2012), nicotine (Brody et al., 2004; Liao et al., 2012), and alcohol (Benegal et al., 2007; Mechtcheriakov et al., 2007). For example, Sim et al. (2007b) reported that cocaine-dependent participants had at least a 10% reduction in GMV, relative to controls, in regions of the premotor cortex bilaterally, the right orbitofrontal cortex, the temporal cortex bilaterally, the left thalamus, and the cerebellum bilaterally. In a study of participants with dependence on two or more substances (Tanabe et al., 2009), it has been shown that subjects had reduced GMV, relative to controls, in the medial OFC bilaterally. Furthermore, the degree of this GMV reduction correlated with decision-making performance on a modified gambling task.
While we found, consistent with most VBM studies that the right LFP and left VMPFC had decreased GMV in substance users, we also found increased striatal and insular GMV in cocaine users. This is not consistent with all prior studies, but it is consistent with some (Ansell et al., 2012; Barrós-Loscertales et al., 2011); which is indicative of mixed findings regarding these regions. The striatal GMV increase was consistent with Ersche and colleagues (Ersche et al., 2011; Ersche et al., 2012) who found similar increase in GMV of the striatum. Also, animal studies showed that cocaine use increases dopamine receptors and mRNA in the striatum (Beveridge et al., 2009; Spangler et al., 1993; Wong et al., 2006), which may explain the observed increase in GMV. Some studies also point to higher GMV in the left insula of smokers, compared to controls (Zhang et al., 2011). Moreover, it has been shown that the GMV of the insular cortex of cocaine addicts is associated with abstinence duration (Connolly et al., 2013). A recent study suggested that there may be sex related differences regarding GMV and addiction (Rando et al., 2013), which may partially explain the inconsistency with earlier work that was observed in our study (our study only examined male participants). Overall, more research on the morphology of the striatum and insula is needed for shedding light on the mixed findings in prior research.
This study also examined the functional activity of four key regions implicated in cocaine addiction. Using PET or fMRI, researchers have consistently shown that abnormal activity in these regions relate to the use of various substances (for reviews, see Goldstein and Volkow, 2002; Goldstein and Volkow, 2011; Hyman and Malenka, 2001; Kalivas and Volkow, 2005; Leshner, 1997; Volkow and Fowler, 2000; Volkow et al., 2002; Volkow et al., 2012; Volkow and Wise, 2005), including to cocaine (Asensio et al., 2010; Breiter et al., 1997; Goldstein et al., 2007; Kilts et al., 2001), heroin (Daumann et al., 2003; Fu et al., 2008), nicotine (McBride et al., 2006; McClernon et al., 2005; McClernon et al., 2009), alcohol (Hermann et al., 2006), NMDA (Daumann et al., 2003), and marijuana (Schweinsburg et al., 2008). Employing the widely-used cue task paradigm, our results suggest that cocaine users show more activation of the striatum, insula, and lateral prefrontal cortex, combined with lower activation in the VMPFC/OFC. This is consistent with previous studies using the cue task paradigm (Goldstein and Volkow, 2002; Goldstein and Volkow, 2011; Hugh et al., 2000; Hyman and Malenka, 2001; Kalivas and Volkow, 2005; Leshner, 1997; Volkow and Fowler, 2000; Volkow et al., 2002; Volkow et al., 2012; Volkow and Wise, 2005). Similarly, in cocaine addicts the striatal system is often hyperactive, whereas the OFC is typically hypoactive during the performance of a Stroop task (Goldstein et al., 2007). This is supported in PET studies that pointed to hyperactive striatum and lateral prefrontal cortex and a hypoactive VMPFC/OFC (Bonson et al., 2002; Grant et al., 1996) as well as in fMRI studies (Bolla et al., 2004; Bolla et al., 2003).
