Abstract
Chronic kidney disease (CKD) is a global problem, which contributes to a significant morbidity and mortality in China. Concomitant inflammatory state further boosts the mortality due to cardiovascular events in patients with CKD undergoing dialysis. There is a general notion that Omega-3 fatty acid including, docosahexaenoic acids (DHA) and eicosapentaenoic (EPA) has certain health benefits perhaps via the regulation of inflammation. However, the anti-inflammatory effect of omega-3 fatty acids in patients with CKD is controversial. We analyzed the data of oral supplementation of omega-3 fatty acid in CKD patients by searching literature on database from inception to August 2016. The analysis included randomized controlled trials (RCTs) derived from multiple databases, and the effect of omega-3 fatty acids supplementation versus the control cohorts were compared. All of the data analysis was calculated by RevMan 5.2. A total of twelve RCTs involving 487 patients were included in the meta-analysis. Among them 254 patients received omega-3 fatty acid and 233 patients served as controls who received placebo. The meta-analysis revealed no statistical significance in serum levels of CRP (SMD, −0.20; 95% CI, −0.44 to 0.05; p=0.11), IL-6 (SMD, 0.00; 95% CI, −0.33 to 0.33; p=0.99) and TNF-α (SMD, 0.14; 95% CI, −0.17 to 0.44; p=0.38) between the omega-3 fatty acids supplementation group and control. This suggested that there is insufficient evidence to conclude the benefit of omega-3 fatty acids oral supplementation in reducing serum levels of CRP, IL-6 and TNF-α in patients with CKD.
Keywords: Omega-3 fatty acids, Chronic Kidney Disease, Dialysis, CRP, IL-6, TNF-α
INTRODUCTION
Chronic kidney disease (CKD) is a worldwide public health problem. There is an increasing incidence and prevalence of kidney failure in CKD. Apparently, the uremic toxins in CKD patients induce inflammatory responses that are mediated by a variety of cytokines, chemokines and other inflammatory molecules. Consequentially, there is an enhanced oxidative stress and tissue injury. In such an inflammatory setting in patients with CKD and those undergoing dialyses may have a higher mortality due to precipitation of undesirable cardiovascular events (1–3). This chronic inflammatory state in patients on hemodialysis can be monitored by various serum biomarkers, such as, C-reactive protein (CRP), interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) (4). Thus, one can envisage that the modulation of inflammatory responses may serve an important target for the treatment of CKD (5), and efforts to prevent or attenuate inflammation would be an important step to delay the progression of CKD.
Omega-3 fatty acids (ω-3 fatty acid or n-3 fatty acid) are long-chain polyunsaturated fatty acids, which consist of α-linolenic acid (ALA, 18:3 n-3), eicosapentaenoic (EPA, 20:5 n-3) and docosahexaenoic acids (DHA, 22:6 n-3). ALA is present in vegetable oil like flaxseed, canola, olive oil, walnuts and so on, whereas EPA and DHA are found in abundance in marine oils and most fish, particularly oily ones such as tuna, salmon and trout etc (6). Omega-3 fatty acids are essential fatty acids since they are not synthesized in the body (7). There is increasing evidence that suggests omega-3 fatty acids participation in the regulation of inflammation and immune response by reducing the production monocyte pro-inflammatory cytokine, such as, interleukin-1 (IL-1), IL-6 and TNF-α (8). In addition, they can also reduce triglyceride (TG) levels (9, 10) and hence the cardiovascular risk (11, 12). In this regard, the American Heart Association (AHA) has issued guidelines for recommending daily supplementation of omega-3 fatty acids in patients with the potential to develop cardiovascular disease (13). Likewise, Improving Global Outcomes (KDIGO) Clinical Practice Guidelines for Glomerulonephritis in 2012 (14) and the Japanese Clinical Practice Guides for IgA nephropathy (IgAN) in 2014 (15) recommended using omega-3 fatty acids in the treatment of these nephritides. In addition, the omega-3 fatty acids also reduce systemic inflammation by lowering the serum levels of TNF-α, IL-6 and CRP (16).
However, controversies have risen since recent studies have challenged the notion of anti-inflammatory effect of omega-3 fatty acids in patients with CKD. Most of the past studies indicate that patients with CKD have low omega-3 fatty acids levels, which expectedly result in an inflammatory co-morbid state (17). In support of this notion certain studies revealed that supplementation of omega-3 fatty acids decreases levels of inflammatory markers in hemodialysis patients (18, 19). However, other studies report no significant effect in lowering inflammation in CKD patients treated with omega-3 fatty acids supplementation (20, 21).
In this meta-analysis study, we report randomized controlled trial (RCT) while focusing on the changes in the serum concentrations of CRP, IL-6 and TNF-α following oral supplementation of omega-3 fatty acid in CKD patients, and evaluated whether or not omega-3 fatty acids improve the inflammatory state.
MATERIALS AND METHODS
Inclusion criteria
In this RCTs study we compared the effect of supplementation of omega-3 fatty acid, DHA and/or EPA, on markers of inflammation between experimental subjects and the controls receiving placebo. The experimental subjects included patients with CKD receiving renal dialysis and who were dialysis-independent. The outcomes parameters included measuring the levels of serum IL-6, TNF-α and CRP. The literature studies excluded were as follows: reviews, meeting summaries, case reports, and non-clinical research articles, non-randomized controlled trials, animal research data, incorrect or incomplete data that cannot be extracted, duplicate publications or poor quality data, and studies lacking inflammatory marker data.
Search strategy
We searched the literature in Pubmed, Embase, Cochrane Central Register of Controlled Trials (CCRCT) and China National Knowledge Infrastructure Database (CNKI) for clinical trials assessing the effects of omega-3 fatty acid on markers of inflammation in CKD patients from inception to August 2016. The following keywords were used: chronic renal failure (CRF), end stage renal disease (ESRD), chronic kidney disease (CKD), uremia, dialysis, hemodialysis (HD) or peritoneal dialysis; combined with omega-3 fatty acid, omega-3 polyunsaturated fatty acid, ω-3 fatty acid, n-3 fatty acid, n-3 polyunsaturated fatty acid, polyunsaturated fatty acids, fish oil, docosahexaenoic acid, eicosapentaenoic acid, DHA or EPA, and C-reactive protein, CRP, interleukin 6, IL-6, tumor necrosis factor alpha, TNF-α and inflammation. In addition, we also screened the references pertaining to the included studies with the aim to identify potential eligibility for RCTs.
