Abstract
Background
Psychological distress is common among people with a substance abuse disorder in treatment. Identifying correlates of psychological distress may serve as points of intervention to improve substance abuse treatment outcomes. Immune function measured as cytokine levels have been associated with psychological distress, but this association remains unexplored among people with a substance abuse disorder in treatment. This study aimed to examine whether cytokine levels in patients treated for a substance use disorder were related to depression, anxiety, and overall psychological distress, and to observe these associations separately among people with a past year alcohol use disorder and those with a past year drug use disorder.
Methods
We collected cross-sectional data from 80 inpatients at five alcohol and substance abuse treatment centers in Norway. We determined alcohol and drug diagnoses, and assessed symptoms of depression, anxiety, and overall psychological distress. We tested blood samples for IL-1, IL-6, TNF-α, INF-γ, and IL-10. We used multivariate linear regressions to examine the associations between cytokine levels and psychological distress measures.
Results
All cytokines were significantly and positively associated with depression score. INF-γ was significantly and negatively associated with anxiety, and IL-6 was significantly and positively associated psychological distress. Among people with only an alcohol use disorder, IL-6 was positively associated with depression and psychological distress scores, and IL-10 was negatively associated with anxiety score. Among people with only a drug use disorder, TNF-α was positively associated with depression score.
Conclusion
The relationship between immune function and psychological distress is robust in the context of substance abuse, and further research is warranted.
Keywords: Depression, anxiety, psychological distress, alcohol abuse, drug abuse, substance abuse treatment, cytokines
Graphical Abstract
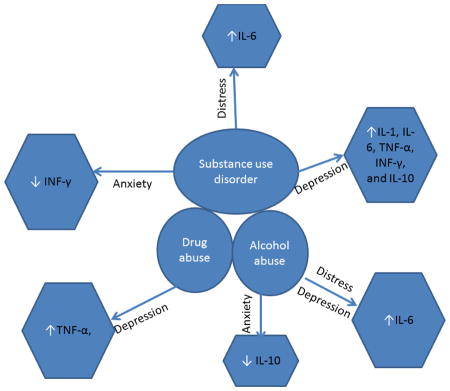
Graphical abstract showing direction of changes in the level of each cytokine across the study groups.
1. INTRODUCTION
Substance use disorders and psychological distress, encompassing symptoms of depression and anxiety, are among the most common and costly disorders challenging healthcare systems worldwide. Psychological distress is also highly prevalent among people struggling with alcohol and illicit drug use disorders. In a nationally representative study of adults residing in the U.S., the 12-month prevalence of major depression and any anxiety disorder among people with a current substance use disorder was 14.5% and 17.7%, respectively [1]. Among people with substance use disorders accessing treatment, comorbid symptoms of depression and anxiety are typically even higher. A multisite study among people admitted to substance abuse treatment facilities in the U.S. observed a past year prevalence of 51.4% for depressive symptoms and 41.6% for anxiety symptoms [2]. In a study of people with substance use disorders in treatment in Norway, 36% had past-year major depression and 78% had any past-year anxiety disorder [3]. The impact of depression and anxiety on treatment outcomes for people with substance use disorders is also well established: those with such comorbidity have a reduced physical and mental health status [4], and are more likely to drop out of treatment and experience relapse [5, 6]. Thus, understanding contributors to psychological distress among people with substance use disorders accessing treatment may help identify targets for interventions to improve treatment outcomes.
Immune function, as measured by circulating cytokine levels, has been associated with depression [7] and, at times, anxiety [8]. Cytokines are a heterogeneous group of signal-transducing proteins secreted by immune cells to regulate immune activity and communicate with the nervous and endocrine systems. Higher circulating and brain inflammatory cytokine levels has been consistently reported in both animal models of depression as well as among depressed human subjects [9–11]. However, debate remains around whether the observed inflammatory augmentation is consequent to or a cause of the depressive state [12, 13], and over which cytokines are potential determinants of depression [9, 10, 14]. Nonetheless, there is biological plausibility suggesting inflammatory cytokines may be mediators of both environmental and genetic factors that may lead to the onset of depressive disorders [15]. In contrast to depression, there is scant and conflicting data on the correlation between immune function and anxiety disorders. One of the first large studies to investigate anxiety disorders and immune function identified elevated levels of C-reactive protein (CRP) in men with a current anxiety disorder, but not in women, and no association with cytokine levels [16]. This is inconsistent with findings from smaller, clinical samples that have identified associations between elevated inflammatory cytokine levels and anxiety disorders [17, 18]. Methodological differences between clinical and general population-based studies notwithstanding, further studies are clearly needed to clarify the relationships between immune function and depression and anxiety.
Changes in immune function are also associated with alcohol and illicit drug use. Alcohol and illicit drugs are well-known immune modulators, and heavy use of both results in increased inflammatory cytokine levels and vulnerability to infections [19–21]. Chronic heavy alcohol use has been shown to increase production of systemic and brain pro-inflammatory cytokines, such as tumor necrosis factors-α (TNF-α), interleukin (IL)-6, and IL-1, and to reduce anti-inflammatory cytokines, such IL-10 [22]. Similar to alcohol abuse, abuse of illicit drugs such as opiates, cannabis, and cocaine is associated with the induction of pro-inflammatory cytokines. Unlike alcohol, however, illicit drugs are understood to exert their immunomodulatory effects via pathways mediated by receptors bound to immune cells [23], which may then result in the production of inflammatory cytokines. Given the associations between immune function and depression and anxiety, changes in immune function due to alcohol or illicit drug abuse may be a biological link between substance abuse and psychological distress, and might help explain the increased prevalence of psychological distress among people with a substance use disorder. Moreover, immunological mechanisms may pose new targets for substance use disorder treatment [24]. However, to warrant advanced investigations into the validity of this causal model and the biological mechanisms that may underlie such a causal pathway, we need to build associational evidence for the relationships between psychological distress and circulating cytokine levels in the context of substance abuse. For both alcohol and drug studies examining the effects of these substances on immune function, experimental animal models and observational human studies have focused on ex-vivo immune cell activity; far fewer studies have examined circulating cytokine profiles among people with alcohol and drug use disorders that would permit observation of associations between substance abuse and circulating cytokines levels.