Considering potential neural recovery after long-term cocaine abstinence, we found that the GMV and activation of some of the four target neural regions (namely, striatum, insula, and dorsolateral prefrontal cortex) correlated positively with the duration of cocaine abstinence. In contrast, neither the GMV, nor functional activity in the left VMPFC, were correlated with years of abstinence. These results support our hypotheses that cortical neural systems sub-serving decision-making may not recover as effectively as sub-cortical regions, even after long-term cocaine abstinence. These findings are consistent with several prior studies. While recovery of neuropsychological functions are expected after a long period of abstinence (e.g., Bell et al., 2014a; McClernon et al., 2005), the persistence of decision-making impairments has been intriguing. For example, it has been shown that alcoholics can achieve long-term abstinence in spite of persistent deficits in decision making as measured by the Iowa Gambling Task (Fein et al., 2004). These findings are consistent with the proposal that decision-making deficits and non-recovering VMPFC reflect a vulnerability or predisposing factor to substance use disorders that may persist with abstinence (Bechara, 2001, 2003). Abstinence in such cases (when decision-making deficits persist), may be possibly attributed to reduction in cues that motivate substance use. This idea, though, should be examined in future research.
Moreover, this study showed that cocaine users also differed from controls in functional connectivity among the abovementioned four key regions, and especially in connectivity between the left insula/right LFP and right striatum. However, the connectivity between the left VMPFC and right striatum did not correlate with abstinence duration. Several studies investigated functional connectivity in addiction disorders using resting state fMRI. For example, it has been found that chronic heroin users show increased functional connectivity between the striatum and ACC, between the striatum and OFC, and between the amygdala and OFC, as well as reduced functional connectivity between the lateral prefrontal cortex and OFC, and between the lateral prefrontal cortex and ACC (Ma et al., 2010). Using arterial spin labeling and resting state fMRI, enhanced regional cerebral blood flow in the left posterior hippocampus in a relapsed group, as compared to early remission and control groups was revealed; using this posterior hippocampus as a seed, increased resting state functional connectivity strength with the posterior cingulate cortex/precuneus in relapsed versus early remission subgroups was also observed (Adinoff et al., 2015). Reviews suggest that the altered functional connectivity in addiction may reflect the underlying psychological dysfunctions associated with reward, affective and cognitive processing, especially the connectivity between the insula and other brain regions (Sutherland et al., 2012). Consistent with this study, previous studies found reduced resting state functional connectivity in cocaine-dependent participants (Kelly et al., 2011) and opioid-dependent patients relative to control subjects (Upadhyay et al., 2010).
Lastly, it is interesting to consider that activity in the PFC in controls also increased after exposure to cocaine pictures; that is, they did not treat cocaine pictures as neutral. This may have happened because cocaine pictures are emotionally charged stimuli, just like the emotional slides in the International Affective Picture System (IAPS). To a normal individual, cocaine cues may evoke many negative emotional responses that are implicit, and not necessarily conscious. The PFC is a key brain region involved in the processing of emotionally competent stimuli, and patients with lesions in the PFC do not trigger, for example, skin conductance responses to IAPS pictures, including pictures of drug paraphernalia (Damasio et al., 1990). On the other hand, cocaine addicts are suspected to have a deficiency in this same PFC region. Therefore, it was reasonable to find that the PFC of normal individuals react (or increase its activity) in response to emotionally charged stimuli such as cocaine cues, whereas cocaine users are less likely to do so.
Overall, our findings provide interesting insights regarding cocaine addiction and possible recovery, and specifically extend prior research by showing possible long-term neural associations with abstinence. The results are fairly consistent with prior research and point not only to similarities and possible shared neural underpinning of addictions to various substances and behaviors (Noel et al., 2013), but also to similarities in terms of possible recovery during abstinence. There are several noteworthy limitations in this study. First, the sample size is relatively small and the participants are all male veterans in their mid-50s. Although we had a large cohort of participants for behavior screening, the relatively strict exclusion criteria, other co-morbidities, the time scope of the study, and body condition disqualified many of them from being scanned, thus reducing the sample size. Essentially, we focused on long term abstinence in an existing cohort and this has reduced the likelihood of meeting our criteria and agreeing again (after many years) to take part in our study. Second, although our previous studies (Hser et al., 2016; Hser et al., 2001; Hser et al., 2006; Nosyk et al., 2014) have demonstrated the reliability and validity of the timeline follow back procedure combined with urine testing, it might be possible that the length of cocaine abstinence measure was biased. Future research may benefit from including a more rigorous biomarker or secondary measure (e.g. caretaker report), in order to strengthen assertions regarding abstinence duration. Third, the functional connectivity analysis only focused on the brain regions showing significant activation in the cue task, but not the whole brain. Fourth, this is a cross-sectional and correlational study, which limits causality arguments. Longitudinal studies should be conducted in the future to replicate and extend the findings from this study. Despite these limitations, these results are compelling in that they suggest that male military veterans cocaine USERS are indefinitely at a higher risk for making lapses in judgment and decision-making leading to possible relapse, if reward salience and craving become more intense. Understanding the neurobiology of long-term cocaine abstinence in vulnerable populations and beyond could help devising better therapeutic strategies that prevent relapse.
