Abstract
Background
The ciprofloxacin-eluting sinus stent (CSS) has unique therapeutic potential to deliver antibiotics to the sinuses. The objective of this study is to evaluate the efficacy of the CSS in eliminating Pseudomonas aeruginosa infection in a rabbit model of sinusitis.
Methods
A ciprofloxacin-eluting sinus stent was created by coating ciprofloxacin/Eudragit RS100 on biodegradable poly-D/L-lactic acid (2mg). After analyzing in vitro inhibition of P. aeruginosa (PAO1 strain) biofilm formation, a total of 8 CSSs (4 shams, 4 CSSs) were placed unilaterally in rabbit maxillary sinuses via dorsal sinusotomy after inducing infection for 1 week with PAO1. Animals were assessed 2 weeks after stent insertion with nasal endoscopy, sinus culture, CT scan, histopathology, and scanning electron microscopy (SEM).
Results
PAO1 biofilm formation was significantly reduced in vitro with exposure to the CSS (p<0.0001). Insertion of the CSS in PAO1 infected rabbits for 2 weeks resulted in significant improvement in sinusitis according to endoscopy scoring (p< 0.0001) and CT scoring (p<0.002). Histology and SEM revealed marked improvement in the structure of the mucosa and submucosa with no detection of biofilm structures in the CSS cohort.
Conclusion
Although the current study included a small sample size, we revealed robust therapeutic efficacy of the ciprofloxacin coated sinus stent by reducing bacterial load and biofilm formation of P. aeruginosa in a preclinical model of sinusitis after placement for two weeks.
Keywords: Sinus stent, Ciprofloxacin, Topical drug delivery, Pseudomonas, PAO1, Chronic sinusitis, Sinusitis, Rabbit
INTRODUCTION
Characterized by impaired mucociliary clearance (MCC) with subsequent compromised microbial elimination, chronic rhinosinusitis (CRS) is known as a multifactorial disease process in which bacterial infection or colonization play a role in the initiation or propagation of the inflammatory response.1–4 The bacteria that colonize the diseased sinuses benefit from damaged epithelium, reduced innate immune mechanisms (e.g., reduced expression of antimicrobial peptides), and a rich nutrient environment to proliferate.5 Bacteria may also form antibiotic-resistant biofilms - a major factor in the refractory nature of CRS.5–9 In particular, recalcitrant CRS patients infected with Pseudomonas aeruginosa have an exuberant inflammatory process and suffer for months to years, which - from a public health perspective - has a tremendous impact on quality of life (many missed work days, need for repeat clinic visits, and continued trips to the operating room for surgical intervention).8,10,11
Long courses of antibiotic therapy are typically required for CRS to ensure sufficient antibiotic exposure to eradicate the microorganisms.5,12 Incomplete treatment with concentrations under the minimal inhibitory concentration (MIC) may aggravate the development of antibiotic resistance.13 Extended-release preparations reduce the number of administrations, improve compliance, and maintain a more constant plasma drug concentration over a prolonged period of time.14 Therefore, drug-eluting implants with prolonged mucosal contact time and sustained drug release may provide a suitable therapeutic option.13,15 Fluoroquinolone antibiotics have been frequently administered for sinus infections, but the U.S. Food and Drug Administration recently updated the warning due to safety concerns regarding tendinitis and tendon rupture with systemic administration.16 However, topical antibiotic delivery could provide effective elimination of multi-drug resistant organisms by providing a drug in a concentration above its MIC at the site of action, while significantly abrogating serious side effects. Ciprofloxacin was chosen as a model drug for incorporation into the stent because of its broad-spectrum activity and potent elimination of P. aeruginosa. It has been used for topical drug delivery in other organ systems such as the, skin, eyes, and ears.17–19
We recently analyzed the in vitro release profile of ciprofloxacin-coated biodegradable poly-D/L-lactic acid (PLLA) material and measured the in vivo drug delivery tolerance and pharmacokinetics of a ciprofloxacin-coated sinus stent (CSS) in a preclinical (rabbit) model.20 Ciprofloxacin was continuously released from the stent at concentrations that exceeded minimum standards considered optimal for P. aeruginosa infections for up to 3 weeks in the rabbit maxillary sinus in vivo. The objective of the current study is to evaluate the therapeutic efficacy of CSS in treating P. aeruginosa infection in a rabbit model of sinusitis.