With the paucity of studies investigating circulating cytokine profiles among people with a substance use disorder, it remains unknown if circulating cytokine profiles could be correlates of psychological distress in this population. Given the associations between both psychological distress and alcohol/drugs with circulating cytokine levels, it is not clear that associations between cytokine levels and psychological distress would be observed among people in treatment for a substance use disorder. Our previous work among adults with alcohol use disorders accessing treatment in Nepal has shown elevated levels of the inflammatory cytokines IL-6, TNF-α, and INF-γ among those with comorbid depression compared to those without [25]. Other studies have shown associations between elevated levels of circulating inflammatory cytokines and depressive symptoms among people with alcohol use disorders [26]. However, these studies are limited in their applicability to people with a drug use disorder seeking treatment. Moreover, studies that have included alcohol measures when investigating the association between immune function and psychological distress typically examine the effect of alcohol only and exclude people with drug use and drug use disorders. Epidemiological studies consistently show that alcohol and drug use disorders frequently co-occur [27], particularly in treatment seeking populations [28]. Identifying an association between circulating cytokine profiles and psychological distress in a group comprised of people with an alcohol and/or drug use disorder in treatment would suggest the association between cytokine levels and psychological distress is robust in this context. Furthermore, given that there are different pathways for the immunomodulatory effects of alcohol and illicit drugs, it would be useful to have information about the association between cytokine levels and psychological distress by alcohol use disorder and drug use disorder separately to observe potential differences in the association across disorders. Therefore, our primary aim was to examine whether cytokine levels in patients treated for a substance use disorder were related to psychological distress, including symptoms of depression and anxiety. Our secondary aim was to observe and describe associations between cytokine levels and psychological distress separately among people with only an alcohol use disorder and those with only a drug use disorder. We hypothesized that higher circulating levels of pro-inflammatory cytokines and lower levels of anti-inflammatory cytokines would be associated with greater symptoms of depression, anxiety, and overall psychological distress among people in treatment for a substance use disorder. We also hypothesize that the profile of cytokines associated with psychological measures would be different when examined separately among people with only an alcohol use disorder and those with only a drug use disorder due to the different pathways by which alcohol and drugs affect immune function.
2. MATERIAL AND METHODS
2.1 Material
We collected cross-sectional data from convenience samples of inpatients at five alcohol and substance abuse treatment centers that are part of the Innland Hospital Trust in Southeastern Norway. We chose these treatment centers based on geographic proximity to the University of Oslo, and facilities for blood draws and storage of biological specimens. We identified and approached these five sites and all agreed to participate. Three of the five sites specialized in the inpatient treatment of drug- and alcohol-dependent persons using a variety of cognitive therapies. One site specialized in the inpatient assessment of co-morbid psychiatric disorders among drug-dependent persons (hereafter referred to as “the Assessment Unit”). The final study site specialized in the treatment of persons with depression. Data were collected from the Assessment Unit between 2003 and 2010. Data from all other sites were collected between 2010 and 2011. All patients are adults (aged 18 or older) and referred to these sites by a medical doctor for the assessment or treatment of a diagnosed substance use disorder or depressive disorder. We approached 148 people, 85 (57%) of whom agreed to participate and provided written informed consent. We were unable to collect blood samples from 5 people. The final analytic sample was thus comprised of 80 people. Thirty-eight participants were from the Assessment Unit and 42 were from the other study sites. Patients of these centers were eligible to participate if they were new or returning patients of the recruitment site and provided informed consent. This study was approved by the Regional Committees for Medical and Health Research Ethics in Norway, and by the Institutional Review Board at the Public Health Institute in the U.S.
2.2 Methods
All study participants were approached by a study staff member who introduced the study, explained the study’s purpose and procedures, and inquired about willingness to participate. We collected data from the Assessment Site among consenting patients through a medical chart review. Data used in this study were collected by healthcare providers as part of the assessment protocol at this site. We collected data from the remaining sites via an interviewer-administered structured questionnaire. Participants were interviewed individually at the treatment center at a time of their choosing. All participants abstained from alcohol or drug use for at least two weeks prior to data and biological sample collection.
Non-fasting blood samples were collected in the morning by a trained nurse. Antecubital venous blood was collected using a 4ml VACUETTE® tube with serum clot activator. After 30 minutes at room temperature, the sample was centrifuged at 2000g for 10 minutes. Serum was decanted into a cryotube in approximately 1–2ml aliquots and immediately stored at −80 C. The frozen samples were transferred on dry ice to the research laboratory at Nordland Hospital in Bodø, Norway, where testing occurred in 2013.
2.3 Measures
2.3.1 Alcohol and drug use disorder measures
All study participants completed the alcohol and drug abuse/dependence sections of the Composite Inventory Diagnostic Interview (CIDI) for current, past-year diagnoses during a face-to-face interview. At the Assessment Site, the interview was conducted by a clinician. At the other study sites, the interview was conducted by a study staff member trained in CIDI administration. The CIDI is a fully-structured interviewer-administered questionnaire and can generate diagnoses based on criteria from the International Classification of Diseases - 10th Edition (ICD-10) and on criteria from the Diagnostic and Statistical Manual for Mental Disorders - 4th Edition (DSM-IV)[29]. The illicit drugs of abuse assessed for in the CIDI were opioids, cannabis, sedatives, cocaine, stimulants, and hallucinogens. We constructed dichotomous (yes/no) variables for past year diagnosis of any alcohol use disorder and drug use disorder, including both abuse and dependence. We also constructed dichotomous (yes/no) variables for past year diagnosis of only an alcohol use disorder and for past year diagnosis of only a drug use disorder. These variables were used to identify participants for inclusion in the analysis to observe associations between cytokine levels and depression, anxiety, and psychological distress among people with only one past-year diagnosed substance use disorder.
