Abstract
Purpose
To investigate the changes in the retinal vasculature in different parts of the fundus in eyes with primary angle closure glaucoma (PACG).
Methods
Healthy subjects and those with PACG were enrolled. Optical coherence tomography and a split-spectrum amplitude decorrelation angiography algorithm were used to quantify the retinal vessel densities in the parafoveal and peripapillary areas.
Results
Thirty-nine eyes from 24 PACG patients and 39 eyes from 20 age- and sex-matched healthy subjects were included. The retinal vessel densities in the parafoveal and peripapillary areas and every sector of the two areas were significantly lower in the PACG eyes than in the healthy eyes. The difference was greater in the peripapillary area (11.75%) than in the parafoveal area (7.55%, P < 0.05). In the PACG eyes, the vessel density in the peripapillary area correlated closely with the intraocular pressure (IOP), but that in the parafoveal area did not. When the PACG eyes were divided into groups with well-controlled and not-well-controlled IOP(≤ 21 mmHg or not), the vessel density and retinal nerve fiber layer thickness in the peripapillary area were much lower in the not-well-controlled eyes (P < 0.05), whereas the vessel density in the parafovea and the ganglion cell complex thickness were similar in the two subgroups.
Conclusion
Retinal vessel density was significantly reduced in PACG eyes. The magnitude of this difference varied between the fundus areas, and was greater in the peripapillary area.
Keywords: optical coherence tomography angiography, primary angle closure glaucoma, retinal vessel density, intraocular pressure
Introduction
Glaucoma is the leading cause of irreversible blindness throughout the world.[1] In 2013, the number of people with glaucoma was estimated to be 64.3 million worldwide, increasing to 76.0 million in 2020.[2] The impairment of the retinal vasculature in glaucoma has attracted increasing attention from ophthalmologists.[3–5]. Chen et al. reported that the peripapillary microcirculation correlated significantly with functional and structural defects in patients with primary open angle glaucoma (POAG).[6] Rao et al. [7] also reported that measurements of the peripapillary vessel density are as useful as measurements of the retinal nerve fiber layer (RNFL) in the diagnosis of both POAG and primary angle closure glaucoma (PACG).
However, our group recently found that the retinal vessels in the peripapillary and parafoveal areas responded differently to increases in blood pressure and oxygen pressure.[8, 9] Elevated intraocular pressure (IOP) is considered the main cause of optic nerve damage in glaucoma,[10] so we hypothesized that the vessel density response to increased IOP may also differ in the two areas. PACG is considered to be a more purely IOP-dependent disease than POAG [11] and therefore provides a better model in which to investigate IOP-induced impairment of the retinal vasculature and structure. The purpose of this study was to investigate the changes in the retinal vascular system in PACG eyes and to compare these changes in different parts of the fundus.
Methods
Ethics
The study protocol was approved by the Institutional Review Board of the Eye & ENT Hospital of Fudan University, Shanghai, China, and conformed to the tenets of the Declaration of Helsinki, and the ethics approval number for this project is KJ2009–16. Written informed consent was obtained from all subjects.
Subjects
We prospectively enrolled subjects diagnosed with PACG who consecutively attended the Glaucoma Clinic at the Eye & ENT Hospital of Fudan University between November 2015 and May 2016. Twenty age- and sex-matched healthy subjects were enrolled as the controls. All the subjects were interviewed regarding their detailed medical histories and underwent thorough ophthalmological examinations, which included best corrected visual acuity (BCVA); the measurement of their refractive status, according to their autorefraction, central corneal thickness, and axial length (AL) using IOLMaster (version 3.01; Carl Zeiss Meditec, Jena, Germany); slit-lamp biomicroscopy; an undilated fundus examination with direct ophthalmoscopy; and the measurement of IOP with a Goldmann applanation tonometer (Haag-Streit, Bern, Switzerland). The PACG patients also underwent a gonioscopic examination with a one-mirror Goldmann lens (Haag Streit Diagnostics, Bern, Switzerland) and visual field tests with a Humphrey Field Analyzer II (Carl Zeiss Meditec, Inc., Dublin, CA, USA) set for the 30-2 threshold test to determine the pattern standard deviation (PSD) and mean deviation (MD). All subjects were instructed to rest in a sitting position for 20 min, and their systemic blood pressure was measured and optical coherence tomography (OCT) images taken. The mean arterial pressure (MAP) was calculated as the diastolic blood pressure plus one-third of the difference between the diastolic blood pressure and the systolic blood pressure. The ocular perfusion pressure was calculated as 2/3MAP − IOP.
