Abstract
Antiretroviral therapy (ART) has been associated with a shift in the epidemiology of HIV-associated cardiomyopathy from a phenotype of primarily left ventricular (LV) systolic dysfunction to LV diastolic dysfunction (DD). Patients with HIV receiving ART have higher rates of DD when compared to age-matched controls and develop DD at a younger age. However, little is known about the natural history and pathogenesis of DD in virally suppressed HIV-infected patients. Current evidence suggests that immune processes modulate the risk for cardiac involvement in HIV-infected persons. Ongoing inflammation appears to have myocardial effects and accelerated myocardial fibrosis appears to be a key mediator of HIV-induced DD. The Characterizing Heart Function on Antiretroviral Therapy (CHART) study aims to systematically investigate determinants, mechanisms, and consequences of DD in HIV-infected patients. We will compare ART-treated, virally suppressed HIV-infected individuals with and without DD and HIV- individuals with DD in regards to (1) systemic inflammation, myocardial stress, and subclinical myocardial necrosis through circulating biomarkers; (2) immune system activation through cell surface receptors; (3) myocardial fibrosis by cardiac magnetic resonance; (4) markers of fibrosis and remodeling, oxidative stress, and hypercoagulability; (5) left atrial function through echocardiography; (6) proteomic and metabolomic profiles; and (7) phenotype signatures derived from clinical, biomarker, and imaging data.
Keywords: human immuno-deficiency virus, diastolic dysfunction, heart failure, pathophysiology
BACKGROUND AND RATIONALE
Introduction
Antiretroviral therapy (ART) has transformed the clinical profile of human immunodeficiency virus (HIV) from an acute infection with a high mortality risk into a treatable, chronic disease with near-normal life expectancy, especially in high-income countries.1 By the year 2030, it is anticipated that 73% of HIV-infected individuals will be aged 50 or older and that 78% will have cardiovascular disease.2 Although cardiac involvement in HIV infection has been long recognized,3 effective combination ART has been associated with a shift in the epidemiology of HIV cardiomyopathy and heart failure (HF) from a phenotype of primarily left ventricular (LV) systolic dysfunction to LV diastolic dysfunction (DD). 4,5 However, there is little information available on the natural history and pathogenesis of DD and in HIV-infected subjects who are virally suppressed on optimal ART therapy. The growing prevalence of DD in HIV is a concern, given the link between DD, HF with preserved ejection fraction (HFpEF), and mortality.
Diastolic Dysfunction and HFpEF in HIV-Infected Persons
In older adults without HIV, DD is frequently present, with a prevalence ranging from 15% to over 30% depending on the definition and the population studied.6–8 DD is associated with increased risk for atrial fibrillation 9 and HF 10; and portends higher risk for all-cause mortality.11 Previous studies have shown that the development of DD is preceded by obesity, hypertension, and metabolic syndrome, conditions that are associated with elevated inflammatory cytokines and endothelial dysfunction.12 Studies in patients with HIV receiving ART have demonstrated declining rates of LV systolic dysfunction,3 but higher rates of DD when compared to age-matched controls (Table 1).13–16 Also, patients with HIV tend to develop DD at a younger age.5 It is still unclear why HIV-infected patients develop DD more frequently and at a younger age. Although early work suggested that certain ART agents may lead to cardiovascular complications, recent studies suggest minimal effect of ART on DD risk.16 On the other hand, a central role for inflammation in the development of DD in the general population has been postulated,12 and there is evidence of persistent immune activation and ongoing inflammation despite ART and viral suppression in HIV-infected patients.17
Table 1.
Diastolic Dysfunction Among HIV Infected Persons on Antiretroviral Therapy in Comparative Studies
| Study | Population | Findings |
|---|---|---|
| Schuster et al 2008 13 | 30 HIV-infected men (age 42.1± 4.7 years, duration of HIV infection 10.4±4.7 years, duration of ART 5.3±2.1 years) and 26 age- matched HIV- controls | HIV-infected patients had similar height-indexed LV mass vs. controls (40.6±9.5 vs 37.5±9.3 g/m; P>0.05), but higher prevalence of DD (abnormal relaxation or pseudonormal filling), 64% vs. 12% (P<0.001). LV systolic function indexes were lower and pulmonary artery pressure was higher in patients with HIV compared with controls. |
| Hsue et al 2010 14 | 196 HIV-infected adults (median age, 47 years; 85% male; median duration of HIV infection 15 years; 82% on ART) and 52 controls | Median BSA-indexed LV mass was higher in HIV-infected vs. HIV- (77.2 vs. 66.5 g/m2; P<0.001). LV ejection fraction was similar. Among HIV-infected, 50% had mild DD vs. 29% in HIV- (P=0.008). HIV-infected patients had an adjusted odds ratio of 2.4 for DD as compared with controls (P=0.019). |
| Luo et al 2014 15 | 325 HIV-infected initially ART-naïve patients, repeat evaluation after 48 weeks of ART, 97 age-matched HIV- controls | HIV-infected patients had a higher prevalence of DD vs. controls (16.5% vs 7.2%, P=0.027) and LV systolic dysfunction (7.3% vs 2.1%, P = .056). DD increased from baseline to week 48 (23.3%; P=0.056 vs. baseline) in HIV-infected patients. |
| Fontes- Carvalho et al 2015 16 | 206 HIV-infected persons (88 ART-naïve and 116 on ART, 41.7±9.4 years, 70.4% male) and 30 controls | Prevalence of DD in HIV-infected patients 23% vs. 3.3% in controls (P=0.01), but not different between ART-naïve (19%) and on-ART (23%) HIV-infected patients. No differences in systolic function. |
ART= antiretroviral therapy; BSA = body surface area; DD = diastolic dysfunction; HIV= human immuno-deficiency virus; LV = left ventricular
Despite ample evidence of high prevalence of DD in HIV-infected patients, epidemiological data on HFpEF in these patients are scarce. In the large Veterans Aging Cohort Study (VACS), the incidence of HFpEF among HIV-infected veterans ranged from 1.11 cases per 1000 patient-years in the 40–49 age group to 8.19 in those age 70 or older, with an overall increased risk of HFpEF (hazard ratio, 1.21; 95% CI, 1.03–1.41) compared to uninfected veterans.18 In that study, HIV-infected veterans had increased risk for HF with preserved (≥50%), borderline (40%–49%), and reduced (<40%) left ventricular ejection fraction (LVEF) compared to their uninfected counterparts.18 Of note, a CD4 count <200 cells/mm3 was associated with an increased risk for both HF with preserved and reduced LVEF.18 In a cohort of 5041 HIV-infected patients receiving care at an urban setting,19 patients with HF (N = 216) were older and more likely to be black, hypertensive, and have diabetes. In that study, ~ 40% of HF cases were classified as HFpEF (LVEF ≥50%).19 Peak HIV viral load ≥100,000 copies/mL and nadir CD4 count <200 cells/mm3 were associated with significantly elevated odds of HF.19 Taken together, these data imply that immune processes modulate risk for HF in HIV-infected persons.
The Role of Inflammation and Immune Activation
Systemic low-level Inflammation has been proposed as a key mechanism in the development of DD and HFpEF in the general population.12 Although ART reduces the degree of immune activation and inflammation in HIV-infected persons,20,21 there is persistent T cell activation 22 and inflammation 23 in these patients. Biomarkers of inflammation remain elevated in the setting of effectively treated and suppressed HIV infection. In a study of 61 HIV-infected patients on ART with undetectable viral load, chronic activated and senescent CD8 cells and apoptotic CD4 and CD8 cells were more frequent and tumor necrosis factor α levels were higher compared to matched controls after a median of 6 years on ART. 17 In HIV-infected patients, T-cell activation is linked to arterial stiffness,24 and expansion of intermediate and non-classical monocytes has been shown,25 which are associated with subclinical atherosclerosis and coronary calcium progression.25 Elevated levels of soluble CD163, a scavenger receptor on monocytes shed by proteolytic cleavage with pro-inflammatory stimulation, have been associated with coronary plaques 26 and arterial inflammation 27 in HIV-infected patients. Increased arterial stiffness has been linked to development of DD in the general population. 28,29
There are several proposed mechanisms for this ongoing immune activation and systemic low-level inflammation in HIV-infected patients, including residual HIV replication,30 gut mucosal injury and microbial translocation,31 co-infections,32 and impaired homeostatic drive.33 Regardless of underlying mechanisms, elevated levels of inflammatory and coagulation biomarkers are strongly predictive of cardiovascular disease and non-AIDS morbidity in HIV-infected patients. Among 9764 HIV-infected patients without history of cardiovascular disease, doubling of baseline interleuking-6 and D-dimer levels was associated with 41% and 45% elevated risk, respectively, for cardiovascular events after a median of 2.6 years.34
This ongoing inflammation in HIV-infected patients appears to have myocardial effects, according to insights offered by cardiac magnetic resonance (CMR) studies. In a study of 28 HIV-infected patients on ART with adequate viral suppression (<200 copies/mL), native T1 relaxation times, relative T2 signal intensity ratio, and early gadolinium enhancement ratio, were all elevated compared to control subjects, indicating myocardial inflammation in HIV-infected patients.35 Another CMR study in 103 HIV-infected persons receiving ART reported similar findings, including subclinical myocardial edema and fibrosis, higher LV mass and lower systolic function, and frequent pericardial effusions,36 pointing to chronic systemic inflammation involving the myocardium and pericardium.
