Abstract
The expression of a wide range of social and affective behaviors, including aggression and investigation, as well as anxiety- and depressive-like behaviors, involves interactions among many different physiological systems, including the neuroendocrine and immune systems. Recent work suggests that the gut microbiome may also play a critical role in modulating behavior and likely functions as an important integrator across physiological systems. Microbes within the gut may communicate with the brain via both neural and humoral pathways, providing numerous avenues of research in the area of the gut-brain axis. We are now just beginning to understand the intricate relationships among the brain, microbiome, and immune system and how they work in concert to influence behavior. The effects of different forms of experience (e.g., changes in diet, immune challenge, and psychological stress) on the brain, gut microbiome, and the immune system have often been studied independently. Though because these systems do not work in isolation, it is essential to shift our focus to the connections among them as we move forward in our investigations of the gut-brain axis, the shaping of behavioral phenotypes, and the possible clinical implications of these interactions. This review summarizes the recent progress the field has made in understanding the important role the gut microbiome plays in the modulation of social and affective behaviors, as well as some of the intricate mechanisms by which the microbiome may be communicating with the brain and immune system.
Keywords: cytokines, endocrine system, gut-brain axis, immune system, lipopolysaccharide, microbiome, social behavior
1. Introduction
A wide variety of experiences can influence physiology and behavior; in order to produce appropriate behavioral responses, neuroendocrine circuits must integrate sensory stimuli with internal physiology. Research has shown that even modest activation of the immune system early in life may increase susceptibility to a range of nervous system disorders and can influence the physiological and behavioral responses to challenges later in life (Bilbo and Schwarz, 2009; Harvey and Boksa, 2012; Knox et al., 2009). Such studies investigating early-life stress, as well as adult stress, often focus on the neuroendocrine and immune systems alone to explain the behavioral phenotype, including aggression, investigation, exploration, and anxiety- and depressive-like behaviors. More recent work, however, suggests that the gut-brain axis (i.e., the bidirectional communication between the brain and gastrointestinal tract) is capable of interacting with these physiological systems and it too may facilitate behavioral responses (Collins and Bercik, 2014; Cryan and O’Mahony, 2011).
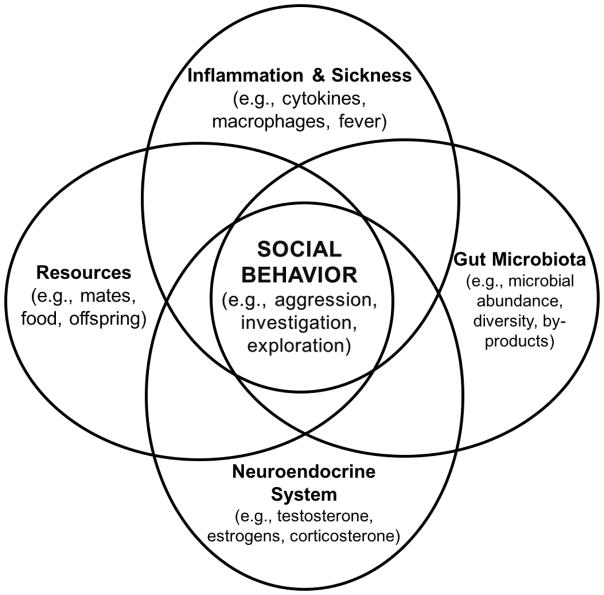
Inside the mammalian body lies a complex ecological community, termed the microbiota, consisting of commensal, symbiotic, and pathogenic bacteria, fungi, and viruses. The microbial genome of these microorganisms is termed the microbiome. This system of living microorganisms is critical for mammalian survival (Lee and Mazmanian, 2010), and interestingly, most of these microbes reside in the large intestine of the gastrointestinal (GI) tract (Wallace et al., 2011). More importantly, the microbiome may play a critical role in modulating behavior by connecting the neuroendocrine and immune systems (Fig. 1) through both direct (e.g., vagus nerve) and indirect (e.g., cytokines) mechanisms. Further, the gut microbiome develops in parallel with the neonatal central nervous system (CNS) via the transmission of signals from the vagus nerve (the major nerve of the parasympathetic nervous system) to the GI tract, therefore perturbations of either system have the potential to affect both (Clarke et al., 2014; Cryan and O’Mahony, 2011; see Table 1).
Figure 1.
Previous work has been aimed at determining the role that the central nervous system, the gut microbiome and the immune systems play in social behavior and adult sensitivity to subsequent stressors, yet some previous studies have overlooked the interactions between these systems and the communication among the systems used to regulate (or deregulate) social behavior. This theoretical model illustrates some of the brain-gut-immune interactions that play a role in behavioral outcomes, including social and affective behaviors (e.g., investigation, aggression, and anxiety- and depression-like behavior). The interactions among these systems are key to understanding how social behavioral changes occur and the potential mechanisms mediating psychopathologies.
Table 1.
Studies investigating the interactions among the microbiome, immune system, neuroendocrine system, and social and affective behaviors.
| Study System | Sex | Manipulation | Behavioral & Physiological Changes | Citation |
|---|---|---|---|---|
| CF-1 mice | M | Treatment with Campylobacter jejuni | Increased anxiety | Goehler et al., 2008 |
| Siberian hamsters | M and F | Antibiotic treatment | Altered gut microbial communities; sexually dimorphic responses: two, but not one, treatment decreased aggression in males; in females, there was decreased aggression after a single treatment | Sylvia et al., 2016 |
| BALB/c mice | M | Antibiotic treatment & SPF conditions | Altered the gut microbiome and increased exploration without an inflammatory response | Bercik et al., 2011 |
| C57BL/6J mice | M | Maternal antibiotics | Reduced locomotion and reduced time exploring at four and eight weeks of age | Tochitani et al., 2016 |
| Sprague-Dawley rats | M | Bifidobacterium infantis | Decreased peripheral pro-inflammatory cytokines and increased tryptophan | Desbonnet et al., 2009 |
| BALB/c mice | M | Lactobacilus rhamnosus | Reduced anxiety- and depression-like behaviors, but vagotomy prevented these changes | Bravo et al., 2011 |
| BALB/c and C57BL/6 mice | M and F | Parental dietary fat consumption during the gestation and lactation | Offspring from parents fed high fat diets had heighted colonic inflammatory responses; increased liver LPS levels; decreased systemic immune response to LPS; lower microbial diversity in the gut; and decreased Firmicutes to Bacteroidetes ratio | Myles et al., 2013 |
| C57Bl/6 mice | M and F | Maternal transplantation with HFD microbiota | HFD females produced pups that vocalized less upon maternal separation, and males exhibited changes in exploration, cognition, and compulsive behaviors | Bruce-Keller et al., 2017 |
| BALB/cAnNTac mice | M | High fat adult diets | Reduced burrowing and decreased memory | Pyndt Jørgensen et al., 2014 |
| Male C57BL/6J and female 129S1/SvImJ mice | M and F | Maternal stress | Altered maternal microbiome, altered offspring microbiome, and altered energy metabolites | Jasarevic et al., 2015 |
| C57BL/6 mice | M and F | Sex differences | Mice pups displayed sexually dimorphic genes in the intestine, some linked to intestinal bowel disease and colorectal cancer; littermates had more similar gut microbial communities; there were differences in the dominant taxa present in males and females | Steegenga et al., 2014 |
| NOD/Jsd (NOD) mice | M and F | Sex differences in GF vs SPF | Different microbial profiles in males and females after puberty; cecal contents transplants successfully altered the microbiome without a damaging immune response | Markle et al., 2013 |
| Sprague-Dawley rats | M | Maternal separation | Increased mucosal conductance and macromolecular permeability in the gut following mild adult acute stress; less exploration of a novel object when compared with control rats. Pre-treatment with a CRH antagonist before the mild stress, eliminated mucosal changes | Soderholm et al., 2002 |
| C57Bl/6 and IL-10−/− mice | M and F | Maternal separation | IL-10−/− mice showed greater severity of colitis in response to maternal separation (e.g., higher concentrations of colonic pro-inflammatory cytokines), and increased colonic permeability when compared with wild type mice | Lennon et al., 2013 |
| Sprague-Dawley rats | M and F | 17β-estradiol, testosterone, progesterone, and 17α-estradiol treatment | 17β-estradiol, but not testosterone, progesterone or 17α-estradiol decreased chloride ion (Cl−) secretion in the female but not male rat distal colon | Condliffe et al., 2001 |
| Sprague-Dawley rats | M | Luminal melatonin | Decrease in gut permeability by way of the nicotinic acetylcholine receptor | Sommansson et al., 2013 |
| Sprague-Dawley rats | M | GF vs SPF | Fewer, smaller and inactive lymph nodes and Peyer’s patches in GF mice | Hoshi et al., 1992 |
| BALB/c mice | M | GF, SPF, and gnotobiotic | High levels of biologically active dopamine and norepinephrine in SPF and gnotobiotic mice; lower and biologically inactive forms of catecholamines in GF mice | Asano et al., 2012 |