Study selection
We included RCTs analyzing oral supplementation of omega-3 fatty acid that affects inflammation parameters in patients with CKD. Two investigators reviewed all the article titles and abstracts independently. The studies that were clearly irrelevant were excluded. For articles, full-text of the articles was examined and evaluated that seem to yield certain degree of certainty. The studies that failed to include inflammation markers were excluded. Study selection and exclusion process is shown in Figure 1.
Figure 1.
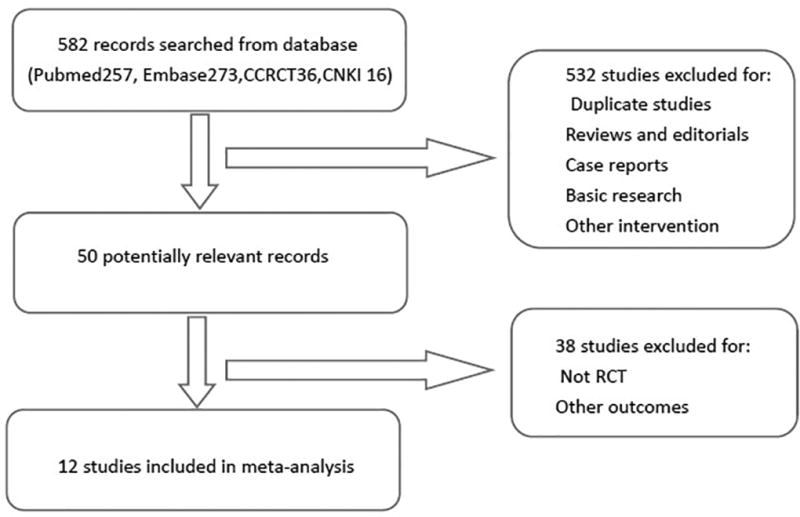
Flow diagram of the literature selection and the exclusion process.
Data extraction and management
The data extraction was collected independently by two investigators: first author's name, study design, publication year, sample size (treatment/control), sex ratio (men/female), measurements of IL-6, TNF-α and CRP levels, duration of follow-up, treatment group (dose of n-3 PUFAs), control group (placebo or other), and kidney disease status. At the same time, the mean with 95% confidence interval was used to calculate the mean ± standard deviation (SD) (22). The data were input to RevMan 5.2 by two investigators independently.
Study quality assessment
We used the Cochrane risk of bias guidelines (23) to assess the quality of studies, which included generation of random sequence, blinding of participants and personnel, blinding of outcome assessment, allocation concealment, incomplete outcome data as well as selective reporting, and other bias. For each study, according to the risk of bias guidelines, we designated as high risk of bias, low risk of bias or unclear risk of bias (Figure 2).
Figure 2.
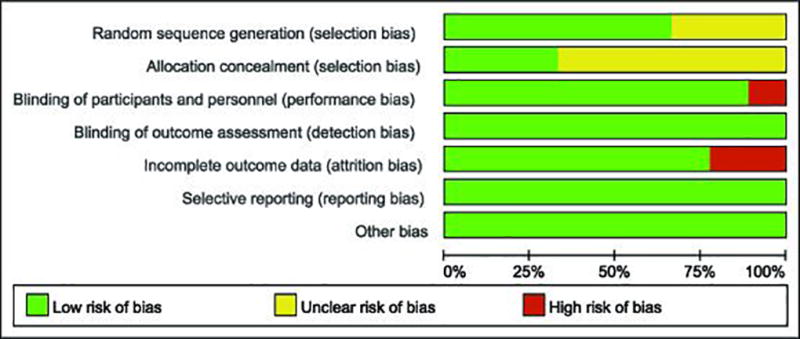
Study quality assessment.
Statistical analysis
For continuous results, we used mean differences (MD) with 95% confidence interval (95% CI). Since the measurement unit is different in the included RCTs, the standardized mean difference (SMD) with corresponding 95% CI to calculate continuous variables was used. Assessment of statistical heterogeneity among trials by using χ2 and I2 statistics was employed (24). If heterogeneity didn't exist among studies, we applied fixed-effect model to calculate pooled effect size, otherwise a random-effect model was used. In addition, the outcomes were designated statistically significant when the p value <0.05.
RESULTS
Study selection
We identified records of 582 patients from Pubmed, Embase, Cochrane Central Register of Controlled Trials (CCRCT) and China National Knowledge Infrastructure Database (CNKI). Out of these 532 irrelevant studies were excluded because of duplication identified by surveying the article's title and abstract. We further excluded 38 studies after retrieving the full texts from the remaining 50 articles. The main reason was because of being non-RCTs or not inclusive of reporting inflammatory markers. Finally, 12 studies were eligible and thus included; the details of the retrieval processes are shown in Figure 1.
Characteristics of included studies and quality assessment
The characteristics of all the studies in this meta-analysis are shown in Table 1. These 12 studies included a total of 487 patients; among which 254 were treated with omega-3 fatty acid and a control group included 233 treated with placebo. These studies also provided the outcomes reflected in the levels of CRP (22, 25–33), IL-6 (25, 27, 28, 32–34) and TNF-α (27, 28, 34, 35). Among these, 4 studies included CKD patients who did not receive dialysis (22, 31, 34, 35). The treatment studies included oral supplementation of omega-3 fatty acid at doses of 0.8 – 6g/d for 2 – 6 months. Additionally, the risk assessments were included in these studies and they are listed in Figure 2.
Table 1.
