Abstract
The hypocretin receptor 1 (HCRTr1) is a critical participant in the regulation of motivated behavior. Previous observations demonstrate that acute pharmacological blockade of HCRTr1 disrupts dopamine (DA) signaling and the motivation for cocaine when delivered systemically or directly into the ventral tegmental area (VTA). To further examine the involvement of HCRTr1 in regulating reward and reinforcement processing, we employed an adeno-associated virus to express a short-hairpin RNA designed to knock down HCRTr1. We injected virus into the VTA and examined the effects of HCRTr1 knockdown on cocaine self-administration and DA signaling in the nucleus accumbens (NAc) core. We determined that the viral approach was effective at reducing HCRTr1 expression without affecting the expression of HCRT receptor 2 or DA-related mRNAs. We next examined the effects of HCRTr1 knockdown on cocaine self-administration, observing delayed acquisition under a fixed-ratio schedule and reduced motivation for cocaine under a progressive ratio schedule. These effects did not appear to be associated with alterations in sleep/wake activity. Using fast scan cyclic voltammetry, we then examined whether HCRTr1 knockdown alters DA signaling dynamics in the NAc core. We observed reduced DA release and slower uptake rate as well as attenuated cocaine-induced DA uptake inhibition in rats with knock down of HCRTr1. These observations indicate that HCRTr1 within the VTA influence the motivation for cocaine, likely via alterations in DA signaling in the NAc.
Graphical Abstract
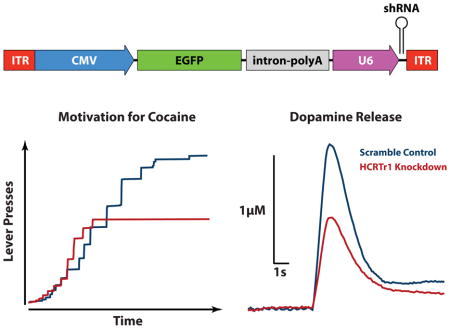
Introduction
The hypocretin/orexin (HCRT) neuropeptide system is recognized to influence arousal-related processes (Hagan et al., 1999; Bourgin et al., 2000; España et al., 2001; Adamantidis et al., 2007). Although initially identified for its participation in governing sleep/wake function, HCRT is increasingly recognized to influence reward and reinforcement (Boutrel et al., 2005; Harris et al., 2007; Smith et al., 2010; Calipari and España, 2012; Mahler et al., 2012). For example, infusions of HCRT-1 peptide increase self-administration of cocaine (España et al., 2011), increase alcohol consumption (Schneider et al., 2007), and reinstate food-based responding (Boutrel et al., 2005). Furthermore, systemic or ventral tegmental area (VTA) delivery of the HCRT receptor 1 (HCRTr1) antagonist, SB-334867, reduces cocaine self-administration (Borgland et al., 2009; España et al., 2010; Hutcheson et al., 2011; Bentzley and Aston-Jones, 2015; Brodnik et al., 2015; Prince et al., 2015), reinstatement of cocaine seeking (Smith et al., 2010), and alters conditioned place preference for morphine (Sharf et al., 2010). These bidirectional effects of HCRT manipulations are consistent with the effects of genetic disruption of HCRT neurotransmission, as animals with complete knockout of HCRT peptides display reduced preference for morphine (Narita et al., 2006; Sharf et al., 2010) and cocaine (Shaw et al., 2016), and mice with constitutive knock out of HCRTr1 receptors show diminished cocaine self-administration (Hollander et al., 2012). Together, these observations indicate that the HCRT system exerts a prominent influence on reward and reinforcement.
The behavioral effects of HCRT manipulations appear to be mediated, in part, through actions on the mesolimbic dopamine (DA) system. HCRT neurons send robust projections to the VTA (de Lecea et al., 1998; Fadel and Deutch, 2002), where HCRT has been shown to potentiate excitatory drive and induce burst firing of DA neurons (Korotkova et al., 2003; Borgland et al., 2006). Furthermore, intra-VTA infusions of HCRT-1 peptide increase DA in the nucleus accumbens (NAc) core under baseline conditions, and promote DA responses to cocaine (España et al., 2011). Consistent with these effects, systemic HCRTr1 blockade via SB-334867 reduces baseline and psychostimulant-induced increases in DA signaling in the NAc (España et al., 2010; Quarta et al., 2010; Prince et al., 2015; Levy et al., 2017). Further, intra-VTA infusion of SB-334867 decreases excitation of DA neurons (Moorman and Aston-Jones, 2010) and disrupts DA responses to cocaine (España et al., 2010). Finally, HCRT knockout mice display disrupted DA signaling in the NAc core under baseline conditions and following cocaine (Shaw et al., 2016). Together, these observations provide strong evidence for HCRT involvement in the regulation of mesolimbic DA signaling that may underlie observed effects on cocaine reinforcement.
The majority of studies assessing HCRTr1 involvement in reward and reinforcement have employed acute, often systemic pharmacological manipulations to HCRT signaling. While this has yielded significant advancements in our understanding of HCRTr1 function, potential issues related to receptor and site specificity exist. For instance, SB-334867 has been criticized because of hydrolytic instability and possible off-target effects at the HCRT receptor 2 (HCRTr2), the A2a adenosine receptor, and 5-HT2a serotonin receptor (Gotter et al., 2012; McElhinny et al., 2012; Lebold et al., 2013). In addition, most pharmacological manipulations of HCRTr1 are systemic, and therefore do not provide information about the contributions of particular brain regions, such as the VTA. To circumvent receptor specificity issues, and to evaluate the importance of the VTA in modulating HCRT effects on behavior, we injected a short-hairpin RNA (shRNA) virus designed to knock down HCRTr1 mRNA (HCRTr1-shRNA-AAV10) directly in the VTA. In addition to allowing for specific targeting of HCRTr1, the viral approach also enabled us to examine the effects of sustained disruption of HCRTr1 on the regulation of cocaine self-administration and DA signaling. We evaluated the effects of the HCRTr1-shRNA-AAV10 virus or the scramble control virus (SCRM-shRNA-AAV10), on the acquisition and maintenance of cocaine self-administration by using a combination of fixed ratio (FR) and progressive ratio (PR) schedules of reinforcement. We next assessed the effects of HCRTr1 knockdown on sleep/wake activity, to confirm that HCRTr1 manipulations do not produce gross deficits in arousal. Finally, to examine whether the effects of HCRTr1 knockdown on behavior are associated with alterations in DA signaling, we used in vivo fast scan cyclic voltammetry (FSCV) to examine DA release and uptake dynamics in the NAc core.
Materials and Methods
Animals
Adult (350–400 g), male Sprague-Dawley rats (Harlan Laboratories, Frederick MD) were housed on a reverse light/dark cycle and given ad-libitum access to food and water. Animals were pair-housed prior to receiving infusions of HCRTr1-shRNA-AAV10 or SCRM-shRNA-AAV10 viruses and subsequently single-housed. All protocols and animal procedures were conducted in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals under the supervision of the Institutional Animal Care and Use Committee at Drexel University College of Medicine.