| C57BL/6J mice | M and F | GF vs SPF | Increased permeability of the BBB during development and adulthood due to fewer tight junction proteins in GF mice | Braniste et al., 2014 |
| C57BL/6N mice | M and F | Maternal Immune challenge and offspring Bacteroidetes fragilis | Pups of immune challenged dams who were given Bacteroidetes fragilis showed recovery of their gut lining, microbial communities, and stereotypic, anxiety-like, and sensorimotor behaviors following treatment | Hsiao et al., 2013 |
| Swiss Webster mice | F | GF vs SPF | Heightened anxiety in GF mice; decreased expression of 5-HT receptor 1A and increased expression of brain-derived neurotrophic factor(BDNF) in the hippocampus in GF mice | Neufeld et al., 2011 |
| Long–Evans rats | M | Treatment with intraventricular propionic acid (PPA) | Hyperactivity; repetitive dystonic behaviors; increased oxidative stress markers and activated microglia | Macfabe et al., 2007 |
| Male Sprague-Dawley rats | M | Treatment with intracerebroventricular NPY | Increased anti-depressive behaviors and if NPY receptors are blocked, the effects are depleted | Ishida et al., 2007 |
| NIH Swiss and Balb/c mice | F | Antibiotic treatment; L. paracasei | Antibiotics increased substance P (SP) immunoreactivity in the colon, but L. paracasei reduced SP in antibiotic-treated mice | Verdu et al., 2006 |
| C57BL/6 mice | M | Vasoactive intestinal peptide | Protected colitis-induced epithelial damage by helping to maintain the integrity and distribution of tight junction proteins | Conlin et al., 2009 |
| Swiss Webster mice | M and F | GF | Less time near a conspecific and less time investigating a novel conspecific compared with a familiar one | Desbonnet et al., 2014 |
Later in life, these systems can influence one another as well. For example, adult house mice challenged with the common GI pathogen, Campylobacter jejuni (C. jejuni), exhibit increased anxiety in a standardized behavioral assay, suggesting that information from the gut may be transferred to the brain to mediate behavior via direct or indirect routes (Goehler et al., 2008). The gut microbiome also plays a direct role in immunity and is therefore likely to explain some of the variation in behavioral responses to infection. The underlying physiological changes that occur during an immune challenge and the potential ways in which crosstalk among the immune system, microbiome, and endocrine system takes place are important factors in explaining how behavior is regulated (Fig. 1). The behavioral effects of both early-life and adult experience on the brain, gut microbiome, and the immune system have often been studied independently, but because these systems do not operate in isolation, it is essential to elucidate the connections and interactions between them. Therefore, this review will focus on the state of current research surrounding the role of the interactions among the gut microbiome, the immune system, and the neuroendocrine system in modulating social and affective behaviors.
2. Overview of the immune system
2.1 Inflammation and the brain
In order for the body to maintain a state of balance in times of immune challenge, it must appropriately integrate information received and convey it to the brain to coordinate proper physiological and behavioral responses. Treatment with lipopolysaccharide (LPS), a cell wall component of gram-negative bacteria, is commonly employed to induce a robust immune response in animals, triggering an increase in circulating glucocorticoids (e.g., cortisol) and pro-inflammatory cytokines (e.g., interleukin [IL]-1β, tumor necrosis factor [TNF]-α), which can impair development of many physiological systems (Bilbo and Schwarz, 2012; French et al., 2013). When LPS is injected into the peritoneal cavity, it enters circulation within 15 minutes and levels stay elevated for at least two hours following injection (Hansen et al., 2000). The release of LPS mimics a bacterial infection, by binding to the toll-like receptor (TLR)-4, which stimulates the activation of transcription factors and subsequent pro-inflammatory mediators in the body, particularly cytokines, IL-1β, IL-2, IL-6, TNF-α, and interferons (IFNs) that act on the brain. These processes initiate the acute-phase response (APR), including fever, lethargy, decreased food intake, and enhanced pain response (Harvey and Boksa, 2012; Perry, 2004; Quan and Banks, 2007). Interestingly, these physiological and behavioral responses to LPS (or other immune challenges) are similar to those described in CNS disorders (e.g., autism spectrum disorder, depression, anxiety) and are of particular interest when thinking about the relationships between the gut-brain axis and the immune system (Cryan and O’Mahony, 2011; Harvey and Boksa, 2012; Perry, 2004; Quan and Banks, 2007).
2.2 Behavioral responses to inflammation
Cytokines play a vital role in the response to immune challenge, as they can mediate changes in behavior, though age can be a particularly important factor in the behavioral response. For example, treatment with LPS can cause an increase in both IL-2 and IL-1α in male and female rats (Costalonga and Zell, 2007), however, the age in which animals are exposed influences their response greatly. In particular, rats treated with IL-2 at three weeks of age exhibit enhanced locomotor activity and greater levels of exploration, whereas rats treated with IL-1α at eight weeks of age show an increase in startle response and an increase in investigative behavior, but no changes in locomotion (Tohmi et al., 2004). This suggests that the release of specific cytokines as a result of immune activation at particular time points in an individual’s life can influence the amount and duration of some behaviors, though the precise mechanisms have not yet been determined. Further, work from our lab suggests that in Siberian hamsters (Phodopus sungorus), LPS exposure in the early postnatal period affects reproductive physiology and behavior in a sex-dependent manner. Specifically, although LPS-treated males show no changes in adult physiology or social behavior, LPS-treated females exhibit heightened levels of pre-copulatory investigation and aggression when paired with male conspecifics and altered reproductive physiology (e.g., abnormal estrous cycles and smaller ovaries) in adulthood. They are, however, capable of successfully reproducing, which suggests that some behavioral changes may not coincide with ultimate changes in fitness in some contexts (Sylvia et al., 2018; Sylvia and Demas, 2017).
One potential mechanism mediating the immediate and long-term response to an immune challenge is changes in the blood brain barrier (BBB). For example, after exposure to LPS during development, cytokines can more easily cross the BBB due to increased permeability, making the brain more vulnerable and possibly more responsive (Shanks et al., 1995; Wong et al., 2004). Further, adult male house mice exposed to prenatal stress have increased expression of cytokines in the hippocampus compared to non-stressed individuals, and they produce greater activation of microglia and astrocytes in response to an immune challenge in adulthood as well (Diz-Chaves et al., 2013). As a consequence of this, neural development and synaptic plasticity can be altered, both of which are extremely important in the regulation of behavioral output (Diz-Chaves et al., 2013; Raber et al., 1998). Furthermore, individuals exposed to postnatal LPS show a greater plasma adrenocorticotropic hormone (ACTH) response to adult restraint stress when compared to control-treated neonates (Bilbo and Schwarz, 2012; Shanks et al., 1995), showing support for the long-term effects of early-life immune activation.
Infection during important developmental periods may also result in subtle alterations in the response to subsequent stressors, making individuals more responsive as adults. For example, in one study, postnatal treatment with E. coli had no effect on immediate memory, however, when adults were later exposed to LPS, they exhibited decreased hippocampal astrocytes, decreased brain IL-1, and impaired recent memory (Bilbo et al., 2005). Further work by Bilbo et al. suggests that these changes in behavior can be reversed. Specifically, rats treated with E. coli as neonates that are given a caspase-1 inhibitor, which prevents the synthesis of the proinflammatory cytokine IL-1, do not show LPS-induced memory impairment (Bilbo, et al., 2005). These data suggest that the behavioral changes seen after a secondary immune challenge may be partially due to the inflammatory response that occurred during the neonatal immune challenge. The lasting physiological and behavioral effects of an early-life immune challenge, however, are not completely understood.
