Abstract
Background
Fetal ethanol (ETOH) exposure can damage the developing central nervous system and lead to cognitive and behavioral deficits, known as fetal alcohol spectrum disorders (FASD). ETOH exposure to mouse pups during early neonatal development was used as a model of ETOH exposure that overlaps the human third trimester “brain growth spurt” - a model that has been widely used to study FASD in rats.
Methods
C57BL/6 male and female mice were exposed to ETOH (4 g/kg/day) on postnatal days (PD) 4–10 by oral intubation. Intubated and non-treated controls were also included. Behavioral testing of the offspring, including open field, elevated plus maze and Morris water maze, was performed on PD 20 – 45.
Results
ETOH exposure during PD 4–10 resulted in hyperactivity and deficits in learning and memory in young mice with no apparent sex differences.
Conclusion
Based on these data, this neonatal intubation mouse model may be useful for future mechanistic and genetic studies of FASD and for screening of novel therapeutic agents.
Keywords: fetal, ethanol, behavior, development
Introduction
Ethanol (ETOH) consumption during pregnancy can result in damage to the developing central nervous system and lead to a variety of cognitive and behavioral deficits including hyperactivity, deficits in inhibitory control, learning, attention and executive functions collectively known as the fetal alcohol spectrum disorders (FASD) (Jacobson, 1998, Riley and McGee, 2005). Many mechanisms have been proposed to help explain the consequences of ETOH’s effects on the developing brain, but there are still many unanswered questions.
Numerous animal models have been used to study FASD. While many studies have used rat models, mouse models have become increasingly useful due to the extensive knowledge of mouse genetics for studies on the underlying mechanisms and etiology of a variety of neurological conditions. Mouse studies using prenatal ETOH exposure to model human 1st and 2nd trimester of CNS development have shown certain behavioral and neuroanatomical changes reported in rats including hyperactivity, learning deficits as well as volume reductions in a variety of brain regions. Voluntary drinking throughout gestation has produced a variety of behavioral impairments and deficits in tasks that tap into hippocampal function or sensorimotor integration with mixed data on activity (Allan et al., 2003; Becker et al., 1993; Brady et al., 2012; Downing et al., 2009; Fish et al., 2016; Sanchez Vega et al., 2013,). While fewer studies have assessed neonatal ETOH exposure on behavioral outcome in mice, Olney and colleagues have shown that a single postnatal ETOH injection can induce neuronal apoptosis (Ikonomidou et al., 2000) and may produce spatial deficits (Wozniak et al., 2004), although there are some inconsistencies in the literature (Lee et al., 2016, Houle et al., 2017). In addition, multiple injections of ETOH has been shown to alter performance on hippocampal and cerebellar tasks (Bearer et al., 2015, Wagner et al., 2014), although again hyperactivity is not uniformly reported (Downing et al., 2009).
The aim of the current study was to examine the effects of neonatal ETOH exposure in C57BL/6 mice using oral intubations as a model of ‘3rd trimester’ ETOH exposure; a period that overlaps the human “brain growth spurt” (Dobbing and Sands, 1979). The validity of this model was assessed by examining a variety of behaviors previously shown to be sensitive to prenatal ETOH exposure in rat and human studies. ETOH was delivered to mouse pups on PD 4–10 by intubation. These offspring were then tested on PD 20–21 for activity in an open field, on PD 25–26 for exploration on an elevated plus maze and on PD 35–45 for spatial learning and memory in a Morris water maze. This neonatal intubation model has been used to show evidence of ETOH induced increases in neuroinflammatory response in hippocampus, cerebellum and cerebral cortex (Drew et al., 2015), and Bearer et al (2015) has shown that a single intubation on PD 5 can alter balance. But to the best of our knowledge, neonatal ETOH exposure on PD 4–10 via intubation in mice has not been used as a model to assess postnatal behavioral outcome.
Materials and Methods
Parent animals
Adult C57BL/6 mice were obtained from Harlan Labs (Indianapolis, IN). Offspring were generated in the University of Kentucky Medical Center’s breeding colony. Male and female pups, representing at least 8 litters from each treatment group, were used in this study. Mice were maintained on a 14:10 h light-dark cycle (lights on at 07:00 h, off at 21:00 h). All procedures were approved by the Animal Care and Use Committee of the University of Kentucky.
Breeding
Adult female and male C57BL/6 mice were caged in a ratio of 2:1 and seminal plugs were examined the next morning as evidence that copulation occurred. If a seminal plug was detected, it was designated gestational day (GD) 0 and the pregnant female was singly housed.
Ethanol administration
ETOH was administered at 09:00 h to mouse pups on postnatal day (PD) 4–10 (as a “3rd trimester exposure” model) (Kelly et al., 1988). The day of birth was designated PD 0. On PD 4, litters were weighed and pseudo randomly culled to 6 pups maintaining 3 males and 3 females when possible. These litters were then assigned to one of three treatment groups: ETOH, intubated control (Maltose) or non-intubated control (NTC). Pups in the ETOH group received 4 g/kg/day of ETOH on PD 4–10, delivered via oral intubation (0.02 ml/g bodyweight) in an artificial milk solution developed to nutritionally mimic rodent milk (Kelly and Lawrence, 2008). This dose of ETOH was chosen because it has been show to produce significant neurotoxicity during the third trimester equivalent and may lead to neurobehavioral deficits (Dursun et al., 2013). Pups in the intubated control group received isocaloric maltose (in the same milk solution) on PD 4–10. Pups were weighed daily and returned to their dam immediately after intubations. Mortality from intubation was low (~ 5%).