METHODS
In Vitro PAO-1 Biofilm Assay with CSS
Biodegradable PLLA tubes were obtained from Zeus Inc (Orangeburg, SC) and laser fine cutting (Laserage Technology Corporation, Waukegan, IL) was employed to create porous stents for the experiments (Figure 1). PLLA polymers were cut to fit into the 24 well plates (25% of the length of the actual stent placed in the in vivo model). CSS stents for the in vitro study were prepared by coating the Eudragit RS 100 polymer (25% weight/volume (w/v) in acetone) with ciprofloxacin solution (15 mg/ml). A total of 500 μg of ciprofloxacin was coated in each CSS stent overnight, which was confirmed by measuring the amount of ciprofloxacin left in the solution after overnight-coating using spectrophotometry. For assessing the inhibition of PAO-1 biofilms, CSS stents were incubated in 2 ml of Luria-Bertani (LB)-Miller broth (Fisher Scientific, Bridgewater, NJ). Ciprofloxacin (1 μg/ml) served as the positive control. PAO-1 bacteria were added at a seeding density of 1 × 104. After 72 hours at 30 °C, crystal violet staining was used to quantify the PAO-1 biofilms. Two ml of 0.1% (w/v) crystal violet was dispensed in each well after removing the CSS and/or culture media and wells were incubated for 15 minutes. The remaining crystal violet was extracted by 2 ml of 30% acetic acid and quantified with a spectrometer at an absorbance of 590 nm.
Figure 1.
PLLA stent measured 0.9 cm in length and 0.25 cm in height, which was trimmed to 0.75 cm in length and inserted into the rabbit’s maxillary sinus.
Induction of PAO-1 Sinusitis in the Rabbit Model
This study was approved by the institutional animal care and use committee (IACUC) at the University of Alabama at Birmingham. Pasteurella-free, female, New Zealand white rabbits (2–4 kg) were used for the study. Before study initiation, rabbits were acclimatized to the animal facility for at least 1 week. Rabbits were anesthetized with [ketamine (20 mg/kg) (MWI, Boise, ID), dextomitor (0.25 mg/kg) (Zoetis Inc., Kalamazoo, MI), buprenorphine (0.03 mg/kg) (Reckitt Benckiser Pharmaceuticals Inc., Richmond, VA), and carprofen (5 mg/kg) (Zoetis Inc., Kalamazoo, MI)] in a warm room. No rabbits were intubated or provided any inhalational anesthetic agents. Nasal endoscopy (1.7mm 30-degree scope, Karl Storz, Tuttlingen, Germany) was performed bilaterally to exclude pre-existing infection or abnormal lesions. A polyurethane glycol dressing (2 cm NasoPore ®,Stryker, Kalamazoo, MI) was inserted into the middle meatus to occlude the maxillary sinus outflow tract. The ipsilateral maxillary sinus was transnasally inoculated through the medial wall of the maxillary sinus (in front of the middle turbinate) with 0.5 mL of wild-type P. aeruginosa (PAO-1) using a 22-guage spinal needle (Becton-Dickinson, Franklin Lakes, NJ) (Figure 2A). The concentration was adjusted to an OD600 = 0.6 in sterile saline (4.0 × 108 colony forming units (CFUs)).
Figure 2. Nasal Endoscopic Examination.
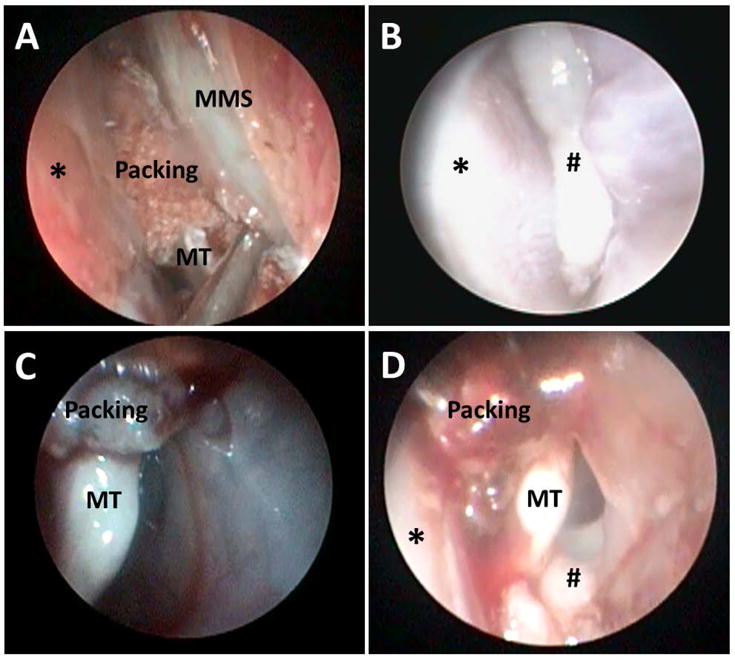
A: Transnasal inoculation of PAO-1 into the left maxillary sinus using a 22-gauge spinal needle after placement of nasal packing (*: Septum, MMS: Medial wall of maxillary sinus, MT: Middle turbinate)
B: Rabbit nasal cavity (left) on week 1 (*: Septum, #: Purulent drainage)
C: Rabbit nasal cavity (left) on week 3 after placement of CSS for 2 weeks (MT: Middle turbinate)
D: Rabbit nasal cavity (left) on Week 3 after insertion of sham stent for 2 weeks (MT: Middle turbinate, *: Septum, #: Purulent drainage from middle meatus)
Insertion of the Ciprofloxacin Coated Sinus Stents (CSS)
As previously described, PLLA stents were fabricated using a 3D printer to conform to the size of the rabbit maxillary sinus, printed at 0.75 cm in length and 0.2 cm in height.16 PLLA stents were rotated in the aqueous ciprofloxacin solution (10 mg/mL) overnight until the solution was completely dry. A total of 2mg of ciprofloxacin was successfully coated on the PLLA stents for the in vivo assay as explained in our previous study.16 Ciprofloxacin coating was identical to the stents created for in vitro studies as the in vivo stents were 4× larger. One week after inoculation, 8 New Zealand white rabbits were randomized to receive either a ciprofloxacin-loaded stent (n = 4) or sham stent (n = 4). The surgical procedure to insert the stent was as follows: 1) a dorsal nasal, vertical, midline incision was created, 2) the unilateral maxillary sinus was entered by creating a small 8mm × 8mm dorsal hole using a trocar, 3) the stent was inserted through the opening, and 4) the periosteum, subcutaneous tissue, and skin were closed with 4-0 Vicryl suture (Ethicon, Inc., Somerville, NJ). Rabbits did not receive any oral or parenteral antibiotics during or after the surgical procedure.