2.3.2 Depression, anxiety, and psychological distress symptom measures
Participants also completed the Symptom Checklist-90 Revised version (SCL-90R)[30]. The SCL-90-R is a 90-item inventory with a 5-point Likert response scale ranging from “not at all” (0) to “extremely (4). The SCL-90-R assesses psychological distress over the previous 7 days. This measure includes nine subscales, including subscales for depressive and anxiety symptoms with 13 and 10 items, respectively. The SCL-90-R also produces the Global Severity Index (GSI), which is the total sum score of all subscales and is a measure of overall psychological distress. We calculated raw scores for the depression and anxiety subscales, and for the overall GSI score. We converted these raw scores into standardized t-scores developed to enable comparisons to a reference group. As our sample was comprised of people with a substance use disorder in treatment, we used the psychiatric inpatient norms presented in the SCL-90-R Administration, Scoring, and Procedures Manual [31]. Weused these t-scores as continuous measures of symptoms of psychological distress. We also calculated a dichotomous variable indicating “caseness” for overall psychological distress according to the recommended cut-off of a t-score>=63 using the GSI score[31].
2.3.3 Medical health measures and demographics
During the face-to-face interview, patients were queried about their medical health status (history and presence of any chronic or acute illnesses) and current use of over-the-counter and prescription medication, including immunosuppressive drugs and anti-depressants. We verified self-reported prescription and disease status through medical chart review at all participating centers. Immune-compromising disorders (liver disease, autoimmune diseases) and chronic infections (HCV, HIV) and acute infections (bronchitis, flu) were also assessed. Finally, basic demographics were measured, including age, gender, marital status, and employment.
2.3.4 Cytokine measures
Cytokine levels were analyzed using a multi-plex enzyme-linked immunosorbent assay (ELISA) kit for 27 cytokines (Human Bio-Plex; Bio-Rad Laboratories Inc., Hercules, CA, USA). We examined the pro-inflammatory cytokines IL-1, IL-6, TNF-α, and INF-γ, and the anti-inflammatory cytokine IL-10. The interassay and intrassay coefficients of variation were <10% for all analyses, and all values were within the standard curve except for one for TNF-α, which was excluded.
3. STATISTICAL ANALYSIS
All cytokine values were log transformed to obtain a normal data distribution prior to statistical analyses. Descriptive statistics are presented as frequencies (%) and means (Standard deviation; SD). To identify potential confounders, we used T-tests and Pearson’s correlations to assess associations between demographic factors and each t-score for depression, anxiety, and psychological distress. We also investigated study site as a potential confounder given the different sites included using ANOVA. We used linear regression to observe associations between cytokine levels and symptoms of depression, anxiety, and psychological distress, and multivariate analysis to adjust for covariates identified as statistically significant in bivariate analysis, for each cytokine individually. In all regression models, scores for depression, anxiety, and psychological distress were the dependent variable. Statistical analyses were performed using STATA 14.0.
4. RESULTS
4.1 Patient characteristics and cytokine profiles
Demographic and mental health characteristics of participants are described in Table 1. Study participants had a mean age of 42 years and were predominantly male (71.3%). An alcohol use disorder was the most common past year substance-use diagnosis (n=30, 37.5%). A past year drug abuse or dependence diagnosis was most common for stimulants (n=21, 26.3%), followed by sedatives (n=18, 22.5%) and cannabis (n=16, 20%). Twenty percent (n=16) of the total sample were case positive for psychological distress. Mean values for all cytokines did not differ significantly between those who were case negative and case positive for symptoms of psychological distress. Demographic and mental health characteristics of participants by study site are presented in Table 2. Levels for each cytokine varied significantly across study site, as did anxiety and psychological distress score.
Table 1.
Demographic, health, and substance use disorder characteristics and cytokine levels of patients (n=80) in 5 Norwegian alcohol & drug treatment centers
| Psychological distress case negative | Psychological distress case positive | ||
|---|---|---|---|
| n=64 | n=16 | ||
| Characteristics | n (%) | n (%) | p-value |
| Demographics | |||
| Age (mean, SD) | 40.7 (12.8) | 47.0 (8.6) | 0.069 |
| Male | 47 (73.4) | 10 (62.5) | 0.387 |
| Single/never married | 44 (68.8) | 8 (50.0) | 0.26 |
| Full-time employment past 6 months | 7 (11.1) | 2 (12.5) | 0.415 |
| Health | |||
| Chronic disease present | 25 (39.1) | 4 (25.0) | 0.295 |
| Weekly/daily over the counter medication use | 31 (48.4) | 1 (6.3) | 0.002 |
| Substance use disorders | |||
| Past year AUD only | 30 (37.5) | 8 (26.7) | 0.248 |
| Past year DUD only | 24 (30.0) | 2 (12.5) | 0.088 |
| Past year comorbid AUD and DUD | 14 (17.5) | 3 (18.8) | 0.883 |
| Cytokines | |||
| IL-6 (mean, SD) | 2.3 (0.42) | 2.4 (0.12) | 0.231 |
| TNF-a (mean, SD) | 3.9 (0.30) | 3.9 (0.47) | 0.784 |
| INF-g (mean, SD) | 4.8 (0.46) | 4.9 (0.46) | 0.279 |
| IL-10 (mean, SD) | 2.6 (0.50) | 2.6 (0.61) | 0.833 |
Note: AUD = Alcohol Use Disorder; DUD = Drug Use Disorder; SCL-90-R=Symptom Checklist 90-Revised; all cytokine values were log transformed.
Table 2.