Blood ethanol concentration (BEC) measurement
For BEC of the pups, separate groups of pups were treated and trunk blood of pups was collected on PD 7 following decapitation 30, 60, 90, 120, 180, 240, 360 and 480 min after ETOH intubation. BECs were determined using an assay kit from Sigma-Aldrich, St. Louis, MO (product number: MAK076). The data were collected from multiple samples (n=4).
Behavioral testing
Offspring were tested for activity in an open field for 2 days (between 08:00 – 12:00 h) on PD 20–22 (Mei et al., 2016), and for activity, and exploration in an elevated plus maze at similar times in the morning on PD 25–27 (Mei et al., 2016). Offspring were then weaned on PD 28, housed with 2–3 same sex littermates and allowed to acclimate for one week prior to testing in the Morris water maze. Spatial learning and memory in a Morris water maze testing was conducted between 12:00– 16:00 h on PD 35–45 (Chen et al., 2013). These tasks and ages were selected to assess the presence of behavioral impairments in both preweaning and adolescence, as have been reported in clinical populations with FASD (Mattson et al., 2011). All behavioral testing was conducted under low ambient light conditions with white noise to reduce extraneous auditory stimuli. Surfaces and holding cages were cleaned before and after testing with Nature’s Miracle© enzymatic cleaning solution to remove animal odors. When males and females were tested on the same day, the males were tested prior to females. Animal movements were recorded using the AnyMaze tracking system (Stoelting Co.).
Open Field (OF)
The OF is commonly used to measure levels of activity, habituation to novel environments and patterns of exploration which may also indicate impulse control or anxiety (Bailey and Crawley, 2009). Each mouse was removed from its home cage and brought into the test room in a clean holding cage for a 10-min habituation period. The OF was a round chamber (diameter 39.4 cm) with opaque white walls and floor. Subjects were tested 30 min daily for two consecutive days. The dependent measures included total distance travelled and distance travelled in the center. The center was defined as a circular zone in the center of the OF with a diameter half of the width of the OF. Measurements related to activity in the center are often used as a measure of anxiety and/or inhibitory control since mice typically display thigmotaxis and avoid the center of open arenas. Additional analyses were run on center exploration when controlling for total distance travelled to produce a preference score.
Elevated Plus Maze (EPM)
The EPM is primarily used as a test for anxiolytic agents although it can also be used to measure exploration (Bailey and Crawley, 2009). The EPM apparatus consisted of a plus-shaped (+) Plexiglas maze with clear walls bordering two of the four arms (30×6 cm). The other two arms had a floor but no walls and were referred to as the open arms. The mouse was placed halfway down one open arm of the maze, facing away from the center, and was then allowed to explore the maze for 5 min. Subjects were tested in a single session between PD 25–27. The dependent measures included overall distance travelled, distance travelled in the open arms and number of entries into the open arms. Additional analyses were conducted looking at open arm exploration controlling for total distance travelled and open arm entries.
Morris Water Maze (MWM)
MWM is used to measure spatial learning and retention (Vorhees and Williams, 2006). The MWM consisted of a round plastic tub (diameter 107.6 cm) filled with water (22–23 °C) made opaque using white non-toxic water-based paint. A platform (15.2 × 15.2 cm) was submerged 0.75cm under the surface of the water in a fixed location. Four visible extra-maze cues were placed at various points around the maze. Each mouse was placed in one of four starting positions on the far side of the pool (120, 150, 210 and 240° from the platform) and allowed to swim until either they found the submerged platform or they reached a ceiling of 60 sec. If they did not find the platform, they were gently guided to the platform. Subjects remained on the platform for 5 sec before being removed for a 5 min intertrial interval (ITI). During maze acquisition, four trials were completed each day for four days. On the fifth day, the platform was removed for a single probe trial at 08:00 h. The mouse was placed in the opposite quadrant and the swim pattern was recorded for 60 sec. Four hours after the probe trial, subjects were tested for reversal learning which consisted of four trials with the platform replaced one quadrant away from its original location. Following reversal learning (ITI 5 min), a single visible platform trial was conducted where the platform’s location was indicated by a visible rod above the surface of the water. The visible platform component was included to ensure that there were no visual or motor deficits potentially contributing to performance on this task following developmental ETOH exposure. MWM testing was conducted over 5 days between PD 35–45.
The dependent measures during acquisition included latency to reach the platform and distance travelled for each trial. For the probe trial, annulas crossings (the number of crossings in the maze location where the platform was placed during acquisition training) and the distance traveled in this region were recorded. For the reversal phase, the dependent measures included latency to reach and distance travelled to the new platform location. Similar measures were also recorded on the visible platform trial.
Statistical analysis
Data were analyzed using SPSS software version 21 (IBM). To avoid potential litter effects, behavioral data from same-sex siblings were averaged together to produce a single data point per sex, per treatment, per litter for each measure (Abbey and Howard, 1973, Wainwright, 1998). Thus, each data point represented the mean of 2–3 same sex siblings. For each analysis, univariate or mixed-factors analysis of variance (ANOVA) was performed with treatment and sex as grouping factors and repeated measures when warranted. Significant interactions were broken down by simple main effect and/or post hoc Tukey test. The Greenhouse-Geisser correction was used to correct for violations in homogeneity of variance when necessary. In some cases, this correction resulted in degrees of freedom that were not whole numbers. If there was no main effect or interaction with sex or across multiple time points (e.g. days, time bins, etc.), the data were collapsed across this variable for ease of presentation.