The Role of Cardio-Metabolic Risk Factors
Cardio-metabolic risk factors such as glucose and lipid metabolism disturbances that induce a pro-inflammatory state and promote DD in the general population are at least equally prevalent in HIV-infected patients. In a meta-analysis of 65 studies comprising over 55,000 HIV-infected patients across 5 continents, the prevalence of metabolic syndrome was 16.7% to 31.3% depending on the definition.37 In a study of 151 HIV-infected but ART-naive, 150 HIV-infected on ART, and 153 HIV-negative adults in Tanzania, more HIV-infected adults on ART had metabolic syndrome versus HIV-negative controls (21.3% vs 7.8%, p=0.008) and elevated lifetime cardiovascular risk (34.7% vs 17.0%, p<0.001), which seems to develop 3–4 years after ART initiation.38 Lipid profiles appear to be altered by both HIV infection and ART.39 Thus, there is an unmet need to elucidate the role of conventional versus HIV-specific risk factors for the development of DD in HIV-infected individuals, and the underlying biological pathways mediating DD risk.
Myocardial Fibrosis as a Potential Mediator of HIV-Induced Diastolic Dysfunction
Besides standard clinical and subclinical structural and functional derangements, CMR can identify focal and diffuse myocardial fibrosis as well as myocardial inflammation and edema.40 T1 mapping allows for quantification of the myocardial extracellular volume fraction, a validated index of extracellular matrix expansion.40,41 In the absence of amyloid or edema, extracellular volume is associated with diffuse interstitial fibrosis, a hallmark of LV remodeling.40 Myocardial inflammation and interstitial fibrosis, both focal and diffuse, have a higher prevalence in HIV-infected patients (Table 2). 35,36,42,43 In addition, HIV-infected patients have increased myocardial lipid content.43,44 Importantly, increased myocardial lipid levels and diffuse myocardial fibrosis (as measured by the extracellular volume index on CMR) both correlate with impaired myocardial function in HIV-infected patients.43 In a large cohort of adults without known HIV infection, 45 myocardial fibrosis quantified by extracellular volume on CMR was higher among patients with HFpEF and those at risk for HFpEF, suggesting that fibrosis precedes HFpEF. A common finding in CMR studies is that HIV-infected patients have impaired LV systolic function (as measured by strain parameters) despite normal LVEF. 35,36,42,43 Of note, impaired systolic function using echocardiographic strain imaging has been shown in patients with HFpEF in two clinical trials.46,47 However, it is important to note that although reduced longitudinal strain is a marker of subclinical myocardial dysfunction, it is certainly not specific for HIV-related myocardial dysfunction and does not necessarily signify a progressive condition.
Table 2.
Myocardial Fibrosis Among HIV Infected Persons in Comparative Studies
| Study | Population | Findings |
|---|---|---|
| Holloway et al 2013 42 | 90 patients with HIV on ART (median age 43 years), duration of HIV infection 7.4±6.0 years; 39 age-matched controls (median age 40 years) without CV disease | Myocardial fibrosis by LGE, predominantly in the LV basal inferolateral wall, was observed in 76% of patients with HIV vs. 13% of controls (P<0.001); peak myocardial systolic and diastolic longitudinal strain were lower in HIV patient; patients with HIV had 47% higher median myocardial lipid levels |
| Thiara et al 2015 43 | 95 patients with HIV (age, 49±10 years; 93% on ART for 9±6 years), duration of HIV infection 14±8 years; 30 matched healthy adults (age, 46±8 years) without known CV disease | Myocardial extracellular volume was 0.28±0.04 in patients with HIV vs. 0.26±0.04 in controls (P=0.02) but focal myocardial scarring (by LGE) was similar (8.6% and 7.7%, respectively; P =0.8); mean LV radial strain was decreased in patients with HIV vs. controls (21.7% ± 8.6% vs. 30.5% ± 14.2%; P=0.004) |
| Luetkens et al 2016 35 | 28 patients with HIV (age, 49±9 years) on ART, duration of HIV infection 9.7±6.9 years; 22 healthy adults (age, 45±16 years) without known CV disease | Myocardial fibrosis by LGE, predominantly in the mid-ventricular and basal inferolateral LV wall, was present in 82.1% of patients with HIV vs. 27.3% of controls (P<0.001); patients with HIV had lower LVEF and global longitudinal ( 17.7±3.4% vs. 20.2±3.2%, P<0.001) and circumferential ( 21.2±4.6% versus 24.7±5.1%; P<0.001) strain; parameters indicating inflammation were elevated in patients with HIV |
| Ntusi et al 2016 36 | 103 patients with HIV without known CV disease (age, 44±10; 13 ART naïve; 29 on protease-inhibitor regimen; 61 on non- nucleoside reverse- transcription inhibitor regimen); 92 healthy controls (age, 45±10) | Patients with HIV had more myocardial areas with elevated short-tau inversion recovery (indicating myocardial injury) vs. controls, higher average native T1 values (969 vs. 956 ms; P=0.01), more frequent LGE (83% vs. 16%; P<0.001), and 9% lower peak diastolic strain rate (P< 0.001); pericardial effusions were more common in patients with HIV |
ART= antiretroviral therapy; CV=cardiovascular; HIV= human immunodeficiency virus; LGE = late gadolinium enhancement; LV = left ventricular; LVEF=left ventricular ejection fraction
Therefore, accelerated myocardial fibrosis appears to be a key mediator of HIV-induced systolic and diastolic dysfunction. This hypothesis is corroborated by biomarker data in patients with HIV. Soluble ST2, which promotes cardiac fibrosis and is linked to DD 48 and mortality in HFpEF,49 is associated with cardiac dysfunction and mortality In HIV-infected patients.50 Galectin-3, which is associated with fibrosis, incident HF, and mortality,51 is elevated in HIV-infected patients.52 Growth differentiation factor 15, which is produced by myocytes and endothelial cells and correlates with cardiac mass and fibrosis,53 predicts all-cause mortality in HIV-infected patients.50 Disturbances in matrix metalloproteinases and their tissue inhibitors have been reported seen in HIV-infected patients 54; these changes have been associated with LV hypertrophy and HF.55 However, the exact mechanisms that accelerate focal and diffuse fibrosis and associated ventricular dysfunction in HIV-infected patients have not been elucidated yet.
Biomarkers of Myocardial Strain and Injury in HIV-Infected Patients
Natriuretic peptides and cardiac troponins are markers of myocardial strain and injury, respectively, and have been associated with risk for HF and subsequent outcomes.56,57 In a nested case-control study, baseline NT-pro-BNP levels were significantly higher among HIV-infected patients vs. controls who experienced subsequent cardiovascular events.58 In HIV-infected women, NT-pro-BNP was elevated compared to controls and predicted mortality.59 In a recent study, N-terminal pro-B-type natriuretic peptide (NT-pro-BNP) was higher and high-sensitivity cardiac troponin I was more frequently detectable in HIV infected patients despite normal LVEF.50 In another study of 155 young, asymptomatic HIV-infected patients with LVEF ≥50% on cardiac computed tomography (except for 2 patients), high-sensitivity cardiac troponin T was detectable in 50%,52 indicating that subclinical myocyte injury is frequently present even among HIV-infected patients at low risk for cardiovascular disease. Overall however, data are limited in HIV-infected patients, and the potential of these markers to identify those at high risk for DD and HFpEF is unknown.