Outcome Measures
A sham version of the stent served as the control to differentiate effects related to the drug from the consequences related to the physical presence of the stent.
Endoscopic examination – 1.7mm 30-degree endoscopy was performed in the nasal cavity before (week 1) and after stent placement (week 3). As there is no validated endoscopic grading system in the animal model, we generated a visual analog scale (VAS) system for three categories (edema, scarring, discharge) as a single mark on a 10-cm visual analogue scale from 0 (none) to 10 (most severe) with a total possible score of 30. Endoscopic examinations from each rabbit were video recorded, de-identified, and aggregated and 3 investigators blinded to the rabbits’ history independently scored them based on a VAS system.
Micro computed tomography (CT) scanning - All rabbits were scanned at week 1 to confirm the presence of sinusitis after the inoculation of PAO-1. After implantation, micro CT scanning was repeated at week 3. Micro CT scanning was performed at the UAB small animal imaging shared facility using SPECT/CT (X-SPECT system, Gamma Medica, Northridge, CA). CT findings of sinus opacification were scored as follows: 1: mild; 2: moderate; 3: severe; 4: very severe, based on previous radiological grading methods in experimental rabbit sinusitis.21 Absence of any opacification was scored as 0.
Bacterial counts (colony forming units) from nasal swabs – A cotton swab (BBL™ CultureSwab™ Plus, Sparks, MD) was inserted into the middle meatus to collect mucus from all rabbits at week 1 (before stent insertion) and week 3. The mucus was then re-suspended in LB-Miller broth overnight (37°C) and shaken at 200 rpm. The next day, 500 μl of the solution was transferred to each well of a 24-well plate. A microplate reader measured the OD at 600 nm (Synergy HK, BIO-TEK Instruments, Winooski, VT). An OD600 of 0.1 was equal to 3 × 108 CFU/ml and OD readings between 0.2 and 0.8 are considered reliable.
Histology and SEM – After micro CT scanning at week 3, rabbits were euthanized and heads were harvested for histologic sectioning with hematoxylin and eosin (H&E) staining (n = 4, 2 rabbits from each group) or SEM (n = 4, 2 rabbits from each group). Ten representative sections of the maxillary sinus were selected and stained with H&E. Slides were evaluated by a veterinary pathologist blinded to the specimen and side. For SEM, the mucosa was extracted with the surrounding bone of the maxillary sinus. The mucosa from one rabbit of each study arm was prepared to study biofilm identification using SEM. 4% glutaraldehyde-fixed mucosal specimens were dehydrated in a series of increasing ethanol concentrations, up to 100%. The mucosa was then critical-point dried in CO2, mounted on aluminum stubs, and prepared with Au-Pd sputter coating to promote surface conductivity and reduce charging artifacts. The PAO-1 biofilms were imaged using a Quanta FEG 650 Scanning Electron Microscope (FEI, Hillsboro, OR) at an accelerating voltage of 20 kV. Photomicrographs were then evaluated for morphological evidence of appropriate-sized (0.5–2 mm diameter) rod-shaped bacteria existing in towers with intertwining water channels.12 Representative pictures, blinded to the study group, were then analyzed by the veterinary pathologist (Trenton R. Schoeb) for the presence of biofilms.
Statistical Analysis
Statistical analyses were conducted using Excel 2016 and GraphPad Prism 6.0 software (La Jolla, Ca) with significance set at P < 0.05. Statistical evaluation utilized unpaired Student t tests. Data is expressed +/− standard error of the mean.
RESULTS
In Vitro PAO-1 Biofilm Assay with CSS
To assess biofilm formation inhibition of CSS against the PAO-1 strain, PAO-1 biofilms were grown for 72 hours in the presence of CSS (coated with 500 μg of ciprofloxacin, n = 3) and 1 μg/ml of ciprofloxacin (positive control, n = 3) (Figure 3). The CSS significantly reduced biofilm mass compared to controls (relative biofilm value compared to control at OD590, CSS = 0.11+/− 0.03, p < 0.0001).