Results
BEC data and body weight
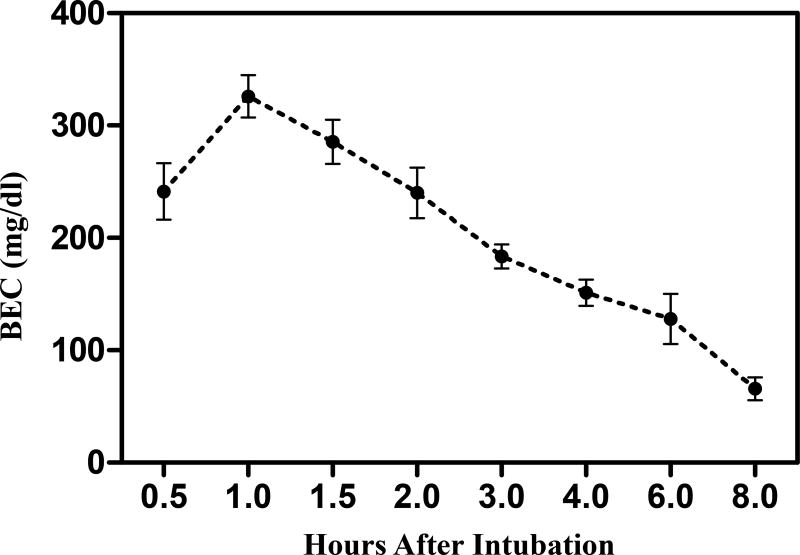
BEC of the separate group of pups was determined on PD 7 (Fig. 1). BEC curves show a peak at 325.9 ± 18.8 mg/dl approximately 1 h after intubation for the pups with ETOH exposure.
Figure 1. BEC of ETOH treated pups.
BECs were measured at 0.5, 1, 1.5, 2, 3, 4, 6 and 8 hours post ethanol intubation (4 g/kg) on PD 7. BECs peaked at approximately 1 h post intubation. Data are expressed as the mean ± S.E.M (N=4).
Offspring body weights on PD 4–10 are presented in Table 1. A mixed factors ANOVA demonstrated a significant interaction of treatment and age on weight, F (3.06, 32.17) =10.84, p < 0.05. Post hoc tests indicated no significant difference in body weights across groups on PD 4 - 9. ETOH exposure significantly decreased body weight when compared with NTC only on PD 10.
Table 1. Offspring body weight during postnatal treatment and behavioral testing.
Offspring body weight (in g) on PD 4–10 (mean ± S.E.M.) and prior to behavioral testing in the OF (PD 20), the EPM (PD 24) and the MWM (PD 35).
| Postnatal Days (PD) |
NTC | Maltose Control | ETOH | |||
|---|---|---|---|---|---|---|
| 4 | 2.57 ± 0.22 | 2.47 ± 0.23 | 2.48 ± 0.19 | |||
| 5 | 3.04 ± 0.25 | 2.93 ± 0.28 | 2.86 ± 0.20 | |||
| 6 | 3.49 ± 0.23 | 3.35 ± 0.30 | 3.20 ± 0.25 | |||
| 7 | 3.95 ± 0.21 | 3.79 ± 0.29 | 3.65 ± 0.22 | |||
| 8 | 4.39 ± 0.22 | 4.20 ± 0.35 | 4.02 ± 0.28 | |||
| 9 | 4.85 ± 0.22 | 4.64 ± 0.42 | 4.46 ± 0.32 | |||
| 10 | 5.30 ± 0.22 | 5.08 ± 0.45 | 4.76 ± 0.32© | |||
| M | F | M | F | M | F | |
| 20 | 9.52 ± 0.36 | 9.46 ± 0.56 | 10.57 ± 0.26 | 10.13 ± 0.24 | 8.23 ± 0.50 # | 7.98 ± 0.34* |
| 24 | 14.11 ± 1.10 | 12.58 ± 0.85 | 11.49 ± 0.61 | 11.28 ± 0.77 | 10.66 ± 0.77 | 10.83 ± 0.74 |
| 35 | 19.46 ± 0.42 | 16.60± 0.45 | 22.01 ± 0.48© | 18.18 ± 0.29 | 18.15 ± 0.72# | 15.79 ± 0.62 |
indicates a significant difference from the same sex non-treated controls (NTC) within each age. P’s < 0.05.
indicates a significant difference from maltose-treated controls. P’s < 0.05.
indicates a significant difference from both controls. P’s < 0.05 (N=8).
ETOH exposure also reduced body weight at the time of OF (PD 20), EPM (PD 24), and MWM (PD 35) testing (Table 1). There were significant interactions between treatment and age, F (4, 54) = 3.69, p < 0.05, and sex and age, F (2, 54) = 4.86, p < 0.05. Therefore, post hoc tests were conducted for males and females separately. For males, ETOH treatment resulted in lower body weights on PD 20 and PD 35, but not on PD 24, relative to maltose-treated controls (p < 0.05). Ethanol-treatment did not result in significant differences in body weight compared to NTC. Additionally, maltose treated offspring weighed more than NTC on PD 35, immediately prior to testing in MWM, but did not differ from NTC before OF (PD 20) or EPM (PD 24) testing. Among females, ETOH treatment resulted in a reduction in body weight on PD 20 relative to both NTC and maltose controls with no body weight differences on PD 24 or PD 35.