Characteristics of included studies
| Stud(year) | Study design |
No.patients | Gender (M/F) |
Biomarkers | Duration (months) |
Intervention | Treatment modality |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Himmelfarb et al 2007[32] | RCT | T:31 | T:23/8 | IL-6 and CRP | 2 months | T:(0.8g DHA)/day | hemodialysis |
| C:32 | C:17/15 | C: sunflower oil | |||||
|
| |||||||
| Saifullah et al 2007[30] | RCT | T:15 | T:11/4 | CRP | 3 months | T: (1.3g ω3FA)/day | hemodialysis |
| C:8 | C:7/1 | C: soybean/corn oil | |||||
|
| |||||||
| Madsen et al 2007[31] | RCT | T:22 | T:13/9 | CRP | 2 months | T: (2.4g ω3FA)/day | nondialysis |
| C:24 | C:17/7 | C: olive oil | |||||
|
| |||||||
| Bowden et al 2009[26] | RCT | T:18 | T:11/7 | CRP | 6 months | T: (6g Fish oil)/day | hemodialysis |
| C:15 | C:8/7 | C: corn oil | |||||
|
| |||||||
| Mori et al 2009[22] | RCT | T:20 | T:12/8 | CRP | 2 months | T: (4g ω3FA )/day | nondialysis |
| C:15 | C:8/7 | C: olive oil | |||||
|
| |||||||
| Kooshki et al 2010[28] | RCT | T:17 | T:10/7 | CRP,TNF-α and IL6 | 2.5months | T:(1.24g EPA, 0.84g DHA)/day | hemodialysis |
| C:17 | C:11/6 | C: triglyceride oils | |||||
|
| |||||||
| Mat Daud et al 2012[29] | RCT | T:31 | T:20/11 | CRP | 6 months | T: (1.8g EPA, 0.6g DHA)/TIW | hemodialysis |
| C:32 | C:12/20 | C: olive oil | |||||
|
| |||||||
| Deike et al 2012[34] | RCT | T:17 | T:8/9 | IL-6 and TNF-α | 2 months | T: (2.4g Fish oil)/day | nondialysis |
| C:14 | C:9/5 | C: safflower oil | |||||
|
| |||||||
| Gharekhani et al 2014[27] | RCT | T:25 | T:13/12 | CRP, IL-6 and TNF-α | 4 months | T: (1.08g EPA, 0.72g DHA)/day | hemodialysis |
| C:20 | C:12/8 | C: paraffin oil | |||||
|
| |||||||
| Hung et al 2014[25] | RCT | T:17 | T:14/3 | CRP and IL-6 | 3 months | T: (2.9g ω3FA )/day | hemodialysis |
| C:17 | C:13/4 | C: placebo | |||||
|
| |||||||
| Mirhashemi et al 2016[35] | RCT | T:30 | T:11/19 | TNF-α | 3 months | T: (1g ω3FA )/day | nondialysis |
| C:30 | C:10/20 | C: placebo | |||||
|
| |||||||
| Deger et al 2016[33] | RCT | T:11 | T:9/2 | CRP and IL-6 | 3 months | T: (2.9g ω3FA )/day | hemodialysis |
| C:9 | C:8/1 | C: placebo | |||||
Note: T: omega-3 fatty acid group C: placebo group
Omega-3 fatty acids and CRP
Ten studies described the effect of omega-3 fatty acid supplementation on the serum CRP levels (22, 25–33). The pooled results of seven trials indicated that omega-3 fatty acids intake had no statistical difference in the levels of serum CRP (SMD, −0.20; 95% CI, −0.44 to 0.05; p=0.11; Figure 3), and there was no heterogeneity among these studies (heterogeneity χ2 =5.92, p = 0.43, I2 = 0%; Figure 3). In the study performed by Madsen, CRP changes were calculated as median with interquartile range. The results for the group of omega-3 fatty acids supplementation and control groups were: (2.46 versus 1.47 mg/L; p =0.06) and (3.27 versus 3.14 mg/L; p = 0.12), respectively (30). Overall, it indicated that the change of serum CRP level was not different among the two groups. In an another study performed by Himmelfarb et al. where serum CRP data were expressed in the form of a histogram, and it also indicated no significant difference in the treatment and control group (32).
Figure 3.
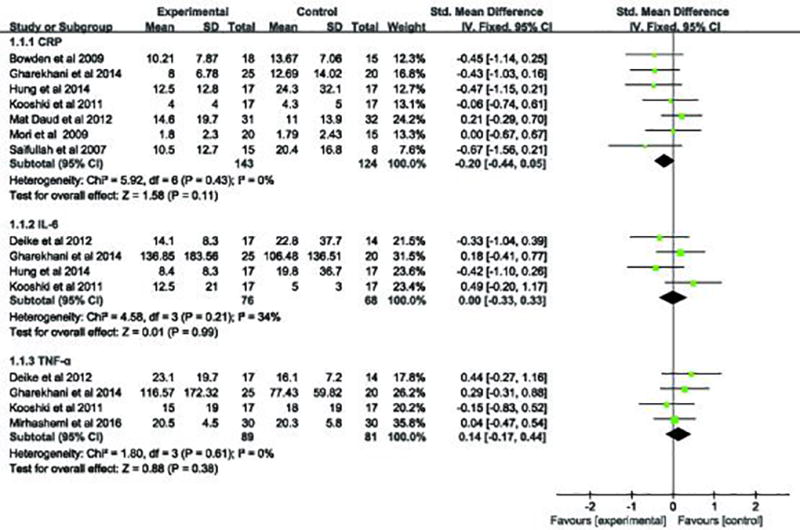
Forest plot of studies comparing the role of omega-3 fatty acids supplementation versus placebo on serum inflammation markers in CKD patients.
Omega-3 fatty acids and IL-6
Six studies were included pertaining to the changes in serum IL-6 levels (25, 27, 28, 32–34). Four articles were included in the pooled analysis, which suggested that serum IL-6 level were not significantly different in the group receiving omega-3 fatty acids supplementation compared with the controls (SMD, 0.00; 95% CI, −0.33 to 0.33; p=0.99; Figure 3). A low heterogeneity was detected in these studies (heterogeneity χ2 =4.58, P= 0.21, I2 = 34%; Figure 3). One study performed by Himmelfarb et al. was not included in pooled analysis since it reported serum IL-6 data as a histogram (32). However, in this study, IL-6 value was significantly reduced in the treatment group but it was not significantly different from the control group.