THE CHARACTERIZING HEART FUNCTION ON ANTIRETROVIRAL THERAPY (CHART) STUDY
Objective
The purpose of the CHART study is to investigate the determinants, mechanisms, and consequences of DD in HIV-infected patients. We will compare ART-treated, virally suppressed HIV-infected individuals with and without DD and HIV- individuals with DD in regards to (1) persistent systemic inflammation through circulating biomarkers; (2) immune system activation through cell surface receptors; (3) myocardial fibrosis by CMR; (4) circulating biomarkers of fibrosis and remodeling, oxidative stress, and hypercoagulability; (5) left atrial function through echocardiography; (6) myocardial stress and subclinical necrosis through circulating biomarkers; (7) proteomic and metabolic profiles; and (8) clinical, biomarker, and imaging data to derive phenotype signatures.
Study Design
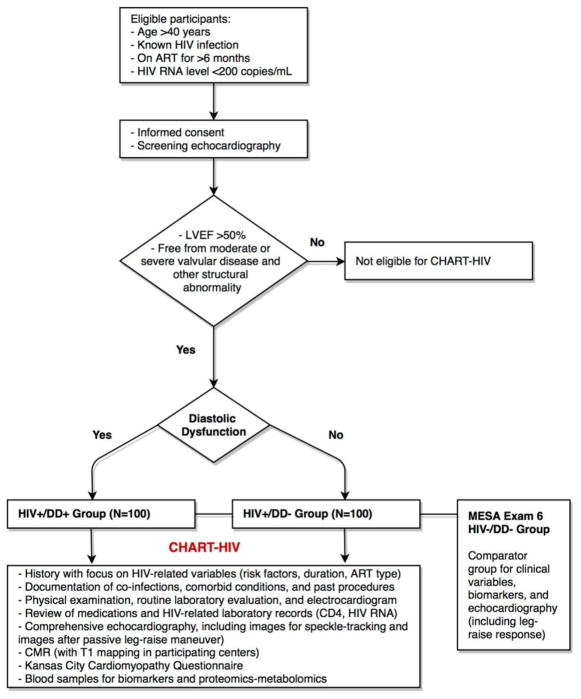
CHART is a prospective case-control study; the design is summarized in Figure 1.
Figure 1. Overview of Study Design of the Characterizing Heart function on Anti-Retroviral Therapy (CHART) Study.
ART: antiretroviral therapy; CD4: cluster of differentiation 4; CMR: cardiac magnetic resonance; DD: diastolic dysfunction; HIV: human immunodeficiency virus; LVEF: left ventricular ejection fraction; RNA: ribonucleic acid
Hypothesis
The primary hypothesis is that HIV-infected/DD+ participants will demonstrate a higher degree of systemic inflammation than the HIV-infected/DD- group despite similar ART and viral suppression. We also hypothesize that a higher fibrosis burden will be present in the HIV-infected/DD+ group and that left atrial function will be impaired compared to HIV-infected/DD− and HIV−/DD+ participants.
Enrolling Centers
The Heart Failure Clinical Research Network (HFN) is a clinical research initiative funded by the National Heart Lung and Blood Institute (NHLBI) with the aim of improving our understanding and management of HF and discover innovative treatment options for HF. The HFN provides a platform for collaborative research by bringing together many centers of excellence across North America. The HFN consists of 9 Regional Clinical Centers and their affiliated sites whose investigators provide scientific leadership in the development of the scientific agenda. The primary goal is to conduct multiple clinical trials to evaluate treatments and strategies to improve management of acute and chronic HF. Ancillary sites can participate in specific HFN protocols. The Coordinating Center at Duke Clinical Research Institute coordinate regulatory, data, and clinical operations. Additional information about the HFN is available at www.hfnetwork.org. For the CHART Study, patients will be enrolled at 11 centers (University of Pennsylvania Health System, PA; University Hospitals of Cleveland Medical Center, OH; Duke University Medical Center, NC; Brigham and Women's Hospital, MA; Mayo Clinic, MN; Washington University School of Medicine, MO; Thomas Jefferson University, PA; University of Vermont Medical Center, VT; Tufts University Medical Center, MA; Emory University Hospital, GA).
Study Populations
We plan to enroll a minimum of 200 ART-treated, virally suppressed HIV-infected subjects with (a) no DD (HIV-infected/DD- cohort; N=100) and (b) with DD (HIV-infected/DD+ cohort; N=100). These groups will be compared to HIV- subjects with DD (HIV−/DD+ cohort) from the Multi-Ethnic Study of Atherosclerosis (MESA) study, an NIH-funded longitudinal cohort.60 MESA participants were aged 45–84 and free of cardiovascular disease at enrollment (2000–2002). MESA Exam 6 will evaluate 3500+ participants with comprehensive echocardiography and blood samples for biomarkers; this will form the HIV−/DD+ cohort for the present study. We will select 100 age- and sex-matched participants from MESA without DD based on the CHART criteria for DD outlined below. Importantly, CHART and MESA will have an identical dynamic echocardiography protocol, with a single core laboratory for both studies, and thus we expect these data to be fully comparable. However, MESA participants will not have CMR data for comparison.
Eligibility Criteria
The enrollment criteria are summarized in Table 3. Although DD may occur in younger HIV-infected persons, most HIV studies of DD have included patients >40 years old.13–16 Younger patients with HIV have less comorbidity that contributes to DD. Because the main concern in HIV is the effect of DD on long term complications, and in order to elucidate the role of traditional vs. HIV-specific factors, we will enroll middle-aged and older HIV-infected individuals to compare with HIV- subjects with DD.
Table 3.
Main Inclusion and Exclusion Criteria
| HIV-infected/DD- (N=100) | HIV-infected/DD+(N=100) | HIV−/DD+ (MESA Study) | |
|---|---|---|---|
|
| |||
| Inclusion Criteria | |||
|
| |||
| Age | >40 years | >40 years | >40 years |
| HIV antibody | Positive | Positive | Negative/unknown |
| ART | >6 months | >6 months | Not applicable |
| HIV RNA level | <200 copies/mL | <200 copies/mL | Not applicable |
| LVEF | >50% | >50% | >50% |
| Diastolic dysfunction* | Absent | Present | Present |
|
| |||
| Exclusion Criteria | |||
|
| |||
| History of AIDS | Any documented CD4 cell count of less than 200 or AIDS-defining opportunistic infections | ||
| LVEF | Past LVEF <50% | ||
| Valve | Moderate or severe valve stenosis or regurgitation, or past repair or replacement | ||
| Coronary disease | History of percutaneous or surgical revascularization or active angina | ||
| Arrhythmias | Persistent atrial fibrillation | ||
| Blood pressure | >160 mmHg systolic or >100 mmHg diastolic BP | ||
| Comorbidity | Comorbid inflammatory disease (e.g. rheumatoid arthritis or systemic lupus erythematosus) | ||
| Cancer | Active cancer or cancer chemotherapy treatment in the prior year | ||
| Medications | Chronic use of steroid or anti-inflammatory therapy | ||
| CMR eligibility | Contraindication to CMR or gadolinium injection | ||
AIDS: acquired immune deficiency syndrome; ART= antiretroviral therapy; BP: blood pressure; CD4: cluster of differentiation 4; CMR: cardiac magnetic resonance; HIV= human immuno-deficiency virus; LVEF = left ventricular ejection fraction; RNA: ribonucleic acid * As defined in Table 3.
Definition of Diastolic Dysfunction
DD will be defined according to the criteria shown in Table 4, which are based on a combination of reduced early diastolic relaxation and evidence of cardiac structural abnormalities that are known to be associated with DD. We chose these criteria instead of the 2016 American Society of Echocardiography (ASE) recommendations for the diagnosis of DD 61 because we sought to identify DD prior to the onset of symptomatic HFpEF; the 2016 ASE diastolic function recommendations are more specific for overt elevation of LV filling pressures.62 Furthermore, E/e’ and PASP may be elevated for reasons other than DD in the setting of HIV, such as a high output state due to hepatitis C-related liver disease (for E/e’ or PASP) or HIV-related pulmonary vascular disease (for PASP).
Table 4.
