Abstract
Objective
To examine the incidence of gout over the last 20 years and evaluate possible changes in associated comorbid conditions.
Patients and Methods
The medical records of all adults with a diagnosis of incident gout in Olmsted County, MN during two time periods (January 1st 1989 – December 31st 1992 and January 1st 2009 – December 31st 2010) were reviewed. Incident cases had to fulfill at least 1 of 3 criteria: the American Rheumatism Association (ARA) 1977 preliminary criteria for gout, the Rome or New York criteria.
Results
A total of 158 patients with new-onset gout were identified during 1989–1992 and 271 patients during 2009–2010, yielding age- and sex-adjusted incidence rates of 66.6/100,000 (95% CI: 55.9 – 77.4) in 1989–1992 and 136.7/100,000 (95% CI: 120.4 – 153.1) in 2009–2010. The incidence rate ratio was 2.62 (95% CI: 1.80 – 3.83). At the time of their first gout flare, patients diagnosed with gout in 2009–2010 had higher prevalence of comorbid conditions compared with 1989–1992, including hypertension (69% vs. 54%), diabetes mellitus (25% vs. 6%), renal disease (28% vs. 11%), hyperlipidemia (61% vs. 21%) and morbid obesity (BMI≥ 35 kg/m2) (29% vs. 10%).
Conclusion
The incidence of gout has more than doubled over the recent 20 years. This increase together with the more frequent occurrence of comorbid conditions and cardiovascular risk factors represents a significant public health challenge.
Keywords: gout, incidence, epidemiology, comorbidity
Introduction
Gout is the most common inflammatory arthritis in the Unites States (1). Several studies have demonstrated an increasing prevalence over the recent decades (2–5). Not surprisingly, the life lived with disability due to gout has increased dramatically over the last 20 years (6).
Whether there has been an actual increase in the incidence of gout in recent years is unclear. Previous population-based studies using the Rochester Epidemiology Project (REP) have suggested that the incidence of gout nearly doubled between 1977–1978 and 1995–1996 (4), but population-based data about gout incidence in the United States over the last 20 years is limited (7, 8).
While the morbidity associated with gout itself is concerning, patients with gout also have a higher likelihood of suffering from various comorbid conditions such as obesity, hyperlipidemia, renal disease and hypertension (9). Possible changes in the prevalence of these comorbidities among individuals with gout would have tremendous impact on the overall morbidity of patients with gouty arthritis.
The purpose of the present study was to examine trends in the incidence of gout in a United States based population, and determine whether the prevalence of some comorbid conditions having major effects on patient well-being and survivorship among affected patients has changed.
Patients and Methods
Olmsted County, Minnesota is well suited for population-based studies through the resources of the REP (10). The REP is a medical record linkage system that enumerates the population and provides ready access to the medical records from all health care providers in Olmsted County, Minnesota, including the Mayo Clinic, the Olmsted Medical Center and their affiliated hospitals, local nursing homes and the few private practitioners. The REP ensures the full capture of all the medical records, both inpatient and outpatient, for the population. The REP ensures virtually complete ascertainment of all clinically recognized cases of gout among the residents of Olmsted County, Minnesota. This study was approved by the Institutional Review Boards of the Mayo Clinic (12-007239) and the Olmsted Medical Center (018-OMC-15).
Using the REP, adult (age ≥ 18 years) Olmsted County residents with the potential diagnosis of gout were retrieved using diagnostic codes (International Classification of Diseases Version 9: code 274.x) during two different time periods (January 1st 1989– December 31st 1992) and (January 1st 2009– December 31st 2010). The medical records (inpatient and outpatient) of each potential case were then reviewed for ascertainment of gout diagnosis. Cases were identified as incident at the earliest date the case fulfilled at least one of three proposed gout criteria: the American Rheumatism Association (ARA) 1977 preliminary criteria for gout (11), the Rome criteria (12) or the New York criteria (13) (table 1).
Table 1.