Demographic, health, and substance use disorder characteristics and cytokine levels of patients at each study site
| The Assessment Unit | Bla Kors Eina | Sorlighau gen | Modum Bad | Riisby | p- value | |
|---|---|---|---|---|---|---|
| n=38 | n=15 | n=8 | n=4 | n=15 | ||
| n(%) | n(%) | n(%) | n(%) | n(%) | ||
| Demographics | ||||||
| Age (mean, SD) | 35.8 (10.4) | 50.9(10.2) | 32.6 (8.1) | 52.8(5.8) | 50.8(8.5) | - |
| Male | 30 (79.0) | 15(100.0) | 4 (50.0) | 2 (50.0) | 6 (40.0) | - |
| Single/never married | 29 (76.3) | 11 (73.3) | 4 (50.0) | 1 (25.0) | 7 (46.7) | - |
| Full-time employment past 6 months | 5 (13.5) | 3 (20.0) | 0 (0.0) | 0 (0.0) | 1 (6.7) | - |
| Health | ||||||
| Chronic disease present | 15 (39.5) | 4 (26.7) | 4 (50.0) | 0 (0.0) | 6 (40.0) | - |
| Weekly/daily over the counter medication use | 20 (52.6) | 6 (40.0) | 2 (25.0) | 0 (0.0) | 4 (26.7) | - |
| Substance use disorders | ||||||
| Past year AUD only | 8 (21.1) | 10 (66.7) | 1 (12.5) | 2 (50.0) | 9 (60.0) | - |
| Past year DUD only | 17 (44.7) | 0 (0.0) | 4 (50.0) | 0 (0.0) | 3 (20.0) | - |
| Past year comorbid AUD and DUD | 9 (23.7) | 1 (6.7) | 1 (12.5) | 1 (25.0) | 2 (13.3) | - |
| Cytokines | ||||||
| IL-6 (mean, SD) | 2.2 (0.40) | 1.9(0.28) | 2.6 (0.42) | 2.2(0.21) | 2.7(0.26) | 0.002 |
| TNF-a (mean, SD) | 3.9 (0.29) | 3.6(0.26) | 4.1 (0.30) | 3.5(0.19) | 4.1(0.28) | 0.02 |
| INF-g (mean, SD) | 4.8 (0.44) | 4.4(0.40) | 5.2 (0.25) | 4.9(0.14) | 5.2(0.24) | 0.001 |
| IL-10 (mean, SD) | 2.6 (0.41) | 2.1 (0.53) | 2.8 (0.45) | 2.6 (0.20) | 3.0 (0.46) | 0.003 |
| Psychological distress measures | ||||||
| Depression score (mean, SD) | 49.2 (8.2) | 46.1(8.3) | 43.1 (6.0) | 46.5(8.3) | 54.9(12.7) | 0.15 |
| Anxiety score (mean, SD) | 49.9 (5.4) | 45.7(5.8) | 46 (6.9) | 47.5(0.6) | 45.6(9.6) | 0.03 |
| Psychological distress score (mean, SD) | 51.9 (8.8) | 48.0(10.1) | 50.5(11.4) | 69.5(5.0) | 58.1(12.5) | 0.01 |
Note: No statistical tests for differences were performed for demographics, health measures, and substance use disorders due to small cell sizes.
No demographics or health characteristics were statistically significantly associated with depression score (data not shown). Age was associated with anxiety score (rho=−0.27, p=0.02), and anxiety scores were higher among those with a chronic illness compared to those without (46.5 vs. 50.1, p=0.02). Psychological distress scores were higher among those with less than weekly or daily over-the-counter medication use compared to those with weekly or daily use (55.8 vs. 49.1, p=0.007) (Table 3).
Table 3.
Associations between demographic, health variables, and measures of psychological distress
| Depression score | Anxiety score | Psychological distress score | |
|---|---|---|---|
| mean (SD) | mean (SD) | mean (SD) | |
| Age, rho (p-value) | 0.02 (0.87) | −0.27 (0.02) | −0.04 (0.71) |
| Gender, mean | |||
| Male | 49.1 | 48.2 | 52.7 |
| Female | 48.4 | 46.7 | 54.0 |
| Marital Status | |||
| Single/never married | 49.0 | 48.4 | 52.5 |
| Divorced/widowed | 49.7 | 46.9 | 55.7 |
| Married | 47.2 | 46.3 | 51.3 |
| Employment | |||
| None | 50.2 | 47.5 | 53.4 |
| Part-time | 46.7 | 48.8 | 52.7 |
| Full-time | 46.7 | 47.2 | 52.4 |
| Chronic illness | |||
| No | 48.3 | 46.5* | 53.5 |
| Yes | 50.1 | 50.1 | 52.4 |
| Weekly/daily over the counter medication use | |||
| No | 49.3 | 47.6 | 55.8* |
| Yes | 48.4 | 48.8 | 49.1 |
p-value<0.05
4.2 Associations between cytokine profiles and psychological distress measures
Bivariate and multivariate associations between level of each cytokine and symptoms of depression, anxiety, and psychological distress are presented in Table 4. Multivariate models for anxiety controlled for age, having a chronic illness, and study site; for psychological distress score, models controlled for weekly/daily over-the-counter medication use, and study site. As no covariates were associated with depression score, and scores did not vary by study site, none were included in the models for depression score. All cytokines were significantly and positively associated with depression score except for INF-γ which only approached statistical significance (p=0.07). Only INF-γ was significantly and negatively associated with anxiety in adjusted analysis. For psychological distress, IL-6 was significantly and positively associated in both unadjusted and adjusted models.
Table 4.
Unadjusted and adjusted associations between cytokine levels and symptoms of depression, anxiety, and psychological distress
| Cytokine (pg/ul) | SCL-90-R depression score | SCL-90-R anxiety score | SCL-90-R Psychological distress score | ||
|---|---|---|---|---|---|
| Unadjusted* β (SE) | Unadjusted β (SE) | Adjusted β (SE) | Unadjusted β (SE) | Adjusted β (SE) | |
| IL-6 | 0.15 (0.05) | −0.04 (0.04) | −0.03 (0.04) | 0.14 (0.05) | 0.12 (0.06) |
| TNF-a | 0.14 (0.06) | −0.04 (0.05) | −0.04 (0.06) | 0.10 (0.07) | 0.12 (0.08) |
| INF-g | 0.09 (0.07) ~ | −0.07 (0.03) | −0.08 (0.04) | 0.09 (0.05) ~ | 0.05 (0.06) |
| Il-10 | 0.09 (0.04) | −0.02 (0.03) | −0.03 (0.03) | 0.06 (0.05) | 0.002 (0.05) |
Note: Bold indicates p-value≤0.05
No adjusted model for Depression score because no covariates significant in bivariate analysis
Anxiety adjusted for age, chronic illness, and study site
Psychological distress adjusted for weekly/daily over the counter medication use and study site
p-value=0.07
4.3 Associations between cytokine profiles and psychologic distress measures by substance use disorder
Mean values for all cytokines did not differ significantly between people with only a past year alcohol use disorder diagnosis compared to those with only a past year drug use disorder (data not shown). Among people with only a past year alcohol use disorder, IL-6 was positively associated with depression score (β=0.15, p=0.04), and psychological distress score (β=0.20, p=0.04). IL-10 was negatively associated with anxiety score (β=-0.20, p=0.002). Among people with only a past year drug use disorder, TNF-α was positively associated with depression score (β=0.18, p=0.03). No cytokines were statistically significantly associated with anxiety or psychological distress score in this group.