Figure 3.
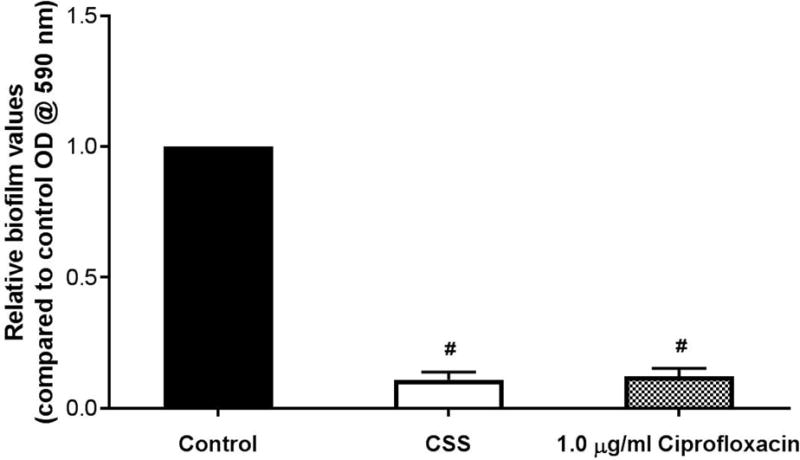
Effect of ciprofloxacin-coated sinus stent on Pseudomonas aeruginosa PAO-1 biofilms: CSS (coated with 500 μg of ciprofloxacin) and 1 μg/ml of ciprofloxacin (positive control) significantly reduced biofilm mass. #: Statistical significance when compared to control (p < 0.0001) in reducing PAO-1 biofilm formation (n = 3, respectively).
Nasal Endoscopic Examination
Nasal endoscopies were performed in all rabbits (n = 8) on week 0, week 1 (induction of acute sinusitis with PAO-1, before the placement of stents), and week 3 (after insertion of the stents) (Figure 2). The inferior, middle and superior meati were inspected bilaterally. There was no evidence of purulent drainage or signs of infection on week 0. All rabbits demonstrated significant purulent drainage from the nostril where PAO-1 was inoculated by week 1 (Figure 2B). By week 3, a marked difference was noticed between the 2 groups. For those rabbits who had CSS at week 1 (n = 4), purulent drainage from the middle meatus was almost cleared in the CSS cohort when compared to the sham stent cohort (n = 4) by week 3 (Figure 2C and 2D). Substantial improvement was also noticed on the nasal endoscopic examinations after placement of the CSS. Rabbits who had insertion of the CSS at week 1 demonstrated significantly lower VAS scores at week 3 compared to week 1 (week 1 = 28.7 +/− 0.38, week 3 = 16.3 +/− 0.88, p < 0.0001). However, there was no difference in rabbits who received the sham stent (week 1 = 28.5 +/− 0.45, week 3 = 27.6 +/− 0.43, p = 0.08).
Micro CT
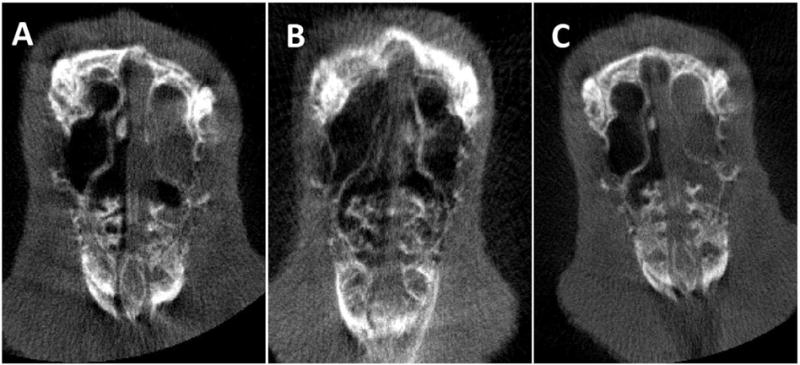
Micro CT examination was also performed in rabbits (n = 8) before and after insertion of the sham stents and CSS (Figure 4) at week 1 and week 3. All rabbits demonstrated complete or near-complete opacification of the ipsilateral sinuses at week 1. The CSS group had marked improvement of opacification by week 3 compared to the sham group at week 3 (Figure 4B and 4C). Rabbits in the CSS cohort demonstrated significantly lower CT scores at week 3 compared to week 1 (week 1 = 3.8 +/− 0.37, week 3 = 1.0 +/− 0.41, p < 0.002). There was no difference in the CT scores of the sham cohort (week 1 = 3.3 +/− 0.41, week 3 = 3.0 +/− 0.41, p = 0.47).
Figure 4. Micro CT.
A: Induction of acute sinusitis with PAO-1 on week 1 – Complete opacification in the left maxillary sinus.
B: Post-implantation of the ciprofloxacin coated sinus stent at week 3 – Clearance of opacification in the left maxillary sinus.
C: Post-implantation of the sham sinus stent at week 3 – Complete opacification in the left maxillary sinus.