Preparation of Viral Vectors
We utilized shRNA viruses, which have been shown to modulate the expression of target proteins (Budygin et al., 2016). Vectors were created at the University of Buffalo, under the direction of Dr. Caroline E. Bass. The HCRTr1-shRNA-AAV10 viruses (Fig. 1A) produce shRNAs, driven by the mouse U6 promoter that targets and degrades the HCRTr1 mRNA. To effectively attenuate HCRTr1 expression, we generated three HCRTr1 shRNAs that target the following HCRTr1 sequences: (TGGTGCGGAACTGGAAGCGA, TGGCGCGATTATCTCTATCCG, and TAGCCAATCGCACACGGCTCT), as previous research has shown that such a combinatorial approach can produce a stronger cumulative effect (Bahi et al., 2005). The shRNAs consist of 20 – 21 nucleotide target sequences, a loop sequence (ACTCGAGA) containing an XhoI site, and the antisense to the HCRTr1 target sequence. In the scramble control virus the shRNA does not target any known RNA sequence in the rat genome. The constructs also produce an enhanced green fluorescent protein (EGFP) which is driven by a human cytomegalovirus promotor (CMV). Both the CMV promotor and AAV2/10 capsid are highly selective for neurons, a finding which as has been previously reported (Tanguy et al., 2015). Consequently, it is likely that the virus used here preferentially transfects neuronal cells of the VTA which include both DA and GABA neurons, as well as the relatively small population of glutamate neurons located in this region (Yamaguchi et al., 2007; Dobi et al., 2010). It is important to note that HCRTr1 is expressed in both DA and GABA neurons of the VTA (Korotkova et al., 2002; Korotkova et al., 2003; Narita et al., 2006; Baimel and Borgland, 2015), and thus HCRTr1 knockdown is predicted to reduce HCRTr1 expression in both of these neuronal subtypes. Lastly, the virus is likely to transfect only the cells in the area of injection, as AAV2/10 is not retrogradely transported (Cearley and Wolfe, 2007).
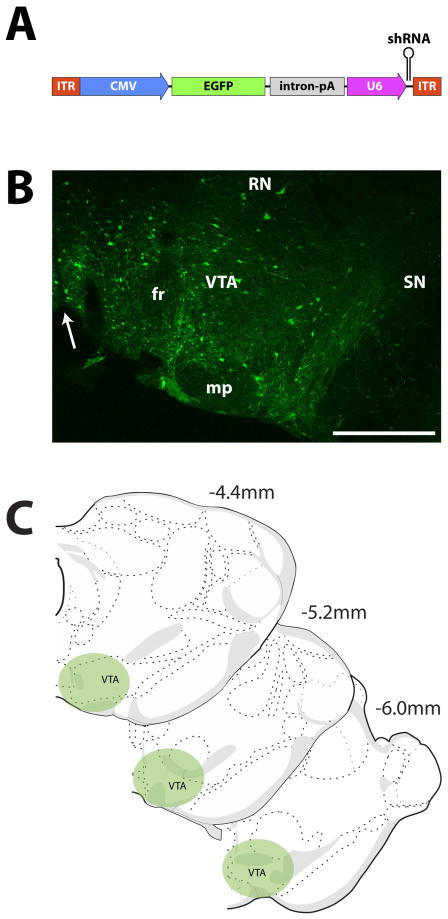
Figure 1. Illustration of viral vector and infusion placement in VTA.
(A) Transgene packaged into the SCRM-shRNA-AAV10 (SCRM) or HCRTr1-shRNA-AAV10 (KD) viruses. Viral vectors were comprised of two noncoding ITRs flanking a CMV promotor that drives expression of the EGFP fluorescent reporter protein, followed by a combined intron/poly A sequence to enhance EGFP expression, and a U6 promotor to drive expression of the shRNA. Viruses differed only in the shRNA sequence used. (B) Example photomicrograph depicting EGFP labeling resulting from injection of the KD virus in the VTA. Arrow indicates midline. (C) Illustration of injection locations across the rostrocaudal extent of the VTA. CMV, human cytomegalovirus promoter; EGFP, enhanced green fluorescent protein; fr, fornix; ITR, AAV inverted terminal repeats (viral sequences for packaging transgene); intron-pA: intron and poly A sequences derived from SV40; mp, mammillary peduncle; RN, Red Nucleus; SN, substantia nigra. U6, mouse U6 promoter for driving shRNA expression; shRNA, short hairpin RNA targeting the HCRTr1 or a scrambled sequence; VTA, ventral tegmental area. Scale bar is 0.5 mm.
Virus Infusion
To deliver the HCRTr1-shRNA-AAV10 or SCRM-shRNA-AAV10 viruses, animals were anesthetized with isoflurane and placed into a stereotaxic frame, with the skull flat. To target VTA, a 1 mm hole was drilled in the skull (−5.25 mm A/P, +0.95 mm M/L relative to bregma) and a glass injection pipette (Sigma Aldrich, St. Louis MO) was lowered into the VTA, reaching a final depth of 7.8 mm ventral to the brain surface. Glass pipettes were cut to an inner diameter of ~30 μM. After waiting 10 min to allow tissue to settle, 0.5 μl of HCRTr1-shRNA-AAV10 or SCRM-shRNA-AAV10 was delivered into the VTA over 10 min using a picospritzer. Figure 1B shows an example infusion location in the general region of the VTA. This region of the VTA has previously been shown to express moderate levels of HCRTr1 (Marcus et al., 2001; Ch’ng and Lawrence, 2015). Following surgery, animals were housed singly, administered ketoprofen (5 mg/kg, s.c.) at 12 hr intervals for 36 hr, and then designated for molecular, behavioral, or neurochemical experiments.
Quantitative Real-Time PCR (qRT-PCR)
Rats received infusion of HCRTr1-shRNA-AAV10 virus in one hemisphere of the VTA and SCRM-shRNA-AAV10 virus into the contralateral hemisphere. After 4 weeks of incubation, animals were anesthetized with isoflurane and rapidly decapitated. Brains were extracted over ice and placed into a matrix slicing guide on ice, with the ventral surface visible. Two coronal cuts were made, with the rostral cut at the caudal portion of the mammillary bodies, and the second cut made 2.0 mm caudal to the first, yielding a single slice (−4.4 to −6.4 mm A/P relative to bregma) encompassing the majority of VTA. The tissue comprising VTA was taken as the area ventrolateral to the periaqueductal grey, ventral to the mesencephalic reticular nucleus, and medial to the substantia nigra (Fig. 1C). In all cases, the HCRTr1-shRNA-AAV10 hemisphere was compared with the contralateral SCRM-shRNA-AAV10 hemisphere from the same animal.