Highlights.
Cocaine users showed higher neural response for processing reward and craving
Cocaine users showed less neural response for inhibitory control and decision-making
Recovery occurred in neural systems of reward, craving, and inhibitory control
Recovery didn’t occur in neural systems related to decision-making
Acknowledgments
YH was supported by National Institute on Drug Abuse (NIDA), Grants R03DA032542-01A1 and P30DA016383. QH was supported by research grants from the National Natural Science Foundation of China (31400959), Entrepreneurship and Innovation Program for Chongqing Overseas Returned Scholars (cx2017049), Fundamental Research Funds for the Central Universities (15XDSKD004), Open Research Fund of the Key Laboratory of Mental Health, Institute of Psychology, Chinese Academy of Sciences (KLMH2015G01), and the Research Program Funds of the Collaborative Innovation Center of Assessment toward Basic Education Quality at Beijing Normal University (2016-06-014-BZK01). We would also like to thank Alexandra Hollihan and Stephanie Castillo who helped with the data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare no conflict of interest.
Publication Statement
This is an original submission that has not been published before and that is not currently under review at any other publication outlet.
Author Contribution Statement
QH, AB, and YH designed the study, QH, XH, MS, DH, and AT collected the data, QH, XH, and OT analyzed the data, QH, XH, OT, AB, and YH wrote the manuscript, all authors approved the final version of the manuscript.
References
- Adinoff B, Gu H, Merrick C, McHugh M, Jeon-Slaughter H, Lu H, Yang Y, Stein EA. Basal Hippocampal Activity and Its Functional Connectivity Predicts Cocaine Relapse. Biol Psychiatry. 2015;78(7):496–504. doi: 10.1016/j.biopsych.2014.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansell EB, Rando K, Tuit K, Guarnaccia J, Sinha R. Cumulative Adversity and Smaller Gray Matter Volume in Medial Prefrontal, Anterior Cingulate, and Insula Regions. Biological Psychiatry. 2012;72(1):57–64. doi: 10.1016/j.biopsych.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asensio S, Romero MJ, Palau C, Sanchez A, Senabre I, Morales JL, Carcelen R, Romero FJ. Altered neural response of the appetitive emotional system in cocaine addiction: an fMRI Study. Addiction biology. 2010;15(4):504–516. doi: 10.1111/j.1369-1600.2010.00230.x. [DOI] [PubMed] [Google Scholar]
- Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, Kalivas PW. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci. 2003;6(7):743–749. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- Barrós-Loscertales A, Garavan H, Bustamante JC, Ventura-Campos N, Llopis JJ, Belloch V, Parcet MA, Ávila C. Reduced striatal volume in cocaine-dependent patients. Neuroimage. 2011;56(3):1021–1026. doi: 10.1016/j.neuroimage.2011.02.035. [DOI] [PubMed] [Google Scholar]
- Bechara A. Neurobiology of decision-making: risk and reward. Semin Clin Neuropsychiatry. 2001;6(3):205–216. doi: 10.1053/scnp.2001.22927. [DOI] [PubMed] [Google Scholar]
- Bechara A. Risky business: emotion, decision-making, and addiction. J Gambl Stud. 2003;19(1):23–51. doi: 10.1023/a:1021223113233. [DOI] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H. Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain. 2000;123(11):2189–2202. doi: 10.1093/brain/123.11.2189. [DOI] [PubMed] [Google Scholar]