Bacterial load
To determine PAO-1 bacterial load within the middle meatus, quantities were calculated using a spectrometer (that reads absorbance at 600 nm) from a swab culture. There was a statistically significant decrease in PAO-1 growth at week 3 for the CSS cohort (OD600 of CSS rabbits (n = 4): Week 1 = 0.83 +/− 0.01, Week 3 = 0.54 +/− 0.08, p = 0.047). However, there was no difference in the PAO1 bacterial load at week 3 for the sham group (OD600 of sham rabbits (n = 4): Week 1 = 0.86 +/− 0.00, Week 3 = 0.98 +/− 0.09, p = 0.25).
Histology and SEM
Histologic analysis was performed at identical sites in all rabbits (n = 4) at the lateral wall of the maxillary sinus (Figure 5A). Compared to control sides, significant submucosal edema with massive hyperplasia of submucosal glands and infiltration of inflammatory cells were noted in the rabbits who received non-drug eluting stents at week 3 (Figure 5D). In contrast, mild submucosal edema with mild hypertrophy of submucosal glands was noted in rabbits in the CSS group at week 3 (Figure 5C). The mucosa of 4 rabbits with either CSS (n=2) or sham stent (n=2) for 2 weeks were analyzed with SEM (Figure 6). Bacterial biofilms were only identified in the sham cohort. No bacterial biofilm towers were observed in the CSS group of rabbits. However, rods consistent with P. aeruginosa were attached to the sinus mucosa. Ciprofloxacin-mediated inhibition of the PAO-1 biofilm mass is reflected in the SEM micrographs. When analyzing the PLLA stents, sham stents were fully coated with inflammatory cells and biofilms while no bacterial colonization or biofilms were observed on the CSS matrix (n = 2).
Figure 5. Histology at Week 3 (Scale shown in right upper).
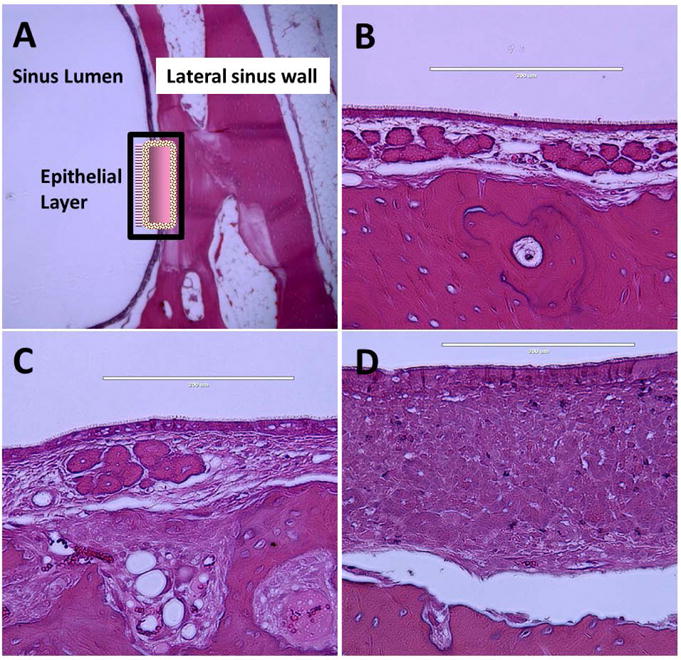
A. Schematic drawing of histologic analysis site - Lateral wall of rabbit maxillary sinus.
B: Control (Contralateral) side of maxillary sinus.
C: Post-implantation of ciprofloxacin coated sinus stent – Mild submucosal edema with mild infiltration of inflammatory cells.
D: Post-implantation of sham sinus stent – Significant submucosal edema with massive hypertrophy/hyperplasia of submucosa glands and infiltration of inflammatory cells.
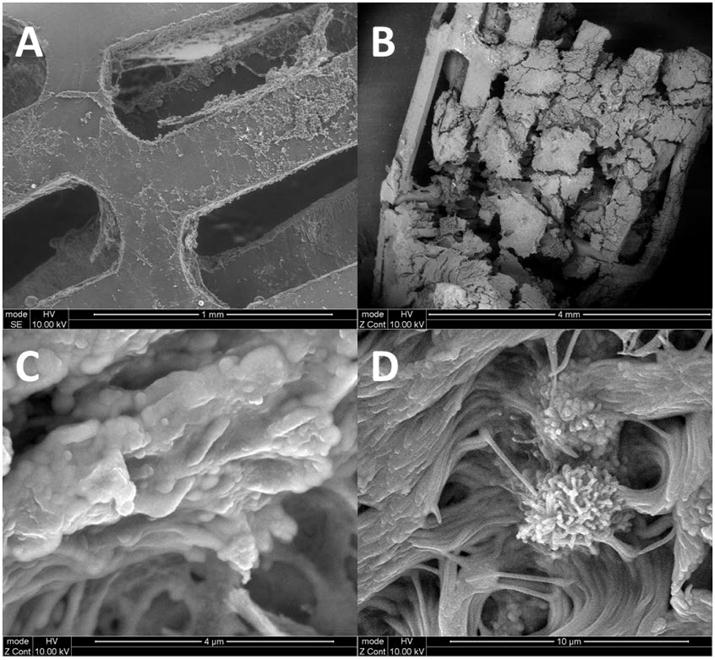
Figure 6. Scanning Electron Microscopy.