Immediately following extraction, tissue was placed into 75 ul of RNAlater (Qiagen Inc., Valenia, CA, USA), and stored at −20° C until processing. Total RNA was purified as previously described (Barson et al., 2013), with resulting A260/A280 ratios between 1.88 and 2.19, indicating high purity. cDNA was reverse transcribed and qRT-PCR was conducted as described previously (Barson et al., 2013), using a StepOnePlus Real-Time PCR System (Applied Biosystems, Grand Island, NY). Expression of HCRTr1, HCRTr2, tyrosine hydroxylase (TH), vesicular monoamine transporter (VMAT), D2 DA receptor (D2R) and the DA transporter (DAT) were quantified relative to cyclophilin-A using the relative quantification method (ΔΔCT). Primers were designed with the NCBI Primer design tool (http://www.ncbi.nlm.nih.gov/tools/primer-blast/; (Ye et al., 2012), and obtained from Invitrogen (Grand Island, NY; Table I).
Table I.
Primer sequences and concentrations used for quantitative real-time polymerase chain reaction.
| Primer | Sequence | Concentration |
|---|---|---|
| Cyclophilin-A | 5′-GTGTTCTTCGACATCACGGCT-3′ (forward) 5′-CTGTCTTTGGAACTTTGTCTGCA-3′ (reverse) |
200 nM |
| HCRTr1 | 5′-GCGAGGTCCAGGGCTCACT-3′ (forward) 5′-GCCTGGTACACCCAGATGCCG-3′ (reverse) |
100 nM |
| HCRTr2 | 5′-GGCAGGACCGCTTCAAAACTGGTAT-3′ (forward) 5′-CCCTCAGACCTCCAGCCAGGT-3′ (reverse) |
200 nM |
| TH | 5′-CCTTCCAGTACAAGCACGGT-3′ (forward) 5′-TGGGTAGCATAGAGGCCCTT-3′ (reverse) |
100 nM |
| VMAT | 5′-AGTCTCTGCTGTCTTGCCAAC-3′ (forward) 5′-TGGTCCAGTGAAGCTCACTTCT-3′ (reverse) |
200 nM |
| D2R | 5′-AGACGATGAGCCGCAGAAAG-3′ (forward) 5′-GCAGCCAGCAGATGATGAAC-3′ (reverse) |
100 nM |
| DAT | 5′-CAACGGTGGCATCTACGTCT-3′ (forward) 5′-CTGAATTGCTGGACGCCGTA-3′ (reverse) |
50 nM |
Immunohistochemistry and Cell Counting
At the conclusion of all but the qRT-PCR experiments, rats were anesthetized with isoflurane and transcardially perfused with 140 ml saline, followed by 150 ml of 10% formalin in 0.01 M phosphate-buffered saline (pH 7.4). Brains were extracted and fixed in 10% formalin solution for a further 60 min, then placed in a solution of 30% sucrose in PBS for 72 hr at 4 °C. Prior to cryosectioning, a tracking mark was made in the right lateral cortex for identification of hemispheres. Using a microtome, 40 μM coronal sections through the VTA were collected, and then stored in a solution of 0.1% sodium azide in 0.01 M PBS, at 4 °C. To identify the site of infusion, sections were incubated overnight with primary antibodies for EGFP (600-101-215, 1:10000, Rockland Antibodies, Gilbertsville, PA) and TH (#AB152, 1:2000, EMD Millipore, Temacula, CA) at 4 °C, followed by Alexa-fluor 488 donkey anti-goat (A11055, 1:1000, Life Technologies, Eugene, OR) and Alexa-fluor 594 donkey anti-rabbit (A21207, 1:1000, Life Technologies) secondary antibodies, respectively for 90 min at 20 °C. To determine cellular density in the transfection sites, sections were incubated with the NeuN primary antibody (ab104225, 1:5000, Abcam, Cambridge, MA), overnight at 4 °C, followed by biotin-SP-conjugated donkey anti-rabbit secondary antibody (#711-065-152, 1:1000, Jackson Labs, West Grove, PA) for 90 min at 20 °C, followed by 60 min with the Avidin-Biotin Complex (Vector Laboratories, Burlingame, CA), and 2–5 min with the DAB reagent (Vector Laboratories). Sections were mounted and coverslipped with fluorescent mounting medium (Vector Laboratories) or with Vectamount (Vector Laboratories).
To determine the magnitude and location of viral expression, EGFP labeled cells were counted in the VTA and adjacent structures across the anterior (+4.4 mm), central (+5.2 mm), and posterior (+6.0 mm) aspects of VTA, relative to bregma. Counts were performed by an experimenter blinded to treatment conditions under 200x magnification using on an Axiovert 135m fluorescence microscope (Zeiss Instruments, Thornwood NY). To be included in analyses rats had to display; 1) an average count of at least 100 EGFP-labeled cells in each hemisphere of the VTA across the 3 rostro-caudal sections sampled; and 2) at least 75% of all EGFP-labeled cells had to be located in the VTA as opposed to adjacent structures.
To examine whether virus infusions resulted in loss of neurons in the VTA, sections containing the VTA were captured at 200x magnification using SpotPro 5.1 Imaging software (Spot Digital, Taipei, Taiwan). Images were taken from the three rostrocaudal (+4.4, +5.2, and +6.0) aspects of VTA centered at approximately 7.7 mm ventral from top of brain and 0.9 mm lateral to midline. This approach resulted in images that encompassed an approximately 1.0 mm x 0.75 mm rectangular area that was bordered medially by midline, laterally by the substantia nigra, and dorsally by the medial reticular nucleus for rostral sections and the red nucleus for caudal sections. Cell counts were analyzed using ImageJ (NIH) by an experimenter blinded to treatment conditions.
Cocaine Self-Administration
Rats received bilateral infusions of either the HCRTr1-shRNA-AAV10 or SCRM-shRNA-AAV10 viruses into the VTA. After 2 weeks of incubation, rats were anesthetized with a ketamine/xylazine (80/10 mg/kg, i.p.), and implanted with jugular catheters as previously described (España et al., 2010; Brodnik et al., 2015; Prince et al., 2015). Immediately following surgery, rats were placed in operant behavioral chambers, where they were housed for the duration of the experiments. On the third day after surgery, rats were placed on a FR1 schedule of reinforcement, whereby single lever presses resulted in delivery of 0.75 mg/kg intravenous (i.v.) cocaine (in saline; National Institute on Drug Abuse). Rats were given 6-hr daily access to a lever, and allowed a maximum of 20 injections per session. Following acquisition of self-administration (10 injections per session for 3 consecutive days), rats were allowed to self-administer on the FR1 schedule until they reached stable responding (3 days of 20 injections per session). The latency to first cocaine injection and rate of intake were monitored during these sessions. Rats were then switched to the PR schedule of reinforcement, with 6-hr access to a lever, and single cocaine (0.75 mg/kg) injections now contingent upon an increasing number of responses (Richardson and Roberts, 1996). The 0.75mg/kg dose was selected because it is on the ascending limb of the “inverted-U” dose-response curve under the PR schedule (Richardson and Roberts, 1996) and to compare to previous observations (España et al., 2010; Brodnik et al., 2015; Prince et al., 2015). Average breakpoint (number of cocaine injections received per session without an intervening 1 hr break), and total lever presses were recorded for 10 days. The timing of catheterization surgeries and behavioral training were selected to ensure that rats were self-administering under the PR schedule at approximately 4 weeks after virus infusion.