Criteria used for diagnosis of gout: 1977 American Rheumatism Association (ARA) preliminary criteria, Rome and New York criteria.
| The 1977 American Rheumatism Association (ARA) preliminary criteria (11) | Rome criteria (12) | New York criteria (13) |
|---|---|---|
|
| ||
| Presence of characteristic urate crystals in the joint fluid * Presence of a tophus proven to contain urate crystals by chemical means or polarized light microscopy * Presence of six of the following clinical, laboratory, and radiographic phenomena:
|
Any two of the following:
|
urate crystals in joint fluid or tissue or tophus * Any two of the following:
|
Demographic and clinical data were abstracted via medical record review. Smoking status (never, current, former) at the time of the incident gout diagnosis was collected. Body mass index (BMI) was calculated using the standing height and weight recorded in the medical record at the time of gout diagnosis. BMI was categorized based on the World Health Organization (WHO) classification: normal (18.5–24.9 kg/m2), overweight (pre-obese) (25–29.9 kg/m2), class I obesity (30–34.9 kg/m2), class II obesity (35–39.9 kg/m2), class III obesity (≥40 kg/m2) (14). Cardiovascular and metabolic risk factors and comorbidities were abstracted from the records based on physician diagnoses and laboratory values (i.e., lipid profile) prior to or at the diagnosis of gout. Concomitant medication use for cardiovascular risk factors and comorbidities were recorded.
Statistical Analysis
Descriptive statistics (means, percentages, etc.) were used to summarize data. Clinical characteristics were compared between the time periods using Wilcoxon rank sum tests and chi-square tests. P-values < 0.05 were considered to be statistically significant. Age- and sex-specific incidence rates were calculated by using the number of incident cases as the numerator and population estimates for adults (age ≥ 18 years) based on decennial census counts as the denominator, with linear interpolation used to estimate population size for intercensal years. Incidence rates were age- and sex- adjusted for the US 2010 white population. In order to compute 95% confidence intervals (95% CI) for incidence rates, it was assumed that the number of incident cases followed a Poisson distribution. Incidence rates were compared between the two time periods using Poisson regression methods. Analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA) and R 3.1.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
A total of 429 patients with incident gout were identified, 158 patients in 1989–1992 and 271 patients in the 2009–2010 time period. While the age and sex distribution of individuals diagnosed with gout remained similar when comparing both time periods (table 2), the proportion of non-whites increased from 6% to 10%. BMI in the 2009–2010 cohort was significantly higher than that of the 1989–1992 cohort (median 30.9 vs. 28.3, respectively, p<0.001). This increase in BMI was observed in both males and females. Moreover, the number of individuals with class II and III obesity tripled between the earlier and the later cohort (29% vs. 10% with BMI ≥ 35 kg/m2 in 2009–2010 vs. 1989–1992, respectively; p<0.001).
Table 2.
Patient characteristics, Major associated co-morbidities and medication use among Olmsted County, Minnesota residents with incident gout in 1989–1992 compared with 2009–2010.
| Characteristic | 1989–1992 (N=158) | 2009–2010 (N=271) | p value |
|---|---|---|---|
| Age at diagnosis, years, mean (SD) | 59.3 (17.9) | 60.0 (17.0) | 0.68 |
| Sex, Male | 116 (73%) | 196 (72%) | 0.81 |
| Race and Ethnicity | |||
| Caucasian | 149 (94%) | 244 (90%) | 0.17 |
| Black or African-American | 1 (1%) | 2 (1%) | |
| Somali | 0 (0%) | 2 (1%) | |
| Asian | 5 (3%) | 12 (4%) | |
| Native Hawaiian/Pacific Islander | 1 (1%) | 0 (0%) | |
| Other | 0 (0%) | 9 (3%) | |
| Unknown | 2 (1%) | 2 (1%) | |
| Smoking | |||
| Never | 64 (42%) | 131 (48%) | 0.068 |
| Current | 17 (11%) | 26 (10%) | |
| Former | 72 (47%) | 106 (39%) | |
| Unknown | 0 (0%) | 8 (3%) | |
| Major Associated Comorbidities: | |||
| Body mass index, kg/m2, median (25th, 75th percentile) | 28.3 (24.9, 32.0) | 30.9 (27.4, 35.8) | <0.001 |
| Obesity (body mass index ≥30 kg/m2) | 56 (37%) | 150 (56%) | <0.001 |
| Hypertension | 86 (54%) | 188 (69%) | 0.002 |
| Heart failure | 10 (6%) | 27 (10%) | 0.20 |
| Diabetes mellitus | 9 (6%) | 68 (25%) | <0.001 |
| Renal disease | 18 (11%) | 77 (28%) | <0.001 |
| Coronary artery disease | 36 (23%) | 53 (20%) | 0.43 |
| Stroke | 7 (4%) | 20 (7%) | 0.24 |
| Hyperlipidemia | 33 (21%) | 164 (61%) | <0.001 |
| Total cholesterol* mg/dL, median (25th, 75th percentile) | 214 (188, 244) | 179 (152, 207) | <0.001 |
| Low-density lipoprotein cholesterol*, mg/dL, median (25th, 75th percentile) | 136 (66, 267) | 96 (74, 126) | <0.001 |
| High density lipoprotein cholesterol*, mg/dL, median (25th, 75th percentile) | 39 (33, 48) | 43 (35, 52) | 0.014 |
| Triglycerides*, mg/dL, median (25th, 75th percentile) | 174 (107, 246) | 142 (103, 212) | 0.075 |
| Serum uric acid level**, mg/dL, mean (SD) | 8.0 (1.8) | 8.2 (2.0) | 0.65 |
| Number tested | 107 | 179 | |
| Medication use: | |||
| Diuretic use | 68 (43%) | 130 (48%) | 0.32 |
| Aspirin | 36 (23%) | 107 (39%) | <0.001 |
| Statin | 7 (4%) | 111 (41%) | <0.001 |
Values in table are n (%) unless otherwise specified.