Behavioral testing
Open field
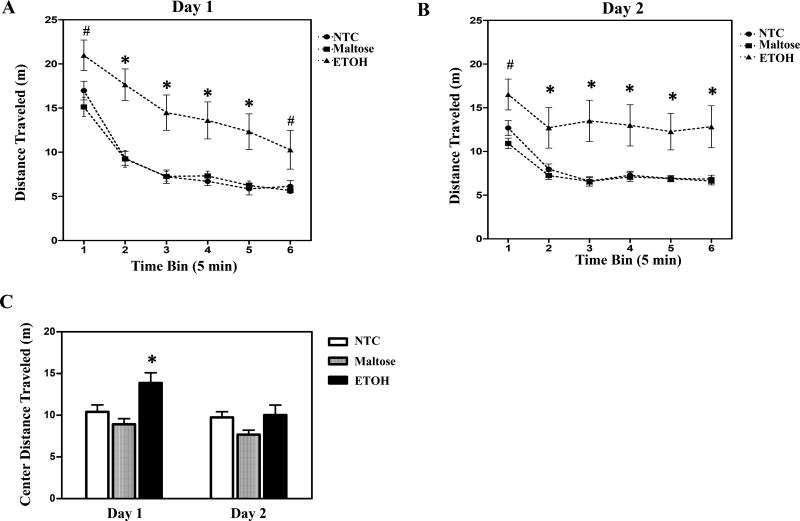
Neonatal ETOH exposure increased activity relative to controls in the OF (Fig. 2). These data are shown collapsed across sex due to the lack of interaction with treatment or a main effect of sex. The repeated measures ANOVA of distance travelled revealed significant main effects of treatment, F (2, 43) = 11.99, p < 0.05 and 5 min block, F (2.67, 114.7) = 79.16, p < 0.05, with significant interactions of day by block, F (3.89, 167.2) = 22.66, p < 0.05. Post hoc tests showed that on day 1 (Fig. 2A) offspring with ETOH exposure traveled more distance across all time blocks relative to maltose-treated controls (p < 0.05), and from bin 2 to 5 compared to NTC. On day 2 (Fig. 2B), a similar pattern was observed. ETOH treated offspring were more active (i.e. traveled more distance) across all time blocks than maltose-treated controls, while they were hyperactive from bin 2 to 6 relative to non-treated controls (p < 0.05).
Figure 2. Open field test.
Neonatal ETOH exposure resulted in hyperactivity on both day1 (A) and day 2 (B). Data is presented, collapsed across sex, as distance travelled (mean ± S.E.M.). ETOH exposed offspring also traveled a greater distance in the center relative to controls on the first day of testing (C). Data is presented, collapsed across sex and the 30-min test session for each day (mean + S.E.M.). *indicates a significant difference from both NTC and maltose-treated controls. (#) indicates a significant difference from maltose-treated animals. N’s = 8 – 9 litters per treatment group. p’s < 0.05.
Additional analyses assessed the effects of ETOH on center exploration and preference, respectively, measured as the total distance travelled in the center zone and adjusted for the total amount of activity [(distance traveled in the center zone/total distance travelled)×100]. As expected, control mice avoided the center zone, with only 19–20% of exploration typically occurring in this zone. ETOH treatment was associated with increased distance traveled in the center zone (Fig. 2C), although this did not reflect a greater preference for open spaces than controls but more reflected the hyperactivity the ETOH exposed animals were displaying. The ANOVA on distance traveled in the center revealed a main effect of treatment, F (2, 43) = 7.99, p < 0.05, sex, F (1, 43) = 5.33, p < 0.05, day, F (1, 43) = 11.02, p < 0.05, and a day by 5 min block interaction, F (5, 215) = 16.96, p < 0.05. Male mice explored greater distance in the center than the females, but there was no sex by treatment interaction. Post hoc tests showed that offspring with ETOH exposure explored significantly more distance in the center than NTC or maltose-treated controls (p < 0.05) on day 1, but not on day 2 (p > 0.05). When adjusting these scores for the total level of activity to create a preference score, a different pattern was apparent. There were no significant differences across groups in center preference on either day 1 or day 2 (data not shown).
Elevated plus maze
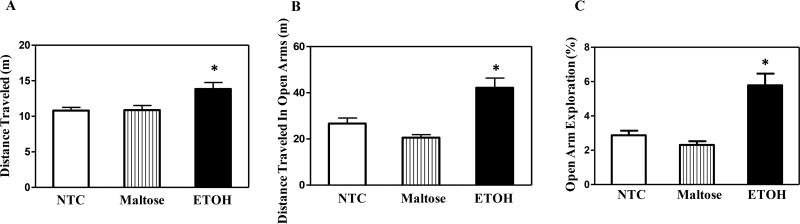
Neonatal ETOH exposure also produced hyperactivity in the EPM relative to both the NTC and the maltose-treated controls (Fig. 3A). The univariate ANOVA of distance travelled revealed a significant main effect of treatment, F (2, 43) = 5.92, p < 0.05, with post hoc tests confirming these findings.
Figure 3. Elevated plus maze test.
ETOH exposed offspring were more active in the EPM (A), traveled more distance (B) and spent a greater percentage of their time in the open arms (C) relative to controls. The data is presented, collapsed across sex, and the 30-min test session. * indicates a significant difference from both controls. N = 8 litters per treatment group. p’s < 0.05.
The ETOH exposed offspring also showed increased exploration of open arms in the EPM (Fig. 3B) and preference for the open arms of the maze (Fig. 3C). Univariate ANOVA for distance travelled in the open arms showed a main effect of treatment, F (2, 43) = 19.19, p < 0.05, with post hoc tests showing that ETOH treatment increased the distance travelled in the open arms relative to both controls (p < 0.05). The univariate ANOVA of preference scores also yielded a significant main effect of treatment, F (2, 43) = 15.40, p < 0.05. As expected, control mice avoided the open arms, with 27% (NTC) of exploration typically occurring in these areas of the maze. ETOH treatment increased open arm preference relative to both controls (p < 0.05). This effect consisted of a ~15% increase in open arm preference with ~42% of all exploration occurring in the open arms.