Sleep/Wake Studies
Rats received bilateral infusion of either HCRTr1-shRNA-AAV10 or SCRM-shRNA-AAV10 into the VTA. After 3 weeks of incubation, rats were anesthetized with isoflurane, and placed into a stereotaxic frame. Two electroencephalographic (EEG) screw electrodes (Plastics One, Roanoke VA), were implanted bilaterally above the frontal cortex (+1.3 mm A/P; ±1.3 mm M/L; −1.0 mm D/V) and a third electrode was implanted above the hippocampus (2.7 A/P; +3.2 mm M/L; −1.0 mm D/V). Additionally, two electromyographic (EMG) wire electrodes were implanted into the dorsal neck muscle. EMG electrodes consisted of 100 mm insulated stainless steel wires (Cooner Wire, Chatsworth, CA) with 2 mm of exposed wire directly in contact with muscle. Electrodes were routed through a connector (Plastics One), and cemented into place on the skull. Timing of EEG/EMG surgeries was selected to ensure that rats were tested for sleep/wake activity 4 weeks after virus infusion. After 1 week of recovery, rats were placed into recording chambers with food and water available ad-libitum, and then connected to an electrophysiology headstage. After 16 hr of acclimation, baseline EEG (0.3–100.0 Hz bandpass) and EMG signals (1.0–50.0 Hz bandpass) were amplified, filtered, and recorded using Labchart 7 (AD Instruments, Colorado Springs, CO) for 48 hr. Data were analyzed using Sirenia Sleep Pro (Pinnacle Technology, Lawrence, KS) to obtain measures of waking, rapid eye movement sleep (REM), and nonREM (NREM) sleep (Supplemental Figure 1).
Fast Scan Cyclic Voltammetry
Rats received unilateral infusion of either HCRTr1-shRNA-AAV10 or SCRM-shRNA-AAV10 into the VTA. After 4 weeks of incubation, rats were anesthetized with isoflurane, implanted with an acute jugular catheter, and placed in a stereotaxic frame. Rats were implanted with a bipolar stimulating electrode (Plastics One) in the VTA (+5.2 mm A/P, +1.1 mm M/L, −7.5 to −8.0 mm D/V), a carbon fiber microelectrode in the core of the NAc (−1.3 mm A/P, +1.3 mm M/L, −6.5 to −7.0 mm D/V), and a reference electrode in the contralateral cortex (−2.5 mm A/P, −2.5 mm M/L, −2.0 mm D/V). Monophasic electrical pulse trains (60 Hz, 4 ms, 600 mA, 60 pulses) in the VTA were used to evoke DA release in the NAc, in accordance with established methods (Prince et al., 2015; Levy et al., 2017). During baseline recordings, electrically-evoked DA release was elicited every 5 min for a minimum of 30 mins or until a stable baseline recording was obtained (DA release within 10% across three consecutive stimulations). After obtaining 15 min of stable baseline recordings, animals received a 2 s, ~200 μl i.v. cocaine injection (1.5 mg/kg). The 1.5mg/kg dose of cocaine was used to compare with previous FSCV studies (España et al., 2010; España et al., 2011; Prince et al., 2015; Levy et al., 2017) and because this dose produces sufficient DA uptake inhibition (España et al., 2008) to assess reductions in cocaine effects following disruptions to HCRTr1. Electrically-evoked DA release was elicited 30 sec, 1 min, and 5 min post cocaine delivery, and every 5 min thereafter for 60 min.
The electrode potential was linearly scanned (−0.4 to 1.2 V and back to −0.4 V vs Ag/AgCl) and cyclic voltammograms were recorded every 100 ms with a scan rate of 400 V/s using a voltammeter/amperometer (Chem-Clamp; Dagan Corporation, Minneapolis, MN). Quantification of electrically-evoked DA release was achieved by comparing current at the peak oxidation potential for DA in consecutive voltammograms with calibration factors obtained from electrodes exposed to 3 μM DA. DA overflow curves were fitted to a Michaelis-Menten-based kinetic model using Demon Voltammetry and Analysis software (Yorgason et al., 2011) written in LabVIEW language (National Instruments, Austin, TX). DA uptake rates prior to cocaine delivery were modeled by setting the affinity of DA for the DA transporter (DAT) between 0.16 – 0.20 μM and then fitting the overflow curve to establish a baseline, maximal uptake rate (Vmax) for each subject. Baseline release and uptake were expressed as the average of 3 collections that occurred prior to the injection of cocaine. Following cocaine injection, Vmax was held constant for the remainder of the experiment and changes in DA uptake rate due to cocaine-induced uptake inhibition were calculated as a change in the apparent affinity for the DAT and defined as (Km).
Data selection
Data were included in analyses only when EGFP labeling was confined to the VTA without significant transfection of adjacent regions including the substantia nigra, red nucleus, or medial reticular nucleus. Across all studies, 3 HCRTr1 knockdown and 3 scramble rats were excluded due to inadequate EGFP expression or misplacement of the virus infusion.
Statistical Analysis
Paired student’s t-tests were used to measure effects of HCRTr1-shRNA-AAV10 or SCRM-shRNA-AAV10 on mRNA expression, NeuN, and TH cell counts. Acquisition of cocaine self-administration, time to first injection, and rate of intake were analyzed using independent student’s t-test to compare between virus treatments. The overall effects of HCRTr1 knockdown on motivation were assessed using a mixed design repeated-measures ANOVA, with viral treatment as the between-subjects variable and days as the within-subjects variable. Independent student’s t-test were conducted to compare the effects of viral treatment on time spent in each sleep/wake state and to measure differences in DA release and uptake (Vmax) prior to cocaine in FSCV experiments. Stimulated DA release, DA per pulse [DAp] and cocaine-induced DA uptake inhibition (Km) across the course of the experiment were assessed using mixed design, repeated measures ANOVAs with viral treatment as the between-subjects variable and time as the within-subjects variable. Planned comparisons for each of these DA measures were conducted using independent student’s t-tests during the first 15 min following cocaine delivery based on previous observations indicating significant effects of HCRT manipulations on DA signaling at those time points (España et al., 2010; España et al., 2011; Prince et al., 2015). Planned comparisons were not conducted at other time points. All data were analyzed using SPSS (SPSS Inc, Chicago, IL).