Available lipid values for 1989–1992 and 2009–2010, respectively: Total cholesterol n=126 and n=215; low density and high density lipoprotein cholesterol n=79 and n=215; triglycerides n=116 and n=216.
at incidence date to 2 weeks post incidence date; n=107 for 1989–1992 and n=179 for 2009–2010.
Abbreviations: SD: standard deviation
In addition, there were notably higher rates of cardiovascular risk factors and comorbidities in the 2009–2010 cohort, including hyperlipidemia, diabetes mellitus, hypertension and renal disease (table 2). The use of aspirin increased from 23% to 39% between 1989–1992 and 2009–2010 cohorts (p<0.01), and the use of statins increased from 4% to 41% between these two time periods. Of note, the serum levels of total and LDL cholesterol were significantly lower in the 2009–2010 cohort than the 1989–1992 cohort, while HDL levels were higher in the more recent cohort.
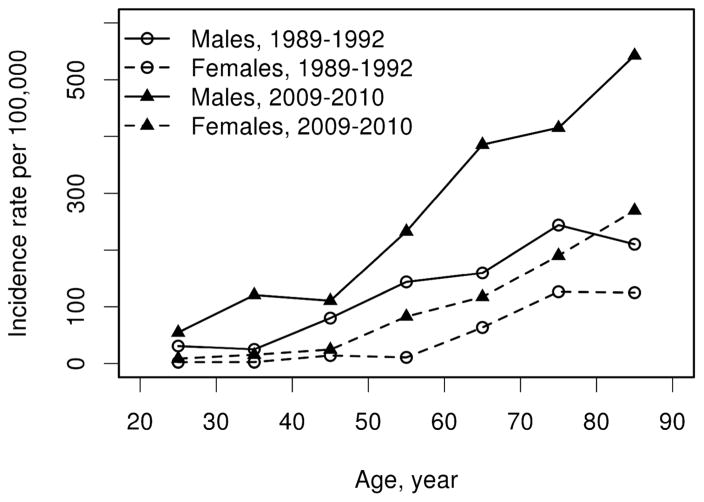
Using the combined criteria set, the age- and sex adjusted incidence rate of gout among adults (age ≥ 18 years) in the 1989–1992 time period was 66.6/100,000 (95% confidence interval [CI]: 55.9 – 77.4) compared with 136.7/100,000 (95% CI: 120.4 – 153.1) in the 2009–2010 time period. This constitutes a more than two-fold increase of incident gout within 20 years (rate ratio: 2.62; 95% CI: 1.80 – 3.83; p<0.001). This increase affected both sexes to a similar extent (figure 1) and involved all age groups (table 3).
Figure 1.
Incidence of gout among adult (age ≥ 18 years) Olmsted County, Minnesota residents in 1989–1992 and 2009–2010 based on the earliest date of fulfillment of 1977 American Rheumatism Association, Rome or New York criteria according to age and sex.
Table 3.