Criteria for Diastolic Dysfunction for HIV-Infected Participants
|
Research Procedures
Clinical Data Collection
Detailed clinical history, physical examination, routine laboratory, medications, and other clinical data will be collected from all participants, as well as blood samples for biomarker determinations (Figure 1). All participants will also undergo an electrocardiogram and complete a Kansas City Cardiomyopathy Questionnaire for quality of life assessment.
Echocardiography
Each participant will undergo comprehensive transthoracic echocardiography. Speckle-tracking will be used to evaluate ventricular and atrial mechanics.63 Passive leg raise will be used to determine the ability of the left atrium to augment its reservoir, conduit, and contractile functions in response to increased preload.64 The effect of leg raise on standard diastolic function parameters will also be examined. Echocardiograms will be centrally interpreted at the Northwestern University Cardiovascular Imaging Core Laboratory.
Cardiac Magnetic Resonance
Each participant will undergo a standard CMR examination, including cine imaging using a steady-state free precession sequence and delayed contrast-enhancement imaging after IV extracellular gadolinium administration using a segmented inversion recovery sequence. Delayed enhancement will allow assessment of the presence, location, and extent of focal myocardial scarring. Myocardial T1 mapping will also be performed at sites capable of obtaining shortened modified Look-Locker inversion recovery 65 images at 1.5 Tesla. Native T1 values and extracellular volume fraction will be assessed. Subjects undergoing T1 mapping will have a hematocrit determination on the day of CMR for calculation of extracellular volume fraction. The Duke Cardiac Magnetic Resonance Core will serve as the core lab.
Biomarkers
Multiple domains of biomarkers, including fibrosis and remodeling, arterial inflammation, hyper-coagulability, and oxidative stress, will be evaluated (Table 5). Samples will be stored at −80º C at the Heart Failure Network core biobank at the University of Vermont.
Table 5.
Study Biomarkers
| Inflammation | Interleukin-6, high-sensitivity C-reactive protein |
| Immune activation | sCD163, sCD14, monocyte expression of CD14 and CD16, T-cell activation and subsets: Th1, Treg, CD4+, CD8+ T cell activation |
| Coagulation | D-dimer, fibrinogen |
| Arterial inflammation | Oxidized low-density lipoprotein, lipoprotein-associated phospholipase A2 |
| Fibrosis and remodeling | soluble ST2, GDF-15, CITP:MMP-1 ratio, Galectin-3, TIMP-1, PIIINP, PINP, PICP |
| Oxidative stress | Myeloperoxidase |
| Stress | N-terminal pro-B-type natriuretic peptide |
| Necrosis | High-sensitivity troponin I |
CITP: carboxyl-terminal telopeptide of collagen type I; GDF: growth differentiation factor; MMP: matrix metalloproteinase; TIMP: tissue inhibitor of metalloproteinase; PICP: carboxyl-terminal peptide of procollagen type I; PINP: amino-terminal peptide of procollagen type I; PIIINP: amino-terminal peptide of procollagen type III
Metabolomics and Proteomics Profiling
For targeted profiling, tandem flow injection mass spectrometry will be used, including 45 acylcarnitine species (mitochondrial metabolism), 15 amino acids, and a set of conventional analytes (lipids, glucose, ketones, non-esterified fatty acids and CRP).66 For non-targeted profiling, gas chromatography and liquid chromatography will be used.67,68 Identification of non-targeted metabolite peaks will be performed using a retention-time-locked metabolite library. Peaks will be identified by matching exact masses to a database of >40,000 metabolites (Human Metabolome Database).69 Proteomic profiling of 1310 proteins will be performed using a DNA aptamer-based platform to target proteins (SomaLogic, Inc), as well as other targeted protein arrays and traditional protein assays. To identify novel proteins or metabolites that differentiate HIV+/DD+ and HIV+/DD− subjects, we will perform targeted analyses of fibrosis and inflammation-related proteins and the full array of metabolomics data. Profiling will be conducted at the Duke Molecular Physiology Institute.
Sample Size and Power Calculations
The primary comparison for power calculation purposes is the difference in log-transformed interleukin (IL)-6 levels between HIV-infected/DD+ and HIV-infected/DD- groups. Log-transformed IL-6 is expected to have a standard deviation (SD) of approximately 0.75.70 Sample sizes of 100 subjects in each group will provide 80% and 90% power to detect differences of 0.30 and 0.35, respectively, in a 2-sample t-test with a 2-sided type I error of 0.05. These calculations suggest that comparisons of continuous variables between HIV-infected/DD− and HIV−infected/DD+ groups will have 80% and 90% power to detect differences of 0.40 and 0.46 SD, respectively. Comparisons with the HIV−/DD+ cohort will have more power due to the larger number of HIV−/DD+ subjects available in MESA.
Analytic Plan
Quantitative imaging (CMR and echocardiography) parameters and circulating biomarkers with skewed distributions will be appropriately transformed to normalize distributions. Unadjusted comparisons between groups will be based on t-tests (for comparisons that include only the HIV+/DD+ vs. HIV+/DD− groups, e.g. CMR data) and analysis of variance (for HIV+/DD+ vs. HIV+/DD− vs. HIV−/DD− comparisons). For adjusted comparisons, we will use linear regression models with the parameter of interest as the outcome, study group as the main exposure, and clinical characteristics as covariates (including demographics, clinical history items, HIV-related blood work, biochemistry, and medication classes). For discrete parameters (e.g. presence of fibrosis), we will use chi-squared tests for unadjusted comparisons and logistic regression models for adjusted comparisons following a similar approach as described above.
For proteins and metabolites, multivariable generalized linear regression models, adjusted for potential confounders, after transforming variables appropriately, will be used to test for differences in mean values of analytes between HIV-infected/DD+ and HIV-infected/DD- subjects. Analytes with >25% values as zero (e.g. below the lower limit of quantification) will be analyzed as binary traits. Benjamini and Hochberg false discovery rate will be used for multiple omics data comparisons.71
DISCUSSION
The CHART study seeks to better understand DD, one of the major predisposing risk factors for development of HFpEF and other cardiovascular disease, in HIV-infected patients receiving contemporary ART. Similar to other individuals with chronic inflammatory conditions, HIV infection can serve as a model to better understand the role of inflammation in DD. Furthermore, understanding DD pathogenesis in HIV will allow for identification of treatment targets for patients at risk of HFpEF. The CHART study will determine clinical, imaging and molecular characteristics of this pathogenesis, and enable evaluation of general vs. HIV-specific components.
Our enrollment criteria maximize the ability to detect impaired LV relaxation associated with structural heart disease. Such impairment is indicative of chronically elevated left atrial pressure (left atrial size) or presence of LV hypertrophy. We excluded E/e' ratio and elevated pulmonary artery pressure as indicators of DD because of the possibility of non-DD causes of increases in these values in patients with HIV (e.g., high output state from co-infection; HIV-associated pulmonary hypertension etc.) and also because these parameters are elevated in patients with overt, symptomatic HFpEF. However, it is important to acknowledge that many different mechanisms can contribute to DD such as relaxation abnormalities, changes in myocardial stiffness, elevation of central blood volume, ventricular interaction, reductions in longitudinal shortening, and atrial pathology, among others. Therefore, echocardiographic assessment and classification of DD, no matter how careful, is a necessary simplification of a complex condition for the practical purposes of a clinical study.
Echocardiography in CHART will assess standard DD parameters; provide a comprehensive cardiac assessment; and provide novel metrics of cardiac reserve by combining speckle-tracking with dynamic preload maneuvers. In patients with HFpEF, left atrial dysfunction leads to pulmonary hypertension, reduced exercise capacity, and poor clinical outcomes.72 Altered response to preload, e.g. with leg raising, can detect diminished left atrial reserve and help identify patients who have an exaggerated rise in LV filling pressure when preload is increased.64,73 Besides reports of left atrial dilatation,74 little is known about the role of left atrial function in DD in HIV-infected patients.
There remains a knowledge gap about factors that lead to elevated risk for DD and HFpEF in HIV infection, and other pathways have also been proposed, e.g. mitochondrial toxicity 75 and endoplasmic reticulum stress.76 Of note, older ART regimens, such as the nucleoside reverse transcriptase inhibitors zidovudine and stavudine, have been associated with mitochondrial toxicity,77 but the impact of newer classes of medications, such as integrase inhibitors, on DD and HF in HIV remains unknown. The broad representation of pathways included in the proteomics and metabolomics panels will enable testing of many known pathways and identification of novel mechanisms. High throughput -omics approaches have identified novel markers and mechanisms in general, but have not been specifically applied to investigation of DD and HF pathogenesis in HIV to date.