Results
HCRTr1-shRNA-AAV10 successfully reduces HCRTr1 in VTA
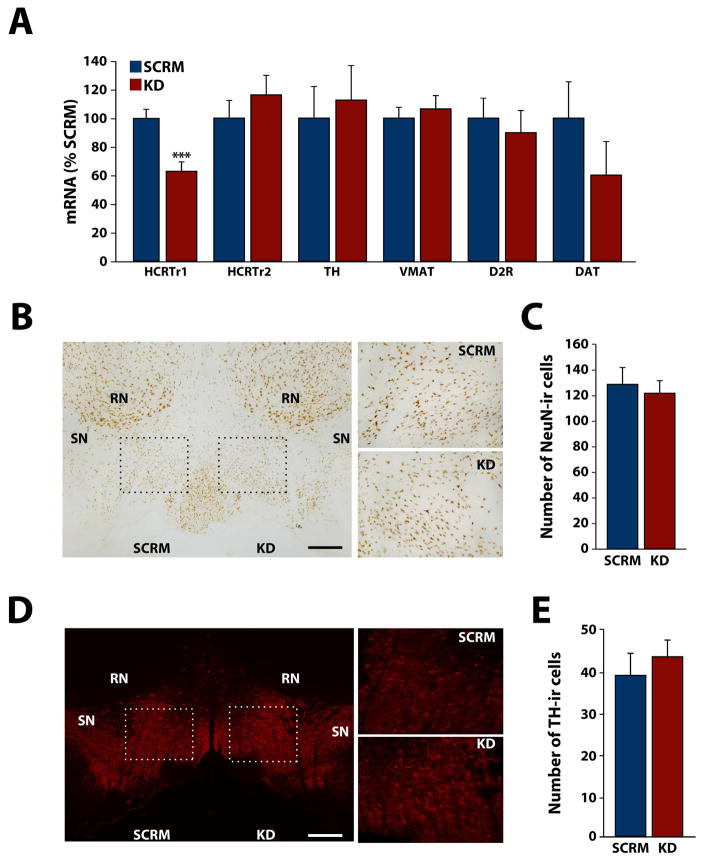
To assess the effectiveness of viral transfection, rats were injected intra-VTA with HCRTr1-shRNA-AAV10 (n=10) in one hemisphere and SCRM-shRNA-AAV10 (n=10) in the contralateral hemisphere. Four weeks after virus injection, we observed that 0.5 μl infusions of either virus produced robust expression of EGFP in the VTA (Fig. 1B and C) with little encroachment into adjacent regions including the substantia nigra. Injections of HCRTr1-shRNA-AAV10 significantly knocked down HCRTr1 receptor mRNA by nearly 40% in the VTA relative to SCRM-shRNA-AAV10 (t(9) = 4.5, p < 0.001; Fig. 2A). Importantly, HCRTr1-shRNA-AAV10 did not alter HCRTr2, TH, VMAT or D2R mRNA, suggesting that our approach did not indiscriminately alter DA neurons. Although not significant, there was a notable decrease in DAT mRNA levels (t(9) = 2.23, p = 0.083).
Figure 2. HCRTr1-shRNA-AAV10 reduces HCRTr1 expression in the VTA.
(A) Shown are the mean ± SEM of mRNA levels for HCRTr1, HCRTr2, TH, VMAT, D2R, and DAT in the VTA, following injection of SCRM-shRNA-AAV10 (SCRM) or HCRTr1-shRNA-AAV10 (KD) virus. (B) Example photomicrographs depicting NeuN staining in the VTA following injection of SCRM or KD virus. Left panel; 50x magnification image depicting the VTA regions used for NeuN-immunoreactive (ir) cell counts. Dashed rectangles indicate the regions used for analysis (see Methods and Materials). Right panels; 200x representation of the dashed rectangular regions shown in the Left panel. (C) Shown are mean ± SEM average number of NeuN-ir cells in the VTA. (D) Example photomicrographs depicting TH staining in VTA following injection of SCRM or KD virus. Left panel; 50x magnification image depicting the VTA regions used for TH-ir cell counts. Dashed rectangles indicate the regions used for analysis (see Methods and Materials). Right panels; 200x representation of the dashed rectangular regions shown in the Left panel. (E) Shown are mean ± SEM average number of TH-ir cells in the VTA. DAT, dopamine transporter; D2R, D2 DA receptor; RN, red nucleus; SN, substantia nigra; TH, tyrosine hydroxylase; VMAT, vesicular monoamine transporter. Scale bars are 0.5 mm. *** p < 0.001.
To assess for potential toxicity, a separate group of animals was transfected as described above (n=4 per virus). After 4 weeks of incubation, coronal sections containing VTA were immunostained for NeuN as an established measure of neuronal density, and TH as a marker of DA synthesizing neurons. As shown in figure 2B and C, HCRTr1-shRNA-AAV10 did not affect NeuN-immunoreactive (ir) cell counts in the VTA relative to SCRM-shRNA-AAV10 (t(3) = 1.02, p = 0.38). Additionally, HCRTr1-shRNA-AAV10 did not affect TH-ir cell counts relative to SCRM-shRNA-AAV10 (t(3) = 1.02, p = 0.38, Fig. 2D and E). Together these observations suggest that HCRTr1 knockdown is not associated with toxicity to VTA DA neurons.