5. DISCUSSION
In our sample of people with alcohol and drug use disorders in treatment, we observed positive associations between the pro-inflammatory cytokines IL-6, TNF-α, INF-γ, and the anti-inflammatory cytokine IL-10 and symptoms of depression. For symptoms of anxiety, we observed a negative association with INF-γ, and for psychological distress we observed a positive association with IL-6. When we disaggregated the sample by past-year alcohol or drug use disorder, IL-6 was positively associated with both symptoms of depression and psychological distress, and IL-10 was negatively associated with symptoms of anxiety among people with only a past year alcohol use disorder. Among people with only a past year drug use disorder, TNF-α was positively associated with depressive symptoms.
Our observation of positive relationships between pro-inflammatory cytokines and measures of depression and psychological distress supports our hypothesis that pro-inflammatory cytokines would be associated with greater levels of depression and psychological distress. This observation is also consistent with previous work from both animal and human studies [32], and similar associations have also been observed among treatment-seeking alcohol dependent populations [25]. Further, the effect sizes of the cytokines we identified to be significantly associated with the psychological distress measures were consistent across the outcomes, such as the association between IL-6 and the depression and psychological distress scores, supporting a robust relationship. On the other hand, our observation of a positive association between the anti-inflammatory cytokine IL-10 and symptoms of depression and psychological distress does not our support our hypothesis that anti-inflammatory cytokine IL-10 would be negatively associated with these mental health measures. This observation is in contrast with some previous studies and consistent with others. In a study among patients with cardiovascular risk factors, Meyer et al identified a positive association between IL-10 and symptoms of depression, controlling for several covariates including other cytokine levels [33]. Conversely, a meta-analysis of cytokines in major depression reported no significant differences in IL-10 levels between depressed and non-depressed people [9]. A potential explanation may be that the patients in our sample had not been using alcohol or illicit drugs in at least the past two weeks and were in a treatment setting, so that IL-10 levels were in the process of rebounding, and symptoms of depression and psychological distress had not yet completely subsided. Our finding of a negative association between INF-y and symptoms of anxiety also does not support our hypothesis of a positive association between pro-inflammatory cytokines and anxiety. Interestingly, some laboratory animal studies have reported an increase in symptoms of anxiety in mice deficient for the INF- γ gene [34, 35]. However, INF- γ is known to induce the metabolism of tryptophan to kynurenine, which has been associated with increased anxiety, and this would suggest a positive relationship between INF- γ and symptoms of anxiety [36]. Further work is needed examining the association between INF- γ and symptoms of anxiety in human subjects and among those with substance use disorders to better understand this relationship.
In our disaggregated analysis, the differences in associations between cytokine level and measures of psychological distress between people with an alcohol use disorder only and people with a drug use disorder only is consistent with our hypothesis. In particular it is interesting that only TNF-α was associated with depression in the drug use disorder only group. This finding supports the idea that the different pathways of the immunomodulatory effects between alcohol and drugs may lead to different cytokine profiles between these two groups. The associations we observed between circulating cytokine levels and measures of psychological distress among people with an alcohol use disorder only were consistent with our observations in the entire sample. No identified association between circulating cytokine levels and symptoms of anxiety among people with an alcohol use disorder, or with symptoms of anxiety or psychological distress among people with a drug use disorder only, could reflect the smaller sample sizes of these groups. Overall, the fact that our finding diverged from the literature could reflect our unique study population, or differences in measures of symptoms of depression, anxiety, and psychological distress. Longitudinal studies with measurements with several time-points beginning at the initiation of treatment could help clarify these inconsistencies.
It is well-understood that psychological distress is more common among people with substance use disorders [37] and that this comorbidity reduces the likelihood of positive treatment outcomes [4–6, 38]. Explanations for this comorbidity include the “self-medication” hypothesis [39], overlapping neurobiological pathways [40], and common genetic risk factors [41]. There is a substantial and growing body of evidence showing that inflammatory markers are associated with psychiatric disorders related to measures of psychological distress. Anxiety disorders such as Panic disorder and Post-traumatic Stress Disorder have been shown to be associated with a generalized inflammatory state [17, 18], although the direction of the relationship remains uncertain. A meta-analysis by Howren et al demonstrated that CRP, IL-1, and IL-6 are positively associated with depression in clinical and community samples, and that evidence supports multiple and bidirectional pathways between depression and inflammation [42]. It is important to note, nonetheless, that several studies have not observed associations between psychiatric disorders or their symptoms and cytokine levels. For example, a large population-based study in the Netherlands did not observe an association between any inflammatory marker and a current or remitted anxiety disorder among women[16]. Also, a meta-analysis by Dowlati et al [9] showed higher concentrations of IL-6 and TNF-α among depressed subjects compared to controls, but did not observe any differences for a host of other cytokines. Thus, the literature remains mixed on precisely which cytokines are of importance to psychiatric symptoms, and the direction of these relationships.