PACG was defined according to established criteria, [12] as follows: 1) eyes had occldable angles (the posterior trabecular meshwork was not seen with gonioscopy for more than 270 degrees in the primary position) or iridotrabecular contact in at least three quadrants on gonioscopy, together with a variable amount of peripheral anterior synechiae; IOP > 21 mmHg or a previous history of acutely elevated IOP; 2) glaucomatous optic neuropathy: the loss of the neuroretinal rim with a vertical cup-to-disc ratio (C/D) of > 0.7 or vertical C/D asymmetry of > 0.2, and/or notching attributable to glaucoma; 3) associated visual field defects on static automated perimetry (SITA standard algorithm with a 30-2 test pattern; Humphrey Visual Field Analyzer II; Carl Zeiss Meditec): a glaucoma hemifield test result outside the normal limits or a cluster of ≥ 3 non-edge contiguous points on the pattern deviation plot that did not cross the horizontal meridian, demonstrating abnormal sensitivity at P < 5% with at least one of the points with P < 1%, or PSD < 0.05. Healthy subjects were included if their BCVA was ≥ 16/20, their spherical equivalence (SE) was between and −6 D, and their AL was 20–25 mm. Subjects with any of the following were excluded: a history of ocular surgery or trauma; signs of myopic degeneration or a pathological form of myopia; any other ophthalmic disease; or the presence of any systemic disease (e.g., diabetes mellitus, hypertension, and migraine) or use of any medication that might affect blood flow. Individuals with BCVA < 16/20, IOP > 21 mmHg, high hyperopia or myopia (greater than or –6 diopters of SE), a history of systemic disease, use of medication that might affect blood flow, or a family history of glaucoma in a first-degree relative were also excluded from the control group.
OCT-A
OCT scans were obtained with a spectral domain system (RTVue-XR Avanti; Optovue, Fremont, CA, USA, software version 2015.1.0.90) by a single observer (Y.Z.). This system has an A-scan rate of 70 kHz, with a light source centered at a wavelength of 840 nm and a bandwidth of 45 nm. If both eyes met the inclusion criteria, then both eyes were included. Retinal vessel density and thickness were determined on the same visit. Three-dimensional OCT angiographic scans were performed and each imaging session included two sets of scans. The A-scans (304 × 304) and two consecutive B-scans were captured at each fixed position and processed with the SSADA algorithm to visualize the retinal vasculature with high resolution.[13]
For OCT-A, optic disc (4.5 × 4.5 mm) and macular scans (6 × 6 mm) were performed, and the images with a signal strength index > 40 and no residual motion artifacts were saved for further analysis. En-face retinal angiograms were created by projecting the flow signal internally to the retinal pigment epithelium. These procedures were performed with the RTVue-XR Avanti software. This software automatically calculates the vessel density of the parafovea (an annulus with an outer diameter of 3 mm and an inner diameter of 1 mm) and the peripapillary area (a 700 μm-wide elliptical annulus extending outward from the optic disc boundary) [14] (Figure 1).
Figure 1.
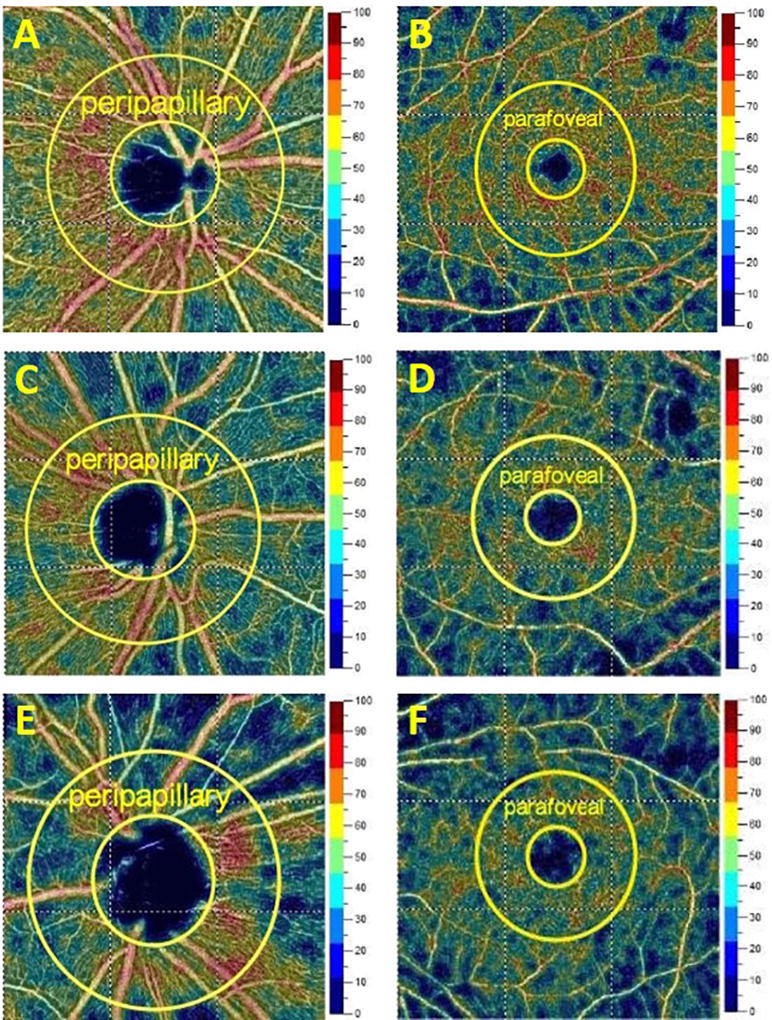
Representative optical coherence tomography angiographic images of the peripapillary and parafoveal areas in healthy eyes (A: peripapillary; B: parafoveal), and in eyes with primary angle closure glaucoma and well-controlled intraocular pressure (C: peripapillary; D: parafoveal) or not-well-controlled intraocular pressure (E: peripapillary; F: parafoveal). IOP, intraocular pressure; PACG, primary angle closure glaucoma.