HCRTr1 knockdown disrupts cocaine self-administration
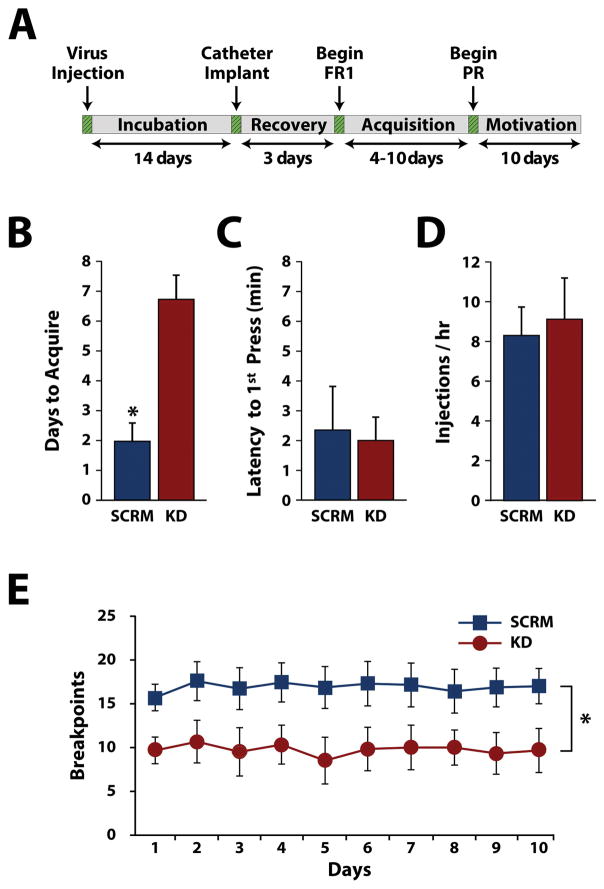
To examine the involvement of HCRTr1 in the VTA on cocaine self-administration, rats received HCRTr1-shRNA-AAV10 (n=7) or SCRM-shRNA-AAV10 (n=6) bilaterally into the VTA. After 2 weeks of incubation, animals were implanted with jugular catheters and placed on an FR1 schedule to acquire cocaine self-administration (Fig. 3A). As shown in Figure 3B, HCRTr1 knockdown significantly delayed acquisition of cocaine self-administration, relative to scramble controls (t(11) = 2.27, p = 0.044). Further, as shown in figure 3C and D, no differences were observed between virus treatments for latency to first lever press (t(11) = 0.44, p = 0.62) nor the rate of cocaine intake (t(11) = 0.51, p = 0.52). Following testing on the FR1 schedule, rats were switched to a PR schedule to assess HCRTr1 knockdown effects on motivation for cocaine. As indicated in figure 3E, over the 10 days of testing, HCRTr1 knockdown produced significantly lower breakpoints relative to scramble controls. Two-way repeated measures ANOVA indicated a significant effect of viral treatment (F(1,11) = 5.5, p = 0.039) but no significant effect of time (F(9,99) = 0.425, p = 0.92) or treatment X time interaction (F(9,99) = 0.649, p = 0.753). The total number of lever presses was not significantly different between groups (data not shown).
Figure 3. HCRTr1 knockdown delays acquisition of cocaine self-administration and reduces motivation for cocaine.
(A) Timeline of surgical procedures and experimental testing. Shown are the mean ± SEM (B) number of days required to reach acquisition criteria, (C) latency to first lever press, and (D) intake rate (i.e., injections/hr) under a fixed-ratio 1 schedule of reinforcement for rats treated with SCRM-shRNA-AAV10 (SCRM) or HCRTr1-shRNA-AAV10 (KD). (E) Shown are the mean ± SEM breakpoints across the 10-day progressive ratio schedule for SCRM and KD animals. * p < 0.05.
HCRTr1 knockdown does not alter sleep/wake behavior
To examine whether the effects of HCRTr1 knockdown on self-administration were associated with alterations in sleep/wake activity, rats received SCRM-shRNA-AAV10 or (n=6) or HCRTr1-shRNA-AAV10 (n=6) bilaterally into the VTA. After 3 weeks of incubation, rats were implanted with EEG/EMG electrodes, and then after 1 week of recovery, sleep/wake activity was recorded for 24 hr. As shown in figure 4, HCRTr1 knockdown did not affect total time spent in waking (t(10) = 1.23, p = 0.25), NREM (t(10) = 0.57, p = 0.58) or REM sleep (t(10) = 1.05, p = 0.32). Further, no differences were observed between virus treatment on the number (waking: t(10 ) = 0.42, p = 0.66; NREM: t(10) = 0.36, p = 0.73; REM: t(10) = .62, p = 0.55) or duration (waking t(10 ) = 0.13, p = 0.91; NREM: t(10) = 0.52, p = 0.61; REM: t(10) = 0.42, p = 0.68) of sleep/wake bouts (Supplemental Figure 1). These results suggest that the behavioral effects of HCRTr1 knockdown are not associated with gross alterations to sleep/wake activity.
Figure 4. HCRTr1 knockdown does not alter sleep/wake behavior.
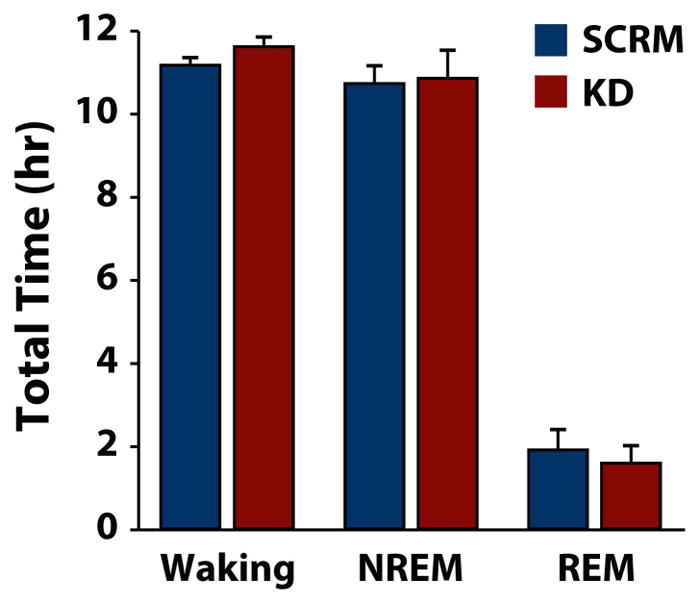
Shown are the mean ± SEM total time spent in waking, non-rapid eye movement (NREM) and rapid eye movement (REM) sleep for rats treated with SCRM-shRNA-AAV10 (SCRM) or HCRTr1-shRNA-AAV10 (KD).