2.3 Connections between the immune system and the microbiome
Much of our understanding of the connections between the microbiome and the host immune system have been the result of studies on germ-free (GF) mice (those with no microbes in or on them) (Hooper et al., 2012). By using this model, we have been able to understand the importance of gut bacterial communities. Further, by supplementing GF mice with specific commensal and pathogenic bacteria, we have learned how different microbes work in concert with the host immune system to mediate responses, both in times of health and disease. The gut also houses its own immune system, which acts to protect against pathogenic bacteria and to create a dynamic environment for beneficial bacteria. For example, immune responses (e.g., virus specific antibody titers and T-cell responses) are significantly decreased in antibiotic-treated C57BL/6 mice exposed to the flu virus; and further, the commensal bacteria in the gut (mainly Lactobacillus and Enterobacter) may play a particularly important role in supporting the immune system in response to the virus (Ichinohe et al., 2011). The host immune system works to relay information to the gut in order to maintain homeostasis, and it may be crucial in supporting the microbiome in times of perturbation, such as those described in more detail in Section 3.
3. Factors that can influence the gut-brain-behavior axis
3.1 Antibiotics and probiotics
To understand the effects of gut microbial alterations on brain and behavior, many studies have looked at GF mice as a model. In doing so, they are only partially able to determine the role that the microbiome plays in the natural function of the mammalian system. However, antibiotics can be used to manipulate the gut microbiome in order to reveal information about how the natural environment in the gut affects physiology and behavior in a variety of species (Archie and Theis, 2011; Gareau, 2014). For example, Bercik et al. found that antibiotics alter the cecal microbiome of male specific pathogen-free (SPF) mice (mice that are known to be free of disease-causing pathogens, as well as opportunistic and commensal organisms), and it also increases exploratory behavior, but these changes are independent of an inflammatory response (i.e., no changes in TNF-α, IL-1β) (Bercik et al., 2011). Further, maternal treatment with antibiotics during pregnancy can influence offspring behavior. For instance, in one study, male C57BL/6J mouse offspring from antibiotic-treated dams showed reduced locomotor activity and exhibited less time exploring than control pups at four weeks of age. This difference in locomotion and exploration was still evident at eight weeks of age, suggesting that these changes were static (Tochitani et al., 2016).
Moreover, treatment with probiotics (live microorganisms used to supplement the host microbiome) may reverse some of the effects of changes in microbial community composition, and it has been found to influence behavior. In particular, Bifidobacteria are known to produce neurochemicals that can modulate behavior. For example, treatment with Bifidobacterium infantis (a common species used in probiotics) is associated with a decrease in peripheral pro-inflammatory cytokines and an increase in tryptophan, (5-HT’s precursor) (Desbonnet et al., 2009), suggesting that reversing the decrease in microbial diversity often associated with antibiotic treatment can aid in the communication between the brain and the immune system to regulate behavior. Interestingly, treatment with Lactobacillus rhamnosus (another common probiotic) reduced anxiety- and depression-like behaviors in male BALB/c mice, however, mice that were vagotomized did not exhibit the same reductions in behavior, suggesting the important role that the vagus nerve plays in communication between the gut and the brain (Bravo et al., 2011).
3.2 Maternal diet and care
During the gestation and lactation periods, females expend considerable energy supplying young with the food and nutrients needed to not only nourish offspring, but also to provide many different bioactive compounds that maintain and facilitate growth, modulate the immune system and protect from disease, and aid in the development of a healthy intestinal microbiota (Zivkovic et al., 2011). In times of decreased energy availability or increased energy demands in rodents (e.g., immune challenge), mothers must work even harder to maintain appropriate maternal care to sustain energy stores and provide the necessary nutrients for their young. Changes in maternal behavior and maternal diet can greatly impact offspring physiology and behavior, and both likely play important roles in the crosstalk between the brain and the microbiome.
It has even been suggested that diet can contribute to the development of CNS disorders and pathological disease in adulthood (Albenberg and Wu, 2014). Specifically, in one study, researchers sought to determine whether parental dietary fat consumption during the gestation and lactation periods would affect the immune system of offspring. They found that offspring from parents fed high fat diets had heighted colonic inflammatory responses, increased liver LPS levels, and decreased systemic immune responses to LPS. Further, offspring from the high fat parental diets also showed altered microbiota. Specifically, high fat groups had lower diversity and a decreased ratio of Firmicutes to Bacteroidetes when compared with low fat diet groups (Myles et al., 2013). These data suggest that the parental microbiome can have a substantial influence on the development of the offspring immune response, which could in turn have drastic effects on behavior. In addition, in another study, conventional female mice were transplanted with high fat diet (HFD) microbiota. HFD females produced pups that vocalized less upon maternal separation than control pups, and male offspring from those HFD females exhibited significant changes in exploratory, cognitive, and compulsive behaviors when compared with male offspring from control dams (Bruce-Keller et al., 2017). Interestingly, in adulthood, changes in diet can also influence the microbiome and behavior. For example, adult male BALB/cAnNTac mice fed a HFD exhibited significantly less burrowing behavior and decreased memory compared with control-treated mice (Pyndt Jørgensen et al., 2014). This study and others like it suggest that diet can play a key role in modulating the gut-brain axis, even in adulthood.
Further, maternal stress can influence the vaginal microbiome of the mother, and in turn, affect the offspring microbiome and behavior. For example, in one study, maternal stress decreased the abundance of bacteria, including Lactobacillus, in the vaginal microbiome, and this altered microbiome was transferred to the neonate colon, along with changes in metabolites particularly important in energy balance (Jašarević et al., 2015). Though this study did not measure any changes in behavior, it is likely they would have seen alterations in behavior that coincide with changes in the microbiome.
3.3 Sex hormones
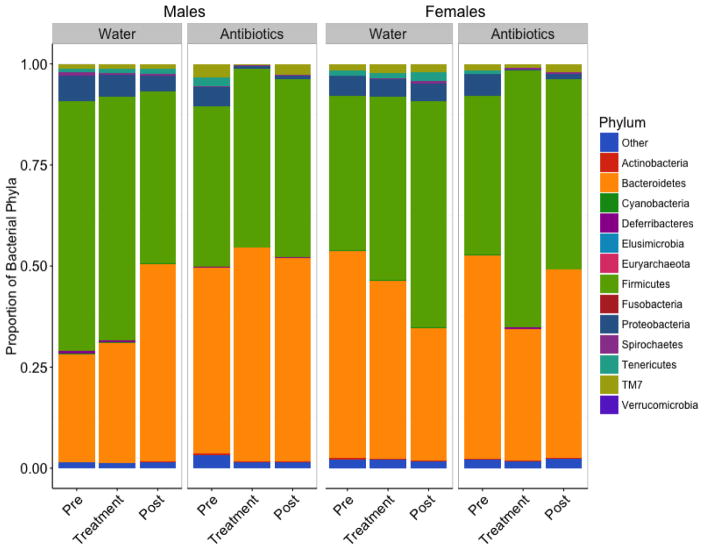
Consideration of the role of sex in the gut-brain axis is extremely important in understanding the development of social behavior, both in the context of normal species-typical behaviors and in health and disease (Foster and McVey Neufeld, 2013). Work in our lab has shown that antibiotic treatment affects the fecal microbial communities in the gut in both sexes (Figure 2), including decreases in the Cyanobacteria, Proteobacteria, and Tenericutes phyla, and that there is a strong sex difference in the behavioral response to antibiotic treatment. Specifically, two, but not one, antibiotic treatment causes marked decreases in aggressive behavior in males, but in females, there is a decrease in aggression after only a single treatment (Sylvia et al., 2016). These findings suggest that females may be more sensitive to stressors, and therefore with a subsequent insult, will exhibit an even more robust change in behavior. It is of further importance to focus on these sex differences because sex hormones (e.g., estradiol and testosterone) affect regulation of motor and sensory function in the GI tract that may be important in the normal function of the gut microbiome (Mulak et al., 2014). Additionally, bowel disorders related to the motility of the GI tract and psychological comorbidities related to these disorders are often more common in women than in men (Mulak et al., 2014), suggesting the intricate role that sex plays in regulation of the gut-brain axis. It has also been suggested that single stressors during the early stages of life do not necessarily produce effects mimicking the entire range of psychopathological disease, but instead, they may produce more subtle changes in behavior that can vary across the sexes (Harvey and Boksa, 2012).