Omega-3 fatty acids and TNF-α
The effect of supplementation of omega-3 fatty acids on serum TNF-α level was also reported in four trials (27, 28, 34, 35). The pooled analysis suggested that TNF-α levels had no significant differences between the experiment and control groups (SMD, 0.14; 95% CI, −0.17 to 0.44; p=0.38; Figure 3). No heterogeneity in these studies was documented (heterogeneity χ2 =1.8, P= 0.61, I2 = 0%; Figure 3).
Omega-3 fatty acids affect serum IL-6, TNF-α and CRP levels in dialysis patients
We also evaluated the data of omega-3 fatty acids supplementation in dialysis patients and their effect on the serum levels of IL-6, TNF-α and CRP. The pooled analyses revealed that serum IL-6, TNF-α and CRP level were not significantly different in patients receiving omega-3 fatty acids versus the controls. The pooled respective results (Figure 4) pertaining to serum IL-6, TNF-α and CRP levels were: (SMD, 0.09; 95% CI, −0.28 to 0.47; p=0.63); (SMD, 0.09; 95% CI, −0.35 to 0.54; p=0.68) and (SMD, −0.23; 95% CI, −0.49 to 0.03; p=0.09).
Figure 4.
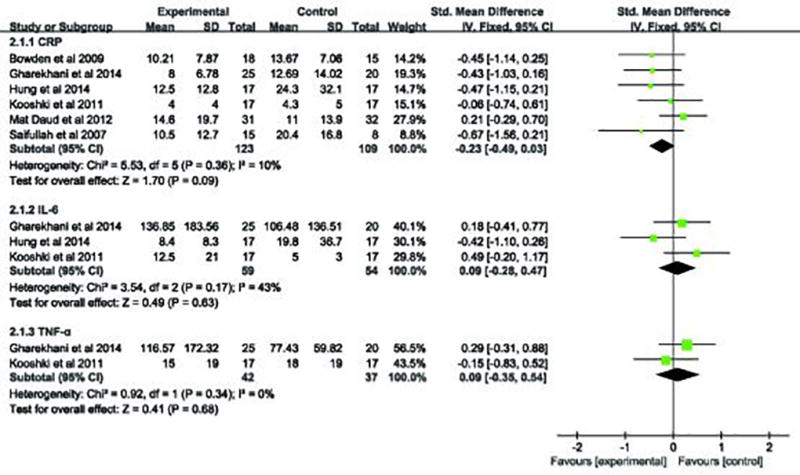
Forest plot of studies comparing the role of omega-3 fatty acids supplementation versus placebo on serum inflammation markers in dialysis patients.
Publication Bias
We used the funnel plots to evaluate the publication bias, as shown in Figure 5, the outcomes of serum IL-6, TNF-α and CRP were symmetric, suggesting that publication bias did not affect the result of our meta-analysis.
Figure 5.
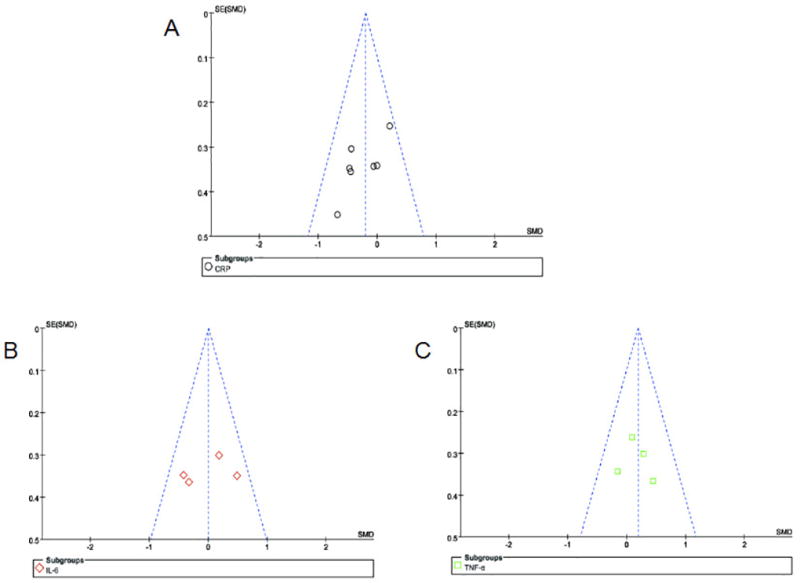
Funnel plots with MD or SMD for studies comparing supplementation of omega-3 fatty acids with placebo for the effect of inflammation in CKD patients. MD: mean differences, SMD: standardized mean difference
DISCUSSION
The meta-analysis data revealed no statistical differences in serum CRP levels between omega-3 fatty acids supplementation group and cohorts in CKD patients. Also, the serum levels of CRP and TNF-α were not statistically different between the dialysis patients receiving omega-3 fatty acids group and control group. Therefore, the current study concluded that oral supplementation of omega-3 fatty acids did not have significant effect in reducing serum levels of IL-6, TNF-α and CRP in patients with CKD and/or those receiving dialysis. This would suggest that the omega-3 fatty acids do not exert obvious beneficial effect by reducing the inflammatory state in patents with CKD or dialysis.
Some of the recent literature reports suggest that micro-inflammation is a salient characteristic of patients with CKD in pre-dialysis or dialysis patients (36–38), which attributes to a relatively high cardiovascular risk in such patients (39, 40). Elevated levels of inflammatory mediators have a potential role in increasing oxidative stress and expression of advanced glycation end (AGE) products (41). It has been reported that the progression of CKD is related to low-grade inflammation, even in patients with moderate renal dysfunctions who may not be yet dialysis-dependent (43–45), suggesting that elevation of inflammatory mediators is associated with the development of chronic renal failure. While the data of certain other recent studies indicate that the lower levels of inflammation biomarkers may rather improve the survival of CKD patients (46–48). Therefore, modulation of micro-inflammation in patients with CKD may be of great clinical significance to delay the progression of CKD.