Despite a growing amount of evidence suggesting that systemic low-level inflammation involving the myocardium and diffuse myocardial fibrosis play a central role in the development of DD in patients with HIV, there are no serial studies to inform us on the rate of progression of these changes in the myocardium and extracellular matrix and importantly, on the clinical significance of these changes. For example, although patients with HIV demonstrate diffuse myocardial fibrosis as indicated by elevated myocardial extracellular volume on CMR, we do not know what threshold of extracellular volume is associated with future development of DD. In addition, the incidence of new-onset HFpEF in patients with HIV and DD has not been reported. The CHART study offers a unique opportunity to study changes in myocardial structure and function over time in HIV-infected patients with or without DD at baseline and their clinical significance. We are currently in the planning stages of a follow-up study in order to preform repeat CMR, echocardiography, and biomarker assessments in CHART participants 2 years after the baseline visit. We are also planning to evaluate for transition from DD- to DD+ phenotype and development of clinical HFpEF in all participants as part of the follow-up study. Finally, an important next step would be to develop interventions to halt the progression of DD in HIV-infected patients.
CONCLUSION
The CHART study seeks to better understand DD in HIV infected individuals who are receiving ART. DD is one of the major predisposing risk factors for development of HFpEF, and a risk factor that has been linked with other cardiovascular diseases and mortality. The CHART study may assist in better understanding of traditional and non-traditional risk factors of DD pathogenesis in both HIV infected and uninfected individuals, identify the role of generic versus HIV-specific DD factors in HIV-infected patients, and in turn may provide impetus for development of screening, prevention, and treatment options for these patients at risk for developing cardiovascular complications, including HF.
Acknowledgments
Funding Sources: The Heart Failure Clinical Research Network is supported by the NHLBI, National Institutes of Health Funding/Support: U10 HL084904 (awarded to the coordinating center) and U01 HL084861, U10 HL110312, U10 HL110337, U10 HL110342, U10 HL110262, U10 HL110297, U10 HL110302, U10 HL110309, U10 HL110336, and U10 HL110338 (awarded to the regional clinical centers). This work is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health.
Footnotes
DISCLOSURES
Javed Butler: Research support from the National Institutes of Health, the European Union, and the Patient Centered Outcomes Research Institute, and consultant to Amgen, Astra-Zeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, CVRx, Janssen, Luitpold, Medtronic, Novartis, Relypsa, Roche, Vifor, and ZS Pharma. Andreas P. Kalogeropoulos: Research support from the American Heart Association and consultant to Roche Diagnostics. Kevin J. Anstrom: None. Priscilla Y. Hsue: Research support from the National Institutes of Health and Pfizer; honoraria received from Gilead. Raymond J. Kim: Research support from Bayer, Stealth Biotherapeutics, and Astellas. Rebecca Scherzer: None. Sanjiv J. Shah: Research support from the National Institutes of Health (R01 HL127028 and R01 HL107577) and the American Heart Association, Actelion, AstraZeneca, Bayer, and Novartis; and consultant to Actelion, Amgen, AstraZeneca, Bayer, Boehringer-Ingelheim, Cardiora, Eisai, Gilead, Ironwood, Merck, MyoKardia, Novartis, Sanofi, and United Therapeutics. Svati H. Shah: Research support from the National Institutes of Health and the American Heart Association, and holds a patent on an unrelated finding. Eric J. Velazquez: Research support from National Institutes of Health, Novartis, Amgen, Pfizer, Alnylam, Philips, GE, Bay Labs; honoraris from Novartis, Amgen, Merck, Pfizer, Expert Exchange, Abiomed. Adrian F. Hernandez: Research support from AstraZeneca, Bayer, Luitpold, Glaxo Smith Kline, Merck, Novartis, Portola Pharmaceuticals and Verily; honorarium from Amgen, Bayer, Boehringer-Ingelheim Boston Scientific, Myokardia, Novartis and Sanofi. Patrice Desvigne-Nickens: None. Eugene Braunwald: For the work under consideration, Dr. Braunwald reports grant support to his institution from Duke University for his role as Chair of the NHLBI Heart Failure Network. For outside the submitted work, Dr. Braunwald reports grant support to his institution from Merck and Company, Astra Zeneca, Novartis, Daiichi Sankyo and Glaxo Smith Kline; personal fees for consultancies with The Medicines Company and Theravance; personal fees for lectures from Medscape and Menarini International; uncompensated consultancies and lectures from Merck and Novartis.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wandeler G, Johnson LF, Egger M. Trends in life expectancy of HIV-positive adults on antiretroviral therapy across the globe: comparisons with general population. Curr Opin HIV AIDS. 2016;11:492–500. doi: 10.1097/COH.0000000000000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smit M, Brinkman K, Geerlings S, Smit C, Thyagarajan K, van Sighem A, de Wolf F, Hallett TB ATHENA observational cohort. Future challenges for clinical care of an ageing population infected with HIV: a modelling study. Lancet Infect Dis. 2015;15:810–818. doi: 10.1016/S1473-3099(15)00056-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Remick J, Georgiopoulou V, Marti C, Ofotokun I, Kalogeropoulos A, Lewis W, Butler J. Heart failure in patients with human immunodeficiency virus infection: epidemiology, pathophysiology, treatment, and future research. Circulation. 2014;129:1781–1789. doi: 10.1161/CIRCULATIONAHA.113.004574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lumsden RH, Bloomfield GS. The Causes of HIV-Associated Cardiomyopathy: A Tale of Two Worlds. BioMed research international. 2016;2016:8196560. doi: 10.1155/2016/8196560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cerrato E, Ascenzo FD’, Biondi-Zoccai G, Calcagno A, Frea S, Grosso Marra W, Castagno D, Omedè P, Quadri G, Sciuto F, Presutti D, Frati G, Bonora S, Moretti C, Gaita F. Cardiac dysfunction in pauci symptomatic human immunodeficiency virus patients: a meta-analysis in the highly active antiretroviral therapy era. Eur Heart J. 2013;34:1432–1436. doi: 10.1093/eurheartj/ehs471. [DOI] [PubMed] [Google Scholar]
- 6.Fischer M, Baessler A, Hense HW, Hengstenberg C, Muscholl M, Holmer S, Döring A, Broeckel U, Riegger G, Schunkert H. Prevalence of left ventricular diastolic dysfunction in the community. Results from a Doppler echocardiographic-based survey of a population sample. Eur Heart J. 2003;24:320–328. doi: 10.1016/s0195-668x(02)00428-1. [DOI] [PubMed] [Google Scholar]
- 7.Redfield MM, Rodeheffer RJ, Jacobsen SJ, Mahoney DW, Bailey KR, Burnett JC. Plasma brain natriuretic peptide to detect preclinical ventricular systolic or diastolic dysfunction: a community-based study. Circulation. 2004;109:3176–3181. doi: 10.1161/01.CIR.0000130845.38133.8F. [DOI] [PubMed] [Google Scholar]