The RNFL was measured along a circle with a diameter of 3.40 mm centered on the optic disc. The thickness of the ganglion cell complex (GCC) was measured with the GCC protocol. GCC and RNFL were then automatically calculated with the RTVue-XR Avanti software as the mean thickness in each area.
Statistical analysis
The statistical analyses were performed with the SPSS software version 16.0 (SPSS Inc., Chicago, IL, USA) and SAS 9.3 (SAS Institute Inc., Cary, NC, USA). For the analysis, the patients with PACG were divided into the well-controlled group (IOP ≤ 21 mmHg) and the not-well-controlled group (IOP > 21 mmHg, even though the maximum tolerated doses of antiglaucoma medications were used). Because the measurements in both eyes of the same subject are usually correlated, and a general linear mixed-model with the subject as the random effect can accommodate the presence of multiple correlated measurements from the same unit,[15, 16] a linear mixed model was used to compare the parameters between the two groups of PACG eyes and the healthy eyes. The nonparametric Mann–Whitney test was used to compare the continuous variables between the PACG and healthy subjects, and the sex distributions in these groups were compared with the χ2 test. The associations between vessel density and other factors were first determined with a univariate linear mixed-model analysis, and if P < 0.05, a multivariable linear mixed-model was applied. The percentage reduction in the retinal vessel density of the PACG eyes was calculated with the following formula: (mean vessel density in healthy eyes − mean vessel density in PACG eyes)/mean vessel density in healthy eyes. The reductions in the retinal vessel density in the well-controlled and not-well-controlled PACG eyes were compared with a linear mixed-model analysis. The significance level was set to P < 0.05.
Results
Forty-five eyes from 29 PACG patients were recruited, and six eyes were excluded because of a poor signal strength index (< 40) or blink artifacts. Thus, a total of 39 eyes of 24 subjects with PACG, including 12 eyes that had had a previous episode of acute angle closure, and 39 eyes of 20 age- and sex-matched healthy subjects were included in the final analysis.
Age (P = 0.095) and sex (P = 0.209) were similar in the healthy and PACG groups, whereas the PACG group had lower BCVA, higher IOP, shorter AL, and thinner GCC and RNFL than the healthy group (all P < 0.05). The PACG eyes had significantly lower vessel densities than the healthy eyes in the peripapillary and parafoveal areas (all P < 0.05; Table 1). The percentage reduction in vessel density was greater in the peripapillary area (11.75%) than in the parafoveal area (7.55%, P < 0.05). When the vessel densities in the different sectors were compared, they were lower in the PACG eyes than in the healthy eyes in all the sectors of the peripapillary and parafoveal areas (all P < 0.05; Table 1).
Table 1.