Figure 2.
The microbial community composition in fecal samples of male and female hamsters across treatment groups. The composition of the male and female gut microbiome is made up of 14 phyla, including one phylum from the Archaea kingdom and a designated ‘Other’ category where no specific organism was found after 16s rRNA sequencing on the Illumina MiSeq platform. Both the male and female gut microbiome in Siberian hamsters is dominated by Firmicutes and Bacteroidetes, yet antibiotic treatment did not affect these highly abundant phyla, and instead most strongly affected phyla in lower abundance (e.g., Cyanobacteria, Proteobacteria, and Tenericutes) (adapted from Sylvia et al., 2016).
Some of the sexually dimorphic differences seen in behavioral response to stress could be due to variation in the hypothalamo-pituitary-adrenal (HPA) response (Handa et al., 1994). For example, female rats have a greater endocrine response to both physiological and psychological stressors, and females show a higher average concentration of glucocorticoids than males (Viau et al., 2005). It is believed that gonadal steroid hormones play a role in regulating HPA negative feedback, taking action in multiple areas of the brain (Handa et al., 1994; Viau, 2002). In an early study, prepubertally castrated males showed a significant increase in circulating glucocorticoids compared to control (intact) males when faced with ether stress. If exogenous testosterone (T) was administered, however, glucocorticoid levels fell back to those seen in control males (Gaskin and Kitay, 1970). More recent data suggest that T not only decreases the amount of circulating glucocorticoids in response to a stressor, but it also decreases circulating ACTH (Handa et al., 1994). Following administration of estradiol, both sexes show an increase in ACTH and glucocorticoid secretion when faced with physiological or psychological stressors, possibly due to impairment of receptor-mediated negative feedback at the level of the hypothalamus. In fact, the paraventricular nucleus (PVN) of the hypothalamus displays estrogen and androgen receptors, further suggesting that sex steroids might be working at the level of the hypothalamus to regulate the release of glucocorticoids, yet the exact role they play has yet to be determined (Handa et al., 1994; Handa and Weiser, 2014).
Moreover, gonadal steroids also play an important role in immune modulation and development, as there is sexual dimorphism in immune responses as well (Naor et al., 2009). Estrogens (e.g., 17β-estradiol) may attenuate inflammatory responses in rats, and in contrast, testosterone may exacerbate inflammation (Sribnick et al., 2005). In many animal models, sex differences in CNS diseases seem to emerge later in life, often after a physiological stressor (Tenk et al., 2008). These studies suggest that the HPA and hypothalamo-pituitary-gonadal (HPG) axes are important in the regulation of appropriate physiological and behavioral responses in the face of infection and inflammation, yet precisely how the immune system, neuroendocrine system, and likely the gut microbiota interact to mediate these responses is yet to be fully determined.
Interestingly, microbes also have the ability to use sex steroid hormones themselves and can manipulate sex steroid receptor signaling to increase their own survival (reviewed in Steeg and Klein, 2016). Research suggests, however, that sex steroids play a greater role in microbial systems after puberty. For example, in one study in C57BL/6 mice, researchers found that at two weeks of age, mice pups displayed sexually dimorphic genes in two segments of the intestine, and in particular, they found that of those genes, some were linked to intestinal bowel disease and colorectal cancer. In their analysis of the intestinal microbiome, although they found no significant difference overall in the colonic luminal microbial composition of males and females at that young age, they did find that there were differences, albeit not significant, in the dominant taxa (i.e., Bacteroidetes and Firmicutes) present in the male and female microbiome that may become more evident after puberty (Steegenga et al., 2014). In another study, researchers found that the microbial profiles of males and females arise after puberty, and cecal contents transplants in both sexes can successfully alter the microbiome of the host without a damaging immune response (Markle et al., 2013).
4. The importance of the gut epithelium in the gut-brain axis
4.1 Factors that can influence gut permeability
The gut lumen is an intricate barrier that allows for homeostasis between the inside of the gut and the molecules in circulation. It acts as a barrier to allow nutrients, electrolytes, and water from inside the lumen to be absorbed into circulation and to prevent pathogens and toxins from entering (reviewed in Kelly et al., 2015). Research shows that the intestinal barrier develops throughout the postnatal period, and changes that take place during this process (e.g., diet, antibiotic use, or exposure to conspecifics) can greatly influence the development of the intestinal barrier and the microbiome (Groschwitz and Hogan, 2009). Early-life stress also affects the permeability of the intestinal barrier. For example, rats exposed to neonatal maternal separation show not only significantly less exploration of a novel object when compared with control rats, but they also show increased mucosal conductance and macromolecular permeability in the gut following mild acute stress (30-min water avoidance) in adulthood, suggesting molecules could more easily pass through the lumen and may have downstream effects. If however, rats were pre-treated with the corticotropin-releasing hormone (CRH) antagonist, α-helical CRH (9–41), 20 min before the mild stress, the mucosal changes were completely eliminated (Soderholm et al., 2002), suggesting that the HPA axis may be mediating some of the changes seen in gut permeability, or “leaky gut” leading to changes in behavioral outputs.
Further work suggests that the immune response may also be capable of modulating stress-induced changes in gut permeability. For example in C57Bl/6 and IL-10−/− mice postnatally exposed to maternal separation, IL-10−/− mice show greater severity of colitis in response to maternal separation (e.g., higher concentrations of colonic pro-inflammatory cytokines), and increased colonic permeability when compared with wild type mice (Lennon et al., 2013). These data suggest that, in the face of early-life stress, animals that can mount an appropriate immune response may be better able to overcome stress; however, the precise mechanisms have not been determined.
Sex steroids can also play an important role in maintaining proper gut permeability, however, results in this area is not consistent. Estrogens may play a particularly important role in this regulation. Estrogen receptor beta (ER-β) is the most abundant estrogen receptor in the colon, and epithelial cells in normal colons of Sprague-Dawley rats show significantly higher ERβ expression in both sexes when compared with cells from malignant tumors in the colon. These data suggest that ERβ may play a particularly important role in protection against disease in the colon (Konstantinopoulos et al., 2003). Further work has shown that 17β-estradiol, but not testosterone, progesterone, or 17α-estradiol, has a significant effect on chloride ion (Cl−) secretion in the female rat distal colon. Interestingly, however, when males were treated with 17β-estradiol, it did not alter Cl− secretion in the colon (Condliffe et al., 2001). These data provide evidence that sex may be a critical modulator of the gut epithelium and may play a vital role in the crosstalk between the brain, behavior, and the microbiome. Moreover, melatonin may play a role in maintaining a strong gut lining as well. In one study, administration of luminal melatonin caused a significant decrease in gut permeability by way of the nicotinic acetylcholine receptor in male Sprague Dawley rats (Sommansson et al., 2013), though the same investigation has not been done in females. All of these studies suggest the intricate relationship between the brain and the gastrointestinal tract, including the gut lining.
4.2 A vulnerable epithelium can lead to changes in physiology and behavior
Though the gut has extensive tolerance mechanisms to prevent the translocation of gut microbes from inside the intestines to outside circulation, these control systems can be broken down, allowing bacteria to elicit damaging changes to the host immune system, and thus behavior. The weakening of the gut epithelium is the basis for many different pathologies and the behavioral comorbidities that go along with them. For example, in times of chronic stress, the gut epithelial layer becomes more permeable (“leaky”), which leads to increased movement of endotoxins from inside the gut to outside creating low-grade inflammation over long periods of time. This chronic, low-grade inflammation is characteristic of many different diseases, including type II diabetes, depression, and autoimmune diseases (De Punder and Pruimboom, 2015). One study found that the increase in circulating LPS as a consequence of a weakened gut lining creates an environment with heightened levels of immunoglobulin [Ig]-M (IgM) and IgA, important antibodies produced during the immune response. These physiological changes are also present in major depressive disorder (MDD) and may contribute to the behavioral abnormalities seen in MDD patients (Maes et al., 2008). Moreover, some studies suggest that treatment with probiotics can correct some of changes associated with increased gut permeability. In particular, offspring of immune-challenged dams who received oral treatment of Bacteroides fragilis showed decreases in gut permeability, recovered microbial composition, and recovery of stereotypic, anxiety-like, and sensorimotor behaviors (Hsiao et al., 2013). These behavioral changes resemble those in many neurodevelopmental disorders seen in humans as well.