HCRTr1 knockdown disrupts DA signaling
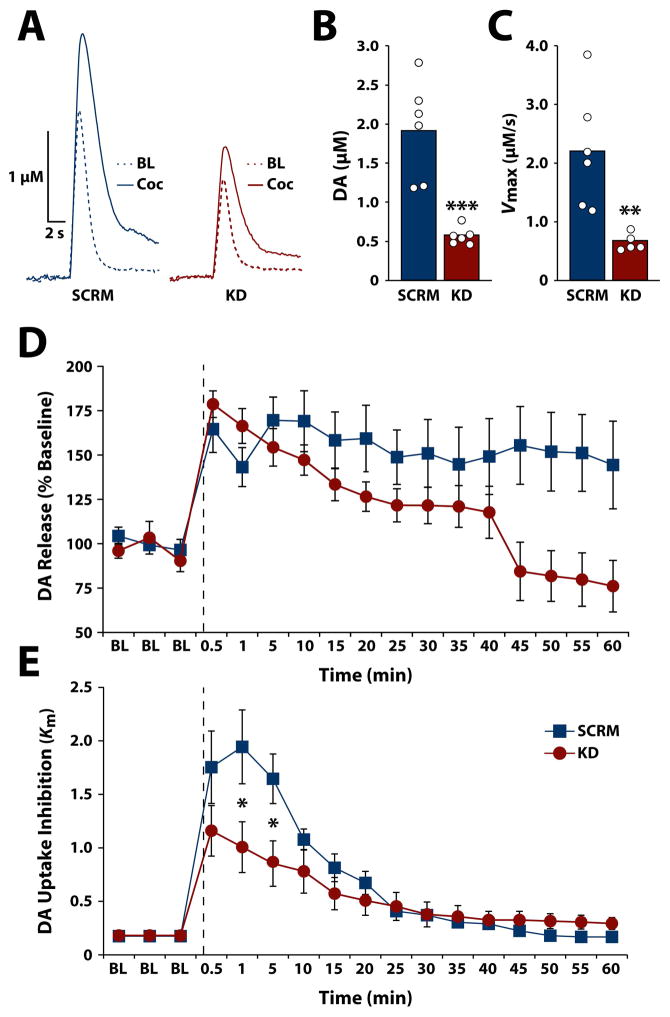
To examine the extent to which HCRTr1 knockdown in VTA impacts DA signaling, rats received unilateral infusion of HCRTr1-shRNA-AAV10 (n=7) or SCRM-shRNA-AAV10 (n=6). After 4 weeks of incubation, DA release and uptake were monitored in the NAc core using FSCV. As shown in figure 5 and Supplemental Figure 2, HCRTr1 knockdown significantly reduced stimulated DA release (t(10) = 5.15, p < 0.001) and reduced maximal uptake (t(10) = 3.73, p = 0.0038) under baseline conditions relative to scramble controls. Importantly, however, when expressed as a percent of baseline, HCRTr1 knockdown did not reduce DA release following cocaine delivery (Fig. 5D). Mixed design, repeated measures ANOVA, with viral treatment as the between-subjects variable and time as the within-subjects variable revealed no effect of virus (F(1,10) = 2.82, p = 0.124), but did indicate a significant effect of time (F(16,160) = 9.4, p < 0.001) and a virus x time interaction (F(16,160) = 3.96, p < 0.001). Planned comparisons did not reveal significant differences between groups following cocaine delivery, indicating that cocaine exerts proportionally similar effects on DA release in both scramble and HCRTr1 knockdown animals.
Figure 5. HCRTr1 knockdown disrupts DA signaling under baseline conditions and in response to cocaine.
(A) Example traces of stimulated DA release prior to (BL) and following 1.5 mg/kg i.v. cocaine (Coc) in rats treated with SCRM-shRNA-AAV10 (SCRM) or HCRTr1-shRNA-AAV10 (KD). Shown are the raw data points as well as the mean for (B) stimulated DA release and (C) DA uptake rate (Vmax) in the NAc under baseline conditions prior to cocaine for SCRM and KD animals. Shown are the mean ± SEM for (D) stimulated DA release expressed as a percent of baseline and (E) cocaine-induced DA uptake inhibition (Km), across the experimental session for SCRM and KD animals. Dashed lines indicate time of 1.5 mg/kg i.v. cocaine injection. * p < 0.05, ** p < 0.01, *** p < 0.001.
Nevertheless, HCRTr1 knockdown significantly attenuated cocaine-induced inhibition of DA uptake (Km; Fig 5E). Mixed design ANOVA revealed no significant effect of virus (F(1,10) = 1.64, p = 0.23) but did indicate a significant effect of time (F(16,160) = 29.97, p < 0.001), and virus x time interaction (F(16, 160) = 4.06, p < 0.001). Subsequent analysis indicated that HCRTr1 knockdown significantly reduced cocaine-induced DA uptake inhibition at the 1 min (t(10) = 2.23, p = 0.049) and 5 min (t(10) = 2.53, p = 0.03) post-cocaine time points.
Discussion
In the present studies, we used a viral shRNA approach to determine the degree of VTA HCRTr1 involvement in the regulation of cocaine self-administration and DA signaling in the NAc. We demonstrate that HCRTr1 knockdown disrupts acquisition of cocaine self-administration and reduces motivation for cocaine, without affecting sleep/wake activity. Further, HCRTr1 knockdown also disrupts DA release and uptake under baseline conditions and attenuates the effects of cocaine on DA uptake. These observations indicate that HCRTr1 influence the motivation for cocaine likely via actions on mesolimbic DA signaling.
HCRTr1 knockdown alters the motivation for cocaine
Numerous observations suggest that HCRTr1 influences motivated behavior, particularly as it relates to drugs of abuse. However, the majority of studies examining HCRT regulation of motivated behavior have employed systemic delivery of HCRT agents, and consequently the specific neural structures involved in these actions is unclear. While limited observations suggests that the VTA may participate in HCRT-mediated effects on behavior (España et al., 2010; Moorman and Aston-Jones, 2010), those observations relied on SB-334867, which has been criticized for potential off-target effects (Gotter et al., 2012). To address these pontential limitations, we employed knock down of HCRTr1 specifically within the VTA and examined the effects on cocaine self-administration. Results indicate that HCRTr1 knockdown delayed acquisition of cocaine self-administration without affecting cocaine intake on an FR1 schedule. Moreover, HCRTr1 knockdown also reduced the motivation for cocaine under a PR schedule. However, it must be noted that self-administration was examined for only one dose of cocaine, which could influence the magnitude of HCRTr1 knockdown effects (Brodnik et al., 2015). Nevertheless, when considered with the existing literature, the present findings indicate that HCRTr1 in the VTA may be critical for establishing and maintaining motivation for cocaine.
HCRTr1 in the VTA are necessary for normal DA signaling
Previous reports suggest that HCRT regulation of cocaine self-administration is likely mediated via actions on the mesolimbic DA system. For example, intra-VTA infusions of HCRT-1 peptide increase DA in the NAc (España et al., 2011), while HCRTr1 antagonists into the VTA decrease DA (España et al., 2010). These observations are consistent with the present findings indicating that HCRTr1 knockdown in the VTA reduces DA release and uptake under baseline conditions and attenuates cocaine-induced DA uptake inhibition.
Given the importance of NAc DA for supporting motivation for both cocaine (Phillips et al., 2003) and natural (Syed et al., 2016) rewards, it is possible that the effects of HCRTr1 knockdown on baseline DA neurotransmission may have generalized effects on behavior. Indeed, previous observations indicate that HCRTr1 blockade reduces motivation for sucrose in sated but not food restricted rats (España et al., 2010) and affects self-administration of highly palatable foods but not regular chow (Borgland et al., 2009). Importantly, these effects of HCRTr1 manipulations do not appear to be associated with generalized reductions in arousal or sedation (Brodnik et al., 2015), which is consistent with our current findings.