Some of the literature studies indicate that omega-3 fatty acids have beneficial effect in several inflammation-mediated diseases, such as, Crohn's disease (49), rheumatoid arthritis (50), IgAN (51) and various skin diseases (52). Also, some of the review articles (7, 10) mentioned the effects of omega-3 fatty acids supplementation in CKD patients who are dialysis-dependent as well as -independent. One of the problems seems to be the uremia-associated taste alterations in CKD patients undergoing hemodialysis, and as a result there would be inadequate supplementation of omega-3 fatty acids (53). Also, hemodialysis may directly affect omega-3 fatty acids bioavailability since these patients are under increased oxidative stress, and this will result in low levels of omega-3 fatty acids (54). Nevertheless, certain studies pertaining to endothelial biology and type 2 diabetes indicate beneficial effect of omega-3 fatty acids by modulation of inflammation (55–57). The mechanism by which omega-3 fatty acids exert their anti-inflammatory effect may be by possibly modulating AMP-activated protein kinase (AMPK) or cellular signaling mediated via silencing information regulator1 (SIRT1); the latter apparently leads to deacetylation of nuclear factor of kappaB (NF-kB) and competitive inhibition of arachidonic acid that is involved in its conversion to pro-inflammatory intermediaries (58). In addition, the omega-3 fatty acids can alter the composition of cell membrane phospholipid fatty acid (59). Furthermore, Micallef et al. reported that omega-3 fatty acids are inversely related to CRP levels in healthy individuals (60), and they also reduce CRP and IL-6 levels in healthy older adults (61). Likewise, omega-3 fatty acids can decrease serum CRP concentrations in various chronic diseases (62) and patients with diabetic nephropathy (63). In addition, Tayyebi-Khosroshahi et al. also reported that omega-3 fatty acids significantly decrease the levels of TNF-α in hemodialysis patients (64). However, other studies indicated no significant effect on the inflammatory state in CKD patients with supplementation of omega-3 fatty acids (20, 21, 65). The present meta-analysis study indicate that there are no significant differences in serum IL-6, TNF-α and CRP levels between the untreated and patients treated with omega-3 fatty acid supplementation. This could be due to several variables, including the study duration, the number of studied patients, EPA/DHA ratios and dosage of omega-3 fatty acids used, as well as the baseline levels of inflammation markers. In addition, the existing medical status or a given patient undergoing other treatments may be additional factors influencing the outcome (66). Another reason for the failure of omega-3 fatty acids supplementation to reduce inflammation in CKD/HD patients may due to the extent of inflammatory response which may too strong to be overcome by the anti-inflammatory effect of omega-3 fatty acids in CKD, especially those patients undergoing hemodialysis.
In terms of side effects of omega-3 fatty acids in CKD patients, there have been three trials reported in the literature (25, 34). The meta-analysis of these studies indicated no obvious alarming side effects related to omega-3 fatty acids supplementation. Our meta-analysis study also included a report by Saifullah et al. (30), which indicated minimal gastrointestinal disturbances in the group of patients taking fish oil. Also, none of the patients discontinued the omega-3 fatty acids supplementation due to these minor gastrointestinal side effects.
Finally, although meta-analysis was carefully performed in this study, there may be certain limitations. Primarily, this may be due to the trial size being relatively small. In this regard, a total of twelve RCTs involving 487 patients were included in this meta-analysis; with seven studies included in pooled analysis pertaining to the change in serum CRP levels, while four studies described the changes in serum levels of both IL-6 and TNF-α. Secondly, some of the included studies were not double-blinded. Thirdly, some of the results were described as median and range, while others in the form of histograms which could not be included in the meta-analysis. Finally, there was lack of long-term follow-up, and about the effect of omega-3 fatty acids supplementation in CKD patients on inflammatory markers.
CONCLUSION
Present meta-analysis indicates that there is insufficient evidence that provides a statistical significant benefit of omega-3 fatty acids supplementation on serum inflammation biomarkers, including CRP, TNF-α and IL-6 level in patients with CKD. Further large-scale and long-term clinical trials are needed to arrive at concrete conclusions.
Acknowledgments
Declaration of funding interests: This work was supported by grants from the Creative Research Group Fund of the National Foundation Committee of Natural Sciences of China (81470960, 81270812, 81300600), Free Explore Plan of Central South University (2012QNZT146), and a grant from the NIH, USA (DK60635).
Footnotes
Declaration of personal interests: None of the authors have any conflict of interest to declare.