5. Potential mechanisms mediating gut-brain-behavior communication
5.1 The immune system
The way in which microbes communicate with the brain to influence behavior is not completely understood, though the immune system may play a particularly important role in this crosstalk (Lee and Mazmanian, 2010). GF rats, for example, exhibit altered development of many aspects of the immune system, including fewer, smaller, and inactive lymph nodes and Peyer’s patches (lymphatic tissue found in the small intestine), prohibiting the immune system from responding appropriately (Hoshi et al., 1992). Further crosstalk may be happening via various microbial-associated molecular patterns (MAMPs) within the gut microbiome, including molecules such as LPS, bacterial lipoprotein (BLP), and flagellin. All of these molecules activate various parts of the immune system (e.g., macrophages), which influence the production of pro-inflammatory cytokines, leading to changes in physiology and behavior (reviewed in detail in Sampson and Mazmanian, 2015). The gut has, however, a set of mechanisms to prevent the activation of the immune system by these MAMPs, though in times of increased loads (e.g., long-term inflammation), the gut lining can become more vulnerable and subject to the translocation of endotoxins into the systemic system, often leading to disease (as discussed in Section 4.2).
5.2 Neurotransmitters
In addition, neurotransmitters, such as serotonin (5-HT; 5-hydroxytryptamine), dopamine, norepinephrine, epinephrine, gamma-aminobutyric acid (GABA), and acetylcholine are produced in the gut, where they can elicit changes in physiology and behavior, however, the exact mechanisms are not known (Gareau, 2014). For example, in one study, GF mice exhibited greater anxiety-like behavior, decreased expression of 5-HT receptor 1A (5-HT 1A), and increased expression of brain-derived neurotrophic factor (BDNF) in the hippocampus compared with SPF (Neufeld et al., 2011). This decrease in 5-HT 1A receptors may prevent the functioning of the serotonergic neurons, and other studies have provided evidence that the 5-HT 1A receptors are particularly important in the modulation of anxiety-like behaviors (Li et al., 2004; Savitz et al., 2009). It is likely, therefore, that the decrease in 5-HT 1A receptors would produce an increase in anxiety-like behavior in these GF mice.
Further work on catecholamines has helped us to understand more about their action in the gut. For example, in one study, catecholamine levels in the GI tract were compared across SPF mice, gnotobiotic (mice inoculated with individual known strains of microorgansms), and GF mice. There are high levels of biologically active dopamine and norepinephrine in SPF mice and gnotobiotic mice inoculated with a mixture of Clostridium species, however in GF mice, catecholamine levels were lower and of biologically inactive forms (Asano et al., 2012), suggesting that these molecules may be important in regulating behavioral outputs.
Further, the presence of the gut microbiome is vital to maintaining the integrity of the blood brain barrier during development. Notably, GF mice exhibit increased permeability of the BBB during development and during adulthood, suggesting that the presence of particular microbes aids in the development of a resilient BBB. This increased BBB permeability in GF mice may be due to fewer tight junction proteins, as further discussed in Section 5.4 (Braniste et al., 2014).
A more direct route of interaction between the gut microbiome and the brain could include changes in serotonin and its precursors. For instance, it is possible that due to the increased permeability of the BBB during development, 5-HT could cross the BBB; it is more likely, however, that the amino acid tryptophan (5-HT’s precursor), which can more easily pass the BBB, especially when compromised, would initiate the production of 5-HT in the brain directly (O’Mahony et al., 2014), which could greatly influence physiology and behavior.
5.3 Bacterial by-products
Bacterial by-products can also influence the brain and behavior, however, research in this area is still in its infancy. By-products, such as short chain fatty acids (SFAs) (e.g., acetate, butyrate, propionate) can both interfere with immune responses and CNS function (Borre et al., 2014a), as well aid in the normal development of cells within the brain (Borre et al., 2014b). For example, rats treated with intraventricular infusions of propionic acid (PPA) exhibit hyperactivity, along with repetitive dystonic behaviors, such as repetitive, full body turning, and forelimb or hindlimb repeated adduction and extension, suggesting PPA may play a role in mediating some of these behaviors. Further, rats treated with PPA also show increased oxidative stress markers and activated microglia, suggesting that there is a neuroinflammatory process happening in response to the PPA treatment (Macfabe et al., 2007). In contrast, SFAs can be particularly important in the proper development and maintenance of microglia in the brain. For example, defective microglia in GF mice can be restored by SFA treatment (sodium propionate, sodium butyrate, and sodium acetate added to drinking water), such that density and morphology are found to be similar to SPF mice after treatment (Erny et al., 2015). These studies provide evidence that there may be an important connection between microbial by-products present in the gut, the immune system, and the CNS. Further work in this area will help us to understand where these by-products come from, where they are active, and how their actions may influence normal species-typical behaviors.
5.4 Neuropeptides
Another important avenue of research aiding in our understanding of the crosstalk among the brain, microbiome, and immune system is neuropeptides, including neuropeptide Y (NPY), substance P (SP), and vasoactive intestinal polypeptide (VIP) (reviewed in Holzer and Farzi, 2014). These biologically active compounds can often work in similar ways, and therefore, understanding the role that they play in this crosstalk is complicated. In one study, rats treated with intracerebroventricular injections of NPY showed increased anti-depressive behaviors (e.g., decreased escape failure in the conditioned avoidance test), and if NPY receptors were blocked, the effects were depleted (Ishida et al., 2007). In a different study, treatment with antibiotics was associated with an increase in SP immunoreactivity in the colon, but administration of Lactobacillus paracasei (a common probiotic) reduced the immunostaining in antibiotic-treated mice (Verdu et al., 2006). Further, VIP can actually protect colitis-induced epithelial damage by helping to maintain the integrity and distribution of tight junction proteins in the lining, suggesting that VIP may play a particularly important role in preventing diseases related to gut permeability (Conlin et al., 2009). Whether or not these bioactive compounds affect the gut-brain axis in a dose-dependent manner is not yet known.
6. Clinical implications of the gut-brain-behavior axis
Changes in the gut microbiome have often been associated with autism spectrum disorders (ASD), and subsets of these patients show gastrointestinal symptoms as well. Interestingly, a number of studies have suggested that targeting the gut microbiota could be a means to treat the behavioral abnormalities related to the disease (Mulle et al., 2013). In addition, many autoimmune diseases and the behavioral comorbidities associated with them, are often sex-specific with higher prevalence in females, and further investigation into the mechanisms mediating these effects is essential (Markle et al., 2013). As mentioned in previous sections, maternal immune activation and maternal separation can have drastic effects on offspring development and behavior, with alterations in the gut microbiome a potential regulator of these changes as well. Further, there have been numerous studies investigating the role of healthy microbiota in ASD in GF models. For example, GF mice exhibited abnormal social behavior, suggesting that a healthy gut microbiome is important in the maintenance of species-typical social behavior. In particular, male GF mice spent significantly less time in a chamber containing a conspecific than they did in an empty chamber, and GF males spent less time investigating novel conspecifics when compared with familiar ones, suggesting social cognition deficits, which are often seen in many patients with ASD (Desbonnet et al., 2014). Importantly, the use of GF mice has helped make major strides in understanding the potential links between gut permeability, composition, and autism spectrum disorders, and the use of prebiotics, probiotics, and antibiotics as potential treatment options for the disorders and their behavioral abnormalities (reviewed in detail in Luczynski et al., 2016).