Morris water maze
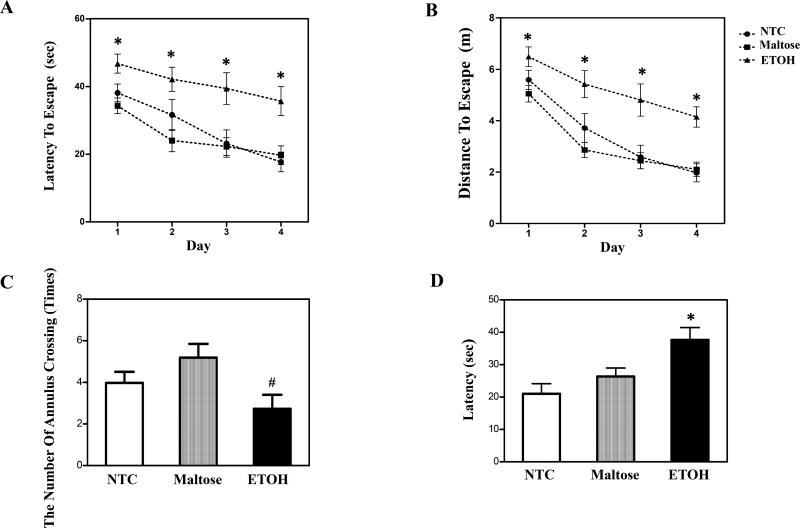
Both latency (Fig. 4A) and the distance (Fig. 4B) traveled to reach the platform were impaired following neonatal ETOH exposure. A subset of mice failed to learn to swim to the platform during acquisition, often floating rather than searching for the platform. Failure to acquire the task was defined as the failure to reach the platform on all 4 trials on acquisition day 4. Neonatal treatment did not significantly influence the number of mice that failed to meet criterion. Adjusted sample sizes for MWM analyses with the removal of subjects unable to acquire the task still represented data from 8–9 litters of mice. The mixed-factors ANOVA of escape latency revealed a significant main effect of treatment, F (2, 41) = 11.30, p < 0.05, and day, F (3, 123) = 17.47, p < 0.05. Post hoc tests confirmed that the ETOH exposed group took longer to find the platform than controls (p < 0.05). The mixed-factors ANOVA of distance travelled to the platform revealed a significant main effect of day, F (3, 123) = 34.82, p < 0.05, and treatment, F (2, 41) = 18.73, p < 0.05. Post hoc tests confirmed that offspring with ETOH treatment traveled greater distance to find the platform (p < 0.05) relative to both controls.
Figure 4. Performance in the Morris Water Maze.
Acquisition of the spatial task across the 4 test days was impaired by neonatal ETOH exposure, shown as increased escape latency (A) and distance (B). The data is collapsed across sex and 4 daily acquisition trials (mean ± S.E.M). The number of annulus crossings was measured in the probe trial (C). Reversal learning of MWM was tested by shifting the platform to a novel location and the latency was measured (D). * indicates a significant difference from both controls. (#) indicates a significant difference from maltose-treated animals. (N=8–9) refers to the number of litters represented per treatment group. p’s < 0.05.
For the probe trial, there was also a significant main effect of treatment, F (2, 41) = 3.66, p < 0.05. Post-hoc analyses revealed a reduction in the number of annulus crossings in the ETOH exposed offspring compared to maltose controls (p < 0.05), but not NTC (p > 0.05) (Fig. 4C). There were no significant treatment effects on the distance explored in the target quadrant.
Performance on the reversal component of the MWM is shown in Fig. 4D. There was a main effect of treatment, F (2, 41) = 6.47, p < 0.05, and day, F (3, 123) = 14.79, p < 0.05 with the ETOH exposed offspring taking longer to reach the platform than the controls. There were no significant interactions or main effects on the distance travelled to reach the platform.
All treatment groups performed similarly on the visual platform component of the MWM (data not shown) indicating that any changes were not due to impairments in vision or swimming.
Discussion
This study was designed to investigate the effects of neonatal ETOH exposure on activity in an OF, the EPM and spatial learning and retention in a MWM in male and female C57BL/6 mice. Our results showed that neonatal ETOH exposure resulted in hyperactivity and deficits in learning and memory in young mice with no sex differences observed. These results are similar to those in clinical studies and so provide strong face validity for this mouse model of FASD. The effects observed in the current study are also very similar to those that have been reported in a variety of rat models for fetal ETOH effects, including those based on gestational exposure alone (Carneiro et al., 2005, Hofmann et al., 2005), postnatal exposure alone (Thomas et al., 2007, Lewis et al., 2012), and combined pre and postnatal exposure (Cronise et al., 2001, Brocardo et al., 2012), and so provide strong support for this mouse model.
Hyperactivity appeared to be a consistent finding in two different paradigms (OF and EPM) and at two different ages in the current study. This suggests that this effect generalizes across different paradigms at least in the young mouse. Hyperactivity is one of the more frequently observed behavioral characteristics in both rodent models and in clinical populations with FASD and represents a key focus of drug development efforts for FASD (Koren, 2015). It should be noted that Downing and colleagues administered ETOH in utero to C57/BL6J mice and did not observe any evidence of hyperactivity (Downing et al., 2009). While the dose used by Downing et al was slightly lower than in the current study (3 g/kg/day vs 4 g/kg/day), the peak BEC’s were fairly similar. This may provide evidence that the “3rd trimester” exposure model is more sensitive to the behavioral teratogenic effects of ETOH on activity in this C57 mouse model. Additional research would be needed to support this hypothesis.