- 8.Abhayaratna WP, Marwick TH, Smith WT, Becker NG. Characteristics of left ventricular diastolic dysfunction in the community: an echocardiographic survey. Heart. 2006;92:1259–1264. doi: 10.1136/hrt.2005.080150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsang TSM, Gersh BJ, Appleton CP, Tajik AJ, Barnes ME, Bailey KR, Oh JK, Leibson C, Montgomery SC, Seward JB. Left ventricular diastolic dysfunction as a predictor of the first diagnosed nonvalvular atrial fibrillation in 840 elderly men and women. J Am Coll Cardiol. 2002;40:1636–1644. doi: 10.1016/s0735-1097(02)02373-2. [DOI] [PubMed] [Google Scholar]
- 10.Kane GC, Karon BL, Mahoney DW, Redfield MM, Roger VL, Burnett JC, Jacobsen SJ, Rodeheffer RJ. Progression of left ventricular diastolic dysfunction and risk of heart failure. JAMA. 2011;306:856–863. doi: 10.1001/jama.2011.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halley CM, Houghtaling PL, Khalil MK, Thomas JD, Jaber WA. Mortality rate in patients with diastolic dysfunction and normal systolic function. Arch Intern Med. 2011;171:1082–1087. doi: 10.1001/archinternmed.2011.244. [DOI] [PubMed] [Google Scholar]
- 12.Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 13.Schuster I, Thöni GJ, Edérhy S, Walther G, Nottin S, Vinet A, Boccara F, Khireddine M, Girard P-M, Mauboussin J-M, Rouanet I, Dauzat M, Cohen A, Messner-Pellenc P, Obert P. Subclinical cardiac abnormalities in human immunodeficiency virus-infected men receiving antiretroviral therapy. Am J Cardiol. 2008;101:1213–1217. doi: 10.1016/j.amjcard.2007.11.073. [DOI] [PubMed] [Google Scholar]
- 14.Hsue PY, Hunt PW, Ho JE, Farah HH, Schnell A, Hoh R, Martin JN, Deeks SG, Bolger AF. Impact of HIV infection on diastolic function and left ventricular mass. Circ Heart Fail. 2010;3:132–139. doi: 10.1161/CIRCHEARTFAILURE.109.854943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo L, Zeng Y, Li T, Lv W, Wang H, Guo F, Han Y, Xie J, Qiu Z, Li Y, Song X, Zhu T, Zhang X, Li L, Ye Y, He Y, Lu H, Huang A, Tang X, Wang H, Zhang T, Gao G, Lei J, Wu X, Sun Y, Bai J, Li K China AIDS Clinical Trial 0810 Group. Prospective echocardiographic assessment of cardiac structure and function in Chinese persons living with HIV. Clin Infect Dis. 2014;58:1459–1466. doi: 10.1093/cid/ciu086. [DOI] [PubMed] [Google Scholar]
- 16.Fontes-Carvalho R, Mancio J, Marcos A, Sampaio F, Mota M, Rocha Gonçalves F, Gama V, Azevedo A, Leite-Moreira A. patients with HIV have impaired diastolic function that is not aggravated by anti-retroviral treatment. Cardiovasc Drugs Ther. 2015;29:31–39. doi: 10.1007/s10557-015-6573-x. [DOI] [PubMed] [Google Scholar]
- 17.Pedersen KK, Pedersen M, Gaardbo JC, Ronit A, Hartling HJ, Bruunsgaard H, Gerstoft J, Ullum H, Nielsen SD. Persisting inflammation and chronic immune activation but intact cognitive function in HIV-infected patients after long-term treatment with combination antiretroviral therapy. J Acquir Immune Defic Syndr. 2013;63:272–279. doi: 10.1097/QAI.0b013e318289bced. [DOI] [PubMed] [Google Scholar]
- 18.Freiberg MS, Chang C-CH, Skanderson M, Patterson OV, DuVall SL, Brandt CA, So-Armah KA, Vasan RS, Oursler KA, Gottdiener J, Gottlieb S, Leaf D, Rodriguez-Barradas M, Tracy RP, Gibert CL, Rimland D, Bedimo RJ, Brown ST, Goetz MB, Warner A, Crothers K, Tindle HA, Alcorn C, Bachmann JM, Justice AC, Butt AA. Association between HIV infection and the risk of heart failure with reduced ejection fraction and preserved ejection fraction in the antiretroviral therapy era: results from the veterans aging cohort study. JAMA Cardiology. 2017;2:536–546. doi: 10.1001/jamacardio.2017.0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steverson AB, Pawlowski AE, Schneider D, Nannapaneni P, Sanders JM, Achenbach CJ, Shah SJ, Lloyd-Jones DM, Feinstein MJ. Clinical characteristics of HIV-infected patients with adjudicated heart failure. Eur J Prev Cardiol. 2017;24:1746–1758. doi: 10.1177/2047487317732432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krebs SJ, Ananworanich J. Immune activation during acute HIV infection and the impact of early antiretroviral therapy. Curr Opin HIV AIDS. 2016;11:163–172. doi: 10.1097/COH.0000000000000228. [DOI] [PubMed] [Google Scholar]
- 21.Bartovská Z, Beran O, Rozsypal H, Holub M. Antiretroviral treatment of HIV infection does not influence HIV-specific immunity but has an impact on non-specific immune activation. Curr HIV Res. 2011;9:88–94. doi: 10.2174/157016211795569078. [DOI] [PubMed] [Google Scholar]
- 22.Vinikoor MJ, Cope A, Gay CL, Ferrari G, McGee KS, Kuruc JD, Lennox JL, Margolis DM, Hicks CB, Eron JJ. Antiretroviral therapy initiated during acute HIV infection fails to prevent persistent T-cell activation. J Acquir Immune Defic Syndr. 2013;62:505–508. doi: 10.1097/QAI.0b013e318285cd33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rönsholt FF, Ullum H, Katzenstein TL, Gerstoft J, Ostrowski SR. Persistent inflammation and endothelial activation in HIV-1 infected patients after 12 years of antiretroviral therapy. PLoS ONE. 2013;8:e65182. doi: 10.1371/journal.pone.0065182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ross AC, Rizk N, O’Riordan MA, Dogra V, El-Bejjani D, Storer N, Harrill D, Tungsiripat M, Adell J, McComsey GA. Relationship between inflammatory markers, endothelial activation markers, and carotid intima-media thickness in HIV-infected patients receiving antiretroviral therapy. Clin Infect Dis. 2009;49:1119–1127. doi: 10.1086/605578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zungsontiporn N, Tello RR, Zhang G, Mitchell BI, Budoff M, Kallianpur KJ, Nakamoto BK, Keating SM, Norris PJ, Ndhlovu LC, Souza SA, Shikuma CM, Chow DC. Non-Classical Monocytes and Monocyte Chemoattractant Protein-1 (MCP-1) Correlate with Coronary Artery Calcium Progression in Chronically HIV-1 Infected Adults on Stable Antiretroviral Therapy. PLoS ONE. 2016;11:e0149143. doi: 10.1371/journal.pone.0149143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burdo TH, Lo J, Abbara S, Wei J, DeLelys ME, Preffer F, Rosenberg ES, Williams KC, Grinspoon S. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis. 2011;204:1227–1236. doi: 10.1093/infdis/jir520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Subramanian S, Tawakol A, Burdo TH, Abbara S, Wei J, Vijayakumar J, Corsini E, Abdelbaky A, Zanni MV, Hoffmann U, Williams KC, Lo J, Grinspoon SK. Arterial inflammation in patients with HIV. JAMA. 2012;308:379–386. doi: 10.1001/jama.2012.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mottram PM, Haluska BA, Leano R, Carlier S, Case C, Marwick TH. Relation of arterial stiffness to diastolic dysfunction in hypertensive heart disease. Heart. 2005;91:1551–1556. doi: 10.1136/hrt.2004.046805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fernandes VRS, Polak JF, Cheng S, Rosen BD, Carvalho B, Nasir K, McClelland R, Hundley G, Pearson G, O’Leary DH, Bluemke DA, Lima JAC. Arterial stiffness is associated with regional ventricular systolic and diastolic dysfunction: the Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28:194–201. doi: 10.1161/ATVBAHA.107.156950. [DOI] [PubMed] [Google Scholar]