7. Conclusions and future directions
Although we are beginning to determine a considerable amount about how the brain, immune system, and microbiome modulate behavior, there is still much to be learned. Because these systems do not work in isolation, it is essential to focus on how their connections work in concert to create a behavioral phenotype (Fig. 1). Further, early-life experience, including immune activation, diet, and stress, can influence the production of species-typical behaviors and potential psychopathologies later in adulthood and are therefore important to investigate in the context of the gut-brain axis. Further investigation into how the microbiome is modulating these social and affective behavioral changes could be a key factor in understanding the evolution of individuals within a species and could be of great clinical potential as well. Thus, collaboration among both basic and clinical scientists to determine the precise physiological mechanisms underlying neuroendocrine-immune-microbiome-behavior interactions will be critical for future progress in the field.
Highlights.
The gut microbiome may play a critical role in modulating behavioral responses.
Gut microbes may communicate with the brain via both neural and humoral pathways.
The neuroendocrine system and microbiome interact to influence social behaviors.
The gut-brain-behavior axis can have important clinical implications.
Acknowledgments
This work was supported by the National Institute of Child Health and Human Development (T32HD49336), the National Science Foundation (IOS 1656414), and Indiana University.
Footnotes
Conflict of interest
The authors declare no conflict of interest, financial or otherwise.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albenberg LG, Wu GD. Diet and the Intestinal Microbiome: Associations, Functions, and Implications for Health and Disease. Gastroenterology. 2014;146:1564–1572. doi: 10.1053/j.gastro.2014.01.058.Diet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archie Ea, Theis KR. Animal behaviour meets microbial ecology. Anim Behav. 2011;82:425–436. doi: 10.1016/j.anbehav.2011.05.029. [DOI] [Google Scholar]
- Asano Y, Hiramoto T, Nishino R, Aiba Y, Kimura T, Yoshihara K, Koga Y, Sudo N. Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am J Physiol Gastrointest Liver Physiol. 2012;303:1288–1295. doi: 10.1152/ajpgi.00341.2012. [DOI] [PubMed] [Google Scholar]
- Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, Deng Y, Blennerhassett P, MacRi J, McCoy KD, Verdu EF, Collins SM. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology. 2011;141:599–609. doi: 10.1053/j.gastro.2011.04.052. [DOI] [PubMed] [Google Scholar]
- Bilbo, Staci D, Biedenkapp JC, Der-Avakian A, Watkins LR, Rudy JW, Maier SF. Neonatal Infection-Induced Memory Impairment after Lipopolysaccharide in Adulthood Is Prevented via Caspase-1 Inhibition. J Neurosci. 2005;25:8000–8009. doi: 10.1523/JNEUROSCI.1748-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Levkoff LH, Mahoney JH, Watkins LR, Rudy JW, Maier SF. Neonatal infection induces memory impairments following an immune challenge in adulthood. Behav Neurosci. 2005;119:293–301. doi: 10.1037/0735-7044.119.1.293. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Schwarz JM. The immune system and developmental programming of brain and behavior. Front Neuroendocrinol. 2012;33:267–286. doi: 10.1016/j.yfrne.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Schwarz JM. Early-life programming of later-life brain and behavior: a critical role for the immune system. Front Behav Neurosci. 2009;3:14. doi: 10.3389/neuro.08.014.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borre YE, Moloney RD, Clarke G, Dinan TG, Cryan JF. The Impact of Microbiota on Brain and Behavior: Mechanisms & Therapeutic Potential. In: Lyte M, Cryan JF, editors. Microbial Endocrinology: The Microbiota-Gut-Brain Axis in Health and Disease. Springer; New York, NY: 2014a. p. 403. [DOI] [PubMed] [Google Scholar]
- Borre YE, O’Keeffe GW, Clarke G, Stanton C, Dinan TG, Cryan JF. Microbiota and neurodevelopmental windows: Implications for brain disorders. Trends Mol Med. 2014b:1–10. doi: 10.1016/j.molmed.2014.05.002. [DOI] [PubMed] [Google Scholar]
- Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Tóth M, Korecka A, Bakocevic N, Ng LG, Kundu P, Gulyás B, Halldin C, Hultenby K, Nilsson H, Hebert H, Volpe BT, Diamond B, Pettersson S. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. 2014:6. doi: 10.1126/scitranslmed.3009759.The. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci. 2011;108:16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Fernandez-Kim SO, Townsend RL, Kruger C, Carmouche R, Newman S, Salbaum JM, Berthoud HR. Maternal obese-Type gut microbiota differentially impact cognition, anxiety and compulsive behavior in male and female offspring in mice. PLoS One. 2017;12:1–20. doi: 10.1371/journal.pone.0175577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke G, O’Mahony SM, Dinan TG, Cryan JF. Priming for health: Gut microbiota acquired in early life regulates physiology, brain and behaviour. Acta Paediatr Int J Paediatr. 2014;103:812–819. doi: 10.1111/apa.12674. [DOI] [PubMed] [Google Scholar]
- Collins SM, Bercik P. The Relationship Between Intestinal Microbiota and the Central Nervous System in Normal Gastrointestinal Function and Disease. Gastroenterology. 2014:2003–2014. doi: 10.1053/j.gastro.2009.01.075. [DOI] [PubMed] [Google Scholar]
- Condliffe SB, Doolan CM, Harvey BJ. 17β-Oestradiol acutely regulates Cl− secretion in rat distal colonic epithelium. J Physiol. 2001;530:47–54. doi: 10.1111/j.1469-7793.2001.0047m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlin VS, Wu X, Nguyen C, Dai C, Vallance BA, Buchan AMJ, Boyer L, Jacobson K. Vasoactive intestinal peptide ameliorates intestinal barrier disruption associated with Citrobacter rodentium-induced colitis. Am J Physiol Gastrointest Liver Physiol. 2009:G735–G750. doi: 10.1152/ajpgi.90551.2008. [DOI] [PubMed] [Google Scholar]
- Costalonga M, Zell T. Lipopolysaccharide enhances in vivo interleukin-2 production and proliferation by naive antigen-specific CD4 T cells via a Toll-like receptor 4-dependent mechanism. Immunology. 2007;122:124–130. doi: 10.1111/j.1365-2567.2007.02620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, O’Mahony SM. The microbiome-gut-brain axis: From bowel to behavior. Neurogastroenterol Motil. 2011;23:187–192. doi: 10.1111/j.1365-2982.2010.01664.x. [DOI] [PubMed] [Google Scholar]
- De Punder K, Pruimboom L. Stress induces endotoxemia and low-grade inflammation by increasing barrier permeability. Front Immunol. 2015;6:1–12. doi: 10.3389/fimmu.2015.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbonnet L, Clarke G, Shanahan F, Dinan TG, Cryan JF. Microbiota is essential for social development in the mouse. Mol Psychiatry. 2014;19:146–148. doi: 10.1038/mp.2013.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbonnet L, Garrett L, Clarke G, Bienenstock J, Dinan TG. The probiotic Bifidobacteria infantis: An assessment of potential antidepressant properties in the rat. J Psychiatr Res. 2009;43:164–174. doi: 10.1016/j.jpsychires.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Diz-Chaves Y, Astiz M, Bellini MJ, Garcia-Segura LM. Prenatal stress increases the expression of proinflammatory cytokines and exacerbates the inflammatory response to LPS in the hippocampal formation of adult male mice. Brain Behav Immun. 2013;28:196–206. doi: 10.1016/j.bbi.2012.11.013. [DOI] [PubMed] [Google Scholar]