- Bell RP, Foxe JJ, Nierenberg J, Hoptman MJ, Garavan H. Assessing white matter integrity as a function of abstinence duration in former cocaine-dependent individuals. Drug Alcohol Depend. 2011;114(2–3):159–168. doi: 10.1016/j.drugalcdep.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RP, Foxe JJ, Ross LA, Garavan H. Intact inhibitory control processes in abstinent drug abusers (I): a functional neuroimaging study in former cocaine addicts. Neuropharmacology. 2014a;82:143–150. doi: 10.1016/j.neuropharm.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RP, Garavan H, Foxe JJ. Neural correlates of craving and impulsivity in abstinent former cocaine users: Towards biomarkers of relapse risk. Neuropharmacology. 2014b;85:461–470. doi: 10.1016/j.neuropharm.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benegal V, Antony G, Venkatasubramanian G, Jayakumar PN. Imaging study: gray matter volume abnormalities and externalizing symptoms in subjects at high risk for alcohol dependence. Addiction biology. 2007;12(1):122–132. doi: 10.1111/j.1369-1600.2006.00043.x. [DOI] [PubMed] [Google Scholar]
- Beveridge TJ, Smith HR, Nader MA, Porrino LJ. Abstinence from chronic cocaine self-administration alters striatal dopamine systems in rhesus monkeys. Neuropsychopharmacology. 2009;34(5):1162–1171. doi: 10.1038/npp.2008.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla K, Ernst M, Kiehl K, Mouratidis M, Eldreth D, Contoreggi C, Matochik J, Kurian V, Cadet J, Kimes A, Funderburk F, London E. Prefrontal cortical dysfunction in abstinent cocaine abusers. J Neuropsychiatry Clin Neurosci. 2004;16(4):456–464. doi: 10.1176/appi.neuropsych.16.4.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, London ED, Kiehl KA, Mouratidis M, Contoreggi C, Matochik JA, Kurian V, Cadet JL, Kimes AS, Funderburk FR, Ernst M. Orbitofrontal cortex dysfunction in abstinent cocaine abusers performing a decision-making task. Neuroimage. 2003;19(3):1085–1094. doi: 10.1016/s1053-8119(03)00113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonson KR, Grant SJ, Contoreggi CS, Links JM, Metcalfe J, Weyl HL, Kurian V, Ernst M, London ED. Neural systems and cue-induced cocaine craving. Neuropsychopharmacology. 2002 doi: 10.1016/S0893-133X(01)00371-2. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, Goodman JM, Kantor HL, Gastfriend DR, Riorden JP. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19(3):591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Jarvik ME, Lee GS, Smith EC, Huang JC, Bota RG, Bartzokis G, London ED. Differences between smokers and nonsmokers in regional gray matter volumes and densities. Biological psychiatry. 2004;55(1):77–84. doi: 10.1016/s0006-3223(03)00610-3. [DOI] [PubMed] [Google Scholar]
- Connolly CG, Bell RP, Foxe JJ, Garavan H. Dissociated Grey Matter Changes with Prolonged Addiction and Extended Abstinence in Cocaine Users. PLOS ONE. 2013;8(3):e59645. doi: 10.1371/journal.pone.0059645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousijn J, Wiers RW, Ridderinkhof KR, van den Brink W, Veltman DJ, Goudriaan AE. Grey matter alterations associated with cannabis use: results of a VBM study in heavy cannabis users and healthy controls. Neuroimage. 2012;59(4):3845–3851. doi: 10.1016/j.neuroimage.2011.09.046. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Tranel D, Damasio H. Individuals with sociopathic behavior caused by frontal damage fail to respond autonomically to social stimuli. Behavioural Brain Research. 1990;41(2):81–94. doi: 10.1016/0166-4328(90)90144-4. [DOI] [PubMed] [Google Scholar]
- Daumann J, Fimm B, Willmes K, Thron A, Gouzoulis-Mayfrank E. Cerebral activation in abstinent ecstasy (MDMA) users during a working memory task: a functional magnetic resonance imaging (fMRI) study. Cognitive brain research. 2003;16(3):479–487. doi: 10.1016/s0926-6410(03)00075-2. [DOI] [PubMed] [Google Scholar]
- Droutman V, Read SJ, Bechara A. Revisiting the role of the insula in addiction. Trends in Cognitive Sciences. 2015;19(7):414–420. doi: 10.1016/j.tics.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Barnes A, Jones PS, Morein-Zamir S, Robbins TW, Bullmore ET. Abnormal structure of frontostriatal brain systems is associated with aspects of impulsivity and compulsivity in cocaine dependence. Brain. 2011;134(Pt 7):2013–2024. doi: 10.1093/brain/awr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Jones PS, Williams GB, Turton AJ, Robbins TW, Bullmore ET. Abnormal Brain Structure Implicated in Stimulant Drug Addiction. Science. 2012;335(6068):601–604. doi: 10.1126/science.1214463. [DOI] [PubMed] [Google Scholar]