A: Ciprofloxacin coated sinus stent at week 3 – No evidence of bacterial colonization or biofilms.
B: Sham stent at week 3 - Completely coated with inflammatory cells and biofilms.
C: Sinus mucosa covered with biofilm-forming P. aeruginosa.
D: Sinus mucosa infected with biofilm: Pseudomonas bacillus embedded within a tower matrix.
DISCUSSION
This is the first study to evaluate the in vivo efficacy of an antimicrobial coated sinus stent for the treatment of sinonasal bacterial biofilms. Biofilms are structured communities of bacterial cells that exist in a self-produced polymeric matrix that are adherent to an inert foreign body or living surface, including damaged epithelium.7 Once present, biofilms are difficult to eradicate even with antibiotics, in part because the bacteria at the center of a biofilm are less metabolically active secondary to hypoxia and nutrient limitation and become unresponsive to the cellular mechanisms driving the action of antibiotics.5 Drug-eluting implants with prolonged mucosal contact duration and sustained drug release could provide a suitable therapeutic alternative in treating recalcitrant bacterial biofilms by delivering high doses of drug directly to the site of the disease while minimizing systemic exposure and toxicity.13,15,22,23 The CSS in the present study had clear therapeutic efficacy in reducing the clinical appearance, bacterial load, and biofilm formation of P. aeruginosa in a preclinical model of sinusitis.
Recent improvements in polymer science have led to the development of implants made of bioabsorbable materials for use in the human body, including the sinonasal cavities.22 Implant materials, such as PLLA, are attractive because of their biocompatibility, biodegradability and capability of encapsulating various drugs.13,24 As a foreign body, there is a risk related to insertion in infected sinus cavities – primarily bacterial colonization or biofilm formation on the stent itself.25 One effective way to prevent bacterial colonization is by using antimicrobial-biocompatible polymers or by forming coatings on the polymeric surface.26 Their presence causes the reduction of initial microbial adhesion to surfaces and inactivation of microorganisms already adherent to a surface. Nanotechnology is a promising tool for the preparation of antimicrobial-treated polymers in its various forms, such as, nanoparticles, nanolayers, nanowires.26–28 Data in the current study suggests that antibiotic coated stents prevent bacterial adhesion and inhibit colonization (preclusion of biofilm formation) in this preclinical model. This is well demonstrated in SEM images of the drug-coated PLLA stents when compared to sham stents.
Robust therapeutic efficacy was observed in this study with the CSS as measured by nasal endoscopy, histology and CT scanning. However, planktonic P. aeruginosa was still present on culture and SEM despite previous pharmacokinetic data indicating local tissue levels of ciprofloxacin persisted at levels sufficient to eradicate them. Therefore, the CSS at the current dose (2 mg of ciprofloxacin) did not completely resolve the infection in this experiment. Higher doses of ciprofloxacin may be necessary to eradicate the infection when delivered topically. Chiu et al. found PAO1 was only eradicated when sinuses were irrigated with tobramycin at 400× the MIC for tobramycin for 7 days.29 Based on our previous high-performance liquid chromatography analysis, the CSS coated with 2 mg ciprofloxacin continuously released drug at a concentration approximately 20 times higher than the MIC for PAO1 strain for up to 3 weeks – 20-fold lower than what was observed with topical tobramycin delivery.
The presence of planktonic P. aeruginosa on culture and SEM after CSS placement could be related to the large initial burst of ciprofloxacin with lower sustained delivery demonstrated in our previous pharmacokinetic experiments.20 The initial drug release from the stent is likely related to the water-soluble properties of ciprofloxacin HCL. This phenomenon was present in other sinus stents as well.30 Antibiotic delivery requires a loading dose that is available for later sustained release. Recent understanding regarding the mechanisms underlying burst release have allowed identification of several strategies to control burst.31 Double (two layer) coating, dual coating with additional antimicrobial compounds (for synergism), or complex formation delivery (liposomal delivery) are techniques that may enhance the effectiveness of the stent and, thus warrant further investigation.
Targeting biofilms is a critical aspect of antibiotic delivery in recalcitrant CRS. SEM provides high-resolution images of the mucosal surface and is a well-accepted method to aid the identification of biofilms adherent to sinus mucosa.29 Bacterial biofilms in this study were identified by SEM in the sinus mucosa of rabbits with sham stents, but no similar structures were observed in the CSS cohort. Only small areas of tissue (10-20 mm) were analyzed at any one time so these tissue samples may not truly represent overall findings within the rabbit sinus. Importantly, the exact same location was analyzed in every rabbit.