- Erny D, De Angelis ALH, Jaitin D, Wieghofer P, Staszewski O, David E, Keren-Shaul H, Mahlakoiv T, Jakobshagen K, Buch T, Schwierzeck V, Utermöhlen O, Chun E, Garrett WS, Mccoy KD, Diefenbach A, Staeheli P, Stecher B, Amit I, Prinz M. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. 2015;18:965–977. doi: 10.1038/nn.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster Ja, McVey Neufeld KA. Gut-brain axis: How the microbiome influences anxiety and depression. Trends Neurosci. 2013;36:305–312. doi: 10.1016/j.tins.2013.01.005. [DOI] [PubMed] [Google Scholar]
- French SS, Chester EM, Demas GE. Maternal immune activation affects litter success, size and neuroendocrine responses related to behavior in adult offspring. Physiol Behav. 2013;119:175–184. doi: 10.1016/j.physbeh.2013.06.018. [DOI] [PubMed] [Google Scholar]
- Gareau MG. Microbial Endocrinology: The Microbiota-Gut-Brain Axis in Health and Disease Chapter 17. Adv Exp Med Biol. 2014;817:39–71. doi: 10.1007/978-1-4939-0897-4. [DOI] [PubMed] [Google Scholar]
- Gaskin JH, Kitay JI. Andrenocortical function in the hamster. Sex differences and effects of gonadal hormones. Endocrinology. 1970;87:779–786. doi: 10.1210/endo-87-4-779. [DOI] [PubMed] [Google Scholar]
- Goehler LE, Park SM, Opitz N, Lyte M, Gaykema RPA. Campylobacter jejuni infection increases anxiety-like behavior in the holeboard: Possible anatomical substrates for viscerosensory modulation of exploratory behavior. Brain Behav Immun. 2008;22:354–366. doi: 10.1016/j.bbi.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groschwitz KR, Hogan SP. Intestinal barrier function: Molecular regulation and disease pathogenesis. Clin Rev allergy Immunol. 2009:3–20. doi: 10.1016/j.jaci.2009.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa RJ, Burgess LH, Kerr JE, O’Keefe JA. Gonadal Steroid Hormone Receptors and Sex Differences in the Hypothalamo-Pituitary-Adrenal Axis. Horm Behav. 1994;28:464–476. doi: 10.1006/hbeh.1994.1044. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Nunley KM, Lorens Sa, Louie JP, McGivern RF, Bollnow MR. Androgen regulation of adrenocorticotropin and corticosterone secretion in the male rat following novelty and foot shock stressors. Physiol Behav. 1994;55:117–124. doi: 10.1016/0031-9384(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Weiser MJ. Gonadal steroid hormones and the hypothalamo – pituitary – adrenal axis. Front Neuroendocrinol. 2014;35:197–220. doi: 10.1016/j.yfrne.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen MK, Nguyen KT, Fleshner M, Goehler LE, Gaykema RP, Maier SF, Watkins LR. Effects of vagotomy on serum endotoxin, cytokines, and corticosterone after intraperitoneal lipopolysaccharide. Am J Physiol Regul Integr Comp Physiol. 2000;278:R331–336. doi: 10.1152/ajpregu.2000.278.2.R331. [DOI] [PubMed] [Google Scholar]
- Harvey L, Boksa P. Prenatal and postnatal animal models of immune activation: Relevance to a range of neurodevelopmental disorders. Dev Neurobiol. 2012;72:1335–1348. doi: 10.1002/dneu.22043. [DOI] [PubMed] [Google Scholar]
- Holzer P, Farzi A. Neuropeptides and the Microbiota-Gut-Brain Axis. In: Lyte M, Cryan JF, editors. Microbial Endocrinology: The Microbiota-Gut-Brain Axis in Health and Disease. Springer; New York, New York, NY: 2014. pp. 195–219. [DOI] [Google Scholar]
- Hooper LV, Littman DR, Macpherson AJ. Interactions Between the Microbiota and the Immune System. Science (80- ) 2012;336:1268–1274. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi H, Aijima H, Horie K, Nagata H, Kaneko T, Ikeda T. Lymph Follicles and Germinal Center in Popliteal Lymph Nodes and Other Lymphoid Tissues of Germ-Free and Conventional Rats. Tohoku J Exp Med. 1992;166:297–307. doi: 10.1620/tjem.166.297. [DOI] [PubMed] [Google Scholar]
- Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, Codelli Ja, Chow J, Reisman SE, Petrosino JF, Patterson PH, Mazmanian SK. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155:1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinohe T, Pang IK, Kumamoto Y, Peaper DR, Ho JH, Murray TS, Iwasaki A. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc Natl Acad Sci U S A. 2011;108:5354–5359. doi: 10.1073/pnas.1019378108. doi: 10.1073/pnas.1019378108/-/DCSupplemental.www.pnas.org/cgi/doi/10.1073/pnas.1019378108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida H, Shirayama Y, Iwata M, Katayama S, Yamamoto A, Kawahara R, Nakagome K. Infusion of Neuropeptide Y Into CA3 Region of Hippocampus Produces Antidepressant-Like Effect via Y1 Receptor. Hippocampus. 2007;280:271–280. doi: 10.1002/hipo. [DOI] [PubMed] [Google Scholar]
- Jašarević E, Howerton CL, Howard CD, Bale TL. Alterations in the vaginal microbiome by maternal stress are associated with metabolic reprogramming of the offspring gut and brain. Endocrinology. 2015;156:3265–3276. doi: 10.1210/en.2015-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JR, Kennedy PJ, Cryan JF, Dinan TG. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric. Front Cell Neurosci. 2015:9. doi: 10.3389/fncel.2015.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox AM, Li XF, Kinsey-Jones JS, Wilkinson ES, Wu XQ, Cheng YS, Milligan SR, Lightman SL, O’Byrne KT. Neonatal lipopolysaccharide exposure delays puberty and alters hypothalamic Kiss1 and Kiss1r mRNA expression in the female rat. J Neuroendocr. 2009;21:683–689. doi: 10.1111/j.1365-2826.2009.01885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstantinopoulos PA, Kominea A, Vandoros G, Sykiotis GP, Andricopoulosd P, Varakisb I, Sotiropoulou-Bonikoub G, Papavassilioua AG. Oestrogen receptor beta (ERb) is abundantly expressed in normal colonic mucosa, but declines in colon adenocarcinoma paralleling the tumour’s dedifferentiation. Eur J Cancer. 2003;39:1251–1258. doi: 10.1016/S0959-8049(03)00239-9. [DOI] [PubMed] [Google Scholar]
- Lee YK, Mazmanian SK. Has the microbiota played a critical role in the evolution of the adaptive immune system? Science (80- ) 2010;330:1768–1773. doi: 10.1126/science.1195568.Has. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennon EM, Maharshak N, Elloumi H, Borst L, SEP, Moeser AJ. Early Life Stress Triggers Persistent Colonic Barrier Dysfunction and Exacerbates Colitis in Adult IL-10−/− Mice. Inflamm Bowel Dis. 2013;19:712–719. doi: 10.1097/MIB.0b013e3182802a4e.Early. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Holmes A, Ma L, Van de Kar LD, Garcia F, Murphy DL. Medial Hypothalamic 5-Hydroxytryptamine (5-HT)1A Receptors Regulate Neuroendocrine Responses to Stress and Exploratory Locomotor Activity: Application of Recombinant Adenovirus Containing 5-HT1A Sequences. J Neurosci. 2004;24:10868–10877. doi: 10.1523/JNEUROSCI.3223-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luczynski P, Neufeld KAMV, Oriach CS, Clarke G, Dinan TG, Cryan JF. Growing up in a bubble: Using germ-free animals to assess the influence of the gut microbiota on brain and behavior. Int J Neuropsychopharmacol. 2016;19:1–17. doi: 10.1093/ijnp/pyw020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfabe DF, Cain DP, Rodriguez-capote K, Franklin AE, Hoffman JE, Boon F, Taylor AR, Kavaliers M, Ossenkopp K. Neurobiological effects of intraventricular propionic acid in rats: Possible role of short chain fatty acids on the pathogenesis and characteristics of autism spectrum disorders. Behav Brain Res. 2007;176:149–169. doi: 10.1016/j.bbr.2006.07.025. [DOI] [PubMed] [Google Scholar]
- Maes M, Kubera M, Leunis J. The gut-brain barrier in major depression: Intestinal mucosal dysfunction with an increased translocation of LPS from gram negative enterobacteria plays a role in the inflammatory pathophysiology of depression. Neuroendocrinol Lett. 2008;29:117–124. [pii] [PubMed] [Google Scholar]
- Markle JGM, Frank DN, Mortin-toth S, Robertson CE, Feazel LM, Rolle-kampczyk U, Von Bergen M, Mccoy KD, Macpherson AJ, Danska JS. Sex Differences in the Gut. Science (80- ) 2013;339:1084–1088. doi: 10.1126/science.1233521. [DOI] [PubMed] [Google Scholar]