Clinical and optical coherence tomography angiography characteristics
| Healthy eyes (n = 39) |
PACG eyes (n = 39) |
P* | |
|---|---|---|---|
| Age (years) | 57.13 ± 4.97 | 58.63 ± 7.15 | 0.095 |
| Sex (male:female) | 18:21 | 16:23 | 0.209 |
| BCVA | 0.95 ± 0.09 | 0.6 ± 0.28 | <0.001 |
| IOP (mmHg) | 14.01 ± 2.79 | 20.45 ± 14.79 | <0.001 |
| AL (mm) | 23.46 ± 0.62 | 22.22 ± 0.99 | <0.001 |
| SBP (mmHg) | 119.95 ± 17.72 | 124.71 ± 13.6 | 0.224 |
| DBP (mmHg) | 76.7 ± 9.65 | 73.54 ± 8.10 | 0.232 |
| MAP (mmHg) | 90.85 ± 10.21 | 90.60 ± 8.72 | 0.900 |
| OPP (mmHg) | 39.87 ± 7.06 | 40.48 ± 5.52 | 0.536 |
| Structural variables | |||
| RNFL thickness (µm) | 106.38 ± 9.58 | 91.41 ± 26.16 | <0.001 |
| GCC thickness (µm) | 100.67 ± 8.11 | 89.13 ± 15.49 | <0.001 |
| Vessel density | |||
| Peripapillary area | 63.62 ± 3.40 | 56.15 ± 7.67 | <0.001 |
| Nasal | 61.39 ± 3.94 | 54.61 ± 7.23 | <0.001 |
| Inferior Nasal | 64.40 ± 4.31 | 55.85 ± 10.87 | <0.001 |
| Inferior Temporal | 66.95 ± 5.30 | 60.11 ± 9.58 | <0.001 |
| Superior Temporal | 66.25 ± 5.23 | 58.36 ± 10.09 | <0.001 |
| Superior Nasal | 63.62 ± 5.44 | 55.64 ± 8.48 | <0.001 |
| Temporal | 63.26 ± 4.08 | 59.72 ± 7.10 | <0.001 |
| Parafoveal area | 50.83 ± 3.35 | 46.99 ± 4.42 | <0.001 |
| Temporal | 51.95 ± 4.04 | 48.18 ± 4.57 | <0.001 |
| Superior | 51.60 ± 4.23 | 47.62 ± 5.17 | <0.001 |
| Nasal | 50.57 ± 4.02 | 45.99 ± 6.08 | <0.001 |
| Inferior | 49.06 ± 4.37 | 46.44 ± 5.42 | 0.003 |
| Reduction rate | |||
| Peripapillary area | / | 11.7% | / |
| Parafoveal area | / | 7.55% | / |
Values shown are means ± standard deviations or numbers of subjects.
PACG, primary angle closure glaucoma; BCVA, best corrected visual acuity; IOP, intra-ocular pressure; AL, axial length; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; OPP, ocular perfusion pressure; RNFL, retinal nerve fiber layer; GCC: ganglion cell complex.
P values in bold are statistically significant at P < 0.05.
In the PACG eyes, univariate analyses showed that the peripapillary vessel density was negatively associated with age, AL, IOP, MAP, and PSD, and was positively associated with RNFL, GCC, and MD (all P < 0.05), whereas the parafoveal vessel density correlated negatively with MAP and positively with RNFL (all P < 0.05). Multivariable analyses revealed that the peripapillary vessel density was positively influenced by MD (β = 0.588) and RNFL (β = 0.157), and that the parafoveal vessel density was positively associated with RNFL (β = 0.052) (all P < 0.05; Table 2).
Table 2.
Correlations between vessel density and other variables in primary angle closure glaucoma eyes
| Peripapillary | Univariate analyses
|
Multivariable analyses
|
Parafovea | Univariate analyses
|
Multivariable analyses
|
||||
|---|---|---|---|---|---|---|---|---|---|
| β | P* | β | P* | β | P* | β | P* | ||
| Age | −0.384 | 0.025 | 0.079 | 0.571 | Age | −0.181 | 0.07 | - | - |
| Gender | 0.578 | 0.82 | - | - | Gender | 0.1777 | 0.221 | - | - |
| AL | −2.469 | 0.048 | −1.448 | 0.107 | AL | −0.13 | 0.072 | - | - |
| IOP | −0.411 | 0.016 | 0.081 | 0.572 | IOP | −0.071 | 0.485 | - | - |
| MAP | −0.242 | 0.028 | 0.01 | 0.915 | MAP | −0.128 | 0.046 | −0.083 | 0.206 |
| Attack | 0.871 | 0.754 | - | - | Attack | −0.54 | 0.736 | - | - |
| MD | 0.644 | 0.000 | 0.588 | 0.000 | MD | −0.041 | 0.675 | - | - |
| PSD | −1.351 | 0.000 | −0.281 | 0.373 | PSD | 0.008 | 0.973 | - | - |
| RNFL | 0.185 | 0.000 | 0.157 | 0.038 | RNFL | 0.065 | 0.016 | 0.052 | 0.045 |
| GCC | 0.386 | 0.000 | −0.492 | 0.628 | GCC | 0.076 | 0.103 | - | - |
AL, axial length; IOP, intraocular pressure; MAP, mean arterial pressure; MD, mean deviation; PSD, pattern standard deviation; RNFL, retinal nerve fiber layer; GCC, ganglion cell complex.
P values in bold are statistically significant at P < 0.05.