Incidence of gout among adult (age ≥ 18 years) Olmsted County, Minnesota residents in 1989–1992 and 2009–2010 based on the earliest date of 1977 American Rheumatism Association, Rome or New York criteria according to age and sex.
| Time Period | Female | Male | Total | ||||
|---|---|---|---|---|---|---|---|
| Age Group | N | Rate* | N | Rate* | N | Rate* | |
| 1989–1992 | 18–29 | 1 | 2.4 | 12 | 31.0 | 13 | 16.2 |
| 30–39 | 1 | 2.5 | 10 | 25.2 | 11 | 13.8 | |
| 40–49 | 4 | 14.1 | 22 | 80.1 | 26 | 46.5 | |
| 50–59 | 2 | 10.8 | 27 | 143.8 | 29 | 77.6 | |
| 60–69 | 9 | 63.7 | 20 | 159.7 | 29 | 108.9 | |
| 70–79 | 14 | 126.5 | 18 | 244.0 | 32 | 173.5 | |
| 80+ | 11 | 124.8 | 7 | 210.3 | 18 | 148.3 | |
| Overall** (95% CI) | 42 | 31.3 (21.7, 41.0) | 116 | 104.9 (84.7, 125.1) | 158 | 66.6 (55.9, 77.4) | |
|
| |||||||
| 2009–2010 | 18–29 | 2 | 8.6 | 11 | 54.9 | 13 | 31.0 |
| 30–39 | 3 | 15.4 | 24 | 120.7 | 27 | 68.6 | |
| 40–49 | 5 | 24.7 | 22 | 110.7 | 27 | 67.3 | |
| 50–59 | 17 | 82.8 | 45 | 232.8 | 62 | 155.5 | |
| 60–69 | 15 | 117.3 | 44 | 385.5 | 59 | 243.8 | |
| 70–79 | 15 | 190.3 | 28 | 415.2 | 43 | 294.0 | |
| 80+ | 18 | 269.5 | 21 | 542.6 | 39 | 369.7 | |
| Overall** (95% CI) | 75 | 70.6 (54.5, 86.6) | 196 | 210.2 (180.3, 240.2) | 271 | 136.7 (120.4, 153.1) | |
Rates are reported per 100,000 population
Overall sex-specific rates are age-adjusted and total rates are age- and sex- adjusted to the US white 2010 population
N = number; CI=confidence interval
The significant increase in the incidence of gout was also apparent when applying each of the criteria separately. Using the 1977 ARA preliminary criteria identified 118 incident cases in 1989–1992 and 183 incident cases in 2009–2010; the age- and sex- adjusted incidence rate rose from 49.5 (95% CI: 40.2, 58.7) in the 1989–1992 cohort to 92.7 (95% CI: 79.2, 106.2) in the 2009–2010 cohort (rate ratio: 2.64; 95% CI: 1.69 – 4.13). When the New York criteria were used, 91 incident cases in 1989–1992 and 217 incident cases in 2009–2010 were identified and the age- and sex- adjusted incidence rate increased from 39.0 (95% CI: 30.7 – 47.2) in the 1989–1992 cohort to 110.0 (95% CI: 95.2 – 124.7) in the 2009–2010 cohort (rate ratio: 3.41; 95% CI: 2.13 – 5.44). Finally, applying the Rome criteria (119 incident cases in 1989–1992 and 240 incident cases in 2009–2010) resulted in an age- and sex- adjusted incidence rate of 50.4 (95% CI: 41.0 – 59.7) in the 1989–1992 cohort and 121.3 (95% CI: 105.9 – 136.8) in the 2009–2010 cohort (rate ratio: 2.94; 95% CI: 1.95 – 4.43).
The serum uric acid level at the time of the first attack was not significantly different when comparing both cohorts (median 8.0 in the 1989–1992 versus 8.2 in the 2009–2010 cohort, p=0.65; table 2). Similarly, the use of diuretics at the time of incident gout did not differ significantly (43% in the 1989–1992 versus 48% in the 2009–2010 cohort, p=0.32).
Discussion
This population-based study demonstrates a marked increase in the incidence of gout among residents of Olmsted County, MN, over the last 20 years. The rate of newly diagnosed cases of gout more than doubled within the observed time period for both males and females and across all age groups. Importantly, these results supplement numerous studies which have demonstrated an increase in the prevalence of gout not only in the US, but in various geographic settings (1–5, 15–20).