References
- 1.Zimmermann J, Herrlinger S, Pruy A, Metzger T, Wanner C. Inflammation enhances cardiovascular risk and mortality in hemodialysis patients. Kidney Int. 1999;55:648–658. doi: 10.1046/j.1523-1755.1999.00273.x. [DOI] [PubMed] [Google Scholar]
- 2.Hung A, Pupim L, Yu C, et al. Determinants of C-reactive protein in chronic hemodialysis patients: relevance of dialysis catheter utilization. Hemodial Int. 2008;12:236–243. doi: 10.1111/j.1542-4758.2008.00260.x. [DOI] [PubMed] [Google Scholar]
- 3.Collins AJ, Foley RN, Herzog C, et al. US Renal Data System 2012 Annual Data Report. Am J Kidney Dis. 2013;61:A7, e1–476. doi: 10.1053/j.ajkd.2012.11.031. [DOI] [PubMed] [Google Scholar]
- 4.Panichi V, Scatena A, Migliori M, Marchetti V, Paoletti S, Beati S. Biomarkers of Chronic Inflammatory State in Uremia and Cardiovascular Disease. Int J Inflam. 2012;2012:360147. doi: 10.1155/2012/360147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rangel-Huerta OD, Aguilera CM, Mesa MD, Gil A. Omega-3 long-chain polyunsaturated fatty acids supplementation on inflammatory biomakers: a systematic review of randomised clinical trials. Br J Nutr. 2012;107(suppl 2):S159–S170. doi: 10.1017/S0007114512001559. [DOI] [PubMed] [Google Scholar]
- 6.Lavie CJ, Milani RV, Mehra MR, Ventura HO. Omega-3 polyunsaturated fatty acids and cardiovascular diseases. J Am Coll Cardiol. 2009;54(7):585–94. doi: 10.1016/j.jacc.2009.02.084. [DOI] [PubMed] [Google Scholar]
- 7.Friedman A, Moe S. Review of the Effects of Omega-3 Supplementation in Dialysis Patients. Clin J Am Soc Nephrol. 2006;1(2):182–92. doi: 10.2215/CJN.00740805. [DOI] [PubMed] [Google Scholar]
- 8.Mayer K, Meyer S, Reinholz-Muhly M, et al. Short-time infusion of fish oil based lipid emulsions, approved for parenteral nutrition, reduces monocyte proinflammatory cytokine generation and adhesive interaction with endothelium in humans. Immunol. 2003;171(9):4837–4843. doi: 10.4049/jimmunol.171.9.4837. [DOI] [PubMed] [Google Scholar]
- 9.Mori TA, Beilin LJ. Omega-3 fatty acids and inflammation. Curr Atheroscler Rep. 2004;6(6):461–7. doi: 10.1007/s11883-004-0087-5. [DOI] [PubMed] [Google Scholar]
- 10.Fassett RG, Gobe GC, Peake JM, Coombes JS. Omega-3 polyunsaturated fatty acids in the treatment of kidney disease. Am J Kidney Dis. 2010;56(4):728–42. doi: 10.1053/j.ajkd.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 11.GISSI-Prevenzione Investigators. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Lancet. 1999;354(9177):447–55. [PubMed] [Google Scholar]
- 12.De Caterina R. n-3 fatty acids in cardiovascular disease. N Engl J Med. 2011;364:2439–2450. doi: 10.1056/NEJMra1008153. [DOI] [PubMed] [Google Scholar]
- 13.Kris-Etherton PM, Harris WS, Appel LJ Nutrition Committee. Fish Consumption, Fish, Oil, Omega-3 Fatty Acids, and Cardiovascular Disease. Arterioscler Thromb Vasc Biol. 2003;23(2):e20–30. doi: 10.1161/01.atv.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- 14.Beck L, Bomback AS, Choi MJ, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for glomerulonephritis. Am J Kidney Dis. 2013;62(3):403–41. doi: 10.1053/j.ajkd.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Yuzawa Y, Yamamoto R, Takahashi K, et al. Evidence-based clinical practice guidelines for IgA nephropathy 2014. Clin Exp Nephrol. 2016;20(4):511–35. doi: 10.1007/s10157-015-1223-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferrucci L, Cherubini A, Bandinelli S, et al. Relationship of Plasma Polyunsaturated Fatty Acids to Circulating Inflammatory Markers. J Clin Endocrinol Metab. 2006;91(2):439–46. doi: 10.1210/jc.2005-1303. [DOI] [PubMed] [Google Scholar]
- 17.Rasic-Milutinovic Z, Perunicic G, Pljesa S, et al. Effects of N-3 PUFAs Supplementation on Insulin Resistance and Inflammatory Biomarkers in Hemodialysis Patients. Ren Fail. 2007;29(3):321–9. doi: 10.1080/08860220601184092. [DOI] [PubMed] [Google Scholar]
- 18.Perunicic-Pekovic GB, Rasic ZR, Pljesa SI, et al. Effect of n-3 fatty acids on nutritional status and inflammatory markers in haemodialysis patients. Nephrology (Carlton) 2007;12(4):331–6. doi: 10.1111/j.1440-1797.2007.00777.x. [DOI] [PubMed] [Google Scholar]
- 19.Ewers B, Riserus U, Marckmann P. Effects of Unsaturated Fat Dietary Supplements on Blood Lipids, and on Markers of Malnutrition and Inflammation in Hemodialysis Patients. J Ren Nutr. 2009;19(5):401–11. doi: 10.1053/j.jrn.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 20.Moreira AC, Gaspar A, Serra MA, Simões J, Lopes da Cruz J, Amaral TF. Effect of a Sardine Supplement on C-Reactive Protein in Patients Receiving Hemodialysis. J Ren Nutr. 2007;17(3):205–13. doi: 10.1053/j.jrn.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 21.Hassan KS, Hassan SK, Hijazi EG, Khazim KO. Effects of omega-3 on lipid profile and inflammation markers in peritoneal dialysis patients. Ren Fail. 2010;32(9):1031–5. doi: 10.3109/0886022X.2010.510231. [DOI] [PubMed] [Google Scholar]
- 22.Mori TA, Burke V, Puddey I, et al. The effects of v3 fatty acids and coenzyme Q10 on blood pressure and heart rate in chronic kidney disease: a randomized controlled trial. J Hypertens. 2009;27(9):1863–72. doi: 10.1097/hjh.0b013e32832e1bd9. [DOI] [PubMed] [Google Scholar]