IOP was well controlled in 24 PACG eyes (of 18 subjects), but not in the other 15 eyes (of 13 subjects). The PACG eyes were divided into the well-controlled IOP group (≤ 21 mmHg, mean ± SD: 16.11 ± 2.83) and the not-well-controlled IOP group (> 21 mmHg, mean ± SD: 27.39 ± 6.47). The subjects in the two groups used similar numbers of antiglaucoma medications (P = 0.095), and similar numbers of eyes had a history of acute angle closure (P = 0.336). The not-well-controlled group had higher IOP and thinner RNFL than the well-controlled group (all P < 0.05), but similar GCC (P = 0.215), MD (P = 0.096), and PSD (P = 0.095). Compared with the well-controlled PACG eyes, the not-well-controlled PACG eyes had a lower vessel density in the peripapillary area (P < 0.05), but a similar vessel density in the parafoveal area (P > 0.05; Table 3). The percentage reductions in vessel density were also compared. The percentage reduction in the peripapillary area was greater in the not-well-controlled group than in the well-controlled group (16.74% vs 8.63%, respectively; P < 0.05), but was similar in the parafoveal areas of the two groups (8.07% vs 7.23%, respectively, P = 0.76; Table 3).
Table 3.
Clinical and optical coherence tomography angiography characteristics of eyes with primary angle closure glaucoma and well-controlled or not well - controlled intraocular pressure
| Well-controlled PACG eyes (n = 24) |
Not well - controlled PACG eyes (n = 15) |
P* | |
|---|---|---|---|
| Age (years) | 57.83 ± 7.84 | 59.93 ± 5.91 | 0.877 |
| Sex (male:female) | 10:14 | 6:9 | 0.918 |
| BCVA | 0.64 ± 0.29 | 0.61 ± 0.24 | 0.735 |
| Attack | 0.25 ±0.44 | 0.4 ± 0.51 | 0.336 |
| IOP (mmHg) | 16.11 ± 2.83 | 27.39 ± 6.47 | <0.001 |
| AL (mm) | 22.09 ± 0.93 | 22.42 ± 1.09 | 0.138 |
| SBP (mmHg) | 123.53 ± 14.55 | 126.64 ± 13.74 | 0.574 |
| DBP (mmHg) | 73.67 ± 9.98 | 73.73 ± 5.73 | 0.507 |
| MAP (mmHg) | 90.29 ± 10.44 | 91.36 ± 6.8 | 0.474 |
| OPP (mmHg) | 40.39 ± 5.79 | 40.60 ± 5.25 | 0.138 |
| Number of glaucoma medications | 0.74 ± 1.15 | 1.27 ± 0.9 | 0.095 |
| Visual field test | |||
| MD (db) | −6.42 ± 7.76 | −11.93 ± 9.80 | 0.096 |
| PSD (db) | 3.42 ± 2.70 | 7.01 ± 4.5 | 0.095 |
| Structural variables | |||
| RNFL thickness (µm) | 97.21 ± 28.90 | 82.13 ± 18.32 | 0.02 |
| GCC thickness (µm) | 93.60 ± 15.25 | 81.96 ± 13.42 | 0.215 |
| Vessel density | |||
| Peripapillary area | 58.13 ± 6.94 | 52.97 ± 7.94 | 0.015 |
| Nasal | 56.08 ± 6.61 | 52.26 ± 7.76 | 0.029 |
| Inferior Nasal | 57.46 ± 10.94 | 53.27 ± 10.62 | 0.109 |
| Inferior Temporal | 61.97 ± 8.86 | 57.13 ± 10.24 | 0.036 |
| Superior Temporal | 60.35 ± 9.43 | 55.17 ± 10.60 | 0.025 |
| Superior Nasal | 56.80 ± 8.84 | 53.78 ± 7.79 | 0.1 |
| Temporal | 61.29 ± 5.85 | 57.21 ± 8.35 | 0.077 |
| Parafoveal area | 47.15 ± 5.14 | 46.73 ± 3.09 | 0.556 |
| Temporal | 48.69 ± 5.26 | 47.35 ± 3.2 | 0.334 |
| Superior | 47.71 ± 5.83 | 47.48 ± 4.08 | 0.651 |
| Nasal | 46.05 ± 7.02 | 45.90 ± 4.41 | 0.774 |
| Inferior | 46.72 ± 6.08 | 45.99 ± 4.33 | 0.805 |
| Reduction rate# | |||
| Peripapillary area | 8.63% | 16.74% | 0.014 |
| Parafoveal area | 7.23% | 8.07% | 0.76 |
Values shown are means ± standard deviations or numbers of subjects.
PACG, primary angle closure glaucoma; IOP, intraocular pressure; BCVA, best corrected visual acuity; AL, axial length; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; OPP, ocular perfusion pressure; MD, mean deviation; PSD, pattern standard deviation; RNFL, retinal nerve fiber layer; GCC: ganglion cell complex.
P values in bold are statistically significant at P < 0.05.
When the different sectors were compared, the not-well-controlled PACG eyes had lower vessel densities in the nasal, inferior temporal, and superior temporal sectors of the peripapillary area than the well-controlled eyes (all P < 0.05), whereas the vessel densities were similar in the two groups in the other sectors of the peripapillary area and in all the sectors of the parafoveal area (all P > 0.05; Table 3).