Why are the incidence and prevalence rates of gout increasing at such a remarkable rate? This study was not designed to investigate the underlying cause for this increase, but other authors have suggested changes in risk factors of gout as the main contributing factor (9, 16). Risk factors of gout that have been increasing in the U.S. population over recent years include obesity, age, diabetes mellitus, chronic renal disease, and aspirin use (21–28). Moreover, as the population advances in age, serum uric acid level was found to increase as well (29). Interestingly, despite the significant increases in diagnosis of hypertension, there were no differences in the use of thiazide diuretics noted between time periods. Thus, increased thiazide diuretic use is unlikely to be responsible for the increasing incidence of gout in this population, despite previous reports linking them to gout occurrence (30).
There has been a marked increase in the prevalence of cardiovascular comorbidities among patients with gout. In this study, diseases including obesity, hypertension, diabetes mellitus, chronic renal disease and hyperlipidemia have become significantly more common in patients diagnosed with gout in the more recent years 2009–2010 compared to those diagnosed with gout 20 years ago. The change in the prevalence of obesity in patients with gout is particularly noteworthy. Severe, class II and class III obesity have tripled in this cohort over the past 20 years.
The high prevalence of comorbidities among patients with gout has been demonstrated in several previously published cohorts (21, 22, 28, 31). For example, in the 2007–2008 NHANES cohort, Zhu and colleagues found a prevalence of 74% hypertension, 71% had chronic renal disease, 53% obesity, 26% diabetes mellitus and 11% heart failure among gout patients. Except for chronic renal disease, which was less common in this study, these numbers are comparable to the findings in the 2009–2010 cohort reported in the current study.
This study has a number of strengths. The population-based approach and availability of the complete medical records for all patients offers the advantage of a comprehensive assessment of all patients diagnosed with gout in a defined geographic area, thereby minimizing the risk of referral bias. The use of a systematic approach to case identification utilizing multiple criteria sets in both time periods is another strength of the study.
Inherent limitations of the study relate to its retrospective nature. The assessment and documentation of criteria used to diagnose gout may have changed over time. For example, arthrocentesis with crystal analysis was more commonly performed in more recent years. This may reflect a change in practice patterns. Use of all three proposed criteria sets for gout mitigates against imprecision of case ascertainment (11–13), lowering the dependence on single criteria that may have been assessed or documented differently over time. In addition, a sub-analysis was performed using each criteria set independently, with each showing a significant increase in the incidence of gout over time. Since completion of this study new gout classification criteria have been published (32). These criteria incorporate new imaging modalities including musculoskeletal ultrasound and dual-beam computed tomography. As these imaging methods were not used during the 1989–1992 time period, but were widely available during the 2009–2010 period, the 2015 ACR/EULAR classification criteria were not applied to the current study in order to reduce imbalance in diagnostic assessment resulting in ascertainment bias.
Since the classification of comorbidities was based on physician diagnosis as reflected in the medical record, the possibility of changing documentation patterns over time could affect ascertainment. However, this would not apply to diagnoses of obesity, since classification was based on BMI, and weight and height have been documented for every patient in both cohorts. Finally, the population of Olmsted County is mainly white and the socioeconomic characteristics largely resemble those of the U.S. white population in general (33). Caution has to be exercised when generalizing these findings to populations with a different demographic composition especially in other populations with higher prevalence of African-Americans, since higher incidence rates have been previously reported in the latter population compared to white population (34–36).
In summary, our data demonstrates a significant increase in the incidence of gout over the last 20 years. Gout is the most common inflammatory arthritis in the United States and affects more than 3% of the population (1). This rise in incidence is combined with an alarming increase in comorbidities such as hypertension, hyperlipidemia, diabetes mellitus, renal disease, and a tripling of grade II and II obesity among patients with gout. The importance of these comorbidities cannot be overemphasized; previous studies have demonstrated their significant contribution to the excess mortality and morbidity in patients with gout (37).
These findings emphasize the importance of acknowledging gout as a “sentinel” disease which rarely occurs in isolation but points to a likely aggregation of various cardiovascular risk factors and comorbidities. Thus, in most patients, management of the initial gout flare will only represent a minor component of treatment. A comprehensive, multi-specialty approach is needed to reduce the morbidity and mortality of gout and its associated health conditions in these patients.