In addition to activity, both the OF and EPM tasks used in the current study included a component typically used to assess anxiety: the open arms of the EPM and the center region of the OF. Mice generally avoid these open areas and control mice displayed this species-typical behavior. ETOH exposure increased exploration in these open areas, although interpretation was complicated by the fact that these offspring were hyperactive. To better understand this pattern, activity in the open areas was assessed relative to total activity for each subject and this lead to different patterns on the two tasks. For the OF, the ETOH exposed mice were equally hyperactive in the periphery and in the center and so the increased exploration in the center was probably best explained as hyperactivity. In contrast, EPM exploration showed open arm preference approaching 50% in the ETOH exposed offspring suggesting no real preference or avoidance for the open arms. These EPM findings are consistent with previous studies using young rats with early ETOH exposure (Carneiro et al., 2005).
The disparity in ETOH effects on open area exploration in the OF and EPM is interesting. Although these open areas are both aversive, previous studies suggest that the EPM may be more sensitive to anxiolytic than the OF (Schmitt and Hiemke, 1998, Acevedo et al., 2014), possibly due to the unique combination of open spaces and raised elevation in the EPM (Schmitt and Hiemke, 1998). It should also be noted that there is controversy about the use of many of the standard tests for anxiety, including the OF and EPM and so any conclusions regarding changes in anxiety as a result of the current findings would need additional examination (see Ennaceur, 2014).
ETOH exposure impaired acquisition of the MWM spatial task. Offspring with ETOH exposure had longer swim paths and greater latencies to reach the platform during acquisition of the task. These differences were not observed on the visible platform trial, so the differences in performance displayed by the ETOH exposed offspring relative to the controls could not be due to swimming deficits. Treatment effects were not evident on the first trial, but once evident, were consistent across acquisition and during reversal learning. While the results from the reversal phase might suggest that the ETOH exposed offspring had difficulty extinguishing their originally learned response (to find the platform), this is confounded by the fact that they had acquisition deficits (Vorhees and Williams, 2006). It is also complicated since there were no ETOH effects on the probe trial relative to non-treated controls. One potential interpretation could be that the ETOH exposed offspring took longer but eventually they were able to acquire the task. These findings are consistent with previous 3rd trimester ETOH exposure rodent models reporting deficits in MWM acquisition (Goodlett and Peterson, 1995, Banuelos et al., 2012, Wagner et al., 2014). Deficits in memory and spatial abilities are also commonly reported among clinical populations with fetal ETOH exposure (Doyle and Mattson, 2015) and also represent key targets for intervention.
There were no observable sex differences in outcome as a function of neonatal ETOH exposure. Previous mouse studies with relatively brief, 1 or 3 days, postnatal ETOH exposure paradigms have similarly shown little or no sex differences with one exception after a 2-day binge that was limited to the probe trial (Wagner et al., 2014). It should be noted that all testing was done in young offspring (ages ranging from PD 20 – 45) and it is possible that sex differences could emerge at a later age or in different paradigms. While performance in the MWM was conducted in females who may have entered puberty, estrus cycles were not recorded. This is not a significant concern, in part, because a recent meta-analysis by Becker et al., has provided convincing evidence that estrus cycle has little (or no) effect on variability in numerous behavioral, neuroanatomical, electrophysiological endpoints (Becker et al 2016).
In addition to the overlap in behavioral characteristics displayed by these offspring to rat and clinical studies, one advantage of this mouse intubation model over injection is that it is more clinically relevant route of administration. Moreover, intubation allows for a more consistent and better controlled BEC level in the pups. It should be noted that a relatively high BEC was used in the current study as previous studies have emphasized the importance of peak BECs in ETOH’s behavioral teratogenicity (Kelly et al., 1987) and that higher BECs are typically required for persistent behavioral effects (Allan et al., 2003). Even considering the faster metabolic rate in rodents such as mice and the difference in sensitivity to these high doses, similarly high BEC levels have been reported in human populations (Urso et al., 1981, Jones and Harding, 2013) and other rodent models (Goodlett et al., 1990).
The underlying mechanisms for these behavioral deficits require further study. Normal developmental signaling in neurons (Luo, 2009) and glial cells (Costa et al., 2004) has been shown to be disrupted by neonatal ethanol exposure in a variety of CNS regions including prefrontal cortex, corpus callosum, hippocampus and cerebellum, which control the functions of activity, learning and memory (Alfonso-Loeches and Guerri, 2011). These changes may contribute to the effects observed and further work with this model can help answer these questions. This model may be a useful addition to better understand the molecular, cellular and genetic mechanisms underlying ethanol-induced behavioral deficits in FASD.
Acknowledgments
We thank Jonathan Handshoe, Liz Mirsky, Becky Mirsky, Christina Kallik, and Alyssa Elswick for their assistance in this study.
Funding information: This work was supported by NIH grant AA015407 to JL and AA020051 to GC.
Footnotes
Conflict of Interest: The authors have no conflict of financial or commercial interest to be declared.