Discussion
In this study, the retinal vessel density in PACG eyes was evaluated with OCT-A. Compared with healthy eyes, the PACG eyes had lower vessel densities in both the peripapillary and parafoveal areas. Notably, the reduction in vessel density was much greater in the peripapillary area than in the parafoveal area. When the PACG eyes were divided to two groups according to their IOPs, the not-well-controlled group (> 21 mmHg) had a lower retinal vessel density in the peripapillary area than the well-controlled group (≤ 21 mmHg), but the two groups had similar vessel densities in the parafoveal area.
OCT-A was recently developed to observe the retinal vessel density in glaucoma patients in both the disc and foveal areas, in a noninvasive, rapid, and accurate manner.[17, 18] In this study, the retinal vessel densities in both the parafoveal and peripapillary areas were significantly lower in the PACG eyes than in the healthy eyes, which is consistent with the findings of Rao et al. [19] and Wang et al. [20]. We also found that the reduction in vessel density in the PACG eyes was greater in the peripapillary area (11.75%) than in the parafoveal area (7.55%). Furthermore, the not-well-controlled group had a significantly lower retinal vessel density and higher percentage reduction than the well-controlled group in the peripapillary area, but not in the parafoveal area. Formerly, Rao et al. [19] demonstrated that the vessel density in both the peripapillary and parafoveal areas was reduced in PACG eyes, and in their results, the reduction in the peripapillary vessel density was nearly 14%, whereas it was about 6% in the parafoveal region. This supports our findings, and is also consistent with the findings of other studies, in which the peripapillary vessel density and RNFL display similarly diagnostic accuracy for glaucoma, [7] whereas the vessel density in the parafovea has relatively weak diagnostic utility.[21] The more-severe reductions in both the vessel density and RNFL in the peripapillary area suggest that these are more vulnerable than their counterparts in the macular area in glaucomatous eyes. Therefore, these parameters should be more sensitive than those in the macular area for the diagnosis of glaucoma and for monitoring disease progression. Why these changes differ in different parts of the fundus is not yet clear. A possible reason is that the macular region of the retina is only supplied by the retinal artery, whereas the optic disc is supplied by the ciliary and retinal arteries. Hayreh et al. [22] noted that the choroidal contribution to the optic disc is most susceptible to high IOP, which appears as a reduction in vascular filling at 30 mmHg IOP, whereas the retinal circulation is not as easily impaired, unless IOP is elevated to the central retinal artery pressure, which is about 40–70 mmHg. When IOP increases to 70 mmHg, the disc and peripapillary choroid capillaries are virtually obliterated, whereas the retinal circulation is only slowed.[23] From another perspective, when IOP is elevated, the swelling of the optic disc and nerve [24] is more apparent than any macular edema in the clinical setting. These phenomena may contribute to the different impairment of the retinal vasculature in different parts of the retina in PACG eyes.
Further analysis of the different sectors of the peripapillary area showed that the not-well-controlled group had lower vessel densities in the nasal, inferior temporal, and superior temporal sectors of the peripapillary area than the well-controlled group, but not in the other sectors. When Holló et al. [25] studied the changes in vessel density in POAG eyes, they reported that the reduction in vessel density was most significant in the inferior temporal and superior temporal sectors. Furthermore, both clinical observations and a previous data analysis have shown that RNFL defects are most frequently detected at the inferotemporal and superotemporal meridians.[26] Jonas et al. [27] demonstrated that rim loss predominantly occurs in the inferotemporal and superotemporal disc regions in eyes with modest glaucoma. This may be explained if the thick nerve fibers in the inferior, superior, and nasal disc areas are more sensitive to glaucoma than the thin nerve fibers on the temporal side of the nerve disc.[27] These data are consistent with our findings, and the more-severe damage to the vasculature in the inferior temporal, superior temporal, and nasal sectors may result from the more prominent reduction in RNFL in these sectors. Unfortunately, the scan pattern of RNFL we used did not provide sectional information about RNFL thickness. A future study might tell us more.