- Fein G, Klein L, Finn P. Impairment on a Simulated Gambling Task in Long - Term Abstinent Alcoholics. Alcoholism: Clinical and Experimental Research. 2004;28(10):1487–1491. doi: 10.1097/01.alc.0000141642.39065.9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Acton PD, Maldjian JA, Gray JD, Croft JR, Dackis CA, O’Brien CP, Childress AR. Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biological psychiatry. 2002;51(2):134–142. doi: 10.1016/s0006-3223(01)01269-0. [DOI] [PubMed] [Google Scholar]
- Fu L-p, Bi G-h, Zou Z-t, Wang Y, Ye E-m, Ma L, Yang Z. Impaired response inhibition function in abstinent heroin dependents: an fMRI study. Neuroscience letters. 2008;438(3):322–326. doi: 10.1016/j.neulet.2008.04.033. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Tomasi D, Rajaram S, Cottone LA, Zhang L, Maloney Te, Telang F, Alia-Klein N, Volkow ND. Role of the anterior cingulate and medial orbitofrontal cortex in processing drug cues in cocaine addiction. Neuroscience. 2007;144(4):1153–1159. doi: 10.1016/j.neuroscience.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. American Journal of Psychiatry. 2002 doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nature Reviews Neuroscience. 2011;12(11):652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, Phillips RL, Kimes AS, Margolin A. Activation of memory circuits during cue-elicited cocaine craving. Proceedings of the National Academy of Sciences. 1996;93(21):12040–12045. doi: 10.1073/pnas.93.21.12040. %@ 10027–18424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon CA, Dufault DL, Wesley MJ, Porrino LJ. Elevated gray and white matter densities in cocaine abstainers compared to current users. Psychopharmacology (Berl) 2011;218(4):681–692. doi: 10.1007/s00213-011-2360-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q, Turel O, Bechara A. Brain anatomy alterations associated with Social Networking Site (SNS) addiction. Sci Rep. 2017;7:1–8. doi: 10.1038/srep45064. (paper 45064) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann D, Smolka MN, Wrase J, Klein S, Nikitopoulos J, Georgi A, Braus DF, Flor H, Mann K, Heinz A. Blockade of cue-induced brain activation of abstinent alcoholics by a single administration of amisulpride as measured with fMRI. Alcoholism: Clinical and Experimental Research. 2006;30(8):1349–1354. doi: 10.1111/j.1530-0277.2006.00174.x. [DOI] [PubMed] [Google Scholar]
- Hser YI, Evans E, Huang D, Weiss R, Saxon A, Carroll KM, Woody G, Liu D, Wakim P, Matthews AG. Long-term outcomes after randomization to buprenorphine/naloxone versus methadone in a multi-site trial. Addiction. 2016;111(4):695–705. doi: 10.1111/add.13238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hser YI, Hoffman V, Grella CE, Anglin MD. A 33-year follow-up of narcotics addicts. Archives of General Psychiatry. 2001;58(5):503–508. doi: 10.1001/archpsyc.58.5.503. [DOI] [PubMed] [Google Scholar]
- Hser YI, Stark ME, Paredes A, Huang D, Anglin MD, Rawson R. A 12-year follow-up of a treated cocaine-dependent sample. Journal of Substance Abuse Treatment. 2006;30(3):219–226. doi: 10.1016/j.jsat.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Hu Y, Salmeron B, Gu H, Stein EA, Yang Y. IMpaired functional connectivity within and between frontostriatal circuits and its association with compulsive drug use and trait impulsivity in cocaine addiction. JAMA Psychiatry. 2015 doi: 10.1001/jamapsychiatry.2015.1. [DOI] [PubMed] [Google Scholar]
- Hugh G, John P, Alan B, Jung-Ki C, Lori S, Thomas JR, Betty Jo S, Robert R, Dan K, Elliot AS. Cue-Induced Cocaine Craving: Neuroanatomical Specificity for Drug Users and Drug Stimuli. American Journal of Psychiatry. 2000;157(11):1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC. Addiction and the brain: the neurobiology of compulsion and its persistence. Nature reviews neuroscience. 2001;2(10):695–703. doi: 10.1038/35094560. [DOI] [PubMed] [Google Scholar]