Limitations of the current study include a small sample size and use of an animal model rather than human subjects. The rabbit sinusitis model may not reflect the human in vivo situation in recalcitrant CRS. Microbiota studies using animal models have also shown inter-study variations due to confounding factors (e.g. origin, maternal effects, and environmental conditions).32 However, the advantages of the rabbit model are numerous in that it enables us to control for a number of variables that are inherent in human samples, including genetics, medical history, environmental allergies/pollutants, and medication use. Future studies using the CSS at higher concentrations or dual coating with other antibacterial agents are planned.
CONCLUSION
Although the current study included a small sample size, we revealed robust therapeutic efficacy of the ciprofloxacin coated sinus stent by reducing bacterial load and biofilm formation of P. aeruginosa in a preclinical model of sinusitis after placement of two weeks.
Acknowledgments
This work was supported by National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (1 R01 HL133006-02) and National Institute of Diabetes and Digestive and Kidney Diseases (5P30DK072482-04, CF Research Center Pilot Award) to B.A.W., John W. Kirklin Research and Education Foundation Fellowship Award, UAB Faculty Development Research Award, American Rhinologic Society New Investigator Award, and Cystic Fibrosis Foundation Research Development Pilot grant (ROWE15R0) to D.Y.C,. We would like to show our gratitude to Trenton R. Schoeb, DVM, PhD for reviewing histology slides.
Do-Yeon Cho, MD receives research grant support from Bionorica Inc.
Bradford A. Woodworth, M.D. is a consultant for Olympus and Cook Medical. He also receives grant support from Bionorica Inc. and Cook Medical.
Footnotes
Manuscript to be presented at the American Rhinologic Society Annual Meeting, Chicago, IL, September 9th, 2017.
References
- 1.Ramakrishnan VR, Feazel LM, Gitomer SA, Ir D, Robertson CE, Frank DN. The microbiome of the middle meatus in healthy adults. PLoS One. 2013;8:e85507. doi: 10.1371/journal.pone.0085507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Illing EA, Cho DY, Zhang S, et al. Chlorogenic Acid Activates CFTR-Mediated Cl− Secretion in Mice and Humans: Therapeutic Implications for Chronic Rhinosinusitis. Otolaryngol Head Neck Surg. 2015;153:291–297. doi: 10.1177/0194599815586720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woodworth BA. Resveratrol ameliorates abnormalities of fluid and electrolyte secretion in a hypoxia-Induced model of acquired CFTR deficiency. Laryngoscope. 2015;125(Suppl 7):S1–S13. doi: 10.1002/lary.25335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang S, Skinner D, Hicks SB, et al. Sinupret activates CFTR and TMEM16A-dependent transepithelial chloride transport and improves indicators of mucociliary clearance. PLoS One. 2014;9:e104090. doi: 10.1371/journal.pone.0104090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kennedy JL, Borish L. Chronic rhinosinusitis and antibiotics: the good, the bad, and the ugly. Am J Rhinol Allergy. 2013;27:467–472. doi: 10.2500/ajra.2013.27.3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benninger MS, Ferguson BJ, Hadley JA, et al. Adult chronic rhinosinusitis: definitions, diagnosis, epidemiology, and pathophysiology. Otolaryngol Head Neck Surg. 2003;129:S1–32. doi: 10.1016/s0194-5998(03)01397-4. [DOI] [PubMed] [Google Scholar]
- 7.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 8.Psaltis AJ, Weitzel EK, Ha KR, Wormald PJ. The effect of bacterial biofilms on post-sinus surgical outcomes. Am J Rhinol. 2008;22:1–6. doi: 10.2500/ajr.2008.22.3119. [DOI] [PubMed] [Google Scholar]
- 9.Orlandi RR, Kingdom TT, Hwang PH, et al. International Consensus Statement on Allergy and Rhinology: Rhinosinusitis. Int Forum Allergy Rhinol. 2016;6(Suppl 1):S22–209. doi: 10.1002/alr.21695. [DOI] [PubMed] [Google Scholar]
- 10.Bendouah Z, Barbeau J, Hamad WA, Desrosiers M. Biofilm formation by Staphylococcus aureus and Pseudomonas aeruginosa is associated with an unfavorable evolution after surgery for chronic sinusitis and nasal polyposis. Otolaryngol Head Neck Surg. 2006;134:991–996. doi: 10.1016/j.otohns.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Singhal D, Psaltis AJ, Foreman A, Wormald PJ. The impact of biofilms on outcomes after endoscopic sinus surgery. Am J Rhinol Allergy. 2010;24:169–174. doi: 10.2500/ajra.2010.24.3462. [DOI] [PubMed] [Google Scholar]
- 12.Woodworth BA, Tamashiro E, Bhargave G, Cohen NA, Palmer JN. An in vitro model of Pseudomonas aeruginosa biofilms on viable airway epithelial cell monolayers. Am J Rhinol. 2008;22:235–238. doi: 10.2500/ajr.2008.22.3178. [DOI] [PubMed] [Google Scholar]