- Mulak A, Taché Y, Larauche M. Sex hormones in the modulation of irritable bowel syndrome. World J Gastroenterol. 2014;20:2433–48. doi: 10.3748/wjg.v20.i10.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulle JG, Sharp WG, Cubells JF. The gut microbiome: A new frontier in autism research. Curr Psychiatry Rep. 2013:15. doi: 10.1007/s11920-012-0337-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myles Ia, Fontecilla NM, Janelsins BM, Vithayathil PJ, Segre Ja, Datta SK. Parental dietary fat intake alters offspring microbiome and immunity. J Immunol. 2013;191:3200–9. doi: 10.4049/jimmunol.1301057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naor R, Domankevich V, Shemer S, Sominsky L, Rosenne E, Levi B, Ben-Eliyahu S. Metastatic-promoting effects of LPS: sexual dimorphism and mediation by catecholamines and prostaglandins. Brain Behav Immun. 2009;23:611–21. doi: 10.1016/j.bbi.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld KM, Kang N, Bienenstock J, Foster JA. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol Motil. 2011:23. doi: 10.1111/j.1365-2982.2010.01620.x. [DOI] [PubMed] [Google Scholar]
- O’Mahony SM, Clarke G, Borre YE, Dinan TG, Cryan JF. Serotonin, Tryptophan Metabolism and the Brain-Gut- Microbiome Axis. Behav Brain Res. 2014;277:1–17. doi: 10.1016/j.bbr.2014.07.027. [DOI] [PubMed] [Google Scholar]
- Perry VH. The influence of systemic inflammation on inflammation in the brain: implications for chronic neurodegenerative disease. Brain Behav Immun. 2004;18:407–413. doi: 10.1016/j.bbi.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Pyndt Jørgensen B, Hansen JT, Krych L, Larsen C, Klein AB, Nielsen DS, Josefsen K, Hansen AK, Sørensen DB. A possible link between food and mood: dietary impact on gut microbiota and behavior in BALB/c mice. PLoS One. 2014;9:e103398. doi: 10.1371/journal.pone.0103398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan N, Banks Wa. Brain-immune communication pathways. Brain Behav Immun. 2007;21:727–735. doi: 10.1016/j.bbi.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Raber J, Sorg O, Horn TFW, Yu N, Koob GF, Campbell IL, Bloom FE. Inflammatory cytokines: putative regulators of neuronal and neuro-endocrine function1Published on the World Wide Web on 24 October 1997.1. Brain Res Rev. 1998;26:320–326. doi: 10.1016/S0165-0173(97)00041-6. [DOI] [PubMed] [Google Scholar]
- Sampson TR, Mazmanian SK. Control of brain development, function, and behavior by the microbiome. Cell Host Microbe. 2015;17:565–576. doi: 10.1016/j.chom.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz J, Lucki I, Drevets WC. 5-HT1A receptor function in major depressive disorder. Prog Neurobiol. 2009;88:17–31. doi: 10.1016/j.pneurobio.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanks N, Larocque S, Meaney MJ. Neonatal endotoxin exposure alters the development of the hypothalamic-pituitary-adrenal axis: early illness and later responsivity to stress. J Neurosci. 1995;15:376–84. doi: 10.1523/JNEUROSCI.15-01-00376.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderholm JD, Yates DA, Gareau MG, Yang PC, MacQueen G, Perdue MH. Neonatal maternal separation predisposes adult rats to colonic barrier dysfunction in response to mild stress. Am J Physiol Gastrointest Liver Physiol. 2002;283:1257–1263. doi: 10.1152/ajpgi.00314.2002. [DOI] [PubMed] [Google Scholar]
- Sommansson A, Nylander O, Sjöblom M. Melatonin decreases duodenal epithelial paracellular permeability via a nicotinic receptor-dependent pathway in rats in vivo. J Pineal Res. 2013;54:282–291. doi: 10.1111/jpi.12013. [DOI] [PubMed] [Google Scholar]
- Sribnick EA, Wingrave JM, Matzelle DD, Wilford GG, Ray SK, Banik NL. Estrogen attenuated markers of inflammation and decreased lesion volume in acute spinal cord injury in rats. J Neurosci Res. 2005;82:283–93. doi: 10.1002/jnr.20622. [DOI] [PubMed] [Google Scholar]
- vom Steeg LG, Klein SL. Sex Steroids Mediate Bidirectional Interactions Between Hosts and Microbes. Horm Behav. 2016;88:45–51. doi: 10.1016/j.yhbeh.2016.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steegenga WT, Mischke M, Lute C, Boekschoten MV, Pruis MG, Lendvai A, Verkade HJ, Boekhorst J, Timmerman HM, Plösch T, Müller M. Sexually dimorphic characteristics of the small intestine and colon of prepubescent C57BL/6 mice. Biol Sex Differ. 2014;5:11. doi: 10.1186/s13293-014-0011-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvia KE, Báez Ramos P, Demas GE. Sickness-induced changes in physiology do not affect fecundity or same-sex behavior. Physiol Behav. 2018;184:68–77. doi: 10.1016/j.physbeh.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvia KE, Demas GE. Overcoming neonatal sickness: Sex-specific effects of sickness on physiology and social behavior. Physiol Behav. 2017;179:324–332. doi: 10.1016/j.physbeh.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvia KE, Jewell CP, Rendon NM, St John EA, Demas GE. Sex-specific modulation of the gut microbiome and behavior in Siberian hamsters. Brain Behav Immun. 2016;60:51–62. doi: 10.1016/j.bbi.2016.10.023. [DOI] [PubMed] [Google Scholar]
- Tenk CM, Kavaliers M, Ossenkopp KP. Sexually dimorphic effects of neonatal immune system activation with lipopolysaccharide on the behavioural response to a homotypic adult immune challenge. Int J Dev Neurosci. 2008;26:331–8. doi: 10.1016/j.ijdevneu.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Tochitani S, Ikeno T, Ito T, Sakurai A, Yamauchi T, Matsuzaki H. Administration of non-Absorbable antibiotics to pregnant mice to perturb the maternal gut microbiota is associated with alterations in offspring behavior. PLoS One. 2016;11:1–22. doi: 10.1371/journal.pone.0138293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohmi M, Tsuda N, Watanabe Y, Kakita A, Nawa H. Perinatal inflammatory cytokine challenge results in distinct neurobehavioral alterations in rats: implication in psychiatric disorders of developmental origin. Neurosci Res. 2004;50:67–75. doi: 10.1016/j.neures.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Verdu EF, Bercik P, Verma-Gandhu M, Huang XX, Blennerhassett P, Jackson W, Mao Y, Wang L, Rochat F, Collins SM. Specific probiotic therapy attenuates antibiotic induced visceral hypersensitivity in mice. Neurogastroenterology. 2006;55:182–190. doi: 10.1136/gut.2005.066100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viau V. YOUNG INVESTIGATOR PERSPECTIVES Functional Cross-Talk Between the Hypothalamic-Pituitary-Gonadal and -Adrenal Axes. J Neuroendocrinol. 2002;14:506–513. doi: 10.1046/j.1365-2826.2002.00798.x. [DOI] [PubMed] [Google Scholar]
- Viau V, Bingham B, Davis J, Lee P, Wong M. Gender and puberty interact on the stress-induced activation of parvocellular neurosecretory neurons and corticotropin-releasing hormone messenger ribonucleic acid expression in the rat. Endocrinology. 2005;146:137–146. doi: 10.1210/en.2004-0846. [DOI] [PubMed] [Google Scholar]
- Wallace TC, Guarner F, Madsen K, Cabana MD, Gibson G, Hentges E, Sanders ME. Human gut microbiota and its relationship to health and disease. Nutr Rev. 2011;69:392–403. doi: 10.1111/j.1753-4887.2011.00402.x. [DOI] [PubMed] [Google Scholar]
- Wong D, Dorovini-Zis K, Vincent SR. Cytokines, nitric oxide, and cGMP modulate the permeability of an in vitro model of the human blood-brain barrier. Exp Neurol. 2004;190:446–455. doi: 10.1016/j.expneurol.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Zivkovic AM, German JB, Lebrilla CB, Mills Da. Human milk glycobiome and its impact on the infant gastrointestinal microbiota. Proc Natl Acad Sci U S A. 2011;108(Suppl):4653–4658. doi: 10.1073/pnas.1000083107. [DOI] [PMC free article] [PubMed] [Google Scholar]