- 30.Mavigner M, Delobel P, Cazabat M, Dubois M, L’faqihi-Olive F-E, Raymond S, Pasquier C, Marchou B, Massip P, Izopet J. HIV-1 residual viremia correlates with persistent T-cell activation in poor immunological responders to combination antiretroviral therapy. PLoS ONE. 2009;4:e7658. doi: 10.1371/journal.pone.0007658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tincati C, Douek DC, Marchetti G. Gut barrier structure, mucosal immunity and intestinal microbiota in the pathogenesis and treatment of HIV infection. AIDS Res Ther. 2016;13:19. doi: 10.1186/s12981-016-0103-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Masiá M, Robledano C, Ortiz de la Tabla V, Antequera P, Lumbreras B, Hernández I, Gutiérrez F. Coinfection with human herpesvirus 8 is associated with persistent inflammation and immune activation in virologically suppressed HIV-infected patients. PLoS ONE. 2014;9:e105442. doi: 10.1371/journal.pone.0105442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Damås JK, Landrø L, Fevang B, Heggelund L, Tjønnfjord GE, Fløisand Y, Halvorsen B, Frøland SS, Aukrust P. Homeostatic chemokines CCL19 and CCL21 promote inflammation in human immunodeficiency virus-infected patients with ongoing viral replication. Clin Exp Immunol. 2009;157:400–407. doi: 10.1111/j.1365-2249.2009.03976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nordell AD, McKenna M, Borges ÁH, Duprez D, Neuhaus J, Neaton JD INSIGHT SMART, ESPRIT Study Groups, SILCAAT Scientific Committee. Severity of cardiovascular disease outcomes among patients with HIV is related to markers of inflammation and coagulation. Journal of the American Heart Association. 2014;3:e000844. doi: 10.1161/JAHA.114.000844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luetkens JA, Doerner J, Schwarze-Zander C, Wasmuth J-C, Boesecke C, Sprinkart AM, Schmeel FC, Homsi R, Gieseke J, Schild HH, Rockstroh JK, Naehle CP. Cardiac Magnetic Resonance Reveals Signs of Subclinical Myocardial Inflammation in Asymptomatic HIV-Infected Patients. Circ Cardiovasc Imaging. 2016;9:e004091. doi: 10.1161/CIRCIMAGING.115.004091. [DOI] [PubMed] [Google Scholar]
- 36.Ntusi N, O’Dwyer E, Dorrell L, Wainwright E, Piechnik S, Clutton G, Hancock G, Ferreira V, Cox P, Badri M, Karamitsos T, Emmanuel S, Clarke K, Neubauer S, Holloway C. HIV-1-Related Cardiovascular Disease Is Associated With Chronic Inflammation, Frequent Pericardial Effusions, and Probable Myocardial Edema. Circ Cardiovasc Imaging. 2016;9:e004430. doi: 10.1161/CIRCIMAGING.115.004430. [DOI] [PubMed] [Google Scholar]
- 37.Nguyen KA, Peer N, Mills EJ, Kengne AP. A Meta-Analysis of the Metabolic Syndrome Prevalence in the Global HIV-Infected Population. PLoS ONE. 2016;11:e0150970. doi: 10.1371/journal.pone.0150970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kingery JR, Alfred Y, Smart LR, Nash E, Todd J, Naguib MR, Downs JA, Kalluvya S, Kataraihya JB, Peck RN. Short-term and long-term cardiovascular risk, metabolic syndrome and HIV in Tanzania. Heart. 2016;102:1200–1205. doi: 10.1136/heartjnl-2015-309026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Belury MA, Bowman E, Gabriel J, Snyder B, Kulkarni M, Palettas M, Mo X, Lake JE, Zidar D, Sieg SF, Rodriguez B, Playford MP, Andrade A, Kuritzkes DR, Mehta NN, Lederman MM, Funderburg NT. Prospective Analysis of Lipid Composition Changes with Antiretroviral Therapy and Immune Activation in Persons Living with HIV. Pathogens & immunity. 2017;2:376–403. doi: 10.20411/pai.v2i3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Puntmann VO, Peker E, Chandrashekhar Y, Nagel E. T1 mapping in characterizing myocardial disease: A comprehensive review. Circ Res. 2016;119:277–299. doi: 10.1161/CIRCRESAHA.116.307974. [DOI] [PubMed] [Google Scholar]
- 41.Wong TC, Piehler K, Meier CG, Testa SM, Klock AM, Aneizi AA, Shakesprere J, Kellman P, Shroff SG, Schwartzman DS, Mulukutla SR, Simon MA, Schelbert EB. Association between extracellular matrix expansion quantified by cardiovascular magnetic resonance and short-term mortality. Circulation. 2012;126:1206–1216. doi: 10.1161/CIRCULATIONAHA.111.089409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holloway CJ, Ntusi N, Suttie J, Mahmod M, Wainwright E, Clutton G, Hancock G, Beak P, Tajar A, Piechnik SK, Schneider JE, Angus B, Clarke K, Dorrell L, Neubauer S. Comprehensive cardiac magnetic resonance imaging and spectroscopy reveal a high burden of myocardial disease in patients with HIV. Circulation. 2013;128:814–822. doi: 10.1161/CIRCULATIONAHA.113.001719. [DOI] [PubMed] [Google Scholar]
- 43.Thiara DK, Liu CY, Raman F, Mangat S, Purdy JB, Duarte HA, Schmidt N, Hur J, Sibley CT, Bluemke DA, Hadigan C. Abnormal Myocardial Function Is Related to Myocardial Steatosis and Diffuse Myocardial Fibrosis in HIV-Infected Adults. J Infect Dis. 2015;212:1544–1551. doi: 10.1093/infdis/jiv274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Diaz-Zamudio M, Dey D, LaBounty T, Nelson M, Fan Z, Szczepaniak LS, Hsieh BP-C, Rajani R, Berman D, Li D, Dharmakumar R, Hardy WD, Conte AH. Increased pericardial fat accumulation is associated with increased intramyocardial lipid content and duration of highly active antiretroviral therapy exposure in patients infected with human immunodeficiency virus: a 3T cardiovascular magnetic resonance feasibility study. J Cardiovasc Magn Reson. 2015;17:91. doi: 10.1186/s12968-015-0193-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schelbert EB, Fridman Y, Wong TC, Abu Daya H, Piehler KM, Kadakkal A, Miller CA, Ugander M, Maanja M, Kellman P, Shah DJ, Abebe KZ, Simon MA, Quarta G, Senni M, Butler J, Diez J, Redfield MM, Gheorghiade M. Temporal relation between myocardial fibrosis and heart failure with preserved ejection fraction: association with baseline disease severity and subsequent outcome. JAMA Cardiology. 2017;2:995–1006. doi: 10.1001/jamacardio.2017.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kraigher-Krainer E, Shah AM, Gupta DK, Santos A, Claggett B, Pieske B, Zile MR, Voors AA, Lefkowitz MP, Packer M, McMurray JJV, Solomon SD PARAMOUNT Investigators. Impaired systolic function by strain imaging in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2014;63:447–456. doi: 10.1016/j.jacc.2013.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shah AM, Claggett B, Sweitzer NK, Shah SJ, Anand IS, Liu L, Pitt B, Pfeffer MA, Solomon SD. Prognostic importance of impaired systolic function in heart failure with preserved ejection fraction and the impact of spironolactone. Circulation. 2015;132:402–414. doi: 10.1161/CIRCULATIONAHA.115.015884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.deFilippi C, Daniels LB, Bayes-Genis A. Structural heart disease and ST2: cross-sectional and longitudinal associations with echocardiography. Am J Cardiol. 2015;115:59–B63B. doi: 10.1016/j.amjcard.2015.01.042. [DOI] [PubMed] [Google Scholar]
- 49.Shah KB, Kop WJ, Christenson RH, Diercks DB, Henderson S, Hanson K, Li S-Y, deFilippi CR. Prognostic utility of ST2 in patients with acute dyspnea and preserved left ventricular ejection fraction. Clin Chem. 2011;57:874–882. doi: 10.1373/clinchem.2010.159277. [DOI] [PubMed] [Google Scholar]
- 50.Secemsky EA, Scherzer R, Nitta E, Wu AHB, Lange DC, Deeks SG, Martin JN, Snider J, Ganz P, Hsue PY. Novel Biomarkers of Cardiac Stress, Cardiovascular Dysfunction, and Outcomes in HIV-Infected Individuals. JACC Heart Fail. 2015;3:591–599. doi: 10.1016/j.jchf.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Boer RA, Voors AA, Muntendam P, van Gilst WH, van Veldhuisen DJ. Galectin-3: a novel mediator of heart failure development and progression. Eur J Heart Fail. 2009;11:811–817. doi: 10.1093/eurjhf/hfp097. [DOI] [PubMed] [Google Scholar]