Acknowledgments
Funding: This work was made possible using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG034676 and CTSA Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum. 2011;63:3136–41. doi: 10.1002/art.30520. [DOI] [PubMed] [Google Scholar]
- 2.Portis AJ, Laliberte M, Tatman P, Moua M, Culhane-Pera K, Maalouf NM, et al. High prevalence of gouty arthritis among the Hmong population in Minnesota. Arthritis Care Res. 2010;62:1386–91. doi: 10.1002/acr.20232. [DOI] [PubMed] [Google Scholar]
- 3.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arromdee E, Michet CJ, Crowson CS, O’Fallon WM, Gabriel SE. Epidemiology of gout: is the incidence rising? J Rheumatol. 2002;29:2403–6. [PubMed] [Google Scholar]
- 5.Wallace KL, Riedel AA, Joseph-Ridge N, Wortmann R. Increasing prevalence of gout and hyperuricemia over 10 years among older adults in a managed care population. J Rheumatol. 2004;31:1582–7. [PubMed] [Google Scholar]
- 6.Smith E, Hoy D, Cross M, Merriman TR, Vos T, Buchbinder R, et al. The global burden of gout: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis. 2014;73:1470–6. doi: 10.1136/annrheumdis-2013-204647. [DOI] [PubMed] [Google Scholar]
- 7.Maynard JW, McAdams DeMarco MA, Baer AN, Köttgen A, Folsom AR, Coresh J, et al. Incident gout in women and association with obesity in the Atherosclerosis Risk in Communities (ARIC) Study. Am J Med. 2012;125:717e9–717.e17. doi: 10.1016/j.amjmed.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhole V, de Vera M, Rahman MM, Krishnan E, Choi H. Epidemiology of gout in women: Fifty-two-year followup of a prospective cohort. Arthritis Rheum. 2010;62:1069–76. doi: 10.1002/art.27338. [DOI] [PubMed] [Google Scholar]
- 9.Singh JA, Reddy SG, Kundukulam J. Risk factors for gout and prevention: a systematic review of the literature. Curr Opin Rheumatol. 2011;23:192–202. doi: 10.1097/BOR.0b013e3283438e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester epidemiology project. Am J Epidemiol. 2011;173:1059–68. doi: 10.1093/aje/kwq482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wallace SL, Robinson H, Masi AT, Decker JL, McCarty DJ, Yü TF. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum. 1977;20:895–900. doi: 10.1002/art.1780200320. [DOI] [PubMed] [Google Scholar]
- 12.Kellgren JH, Jeffrey MR, Ball J. The Epidemiology of Chronic Rheumatism. I. Blackwell Scientific Publications; Oxford: 1963. pp. 295–257. [Google Scholar]
- 13.Decker JL. Report from the subcommittee on diagnostic criteria for gout. Population studies of the rheumatic diseases; New York. Proceedings of the Third International Symposium; 1966. pp. 457–458. [Google Scholar]
- 14.Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:9. [PubMed] [Google Scholar]
- 15.Roddy E, Doherty M. Epidemiology of gout. Arthritis Res Ther. 2010;12:223. doi: 10.1186/ar3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuo CF, Grainge MJ, Zhang W, Doherty M. Global epidemiology of gout: prevalence, incidence and risk factors. Nat Rev Rheumatol. 2015;11:649–62. doi: 10.1038/nrrheum.2015.91. [DOI] [PubMed] [Google Scholar]
- 17.Li R, Sun J, Ren LM, Wang HY, Liu WH, Zhang XW, et al. Epidemiology of eight common rheumatic diseases in China: a large-scale cross-sectional survey in Beijing. Rheumatology. 2012;51:721–9. doi: 10.1093/rheumatology/ker370. [DOI] [PubMed] [Google Scholar]
- 18.Kuo CF, Grainge MJ, See LC, Yu KH, Luo SF, Zhang W, et al. Epidemiology and management of gout in Taiwan: a nationwide population study. Arthritis Res Ther. 2015;17:13. doi: 10.1186/s13075-015-0522-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klemp P, Stansfield SA, Castle B, Robertson MC. Gout is on the increase in New Zealand. Ann Rheum Dis. 1997;56:22–6. doi: 10.1136/ard.56.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Annemans L, Spaepen E, Gaskin M, Bonnemaire M, Malier V, Gilbert T, et al. Gout in the UK and Germany: prevalence, comorbidities and management in general practice 2000–2005. Ann Rheum Dis. 2008;67:960–6. doi: 10.1136/ard.2007.076232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Juraschek SP, Miller ER, Gelber AC. Body mass index, obesity, and prevalent gout in the United States in 1988–1994 and 2007–2010. Arthritis Care Res. 2013;65:127–32. doi: 10.1002/acr.21791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu Y, Pandya BJ, Choi HK. Comorbidities of gout and hyperuricemia in the US general population: NHANES 2007–2008. Am J Med. 2012;125:679–687. e1. doi: 10.1016/j.amjmed.2011.09.033. [DOI] [PubMed] [Google Scholar]