While little work has been done examining inflammation and psychological distress among people with a substance abuse disorder, one study among female crack cocaine users undergoing detoxification in Brazil observed an association between an inflammatory state and childhood maltreatment[43]. This analysis presents early evidence to suggest that the immunologic changes associated with the heavy use of alcohol and illicit drugs may serve to partially explain the greater prevalence of psychological distress observed among people with substance use disorders. Directionality, however, cannot be assessed in this study and requires future longitudinal investigations. Further, we stress the preliminary nature of this study and make a call for future research to explore hypotheses related to whether immune function among people with alcohol and substance abuse disorders may be affected by symptoms of depression and psychological distress above and beyond the impact of the alcohol or drug use disorder. These immune function measures might be further explored in substance using populations as potential ways to identify individuals at high risk of having or developing symptoms of depression, anxiety and psychological distress.
There are important limitations of this work that warrant mention. First, our sample size overall and for comparing between alcohol and drug use disorders was small. This may limit our ability to detect associations between circulating cytokine levels and measures of psychological distress, and to identify associations between potential confounders and the outcomes. However, to avoid over-adjusting bias in our analyses, we only included variables in multivariate models with a statistically significant association in bivariate tests. One confounder of note that we did not include is socioeconomic status (SES). We did not observe any statistically significant differences in our measures of SES (full-time employment, 6-month employment status) and measures of psychological distress, and therefore did not include these measures as confounders. Indeed, studies from similar samples in Norway show similarly low levels of SES with minimal variation [38, 44]. However, there is evidence to suggest that SES is associated with measures of immune function [45] and psychological distress [46]. Thus, while SES did not appear to be a confounder in this sample, we acknowledge the importance of SES as a potential confounder that should be considered in future work on this topic. Second in regards to the limitations, the prevalence of “caseness” of psychological distress was low in our sample compared to other in-treatment samples of people with a substance use disorder, which limited our ability to identify associations between “cases” and circulating cytokine levels. Nonetheless, that associations were detected using continuous measures of psychological distress suggests they are robust. Third, we were unable to control for BMI, smoking tobacco, or recency or severity of alcohol and/or drug use. Smoking is known to have an impact on immune function [47], and is also common among people with substance use disorders in treatment and among people with symptoms of psychological distress [48]. BMI has been shown to be associated with several measures of inflammation [49], including C-reactive protein and IL-6 [50]. The recency and severity of alcohol [51] and/or drug use also impacts immune function [21, 52]. More relevantly, recency of alcohol use has been shown to moderate the association between cytokine levels and depressive symptoms among people with AUD in treatment [25]. Fourth, the cytokines investigated were limited, as other cytokines such as Interleukin-17 have been shown to be associated with anxiety [53]. Furthermore, the cytokine values estimated by our test method and equipment may differ from other non-bead based commonly available commercial assays[54]. Variations in sensitivity as well as quantification have been noted even between different immunoassays using the same technology such as those provided by Bio-Rad Laboratories, Linco Inc., and RnD Systems [55]. This issue, however, may be less relevant for between-group comparisions made in our cross-sectional study.
Thus, reported cytokine levels and associations with measures of psychological distress should be taken with these limitations in mind. Finally, we were unable to obtain information about those who refused participation in this study, thereby limiting our ability to gauge how representative our sample is. Relatedly, while all our participants met the criteria for a lifetime SUD diagnosis, not all met the criteria for a past-year diagnosis, so that this was not a completely homogenous sample in terms of recency of severe alcohol and/or drug use. Given these limitations, we view and present this work as an initial exploration into the presence and magnitude of an association between cytokine profile and psychological distress among people with a substance abuse disorder in treatment. We also present our findings as preliminary evidence to encourage further research into this population that will take into account the important confounders, such as a variety of somatic diseases and other psychiatric co-morbidity, that we were unable to control for in this study.
Nonetheless, to the best of our knowledge, this is one of the first studies to examine the associations between immune function measured as circulating cytokine levels and measures of psychological distress among people with a substance use disorder in treatment. That our analysis identified an association between immune function and psychological distress in this group of patients supports efforts to further investigate these associations in larger samples with longitudinal designs and multiple measures of mental health status. Furthermore, our examination of the associations between cytokine levels and anxiety contributes to the limited literature on the relationship between immune function and anxiety, and extends it to an in-treatment population of people with substance use disorders. The observation of associations between cytokine levels and measures of psychological distress among a group of people in treatment for a variety of substance use disorders suggests the relationship between immune function and psychological distress may be robust even given the relationship between immune function and alcohol and drug abuse. Further work conducted among larger samples controlling for more potential confounders should be undertaken to better understand the potential for immune function to serve as points of intervention for depression and anxiety to improve treatment outcomes among people with substance use disorders.
Highlights.
Serum cytokine levels in patients treated for a substance use disorder were examined in relation to depression, anxiety and psychological distress
All tested cytokines (IL-1, IL-6, TNF-α, INF-γ, and IL-10) were associated with depression score.
INF-γ was associated with anxiety and IL-6 with psychological distress.
IL-6 and IL-10 were related to depression in alcohol abuse and TNF-α in drug abuse
Acknowledgments
The authors would like to acknowledge and thank the study participants and cooperating treatment centers for their time and participation.