- Kaag AM, Crunelle CL, van Wingen G, Homberg J, van den Brink W, Reneman L. Relationship between trait impulsivity and cortical volume, thickness and surface area in male cocaine users and non-drug using controls. Drug Alcohol Depen. 2014;144:210–217. doi: 10.1016/j.drugalcdep.2014.09.016. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. American Journal of Psychiatry. 2005;162(8):1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kanai R, Rees G. The structural basis of interindividual differences in human behaviour and cognition. Neuroscience - Nature Reviews. 2011;12(2):231–242. doi: 10.1038/nrn3000. [DOI] [PubMed] [Google Scholar]
- Kelly C, Zuo XN, Gotimer K, Cox CL, Lynch L, Brock D, Imperati D, Garavan H, Rotrosen J, Castellanos FX, Milham MP. Reduced interhemispheric resting state functional connectivity in cocaine addiction. Biol Psychiatry. 2011;69(7):684–692. doi: 10.1016/j.biopsych.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F, Ely TD, Hoffman JM, Drexler KP. Neural activity related to drug craving in cocaine addiction. Archives of general psychiatry. 2001;58(4):334–341. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- Konova AB, Moeller SJ, Tomasi D, Parvaz MA, Alia-Klein N, Volkow ND, Goldstein RZ. Structural and behavioral correlates of abnormal encoding of money value in the sensorimotor striatum in cocaine addiction. European Journal of Neuroscience. 2012;36(7):2979–2988. doi: 10.1111/j.1460-9568.2012.08211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhar MJ, Ritz MC, Boja JW. The dopamine hypothesis of the reinforcing properties of cocaine. Trends in Neurosciences. 1991;14(7):299–302. doi: 10.1016/0166-2236(91)90141-g. [DOI] [PubMed] [Google Scholar]
- Leshner AI. Addiction is a brain disease, and it matters. Science. 1997;278(5335):45–47. doi: 10.1126/science.278.5335.45. [DOI] [PubMed] [Google Scholar]
- Liao Y, Tang J, Liu T, Chen X, Hao W. Differences between smokers and non-smokers in regional gray matter volumes: a voxel-based morphometry study. Addiction biology. 2012;17(6):977–980. doi: 10.1111/j.1369-1600.2010.00250.x. [DOI] [PubMed] [Google Scholar]
- Liu H, Hao Y, Kaneko Y, Ouyang X, Zhang Y, Xu L, Xue Z, Liu Z. Frontal and cingulate gray matter volume reduction in heroin dependence: Optimized voxel-based morphometry. Psychiatry and clinical neurosciences. 2009;63(4):563–568. doi: 10.1111/j.1440-1819.2009.01989.x. [DOI] [PubMed] [Google Scholar]
- Ma N, Liu Y, Li N, Wang CX, Zhang H, Jiang XF, Xu HS, Fu XM, Hu X, Zhang DR. Addiction related alteration in resting-state brain connectivity. NeuroImage. 2010;49(1):738–744. doi: 10.1016/j.neuroimage.2009.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride D, Barrett SP, Kelly JT, Aw A, Dagher A. Effects of expectancy and abstinence on the neural response to smoking cues in cigarette smokers: an fMRI study. Neuropsychopharmacology. 2006;31(12):2728–2738. doi: 10.1038/sj.npp.1301075. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Hiott FB, Huettel SA, Rose JE. Abstinence-induced changes in self-report craving correlate with event-related FMRI responses to smoking cues. Neuropsychopharmacology. 2005;30(10):1940–1947. doi: 10.1038/sj.npp.1300780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClernon FJ, Kozink RV, Lutz AM, Rose JE. 24-h smoking abstinence potentiates fMRI-BOLD activation to smoking cues in cerebral cortex and dorsal striatum. Psychopharmacology. 2009;204(1):25–35. doi: 10.1007/s00213-008-1436-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechtcheriakov S, Brenneis C, Egger K, Koppelstaetter F, Schocke M, Marksteiner J. A widespread distinct pattern of cerebral atrophy in patients with alcohol addiction revealed by voxel-based morphometry. Journal of Neurology, Neurosurgery & Psychiatry. 2007;78(6):610–614. doi: 10.1136/jnnp.2006.095869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-López L, Catena A, Fernández-Serrano MJ, Delgado-Rico E, Stamatakis EA, Pérez-García M, Verdejo-García A. Trait impulsivity and prefrontal gray matter reductions in cocaine dependent individuals. Drug Alcohol Depen. 2012;125(3):208–214. doi: 10.1016/j.drugalcdep.2012.02.012. [DOI] [PubMed] [Google Scholar]