In our group of patients, the reduction in vessel density was closely associated with the reduction in RNFL. In a previous study of primary angle closure (PAC) eyes by Rao et al. [28], the authors reported that high IOP affects the RNFL earlier than it affects the retinal vessel density. However, they only included a group of PAC eyes with a history of high IOP, but normal visual fields and normal peripapillary RNFLs. In contrast, Xiaolei et al. [29] showed that PACG eyes had lower retinal vessel densities than eyes with suspected primary angle closure, but similar RNFLs. Furthermore, 34 PACG eyes included in their study had had an episode of acute angle closure, and the authors also suggested that the retinal edema observed after an acute attack explains the lack of difference in GCC and RNFL between acute PAC and PAC suspect eyes. In our patients, the reduction in vessel density in PACG was associated with a reduction in RNFL in these eyes. Furthermore, the more-severe reduction in vessel density in the peripapillary areas of eyes with not-well-controlled PACG than in those with well-controlled PACG was associated with a thinner RNFL. These data suggest that this vascular impairment is attributable to axon damage and drop-out, with consequent atrophy of the capillaries that nourished the previously present axons. Our study was limited by the small number of subjects, its cross-sectional design, and the inclusion of both eyes of individual subjects. A future study with more subjects and a longer follow-up period might provide more insight into the retinal vascular changes that occur in PACG. Because the damage to the vasculature differs in different parts of the fundus, future studies of the vascular changes in PACG eyes (and perhaps in other pathological conditions) must ensure that the changes compared are in the same areas.
Finally, PACG eyes have a lower retinal vessel density, especially in the peripapillary area, than healthy eyes. Further research is required to extend our knowledge of the changes in the retinal vasculature that occur in PACG eyes.
Supplementary Material
Acknowledgments
Publication of this article was supported, in part, by research grants from the National Major Scientific Equipment Program (2012YQ12008003), the National Key Research & Development Plan (2017YFC0108200), and the Shanghai Committee of Science and Technology (16140901000, 13430710500, and 15DZ1942204); and was also supported by grants R01 EY023285, DP3 DK104397, R01 EY024544, and P30 EY010572 from the National Institutes of Health (Bethesda, MD, USA); and by unrestricted departmental funding from Research to Prevent Blindness (New York, NY, USA). The authors thank the participants and our colleagues who helped with the execution of this study.
Footnotes
Conflict of Interest: David Huang and Yali Jia have significant financial interests in Optovue, Inc. David Huang also has a financial interest in Carl Zeiss Meditec. These potential conflicts of interest have been reviewed and accepted by Oregon Health & Science University. The other authors have no proprietary or commercial interests in any materials discussed in this paper.
References
- 1.Quigley HA. Glaucoma. The Lancet. 2011;377(9774):1367–1377. doi: 10.1016/S0140-6736(10)61423-7. [DOI] [PubMed] [Google Scholar]
- 2.Tham YC, Li X, Wong TY, et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081–90. doi: 10.1016/j.ophtha.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 3.Yarmohammadi A, Zangwill LM, Diniz-Filho A, et al. Relationship between Optical Coherence Tomography Angiography Vessel Density and Severity of Visual Field Loss in Glaucoma. Ophthalmology. 2016;123(12):2498–2508. doi: 10.1016/j.ophtha.2016.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pasquale LR. Vascular and autonomic dysregulation in primary open-angle glaucoma. Curr Opin Ophthalmol. 2016;27(2):94–101. doi: 10.1097/ICU.0000000000000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grunwald JE, Riva CE, Stone RA, et al. Retinal autoregulation in open-angle glaucoma. Ophthalmology. 1984;91(12):1690–4. doi: 10.1016/s0161-6420(84)34091-x. [DOI] [PubMed] [Google Scholar]
- 6.Chen CL, Zhang A, Bojikian KD, et al. Peripapillary Retinal Nerve Fiber Layer Vascular Microcirculation in Glaucoma Using Optical Coherence Tomography-Based Microangiography. Invest Ophthalmol Vis Sci. 2016;57(9):OCT475–85. doi: 10.1167/iovs.15-18909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rao HL, Kadambi SV, Weinreb RN, et al. Diagnostic ability of peripapillary vessel density measurements of optical coherence tomography angiography in primary open-angle and angle-closure glaucoma. Br J Ophthalmol. 2017;101(8):1066–1070. doi: 10.1136/bjophthalmol-2016-309377. [DOI] [PubMed] [Google Scholar]