- 23.Choi HK, Atkinson K, Karlson EW, Curhan G. Obesity, weight change, hypertension, diuretic use, and risk of gout in men: the health professionals follow-up study. Arch Intern Med. 2005;165:742–8. doi: 10.1001/archinte.165.7.742. [DOI] [PubMed] [Google Scholar]
- 24.Choi HK, Ford ES, Li C, Curhan G. Prevalence of the metabolic syndrome in patients with gout: the Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2007;57:109–15. doi: 10.1002/art.22466. [DOI] [PubMed] [Google Scholar]
- 25.Caspi D, Lubart E, Graff E, Habot B, Yaron M, Segal R. The effect of mini-dose aspirin on renal function and uric acid handling in elderly patients. Arthritis Rheum. 2000;43:103–8. doi: 10.1002/1529-0131(200001)43:1<103::AID-ANR13>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 26.Ajani UA, Ford ES, Greenland KJ, Giles WH, Mokdad AH. Aspirin Use Among U.S. Adults Am J Prev Med. 2006;30:74–7. doi: 10.1016/j.amepre.2005.08.042. [DOI] [PubMed] [Google Scholar]
- 27.Gu Q, Burt VL, Dillon CF, Yoon S. Trends in Antihypertensive Medication Use and Blood Pressure Control Among United States Adults With Hypertension Clinical Perspective. Circulation. 2012;126:2105–14. doi: 10.1161/CIRCULATIONAHA.112.096156. [DOI] [PubMed] [Google Scholar]
- 28.Kuo CF, Grainge MJ, Mallen C, Zhang W, Doherty M. Comorbidities in patients with gout prior to and following diagnosis: case-control study. Ann Rheum Dis. 2016;75:210–7. doi: 10.1136/annrheumdis-2014-206410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuzuya M, Ando F, Iguchi A, Shimokata H. Effect of Aging on Serum Uric Acid Levels: Longitudinal Changes in a Large Japanese Population Group. J Gerontol A Biol Sci Med Sci. 2002;57:M660–4. doi: 10.1093/gerona/57.10.m660. [DOI] [PubMed] [Google Scholar]
- 30.McAdams DeMarco MA, Maynard JW, Baer AN, Gelber AC, Young JH, Alonso A, et al. Diuretic use, increased serum urate levels, and risk of incident gout in a population-based study of adults with hypertension: The Atherosclerosis Risk in Communities cohort study. Arthritis Rheum. 2012;64:121–9. doi: 10.1002/art.33315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richette P, Clerson P, Périssin L, Flipo RM, Bardin T. Revisiting comorbidities in gout: a cluster analysis. Ann Rheum Dis. 2015;74:142–7. doi: 10.1136/annrheumdis-2013-203779. [DOI] [PubMed] [Google Scholar]
- 32.Neogi T, Jansen TL, Dalbeth N, Fransen J, Schumacher HR, Berendsen D, et al. 2015 Gout classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis. 2015;74:1789–98. doi: 10.1136/annrheumdis-2015-208237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ, Rocca WA. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc. 2012;87:151–60. doi: 10.1016/j.mayocp.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh JA. Racial and gender disparities among patients with gout. Curr Rheumatol Rep. 2013;15:307. doi: 10.1007/s11926-012-0307-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maynard JW, McAdams-DeMarco MA, Law A, Kao L, Gelber AC, Coresh J, et al. Racial differences in gout incidence in a population-based cohort: Atherosclerosis Risk in Communities Study. Am J Epidemiol. 2014;179:576–83. doi: 10.1093/aje/kwt299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hochberg MC, Thomas J, Thomas DJ, Mead L, Levine DM, Klag MJ. Racial differences in the incidence of gout. The role of hypertension. Arthritis Rheum. 1995;38:628–32. doi: 10.1002/art.1780380508. [DOI] [PubMed] [Google Scholar]
- 37.Krishnan E, Svendsen K, Neaton JD, Grandits G, Kuller LH MRFIT Research Group. Long-term cardiovascular mortality among middle-aged men with gout. Arch Intern Med. 2008;168:1104–10. doi: 10.1001/archinte.168.10.1104. [DOI] [PubMed] [Google Scholar]