FUNDING: This work was supported by the National Institute of Alcohol Abuse and Alcoholism [grant numbers P50AA005595 and K01AA024832] the Southeastern Norway Regional Health Authority [grant number 2016082]; the Innland Hospital Trust; and the Research Council of Norway.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Grant BF, et al. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2004;61(8):807–16. doi: 10.1001/archpsyc.61.8.807. [DOI] [PubMed] [Google Scholar]
- 2.Chan YF, Dennis ML, Funk RR. Prevalence and comorbidity of major internalizing and externalizing problems among adolescents and adults presenting to substance abuse treatment. Journal of Substance Abuse Treatment. 2008;34(1):14–24. doi: 10.1016/j.jsat.2006.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Landheim AS, Bakken K, Vaglum P. Gender Differences in the Prevalence of Symptom Disorders and Personality Disorders among Poly-Substance Abusers and Pure Alcoholics. European Addiction Research. 2003;9(1):8–17. doi: 10.1159/000067732. [DOI] [PubMed] [Google Scholar]
- 4.Lynskey MT. The comorbidity of alcohol dependence and affective disorders: treatment implications. Drug Alcohol Depend. 1998;52(3):201–9. doi: 10.1016/s0376-8716(98)00095-7. [DOI] [PubMed] [Google Scholar]
- 5.Burns L, Teesson M, O'Neill K. The impact of comorbid anxiety and depression on alcohol treatment outcomes. Addiction. 2005;100(6):787–96. doi: 10.1111/j.1360-0443.2005.001069.x. [DOI] [PubMed] [Google Scholar]
- 6.Landheim AS, Bakken K, Vaglum P. Impact of comorbid psychiatric disorders on the outcome of substance abusers: a six year prospective follow-up in two Norwegian counties. BMC Psychiatry. 2006;6:44. doi: 10.1186/1471-244X-6-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Felger JC, Lotrich FE. Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neuroscience. 2013;246:199–229. doi: 10.1016/j.neuroscience.2013.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salim S, Chugh G, Asghar M. Inflammation in anxiety. Adv Protein Chem Struct Biol. 2012;88:1–25. doi: 10.1016/B978-0-12-398314-5.00001-5. [DOI] [PubMed] [Google Scholar]
- 9.Dowlati Y, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67(5):446–57. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 10.Schiepers OJ, Wichers MC, Maes M. Cytokines and major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(2):201–17. doi: 10.1016/j.pnpbp.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Liu Y, Ho RC, Mak A. Interleukin (IL)-6, tumour necrosis factor alpha (TNF-alpha) and soluble interleukin-2 receptors (sIL-2R) are elevated in patients with major depressive disorder: a meta-analysis and meta-regression. J Affect Disord. 2012;139(3):230–9. doi: 10.1016/j.jad.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Stewart JC, et al. A prospective evaluation of the directionality of the depression– inflammation relationship. Brain, Behavior, and Immunity. 2009;23(7):936–944. doi: 10.1016/j.bbi.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valkanova V, Ebmeier KP, Allan CL. CRP, IL-6 and depression: A systematic review and meta-analysis of longitudinal studies. Journal of Affective Disorders. 2013;150(3):736–744. doi: 10.1016/j.jad.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Maes M, et al. The new '5-HT' hypothesis of depression: cell-mediated immune activation induces indoleamine 2,3-dioxygenase, which leads to lower plasma tryptophan and an increased synthesis of detrimental tryptophan catabolites (TRYCATs), both of which contribute to the onset of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(3):702–21. doi: 10.1016/j.pnpbp.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 15.Raison CL, Miller AH. Is Depression an Inflammatory Disorder? Current Psychiatry Reports. 2011;13(6):467–475. doi: 10.1007/s11920-011-0232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vogelzangs N, et al. Anxiety disorders and inflammation in a large adult cohort. Transl Psychiatry. 2013;3:e249. doi: 10.1038/tp.2013.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoge EA, et al. Broad spectrum of cytokine abnormalities in panic disorder and posttraumatic stress disorder. Depress Anxiety. 2009;26(5):447–55. doi: 10.1002/da.20564. [DOI] [PubMed] [Google Scholar]
- 18.Gill JM, et al. PTSD is associated with an excess of inflammatory immune activities. Perspect Psychiatr Care. 2009;45(4):262–77. doi: 10.1111/j.1744-6163.2009.00229.x. [DOI] [PubMed] [Google Scholar]
- 19.Crews FT, et al. Cytokines and alcohol. Alcohol Clin Exp Res. 2006;30(4):720–30. doi: 10.1111/j.1530-0277.2006.00084.x. [DOI] [PubMed] [Google Scholar]
- 20.Crews FT, Nixon K. Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol Alcohol. 2009;44(2):115–27. doi: 10.1093/alcalc/agn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friedman H, Newton C, Klein TW. Microbial Infections, Immunomodulation, and Drugs of Abuse. Clinical Microbiology Reviews. 2003;16(2):209–219. doi: 10.1128/CMR.16.2.209-219.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Szabo G, et al. Reduced alloreactive T-cell activation after alcohol intake is due to impaired monocyte accessory cell function and correlates with elevated IL-10, IL-13, and decreased IFNgamma levels. Alcohol Clin Exp Res. 2001;25(12):1766–72. [PubMed] [Google Scholar]
- 23.Ninkovic J, Roy S. Role of the mu-opioid receptor in opioid modulation of immune function. Amino Acids. 2013;45(1):9–24. doi: 10.1007/s00726-011-1163-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loftis JM, Huckans M. Substance use disorders: psychoneuroimmunological mechanisms and new targets for therapy. Pharmacol Ther. 2013;139(2):289–300. doi: 10.1016/j.pharmthera.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neupane SP, et al. High frequency and intensity of drinking may attenuate elevated inflammatory cytokine levels of major depression in alcohol-use disorders. CNS neuroscience & therapeutics. 2014;20(10):898–904. doi: 10.1111/cns.12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Irwin M, et al. Major depressive disorder, alcoholism, and reduced natural killer cell cytotoxicity. Role of severity of depressive symptoms and alcohol consumption. Arch Gen Psychiatry. 1990;47(8):713–9. doi: 10.1001/archpsyc.1990.01810200021003. [DOI] [PubMed] [Google Scholar]