- 23.Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomized trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hung AM, Booker C, Ellis CD, et al. Omega-3 fatty acids inhibit the up-regulation of endothelial chemokines in maintenance hemodialysis patients. Nephrol Dial Transplant. 2015;30(2):266–74. doi: 10.1093/ndt/gfu283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bowden RG, Wilson RL, Deike E, Gentile M. Fish Oil Supplementation Lowers C-Reactive Protein Levels Independent of Triglyceride Reduction in Patients With End-Stage Renal Disease. Nutr Clin Pract. 2009;24(4):508–12. doi: 10.1177/0884533609335376. [DOI] [PubMed] [Google Scholar]
- 27.Gharekhani A, Khatami MR, Dashti-Khavidaki S, et al. The effect of omega-3 fatty acids on depressive symptoms and inflammatory markers in maintenance hemodialysis patients: a randomized, placebo-controlled clinical trial. Eur J Clin Pharmacol. 2014;70(6):655–65. doi: 10.1007/s00228-014-1666-1. [DOI] [PubMed] [Google Scholar]
- 28.Kooshki A, Taleban FA, Tabibi H, Hedayati M. Effects of Marine Omega-3 Fatty Acids on Serum Systemic and Vascular Inflammation Markers and Oxidative Stress in Hemodialysis Patients. Ann Nutr Metab. 2011;58(3):197–202. doi: 10.1159/000329727. [DOI] [PubMed] [Google Scholar]
- 29.Daud ZA, Tubie B, Adams J, et al. Effects of protein and omega-3 supplementation, during regular dialysis sessions, on nutritional and inflammatory indices in hemodialysis patients. Vasc Health Risk Manag. 2012;8:187–95. doi: 10.2147/VHRM.S28739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saifullah A, Watkins BA, Saha C, Li Y, Moe SM, Friedman AN. Oral fish oil supplementation raises blood omega-3 levels and lowers C-reactive protein in haemodialysis patients—a pilot study. Nephrol Dial Transplant. 2007;22(12):3561–7. doi: 10.1093/ndt/gfm422. [DOI] [PubMed] [Google Scholar]
- 31.Madsen T, Schmidt EB, Christensen JH. The Effect of n-3 Fatty Acids on C-Reactive Protein Levels in Patients With Chronic Renal Failure. J Ren Nutr. 2007;17(4):258–63. doi: 10.1053/j.jrn.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 32.Himmelfarb J, Phinney S, Ikizler TA, Kane J, McMonagle E, Miller G. Gamma-Tocopherol and Docosahexaenoic Acid Decrease Inflammation in Dialysis Patients. J Ren Nutr. 2007;17(5):296–304. doi: 10.1053/j.jrn.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 33.Deger SM, Hung M, Ellis CD, et al. High Dose Omega-3 Fatty Acid Administration and Skeletal Muscle Protein Turnover in Maintenance Hemodialysis Patients. Clin J Am Soc Nephrol. 2016;11:1227–1235. doi: 10.2215/CJN.04150415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deike E, Bowden RG, Moreillon JJ, et al. The Effects of Fish Oil Supplementation on Markers of Inflammation in Chronic Kidney Disease Patients. J Ren Nutr. 2012;22(6):572–7. doi: 10.1053/j.jrn.2011.10.036. [DOI] [PubMed] [Google Scholar]
- 35.Mirhashemi SM, Rahimi F, Soleimani A, et al. Effects of Omega-3 Fatty Acid Supplementation on Inflammatory Cytokines and Advanced Glycation End Products in Patients With Diabetic Nephropathy A Randomized Controlled Trial. Iranian Journal of Kidney Diseases. 2016;10:197–204. [PubMed] [Google Scholar]
- 36.Neade T, Uribarri J. Diet, Inflammation, and Chronic Kidney Disease:Getting to the Heart of the Matter. Semin Dial. 2008;21(4):331–7. doi: 10.1111/j.1525-139X.2008.00445.x. [DOI] [PubMed] [Google Scholar]
- 37.Pupim LB, Himmelfarb J, McMonagle E, Shyr Y, Ikizler TA. Influence of initiation of maintenance hemodialysis on biomarkers of inflammation and oxidative stress. Kidney Int. 2004;65(6):2371–9. doi: 10.1111/j.1523-1755.2004.00656.x. [DOI] [PubMed] [Google Scholar]
- 38.de Vinuesa SG, Goicoechea M, Kanter J, et al. Insulin Resistance, Inflammatory Biomarkers, and Adipokines in Patients with Chronic Kidney Disease: Effects of Angiotensin II Blockade. J Am Soc Nephrol. 2006;17:S206–12. doi: 10.1681/ASN.2006080916. [DOI] [PubMed] [Google Scholar]
- 39.Stenvinkel P. Inflammation in end-stage renal failure: could it be treated? Nephrol Dial Transplant. 2002;17(Suppl 8):33–8. doi: 10.1093/ndt/17.suppl_8.33. [DOI] [PubMed] [Google Scholar]
- 40.Stenvinkel P, Heimbürger O, Paultre F, et al. Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int. 1999;55(5):1899–911. doi: 10.1046/j.1523-1755.1999.00422.x. [DOI] [PubMed] [Google Scholar]
- 41.Lee SM, An WS. Cardioprotective Effects of ω-3 PUFAs in Chronic Kidney Disease. Biomed Res Int. 2013;2013:712949. doi: 10.1155/2013/712949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Landray MJ, Wheeler DC, Lip GY, et al. Inflammation, endothelial dysfunction, and platelet activation in patients with chronic kidney disease: the chronic renal impairment in Birmingham (CRIB) study. Am J Kidney Dis. 2004;43(2):244–53. doi: 10.1053/j.ajkd.2003.10.037. [DOI] [PubMed] [Google Scholar]
- 43.Kaizu Y, Kimura M, Yoneyama T, Miyaji K, Hibi I, Kumagai H. Interleukin-6 May Mediate Malnutrition in Chronic Hemodialysis Patients. Am J Kidney Dis. 1998;31(1):93–100. doi: 10.1053/ajkd.1998.v31.pm9428458. [DOI] [PubMed] [Google Scholar]
- 44.Herbelin A, Ureña P, Nguyen AT, Zingraff J, Descamps-Latscha B. Elevated circulating levels of interleukin-6 in patients with chronic renal failure. Kidney Int. 1991;39(5):954–60. doi: 10.1038/ki.1991.120. [DOI] [PubMed] [Google Scholar]
- 45.Pecoits-Filho R, Gonçalves S, Barberato SH, et al. Impact of Residual Renal Function on Volume Status in Chronic Renal Failure. Blood Purif. 2004;22(3):285–92. doi: 10.1159/000078699. [DOI] [PubMed] [Google Scholar]