- 52.Fitch KV, DeFilippi C, Christenson R, Srinivasa S, Lee H, Lo J, Lu MT, Wong K, Petrow E, Sanchez L, Looby SE, Hoffmann U, Zanni M, Grinspoon SK. Subclinical myocyte injury, fibrosis and strain in relationship to coronary plaque in asymptomatic HIV-infected individuals. AIDS. 2016;30:2205–2214. doi: 10.1097/QAD.0000000000001186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Izumiya Y, Hanatani S, Kimura Y, Takashio S, Yamamoto E, Kusaka H, Tokitsu T, Rokutanda T, Araki S, Tsujita K, Tanaka T, Yamamuro M, Kojima S, Tayama S, Kaikita K, Hokimoto S, Ogawa H. Growth differentiation factor-15 is a useful prognostic marker in patients with heart failure with preserved ejection fraction. Can J Cardiol. 2014;30:338–344. doi: 10.1016/j.cjca.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 54.Mastroianni CM, Liuzzi GM, Ettorre GD’, Lichtner M, Forcina G, Di Campli NF, Riccio P, Vullo V. Matrix metalloproteinase-9 and tissue inhibitors of matrix metalloproteinase-1 in plasma of patients co-infected with HCV and HIV. HIV Clin Trials. 2002;3:310–315. doi: 10.1310/U9LJ-MFF9-ARE1-257H. [DOI] [PubMed] [Google Scholar]
- 55.Polyakova V, Hein S, Kostin S, Ziegelhoeffer T, Schaper J. Matrix metalloproteinases and their tissue inhibitors in pressure-overloaded human myocardium during heart failure progression. J Am Coll Cardiol. 2004;44:1609–1618. doi: 10.1016/j.jacc.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 56.Daniels LB, Maisel AS. Natriuretic peptides. J Am Coll Cardiol. 2007;50:2357–2368. doi: 10.1016/j.jacc.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 57.Nagarajan V, Hernandez AV, Tang WHW. Prognostic value of cardiac troponin in chronic stable heart failure: a systematic review. Heart. 2012;98:1778–1786. doi: 10.1136/heartjnl-2012-301779. [DOI] [PubMed] [Google Scholar]
- 58.Duprez DA, Neuhaus J, Tracy R, Kuller LH, Deeks SG, Orkin C, Stoehr A, Woolley IJ, Neaton JD INSIGHT SMART Group. N-terminal-proB-type natriuretic peptide predicts cardiovascular disease events in HIV-infected patients. AIDS. 2011;25:651–657. doi: 10.1097/QAD.0b013e32834404a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gingo MR, Zhang Y, Ghebrehawariat KB, Jeong J-H, Chu Y, Yang Q, Lucht L, Hanna DB, Lazar JM, Gladwin MT, Morris A. Elevated NT-pro-brain natriuretic peptide level is independently associated with all-cause mortality in HIV-infected women in the early and recent HAART eras in the Women’s Interagency HIV Study cohort. PLoS ONE. 2015;10:e0123389. doi: 10.1371/journal.pone.0123389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 61.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Popescu BA, Waggoner AD. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29:277–314. doi: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 62.Mitter SS, Shah SJ, Thomas JD. A test in context: E/A and e/e’ to assess diastolic dysfunction and LV filling pressure. J Am Coll Cardiol. 2017;69:1451–1464. doi: 10.1016/j.jacc.2016.12.037. [DOI] [PubMed] [Google Scholar]
- 63.Voigt J-U, Pedrizzetti G, Lysyansky P, Marwick TH, Houle H, Baumann R, Pedri S, Ito Y, Abe Y, Metz S, Song JH, Hamilton J, Sengupta PP, Kolias TJ, d’Hooge J, Aurigemma GP, Thomas JD, Badano LP. Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. J Am Soc Echocardiogr. 2015;28:183–193. doi: 10.1016/j.echo.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 64.Obokata M, Negishi K, Kurosawa K, Arima H, Tateno R, Ui G, Tange S, Arai M, Kurabayashi M. Incremental diagnostic value of la strain with leg lifts in heart failure with preserved ejection fraction. JACC Cardiovasc Imaging. 2013;6:749–758. doi: 10.1016/j.jcmg.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 65.Piechnik SK, Ferreira VM, Dall’Armellina E, Cochlin LE, Greiser A, Neubauer S, Robson MD. Shortened Modified Look-Locker Inversion recovery (ShMOLLI) for clinical myocardial T1-mapping at 1.5 and 3 T within a 9 heartbeat breathhold. J Cardiovasc Magn Reson. 2010;12:69. doi: 10.1186/1532-429X-12-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shah SH, Bain JR, Muehlbauer MJ, Stevens RD, Crosslin DR, Haynes C, Dungan J, Newby LK, Hauser ER, Ginsburg GS, Newgard CB, Kraus WE. Association of a peripheral blood metabolic profile with coronary artery disease and risk of subsequent cardiovascular events. Circ Cardiovasc Genet. 2010;3:207–214. doi: 10.1161/CIRCGENETICS.109.852814. [DOI] [PubMed] [Google Scholar]
- 67.Halket JM, Przyborowska A, Stein SE, Mallard WG, Down S, Chalmers RA. Deconvolution gas chromatography/mass spectrometry of urinary organic acids--potential for pattern recognition and automated identification of metabolic disorders. Rapid Commun Mass Spectrom. 1999;13:279–284. doi: 10.1002/(SICI)1097-0231(19990228)13:4<279::AID-RCM478>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 68.Kind T, Wohlgemuth G, Lee DY, Lu Y, Palazoglu M, Shahbaz S, Fiehn O. FiehnLib: mass spectral and retention index libraries for metabolomics based on quadrupole and time-of-flight gas chromatography/mass spectrometry. Anal Chem. 2009;81:10038–10048. doi: 10.1021/ac9019522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wishart DS, Jewison T, Guo AC, Wilson M, Knox C, Liu Y, Djoumbou Y, Mandal R, Aziat F, Dong E, Bouatra S, Sinelnikov I, Arndt D, Xia J, Liu P, Yallou F, Bjorndahl T, Perez-Pineiro R, Eisner R, Allen F, Neveu V, Greiner R, Scalbert A. HMDB 3.0--The Human Metabolome Database in 2013. Nucleic Acids Res. 2013;41:D801–7. doi: 10.1093/nar/gks1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Danesh J, Kaptoge S, Mann AG, Sarwar N, Wood A, Angleman SB, Wensley F, Higgins JPT, Lennon L, Eiriksdottir G, Rumley A, Whincup PH, Lowe GDO, Gudnason V. Long-term interleukin-6 levels and subsequent risk of coronary heart disease: two new prospective studies and a systematic review. PLoS Med. 2008;5:e78. doi: 10.1371/journal.pmed.0050078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behav Brain Res. 2001;125:279–284. doi: 10.1016/s0166-4328(01)00297-2. [DOI] [PubMed] [Google Scholar]
- 72.Rossi A, Gheorghiade M, Triposkiadis F, Solomon SD, Pieske B, Butler J. Left atrium in heart failure with preserved ejection fraction: structure, function, and significance. Circ Heart Fail. 2014;7:1042–1049. doi: 10.1161/CIRCHEARTFAILURE.114.001276. [DOI] [PubMed] [Google Scholar]
- 73.Borlaug BA, Nishimura RA, Sorajja P, Lam CSP, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail. 2010;3:588–595. doi: 10.1161/CIRCHEARTFAILURE.109.930701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mondy KE, Gottdiener J, Overton ET, Henry K, Bush T, Conley L, Hammer J, Carpenter CC, Kojic E, Patel P, Brooks JT SUN Study Investigators. High Prevalence of Echocardiographic Abnormalities among HIV-infected Persons in the Era of Highly Active Antiretroviral Therapy. Clin Infect Dis. 2011;52:378–386. doi: 10.1093/cid/ciq066. [DOI] [PubMed] [Google Scholar]
- 75.Brinkman K, ter Hofstede HJ, Burger DM, Smeitink JA, Koopmans PP. Adverse effects of reverse transcriptase inhibitors: mitochondrial toxicity as common pathway. AIDS. 1998;12:1735–1744. doi: 10.1097/00002030-199814000-00004. [DOI] [PubMed] [Google Scholar]
- 76.Reyskens KMSE, Essop MF. HIV protease inhibitors and onset of cardiovascular diseases: a central role for oxidative stress and dysregulation of the ubiquitin-proteasome system. Biochim Biophys Acta. 2014;1842:256–268. doi: 10.1016/j.bbadis.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 77.Frerichs FCP, Dingemans KP, Brinkman K. Cardiomyopathy with mitochondrial damage associated with nucleoside reverse-transcriptase inhibitors. N Engl J Med. 2002;347:1895–1896. doi: 10.1056/NEJM200212053472320. [DOI] [PubMed] [Google Scholar]