- 27.Stinson FS, et al. Comorbidity between DSM-IV alcohol and specific drug use disorders in the United States: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug and Alcohol Dependence. 2005;80(1):105–116. doi: 10.1016/j.drugalcdep.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 28.Grant BF, et al. The 12-month prevalence and trends in DSM-IV alcohol abuse and dependence: United States, 1991–1992 and 2001–2002. Drug Alcohol Depend. 2004;74(3):223–34. doi: 10.1016/j.drugalcdep.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 29.Andrews G, Peters L. The psychometric properties of the Composite International Diagnostic Interview. Soc Psychiatry Psychiatr Epidemiol. 1998;33(2):80–8. doi: 10.1007/s001270050026. [DOI] [PubMed] [Google Scholar]
- 30.Derogatis LR, Melisaratos N. The Brief Symptom Inventory: an introductory report. Psychol Med. 1983;13(3):595–605. [PubMed] [Google Scholar]
- 31.Derogatis LR. SCL-90-R : symptom checklist-90-R : administration, scoring & procedures manual. Minneapolis, Minn: National Computer Systems, Inc; 1994. [Google Scholar]
- 32.Anisman H, et al. Cytokines as a precipitant of depressive illness: animal and human studies. Curr Pharm Des. 2005;11(8):963–72. doi: 10.2174/1381612053381701. [DOI] [PubMed] [Google Scholar]
- 33.Meyer T, et al. Serum levels of interleukin-6 and interleukin-10 in relation to depression scores in patients with cardiovascular risk factors. Behav Med. 2011;37(3):105–12. doi: 10.1080/08964289.2011.609192. [DOI] [PubMed] [Google Scholar]
- 34.Kustova Y, et al. The influence of a targeted deletion of the IFNgamma gene on emotional behaviors. Brain Behav Immun. 1998;12(4):308–24. doi: 10.1006/brbi.1998.0546. [DOI] [PubMed] [Google Scholar]
- 35.Litteljohn D, et al. Interferon-gamma deficiency modifies the effects of a chronic stressor in mice: Implications for psychological pathology. Brain Behav Immun. 2010;24(3):462–73. doi: 10.1016/j.bbi.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 36.Wirleitner B, et al. Interferon-gamma-induced conversion of tryptophan: immunologic and neuropsychiatric aspects. Curr Med Chem. 2003;10(16):1581–91. doi: 10.2174/0929867033457179. [DOI] [PubMed] [Google Scholar]
- 37.Grant BF, et al. Co-occurrence of 12-month alcohol and drug use disorders and personality disorders in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2004;61(4):361–8. doi: 10.1001/archpsyc.61.4.361. [DOI] [PubMed] [Google Scholar]
- 38.Landheim AS, Bakken K, Vaglum P. What characterizes substance abusers who commit suicide attempts? Factors related to Axis I disorders and patterns of substance use disorders. A study of treatment-seeking substance abusers in Norway. Eur Addict Res. 2006;12(2):102–8. doi: 10.1159/000090430. [DOI] [PubMed] [Google Scholar]
- 39.Lazareck S, et al. A Longitudinal Investigation of the Role of Self-Medication in the Development of Comorbid Mood and Drug Use Disorders. The Journal of clinical psychiatry. 2012;73(5):e588–e593. doi: 10.4088/JCP.11m07345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brady KT, Sinha R. Co-Occurring Mental and Substance Use Disorders: The Neurobiological Effects of Chronic Stress. American Journal of Psychiatry. 2005;162(8):1483–1493. doi: 10.1176/appi.ajp.162.8.1483. [DOI] [PubMed] [Google Scholar]
- 41.Kendler KS, et al. THe structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Archives of General Psychiatry. 2003;60(9):929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- 42.Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71(2):171–86. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- 43.Levandowski ML, et al. Distinct behavioral and immunoendocrine parameters during crack cocaine abstinence in women reporting childhood abuse and neglect. Drug Alcohol Depend. 2016;167:140–8. doi: 10.1016/j.drugalcdep.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 44.Bakken K, Landheim AS, Vaglum P. Axis I and II disorders as long-term predictors of mental distress: a six-year prospective follow-up of substance-dependent patients. BMC Psychiatry. 2007;7:29–29. doi: 10.1186/1471-244X-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gruenewald TL, et al. Association of socioeconomic status with inflammation markers in black and white men and women in the Coronary Artery Risk Development in Young Adults (CARDIA) study. Soc Sci Med. 2009;69(3):451–9. doi: 10.1016/j.socscimed.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lazzarino AI, et al. The combined association of psychological distress and socioeconomic status with all-cause mortality: a national cohort study. JAMA Intern Med. 2013;173(1):22–7. doi: 10.1001/2013.jamainternmed.951. [DOI] [PubMed] [Google Scholar]
- 47.Arnson Y, Shoenfeld Y, Amital H. Effects of tobacco smoke on immunity, inflammation and autoimmunity. Journal of Autoimmunity. 2010;34(3):J258–J265. doi: 10.1016/j.jaut.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 48.Kalman D, Morissette SB, George TP. Co-Morbidity of Smoking in Patients with Psychiatric and Substance Use Disorders. American Journal on Addictions. 2005;14(2):106–123. doi: 10.1080/10550490590924728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cox AJ, West NP, Cripps AW. Obesity, inflammation, and the gut microbiota. Lancet Diabetes Endocrinol. 2015;3(3):207–15. doi: 10.1016/S2213-8587(14)70134-2. [DOI] [PubMed] [Google Scholar]
- 50.Stepanikova I, Oates GR, Bateman LB. Does one size fit all? The role of body mass index and waist circumference in systemic inflammation in midlife by race and gender. Ethn Health. 2017;22(2):169–183. doi: 10.1080/13557858.2016.1235681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Romeo J, et al. Moderate alcohol consumption and the immune system: a review. Br J Nutr. 2007;98(Suppl 1):S111–5. doi: 10.1017/S0007114507838049. [DOI] [PubMed] [Google Scholar]
- 52.Coller JK, Hutchinson MR. Implications of central immune signaling caused by drugs of abuse: mechanisms, mediators and new therapeutic approaches for prediction and treatment of drug dependence. Pharmacol Ther. 2012;134(2):219–45. doi: 10.1016/j.pharmthera.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 53.Liu Y, Ho RC, Mak A. The role of interleukin (IL)-17 in anxiety and depression of patients with rheumatoid arthritis. Int J Rheum Dis. 2012;15(2):183–7. doi: 10.1111/j.1756-185X.2011.01673.x. [DOI] [PubMed] [Google Scholar]
- 54.Tighe P, et al. Utility, reliability and reproducibility of immunoassay multiplex kits. Methods. 2013;61(1):23–29. doi: 10.1016/j.ymeth.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 55.Djoba Siawaya JF, et al. An evaluation of commercial fluorescent bead-based luminex cytokine assays. PLoS One. 2008;3(7):e2535. doi: 10.1371/journal.pone.0002535. [DOI] [PMC free article] [PubMed] [Google Scholar]