- 13.Gao P, Nie X, Zou M, Shi Y, Cheng G. Recent advances in materials for extended-release antibiotic delivery system. J Antibiot (Tokyo) 2011;64:625–634. doi: 10.1038/ja.2011.58. [DOI] [PubMed] [Google Scholar]
- 14.Blasi F, Aliberti S, Tarsia P. Clinical applications of azithromycin microspheres in respiratory tract infections. Int J Nanomedicine. 2007;2:551–559. [PMC free article] [PubMed] [Google Scholar]
- 15.Albu S. Novel drug-delivery systems for patients with chronic rhinosinusitis. Drug Des Devel Ther. 2012;6:125–132. doi: 10.2147/DDDT.S25199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daneman N, Lu H, Redelmeier DA. Fluoroquinolones and collagen associated severe adverse events: a longitudinal cohort study. BMJ Open. 2015;5:e010077. doi: 10.1136/bmjopen-2015-010077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jain RL, Shastri JP. Study of ocular drug delivery system using drug-loaded liposomes. Int J Pharm Investig. 2011;1:35–41. doi: 10.4103/2230-973X.76727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kataria K, Gupta A, Rath G, Mathur RB, Dhakate SR. In vivo wound healing performance of drug loaded electrospun composite nanofibers transdermal patch. Int J Pharm. 2014;469:102–110. doi: 10.1016/j.ijpharm.2014.04.047. [DOI] [PubMed] [Google Scholar]
- 19.Park AH, White DR, Moss JR, Bear M, LeBel C. Phase 3 Trials of Thermosensitive Ciprofloxacin Gel for Middle Ear Effusion in Children with Tubes. Otolaryngol Head Neck Surg. 2016;155:324–331. doi: 10.1177/0194599816645526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cho DY, Hoffman K, Skinner D, et al. Tolerance and pharmacokinetics of a ciprofloxacin-coated sinus stent in a preclinical model. Int Forum Allergy Rhinol. 2016 doi: 10.1002/alr.21892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ozcan KM, Ozcan I, Selcuk A, et al. Comparison of Histopathological and CT Findings in Experimental Rabbit Sinusitis. Indian J Otolaryngol Head Neck Surg. 2011;63:56–59. doi: 10.1007/s12070-011-0120-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parikh A, Anand U, Ugwu MC, Feridooni T, Massoud E, Agu RU. Drug-eluting nasal implants: formulation, characterization, clinical applications and challenges. Pharmaceutics. 2014;6:249–267. doi: 10.3390/pharmaceutics6020249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geller DE. Aerosol antibiotics in cystic fibrosis. Respir Care. 2009;54:658–670. doi: 10.4187/aarc0537. [DOI] [PubMed] [Google Scholar]
- 24.Cho DY, Skinner D, Zhang S, et al. Cystic fibrosis transmembrane conductance regulator activation by the solvent ethanol: implications for topical drug delivery. Int Forum Allergy Rhinol. 2016;6:178–184. doi: 10.1002/alr.21638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Samuel U, Guggenbichler JP. Prevention of catheter-related infections: the potential of a new nano-silver impregnated catheter. Int J Antimicrob Agents. 2004;23(Suppl 1):S75–78. doi: 10.1016/j.ijantimicag.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 26.Polivkova M, Hubacek T, Staszek M, Svorcik V, Siegel J. Antimicrobial Treatment of Polymeric Medical Devices by Silver Nanomaterials and Related Technology. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18020419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roe D, Karandikar B, Bonn-Savage N, Gibbins B, Roullet JB. Antimicrobial surface functionalization of plastic catheters by silver nanoparticles. J Antimicrob Chemother. 2008;61:869–876. doi: 10.1093/jac/dkn034. [DOI] [PubMed] [Google Scholar]
- 28.Siegel J, Polivkova M, Kasalkova NS, Kolska Z, Svorcik V. Properties of silver nanostructure-coated PTFE and its biocompatibility. Nanoscale Res Lett. 2013;8:388. doi: 10.1186/1556-276X-8-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiu AG, Antunes MB, Palmer JN, Cohen NA. Evaluation of the in vivo efficacy of topical tobramycin against Pseudomonas sinonasal biofilms. J Antimicrob Chemother. 2007;59:1130–1134. doi: 10.1093/jac/dkm087. [DOI] [PubMed] [Google Scholar]
- 30.Li PM, Downie D, Hwang PH. Controlled steroid delivery via bioabsorbable stent: safety and performance in a rabbit model. Am J Rhinol Allergy. 2009;23:591–596. doi: 10.2500/ajra.2009.23.3391. [DOI] [PubMed] [Google Scholar]
- 31.Allison SD. Analysis of initial burst in PLGA microparticles. Expert Opin Drug Deliv. 2008;5:615–628. doi: 10.1517/17425247.5.6.615. [DOI] [PubMed] [Google Scholar]
- 32.Nguyen TL, Vieira-Silva S, Liston A, Raes J. How informative is the mouse for human gut microbiota research? Dis Model Mech. 2015;8:1–16. doi: 10.1242/dmm.017400. [DOI] [PMC free article] [PubMed] [Google Scholar]