- 8.Zong Y, Xu H, Yu J, et al. Retinal Vascular Autoregulation during Phase IV of the Valsalva Maneuver: An Optical Coherence Tomography Angiography Study in Healthy Chinese Adults. Front Physiol. 2017;8:553. doi: 10.3389/fphys.2017.00553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu H, Deng G, Jiang C, et al. Microcirculatory Responses to Hyperoxia in Macular and Peripapillary Regions. Invest Ophthalmol Vis Sci. 2016;57(10):4464–4468. doi: 10.1167/iovs.16-19603. [DOI] [PubMed] [Google Scholar]
- 10.Weinreb RN, Khaw PT. Primary open-angle glaucoma. The Lancet. 2004;363(9422):1711–1720. doi: 10.1016/S0140-6736(04)16257-0. [DOI] [PubMed] [Google Scholar]
- 11.Gazzard G, Foster PJ, Devereux JG, et al. Intraocular pressure and visual field loss in primary angle closure and primary open angle glaucomas. Br J Ophthalmol. 2003;87(6):720–5. doi: 10.1136/bjo.87.6.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ko YC, Liu CJ, Hsu WM, et al. Determinants and characteristics of angle-closure disease in an elderly Chinese population. Ophthalmic Epidemiol. 2015;22(2):109–15. doi: 10.3109/09286586.2015.1012270. [DOI] [PubMed] [Google Scholar]
- 13.Jia Y, Tan O, Tokayer J, et al. Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Opt Express. 2012;20(4):4710–25. doi: 10.1364/OE.20.004710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pechauer AD, Jia Y, Liu L, et al. Optical Coherence Tomography Angiography of Peripapillary Retinal Blood Flow Response to Hyperoxia. Invest Ophthalmol Vis Sci. 2015;56(5):3287–91. doi: 10.1167/iovs.15-16655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glynn RJ, Rosner B. Accounting for the correlation between fellow eyes in regression analysis. Arch Ophthalmol. 1992;110(3):381–7. doi: 10.1001/archopht.1992.01080150079033. [DOI] [PubMed] [Google Scholar]
- 16.Rao HL, Pradhan ZS, Weinreb RN, et al. Vessel Density and Structural Measurements of Optical Coherence Tomography in Primary Angle Closure and Primary Angle Closure Glaucoma. Am J Ophthalmol. 2017;177:106–115. doi: 10.1016/j.ajo.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 17.Jia Y, Wei E, Wang X, et al. Optical coherence tomography angiography of optic disc perfusion in glaucoma. Ophthalmology. 2014;121(7):1322–32. doi: 10.1016/j.ophtha.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu H, Yu J, Kong X, et al. Macular microvasculature alterations in patients with primary open-angle glaucoma: A cross-sectional study. Medicine (Baltimore) 2016;95(33):e4341. doi: 10.1097/MD.0000000000004341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rao HL, Pradhan ZS, Weinreb RN, et al. Vessel Density and Structural Measurements of Optical Coherence Tomography in Primary Angle Closure and Primary Angle Closure Glaucoma. American Journal of Ophthalmology. 2017;177:106–115. doi: 10.1016/j.ajo.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Jiang C, Kong X, et al. Peripapillary retinal vessel density in eyes with acute primary angle closure: an optical coherence tomography angiography study. Graefe's Archive for Clinical and Experimental Ophthalmology. 2017;255(5):1013–1018. doi: 10.1007/s00417-017-3593-1. [DOI] [PubMed] [Google Scholar]
- 21.Rao HL, Pradhan ZS, Weinreb RN, et al. Regional Comparisons of Optical Coherence Tomography Angiography Vessel Density in Primary Open-Angle Glaucoma. Am J Ophthalmol. 2016;171:75–83. doi: 10.1016/j.ajo.2016.08.030. [DOI] [PubMed] [Google Scholar]
- 22.Hayreh SS. Optic disc changes in glaucoma. Br J Ophthalmol. 1972;56(3):175–85. doi: 10.1136/bjo.56.3.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayreh S, Perkins E. Fluorescein Angiography. Karger Publishers; 1971. The effects of raised intraocular pressure on the blood vessels of the retina and optic disc; pp. 323–328. [Google Scholar]
- 24.Zimmerman LE, De Venecia G, Hamasaki DI. Pathology of the optic nerve in experimental acute glaucoma. Invest Ophthalmol. 1967;6(2):109–25. [PubMed] [Google Scholar]
- 25.Hollo G. Vessel density calculated from OCT angiography in 3 peripapillary sectors in normal, ocular hypertensive, and glaucoma eyes. Eur J Ophthalmol. 2016;26(3):e42–5. doi: 10.5301/ejo.5000717. [DOI] [PubMed] [Google Scholar]
- 26.Leung CK, Choi N, Weinreb RN, et al. Retinal nerve fiber layer imaging with spectral-domain optical coherence tomography: pattern of RNFL defects in glaucoma. Ophthalmology. 2010;117(12):2337–44. doi: 10.1016/j.ophtha.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 27.Jonas JB, Fernández MC, Stürmer J. Pattern of glaucomatous neuroretinal rim loss. Ophthalmology. 1993;100(1):63–68. doi: 10.1016/s0161-6420(13)31694-7. [DOI] [PubMed] [Google Scholar]
- 28.Rao HL, Pradhan ZS, Weinreb RN, et al. Vessel density and structural measurements of optical coherence tomography in primary angle closure and primary angle closure glaucoma. Am J Ophthalmol. 2017 doi: 10.1016/j.ajo.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 29.Wang X, Jiang C, Kong X, et al. Peripapillary retinal vessel density in eyes with acute primary angle closure: an optical coherence tomography angiography study. Graefes Arch Clin Exp Ophthalmol. 2017;255(5):1013–1018. doi: 10.1007/s00417-017-3593-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