- 46.Memoli B, Salerno S, Procino A, et al. A translational approach to micro-inflammation in end-stage renal disease: molecular effects of low levels of interleukin-6. Clin Sci (Lond) 2010;119(4):163–74. doi: 10.1042/CS20090634. [DOI] [PubMed] [Google Scholar]
- 47.Bologa RM, Levine DM, Parker TS, et al. Interleukin-6 Predicts Hypoalbuminemia, Hypocholesterolemia, and Mortality in Hemodialysis Patients. Am J Kidney Dis. 1998;32(1):107–14. doi: 10.1053/ajkd.1998.v32.pm9669431. [DOI] [PubMed] [Google Scholar]
- 48.Schömig M, Eisenhardt A, Ritz E. The Microinflammatory State of Uremia. Blood Purif. 2000;18(4):327–32. doi: 10.1159/000014457. [DOI] [PubMed] [Google Scholar]
- 49.Belluzzi A, Brignola C, Campieri M, Pera A, Boschi S, Miglioli M. Effect of an enteric-coated fish-oil preparation on relapses in Crohn’s disease. N Engl J Med. 1996;334(24):1557–60. doi: 10.1056/NEJM199606133342401. [DOI] [PubMed] [Google Scholar]
- 50.Kremer JM, Jubiz W, Michalek A, et al. Fish-oil fatty acid supplementation in active rheumatoid arthritis. A double-blinded, controlled, crossover study. Ann Intern Med. 1987;106(4):497–503. doi: 10.7326/0003-4819-106-4-497. [DOI] [PubMed] [Google Scholar]
- 51.Donadio JV, Jr, Bergstralh EJ, Offord KP, Spencer DC, Holley KE. A controlled trial of fish oil in IgA nephropathy. Mayo Nephrology Collaborative Group. N Engl J Med. 1994;331(18):1194–9. doi: 10.1056/NEJM199411033311804. [DOI] [PubMed] [Google Scholar]
- 52.Ziboh VA, Miller CC, Cho Y. Metabolism of polyunsaturated fatty acids by skin epidermal enzymes: Generation of antiinflammatory and antiproliferative metabolites. Am J Clin Nutr. 2000;71:361S–6S. doi: 10.1093/ajcn/71.1.361s. [DOI] [PubMed] [Google Scholar]
- 53.Dobell E, Chan M, Williams P, Allman M. Food preferences and food habits of patients with chronic renal failure undergoing dialysis. J Am Diet Assoc. 1993;93(10):1129–35. doi: 10.1016/0002-8223(93)91644-6. [DOI] [PubMed] [Google Scholar]
- 54.Ikizler TA, Morrow JD, Roberts LJ, et al. Plasma F2-isoprostane levels are elevated in chronic hemodialysis patients. Clin Nephrol. 2002;58(3):190–7. doi: 10.5414/cnp58190. [DOI] [PubMed] [Google Scholar]
- 55.Moertl D, Hammer A, Steiner S, Hutuleac R, Vonbank K, Berger R. Dose-dependent effects of omega-3-polyunsaturated fatty acids on systolic left ventricular function, endothelial function, and markers of inflammation in chronic heart failure of nonischemic origin: A double-blind, placebo-controlled, 3-arm study. Am Heart J. 2011;161(5):915.e1–9. doi: 10.1016/j.ahj.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 56.Lee KW, Blann AD, Lip GY. Effects of omega-3 polyunsaturated fatty acids on plasma indices of thrombogenesis and inflammation in patients post-myocardial infarction. Thromb Res. 2006;118(3):305–12. doi: 10.1016/j.thromres.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 57.Kabir M, Skurnik G, Naour N, et al. Treatment for 2 mo with n3 polyunsaturated fatty acids reduces adiposity and some atherogenic factors but does not improve insulin sensitivity in women with type 2 diabetes: a randomized controlled study. Am J Clin Nutr. 2007;86(6):1670–9. doi: 10.1093/ajcn/86.5.1670. [DOI] [PubMed] [Google Scholar]
- 58.Xue B, Yang Z, Wang X, Shi H. Omega-3 Polyunsaturated Fatty Acids Antagonize Macrophage Inflammation via Activation of AMPK/SIRT1 Pathway. PLoS One. 2012;7(10):e45990. doi: 10.1371/journal.pone.0045990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Calder PC. Omega-3 polyunsaturated fatty acids and inflammatory processes: nutrition or pharmacology. Br J Clin Pharmacol. 2013;75(3):645–62. doi: 10.1111/j.1365-2125.2012.04374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Micallef MA, Munro IA, Garg ML. An inverse relationship between plasma n-3 fatty acids and C-reactive protein in healthy individuals. Eur J Clin Nutr. 2009;63(9):1154–6. doi: 10.1038/ejcn.2009.20. [DOI] [PubMed] [Google Scholar]
- 61.Tsitouras PD, Gucciardo F, Salbe AD, Heward C, Harman SM. High Omega-3 Fat Intake Improves Insulin Sensitivity and Reduces CRP and IL6, but does not Affect Other Endocrine Axes in Healthy Older Adults. Horm Metab Res. 2008;40(3):199–205. doi: 10.1055/s-2008-1046759. [DOI] [PubMed] [Google Scholar]
- 62.Liepa GU, Basu H. C-Reactive Proteins and Chronic Disease: What Role Does Nutrition Play? Nutr Clin Pract. 2003;18(3):227–33. doi: 10.1177/0115426503018003227. [DOI] [PubMed] [Google Scholar]
- 63.Han E, Yun Y, Kim G, et al. Effects of Omega-3 Fatty Acid Supplementation on Diabetic Nephropathy Progression in Patients with Diabetes and Hypertriglyceridemia. PLoS One. 2016;11(5):e0154683. doi: 10.1371/journal.pone.0154683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tayyebi-Khosroshahi H, Houshyar J, Dehgan-Hesari R, et al. Effect of Treatment with Omega-3 Fatty Acids on C-Reactive Protein and Tumor Necrosis Factor-Alfa in Hemodialysis Patients. Saudi J Kidney Dis Transpl. 2012;23(3):500–6. [PubMed] [Google Scholar]
- 65.Hassan KS, Hassan SK, Hijazi EG, et al. Effects of omega-3 on lipid profile and inflammation markers in peritoneal dialysis patients. Renal Failure. 2010;32(9):1031–1035. doi: 10.3109/0886022X.2010.510231. [DOI] [PubMed] [Google Scholar]
- 66.Luu NT, Madden J, Calder PC, et al. Dietary Supplementation with Fish Oil Modifies the Ability of Human Monocytes to Inducenan Inflammatory Response. J Nutr. 2007;137(12):2769–74. doi: 10.1093/jn/137.12.2769. [DOI] [PubMed] [Google Scholar]