- Morie KP, Garavan H, Bell RP, De Sanctis P, Krakowski MI, Foxe JJ. Intact inhibitory control processes in abstinent drug abusers (II): a high-density electrical mapping study in former cocaine and heroin addicts. Neuropharmacology. 2014;82:151–160. doi: 10.1016/j.neuropharm.2013.02.023. [DOI] [PubMed] [Google Scholar]
- Noel X, Brevers D, Bechara A. A neurocognitive approach to understanding the neurobiology of addiction. Curr Opin Neurobiol. 2013;23(4):632–638. doi: 10.1016/j.conb.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosyk B, Li L, Evans E, Huang D, Min J, Kerr T, Brecht ML, Hser YI. Characterizing longitudinal health state transitions among heroin, cocaine, and methamphetamine users. Drug & Alcohol Dependence. 2014;140:69–77. doi: 10.1016/j.drugalcdep.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rando K, Tuit K, Hannestad J, Guarnaccia J, Sinha R. Sex differences in decreased limbic and cortical grey matter volume in cocaine dependence: a voxel-based morphometric study. Addiction biology. 2013;18(1):147–160. doi: 10.1111/adb.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero MJ, Asensio S, Palau C, Sanchez A, Romero FJ. Cocaine addiction: diffusion tensor imaging study of the inferior frontal and anterior cingulate white matter. Psychiatry Res. 2010;181(1):57–63. doi: 10.1016/j.pscychresns.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Schmaal L, Veltman DJ, Nederveen A, van den Brink W, Goudriaan AE. N-Acetylcysteine Normalizes Glutamate Levels in Cocaine-Dependent Patients: A Randomized Crossover Magnetic Resonance Spectroscopy Study. Neuropsychopharmacology. 2012;37(9):2143–2152. doi: 10.1038/npp.2012.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, Nagel BJ, Schweinsburg BC, Park A, Theilmann RJ, Tapert SF. Abstinent adolescent marijuana users show altered fMRI response during spatial working memory. Psychiatry Research: Neuroimaging. 2008;163(1):40–51. doi: 10.1016/j.pscychresns.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim ME, Lyoo IK, Streeter CC, Covell J, Sarid-Segal O, Ciraulo DA, Kim MJ, Kaufman MJ, Yurgelun-Todd DA, Renshaw PF. Cerebellar gray matter volume correlates with duration of cocaine use in cocaine-dependent subjects. Neuropsychopharmacology. 2007a;32(10):2229. doi: 10.1038/sj.npp.1301346. [DOI] [PubMed] [Google Scholar]
- Sim ME, Lyoo IK, Streeter CC, Covell J, Sarid-Segal O, Ciraulo DA, Kim MJ, Kaufman MJ, Yurgelun-Todd DA, Renshaw PF. Cerebellar gray matter volume correlates with duration of cocaine use in cocaine-dependent subjects. Neuropsychopharmacology. 2007b;32(10):2229–2237. doi: 10.1038/sj.npp.1301346. [DOI] [PubMed] [Google Scholar]
- Spangler R, Unterwald EM, Kreek MJ. ‘Binge’cocaine administration induces a sustained increase of prodynorphin mRNA in rat caudate-putamen. Molecular brain research. 1993;19(4):323–327. doi: 10.1016/0169-328x(93)90133-a. [DOI] [PubMed] [Google Scholar]
- Steiger JH, Fouladi RT. Noncentrality interval estimation and the evaluation of statistical models. In: Harlow LL, Mulaik SA, Steiger JH, editors. What if there were no significance tests? Lawrence Erlbaum Associates Publishers; Mahwah, NJ, US: 1997. pp. 221–257. [Google Scholar]