References
- Abbey H, Howard E. Statistical procedure in developmental studies on species with multiple offspring. Dev Psychobiol. 1973;6:329–335. doi: 10.1002/dev.420060406. [DOI] [PubMed] [Google Scholar]
- Acevedo MB, Nizhnikov ME, Molina JC, Pautassi RM. Relationship between ethanol-induced activity and anxiolysis in the open field, elevated plus maze, light-dark box, and ethanol intake in adolescent rats. Behav Brain Res. 2014;265:203–215. doi: 10.1016/j.bbr.2014.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfonso-Loeches S, Guerri C. Molecular and behavioral aspects of the actions of alcohol on the adult and developing brain. Crit Rev Clin Lab Sci. 2011;48:19–47. doi: 10.3109/10408363.2011.580567. [DOI] [PubMed] [Google Scholar]
- Allan AM, Chynoweth J, Tyler LA, Caldwell KK. A mouse model of prenatal ethanol exposure using a voluntary drinking paradigm. Alcohol Clin Exp Res. 2003;27:2009–2016. doi: 10.1097/01.ALC.0000100940.95053.72. [DOI] [PubMed] [Google Scholar]
- Bailey KR, Crawley JN. Anxiety-Related Behaviors in Mice, in Methods of Behavior Analysis in Neuroscience, Methods of Behavior Analysis in Neuroscience (BUCCAFUSCO JJ ed. Boca Raton (FL): 2009. [Google Scholar]
- Banuelos C, Gilbert RJ, Montgomery KS, Fincher AS, Wang H, Frye GD, Setlow B, Bizon JL. Altered spatial learning and delay discounting in a rat model of human third trimester binge ethanol exposure. Behav Pharmacol. 2012;23:54–65. doi: 10.1097/FBP.0b013e32834eb07d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari A, Robbins TW. Inhibition and impulsivity: behavioral and neural basis of response control. Prog Neurobiol. 2013;108:44–79. doi: 10.1016/j.pneurobio.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Bearer CF, Wellmann KA, Tang N, He M, Mooney SM. Choline Ameliorates Deficits in Balance Caused by Acute Neonatal Ethanol Exposure. Cerebellum. 2015;14:413–420. doi: 10.1007/s12311-015-0691-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocardo PS, Boehme F, Patten A, Cox A, Gil-Mohapel J, Christie BR. Anxiety- and depression-like behaviors are accompanied by an increase in oxidative stress in a rat model of fetal alcohol spectrum disorders: Protective effects of voluntary physical exercise. Neuropharmacology. 2012;62:1607–1618. doi: 10.1016/j.neuropharm.2011.10.006. [DOI] [PubMed] [Google Scholar]
- Carneiro LM, Diogenes JP, Vasconcelos SM, Aragao GF, Noronha EC, Gomes PB, Viana GS. Behavioral and neurochemical effects on rat offspring after prenatal exposure to ethanol. Neurotoxicol Teratol. 2005;27:585–592. doi: 10.1016/j.ntt.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Carola V, D'Olimpio F, Brunamonti E, Mangia F, Renzi P. Evaluation of the elevated plus-maze and open-field tests for the assessment of anxiety-related behaviour in inbred mice. Behav Brain Res. 2002;134:49–57. doi: 10.1016/s0166-4328(01)00452-1. [DOI] [PubMed] [Google Scholar]
- Chen CY, Noble-Haeusslein LJ, Ferriero D, Semple BD. Traumatic injury to the immature frontal lobe: a new murine model of long-term motor impairment in the absence of psychosocial or cognitive deficits. Dev Neurosci. 2013;35:474–490. doi: 10.1159/000355874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LG, Vitalone A, Guizzetti M. Signal transduction mechanisms involved in the antiproliferative effects of ethanol in glial cells. Toxicol Lett. 2004;149:67–73. doi: 10.1016/j.toxlet.2003.12.020. [DOI] [PubMed] [Google Scholar]
- Cronise K, Marino MD, Tran TD, Kelly SJ. Critical periods for the effects of alcohol exposure on learning in rats. Behav Neurosci. 2001;115:138–145. doi: 10.1037/0735-7044.115.1.138. [DOI] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Downing C, Balderrama-Durbin C, Broncucia H, Gilliam D, Johnson TE. Ethanol teratogenesis in five inbred strains of mice. Alcohol Clin Exp Res. 2009;33:1238–1245. doi: 10.1111/j.1530-0277.2009.00949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle LR, Mattson SN. Neurobehavioral Disorder Associated with Prenatal Alcohol Exposure (ND-PAE): Review of Evidence and Guidelines for Assessment. Curr Dev Disord Rep. 2015;2:175–186. doi: 10.1007/s40474-015-0054-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dursun I, Jakubowska-Dogru E, Elibol-Can B, van der List D, Chapman B, Qi L, Berman RF. Effects of early postnatal alcohol exposure on the developing retinogeniculate projections in C57BL/6 mice. Alcohol. 2013;47:173–179. doi: 10.1016/j.alcohol.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodlett CR, Marcussen BL, West JR. A single day of alcohol exposure during the brain growth spurt induces brain weight restriction and cerebellar Purkinje cell loss. Alcohol. 1990;7:107–114. doi: 10.1016/0741-8329(90)90070-s. [DOI] [PubMed] [Google Scholar]
- Goodlett CR, Peterson SD. Sex differences in vulnerability to developmental spatial learning deficits induced by limited binge alcohol exposure in neonatal rats. Neurobiol Learn Mem. 1995;64:265–275. doi: 10.1006/nlme.1995.0009. [DOI] [PubMed] [Google Scholar]
- Green ML, Singh AV, Zhang Y, Nemeth KA, Sulik KK, Knudsen TB. Reprogramming of genetic networks during initiation of the Fetal Alcohol Syndrome. Dev Dyn. 2007;236:613–631. doi: 10.1002/dvdy.21048. [DOI] [PubMed] [Google Scholar]