- Sun W, Rebec GV. Repeated Cocaine Self-Administration Alters Processing of Cocaine-Related Information in Rat Prefrontal Cortex. The Journal of Neuroscience. 2006;26(30):8004–8008. doi: 10.1523/JNEUROSCI.1413-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland MT, McHugh MJ, Pariyadath V, Stein EA. Resting state functional connectivity in addiction: lessons learned and a road ahead. Neuroimage. 2012;62(4):2281–2295. doi: 10.1016/j.neuroimage.2012.01.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe J, Tregellas JR, Dalwani M, Thompson L, Owens E, Crowley T, Banich M. Medial orbitofrontal cortex gray matter is reduced in abstinent substance-dependent individuals. Biological psychiatry. 2009;65(2):160–164. doi: 10.1016/j.biopsych.2008.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MJ, Kalivas PW, Shaham Y. Neuroplasticity in the mesolimbic dopamine system and cocaine addiction. British Journal of Pharmacology. 2008;154(2):327–342. doi: 10.1038/bjp.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Goldstein RZ, Telang F, Maloney T, Alia-Klein N, Caparelli EC, Volkow ND. Thalamo-cortical dysfunction in cocaine abusers: implications in attention and perception. Psychiatry Res. 2007;155(3):189–201. doi: 10.1016/j.pscychresns.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay J, Maleki N, Potter J, Elman I, Rudrauf D, Knudsen J, Wallin D, Pendse G, McDonald L, Griffin M. Alterations in brain structure and functional connectivity in prescription opioid-dependent patients. Brain. 2010;133(7):2098–2114. doi: 10.1093/brain/awq138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cerebral cortex. 2000;10(3):318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Goldstein RZ. Role of dopamine, the frontal cortex and memory circuits in drug addiction: insight from imaging studies. Neurobiology of learning and memory. 2002;78(3):610–624. doi: 10.1006/nlme.2002.4099. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D. Addiction circuitry in the human brain. Annual review of pharmacology and toxicology. 2012;52:321. doi: 10.1146/annurev-pharmtox-010611-134625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wise RA. How can drug addiction help us understand obesity? Nat Neurosci. 2005;8(5):555–560. doi: 10.1038/nn1452. [DOI] [PubMed] [Google Scholar]
- Wang X, Li B, Zhou X, Liao Y, Tang J, Liu T, Hu D, Hao W. Changes in brain gray matter in abstinent heroin addicts. Drug Alcohol Depen. 2012;126(3):304–308. doi: 10.1016/j.drugalcdep.2012.05.030. [DOI] [PubMed] [Google Scholar]
- Wong DF, Kuwabara H, Schretlen DJ, Bonson KR, Zhou Y, Nandi A, Brašić JR, Kimes AS, Maris MA, Kumar A. Increased occupancy of dopamine receptors in human striatum during cue - elicited cocaine craving. Neuropsychopharmacology. 2006;31(12):2716–2727. doi: 10.1038/sj.npp.1301194. [DOI] [PubMed] [Google Scholar]
- Wood SMW, Bechara A. The neuroscience of dual (and triple) system in decision making. In: Reyna VF, Zayas V, editors. The Neuroscience of Risky Decision Making. American Psychological Assoication; Washington, DC: 2014. pp. 177–202. [Google Scholar]
- Yang S, Salmeron BJ, Ross TJ, Xi ZX, Stein EA, Yang Y. Lower glutamate levels in rostral anterior cingulate of chronic cocaine users — A 1H-MRS study using TE-averaged PRESS at 3 T with an optimized quantification strategy. Psychiatry Research: Neuroimaging. 2009;174(3):171–176. doi: 10.1016/j.pscychresns.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan K, Qin W, Dong M, Liu J, Sun J, Liu P, Zhang Y, Wang W, Wang Y, Li Q. Gray matter deficits and resting-state abnormalities in abstinent heroin-dependent individuals. Neuroscience letters. 2010;482(2):101–105. doi: 10.1016/j.neulet.2010.07.005. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Zhu Z, Shi J, Zou Z, Yuan F, Liu Y, Lee TM, Weng X. Gray matter density negatively correlates with duration of heroin use in young lifetime heroin-dependent individuals. Brain and cognition. 2009;71(3):223–228. doi: 10.1016/j.bandc.2009.08.014. [DOI] [PubMed] [Google Scholar]
- Zhang X, Salmeron BJ, Ross TJ, Geng X, Yang Y, Stein EA. Factors underlying prefrontal and insula structural alterations in smokers. NeuroImage. 2011;54(1):42–48. doi: 10.1016/j.neuroimage.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]