- Hofmann CE, Patyk IA, Weinberg J. Prenatal ethanol exposure: sex differences in anxiety and anxiolytic response to a 5-HT1A agonist. Pharmacol Biochem Behav. 2005;82:549–558. doi: 10.1016/j.pbb.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Houle K, Abdi M, Clabough EBD. Acute ethanol exposure during late mouse neurodevelopment results in long-term deficits in memory retrieval, but not in social responsiveness. Brain Behav. 2017;7:e00636. doi: 10.1002/brb3.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonomidou C, Bittigau P, Ishimaru MJ, Wozniak DF, Koch C, Genz K, Price MT, Stefovska V, Horster F, Tenkova T, Dikranian K, Olney JW. Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science. 2000;287:1056–1060. doi: 10.1126/science.287.5455.1056. [DOI] [PubMed] [Google Scholar]
- Jacobson SW. Specificity of neurobehavioral outcomes associated with prenatal alcohol exposure. Alcohol Clin Exp Res. 1998;22:313–320. doi: 10.1111/j.1530-0277.1998.tb03654.x. [DOI] [PubMed] [Google Scholar]
- Jones AW, Harding P. Driving under the influence with blood alcohol concentrations over 0.4 g% Forensic Sci Int. 2013;231:349–353. doi: 10.1016/j.forsciint.2013.06.010. [DOI] [PubMed] [Google Scholar]
- Kelly SJ, Goodlett CR, Hulsether SA, West JR. Impaired spatial navigation in adult female but not adult male rats exposed to alcohol during the brain growth spurt. Behav Brain Res. 1988;27:247–257. doi: 10.1016/0166-4328(88)90121-0. [DOI] [PubMed] [Google Scholar]
- Kelly SJ, Lawrence CR. Intragastric intubation of alcohol during the perinatal period. Methods Mol Biol. 2008;447:101–110. doi: 10.1007/978-1-59745-242-7_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly SJ, Pierce DR, West JR. Microencephaly and hyperactivity in adult rats can be induced by neonatal exposure to high blood alcohol concentrations. Experimental neurology. 1987;96:580–593. doi: 10.1016/0014-4886(87)90220-2. [DOI] [PubMed] [Google Scholar]
- Koren G. Pharmacological treatment of disruptive behavior in children with fetal alcohol spectrum disorder. Paediatr Drugs. 2015;17:179–184. doi: 10.1007/s40272-015-0118-4. [DOI] [PubMed] [Google Scholar]
- Lee DH, Moon J, Ryu J, Jeong JY, Roh GS, Kim HJ, Cho GJ, Choi WS, Kang SS. Effects of postnatal alcohol exposure on hippocampal gene expression and learning in adult mice. Genes Genet Syst. 2016;90:335–342. doi: 10.1266/ggs.15-00026. [DOI] [PubMed] [Google Scholar]
- Lewis SJ, Zuccolo L, Davey Smith G, Macleod J, Rodriguez S, Draper ES, Barrow M, Alati R, Sayal K, Ring S, Golding J, Gray R. Fetal alcohol exposure and IQ at age 8: evidence from a population-based birth-cohort study. PLoS One. 2012;7:e49407. doi: 10.1371/journal.pone.0049407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J. GSK3beta in ethanol neurotoxicity. Mol Neurobiol. 2009;40:108–121. doi: 10.1007/s12035-009-8075-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Crocker N, Nguyen TT. Fetal alcohol spectrum disorders: neuropsychological and behavioral features. Neuropsychology review. 2011;21:81–101. doi: 10.1007/s11065-011-9167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei Y, Monteiro P, Zhou Y, Kim JA, Gao X, Fu Z, Feng G. Adult restoration of Shank3 expression rescues selective autistic-like phenotypes. Nature. 2016;530:481–484. doi: 10.1038/nature16971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore EM, Riley EP. What Happens When Children with Fetal Alcohol Spectrum Disorders Become Adults? Curr Dev Disord Rep. 2015;2:219–227. doi: 10.1007/s40474-015-0053-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley EP, McGee CL. Fetal alcohol spectrum disorders: an overview with emphasis on changes in brain and behavior. Experimental biology and medicine. 2005;230:357–365. doi: 10.1177/15353702-0323006-03. [DOI] [PubMed] [Google Scholar]
- Schmitt U, Hiemke C. Combination of open field and elevated plus-maze: a suitable test battery to assess strain as well as treatment differences in rat behavior. Prog Neuropsychopharmacol Biol Psychiatry. 1998;22:1197–1215. doi: 10.1016/s0278-5846(98)00051-7. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Biane JS, O'Bryan KA, O'Neill TM, Dominguez HD. Choline supplementation following third-trimester-equivalent alcohol exposure attenuates behavioral alterations in rats. Behav Neurosci. 2007;121:120–130. doi: 10.1037/0735-7044.121.1.120. [DOI] [PubMed] [Google Scholar]
- Urso T, Gavaler JS, Van Thiel DH. Blood ethanol levels in sober alcohol users seen in an emergency room. Life Sci. 1981;28:1053–1056. doi: 10.1016/0024-3205(81)90752-9. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner JL, Zhou FC, Goodlett CR. Effects of one- and three-day binge alcohol exposure in neonatal C57BL/6 mice on spatial learning and memory in adolescence and adulthood. Alcohol. 2014;48:99–111. doi: 10.1016/j.alcohol.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainwright PE. Issues of design and analysis relating to the use of multiparous species in developmental nutritional studies. J Nutr. 1998;128:661–663. doi: 10.1093/jn/128.3.661. [DOI] [PubMed] [Google Scholar]
- Wozniak DF, Hartman RE, Boyle MP, Vogt SK, Brooks AR, Tenkova T, Young C, Olney JW, Muglia LJ. Apoptotic neurodegeneration induced by ethanol in neonatal mice is associated with profound learning/memory deficits in juveniles followed by progressive functional recovery in adults. Neurobiol Dis. 2004;17:403–414. doi: 10.1016/j.nbd.2004.08.006. [DOI] [PubMed] [Google Scholar]