The mechanisms through which HCRTr1 influences DA signaling are not well-understood. One possibility is that knock down of HCRTr1 in the VTA disrupts DA release by reducing excitatory drive on DA neurons. Indeed there is substantial evidence to suggest that HCRT is responsible for mediating changes in DA neuron excitability, as HCRT-1 peptide exerts an excitatory influence on VTA DA neurons either directly (Korotkova et al., 2003), through suppression of GABAergic input onto DA neurons (Tung et al., 2016), and/or by enhancing glutamatergic drive onto DA neurons (Borgland et al., 2006; Mahler et al., 2013). These HCRT actions have been shown to increase DA neuron firing and bursting in vivo (Moorman and Aston-Jones, 2010; Muschamp et al., 2014), which is known to increase DA at the terminal (Owesson-White et al., 2009; dela Pena et al., 2015). Consistent with these observations, blockade of HCRTr1 reduces DA neuron firing, which is expected to reduce DA in target regions (Moorman and Aston-Jones, 2010). Based on these observations, it is reasonable to suggest that HCRTr1 knockdown results in a similar reduction in the excitability of DA neurons, which could diminish the effectiveness of electrical stimulation and result in reduced DA release as observed herein. Alternatively, HCRTr1 knockdown may exert its effects by differentially engaging distinct pools of releasable DA (Venton et al., 2006; Covey et al., 2013) which may involve synapsin-dependent alterations to presynaptic Ca2+ influx (Pierce et al., 1998; Venton et al., 2006; Federici et al., 2014).
Extensive evidence suggests that DA signaling in the NAc is influenced by a number of second messenger signaling cascades that affect surface localization and functionality of the DAT (Johnson et al., 2005; Mortensen et al., 2008). Thus, in addition to modulating DA neuron firing directly, HCRTr1 knockdown may also influence DA signaling via alterations to the DA terminals. For example, recent observations suggest that HCRT is capable of modulating signaling cascades such as protein kinase C and calmodulin-dependent protein kinase II, which affect the phosphorylation and glycosylation of DAT (Ozcan et al., 2010; Selbach et al., 2010), and may therefore influence DAT surface localization and functionality (Mortensen et al., 2008; Zahniser and Sorkin, 2009). Given that baseline DA uptake is dependent on functional DATs, modifications that alter DAT levels at the cell surface result in changes in baseline DA uptake rates, an effect that can alter psychostimulant potency (Ukairo et al., 2007). Therefore, HCRT-induced changes in DAT function at the terminal may underlie the diminished effects of cocaine at inhibiting DA uptake. We have previously demonstrated that animals treated with a HCRTr1 antagonist (España et al., 2010; Prince et al., 2015; Levy et al., 2017) display reduced cocaine-induced DA uptake inhibition, possibly due to alterations in DAT function. It is possible, therefore, that HCRTr1 knockdown may attenuate the effects of cocaine through such a mechanism. The presents findings offer some evidence for DAT-driven effects, given that HCRTr1 knockdown had little effect on DA release following cocaine but nevertheless reduced cocaine-induced DA uptake inhibition.
Conclusions
We determined that sustained knockdown of HCRTr1 in the VTA alters both DA signaling in the NAc, and cocaine-reinforced behavior, without affecting arousal. These observations offer further evidence in support of the hypothesis that HCRTr1 in the VTA influence motivated behavior through actions on the mesolimbic DA system.
Supplementary Material
References
- Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–424. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahi A, Boyer F, Kolira M, Dreyer JL. In vivo gene silencing of CD81 by lentiviral expression of small interference RNAs suppresses cocaine-induced behaviour. J Neurochem. 2005;92:1243–1255. doi: 10.1111/j.1471-4159.2004.02961.x. [DOI] [PubMed] [Google Scholar]
- Baimel C, Borgland SL. Orexin Signaling in the VTA Gates Morphine-Induced Synaptic Plasticity. J Neurosci. 2015;35:7295–7303. doi: 10.1523/JNEUROSCI.4385-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barson JR, Fagan SE, Chang GQ, Leibowitz SF. Neurochemical heterogeneity of rats predicted by different measures to be high ethanol consumers. Alcoholism, clinical and experimental research. 2013;37(Suppl 1):E141–151. doi: 10.1111/j.1530-0277.2012.01858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzley BS, Aston-Jones G. Orexin-1 receptor signaling increases motivation for cocaine-associated cues. Eur J Neurosci. 2015;41:1149–1156. doi: 10.1111/ejn.12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Chang SJ, Bowers MS, Thompson JL, Vittoz N, Floresco SB, Chou J, Chen BT, Bonci A. Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. J Neurosci. 2009;29:11215–11225. doi: 10.1523/JNEUROSCI.6096-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49:589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Bourgin P, Huitron-Resendiz S, Spier AD, Fabre V, Morte B, Criado JR, Sutcliffe JG, Henriksen SJ, de Lecea L. Hypocretin-1 modulates rapid eye movement sleep through activation of locus coeruleus neurons. J Neurosci. 2000;20:7760–7765. doi: 10.1523/JNEUROSCI.20-20-07760.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, de Lecea L. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc Natl Acad Sci U S A. 2005;102:19168–19173. doi: 10.1073/pnas.0507480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodnik ZD, Bernstein DL, Prince CD, España RA. Hypocretin receptor 1 blockade preferentially reduces high effort responding for cocaine without promoting sleep. Behavioural brain research. 2015;291:377–384. doi: 10.1016/j.bbr.2015.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budygin EA, Oleson EB, Lee YB, Blume LC, Bruno MJ, Howlett AC, Thompson AC, Bass CE. Acute Depletion of D2 Receptors from the Rat Substantia Nigra Alters Dopamine Kinetics in the Dorsal Striatum and Drug Responsivity. Front Behav Neurosci. 2016;10:248. doi: 10.3389/fnbeh.2016.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, España RA. Hypocretin/orexin regulation of dopamine signaling: implications for reward and reinforcement mechanisms. Front Behav Neurosci. 2012;6:54. doi: 10.3389/fnbeh.2012.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cearley CN, Wolfe JH. A single injection of an adeno-associated virus vector into nuclei with divergent connections results in widespread vector distribution in the brain and global correction of a neurogenetic disease. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:9928–9940. doi: 10.1523/JNEUROSCI.2185-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ch’ng SS, Lawrence AJ. Distribution of the orexin-1 receptor (OX1R) in the mouse forebrain and rostral brainstem: A characterisation of OX1R-eGFP mice. Journal of chemical neuroanatomy. 2015;66–67:1–9. doi: 10.1016/j.jchemneu.2015.03.002. [DOI] [PubMed] [Google Scholar]
- Covey DP, Juliano SA, Garris PA. Amphetamine elicits opposing actions on readily releasable and reserve pools for dopamine. PLoS One. 2013;8:e60763. doi: 10.1371/journal.pone.0060763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, Frankel WN, van Den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dela Pena I, Gevorkiana R, Shi WX. Psychostimulants affect dopamine transmission through both dopamine transporter-dependent and independent mechanisms. European journal of pharmacology. 2015;764:562–570. doi: 10.1016/j.ejphar.2015.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobi A, Margolis EB, Wang HL, Harvey BK, Morales M. Glutamatergic and nonglutamatergic neurons of the ventral tegmental area establish local synaptic contacts with dopaminergic and nondopaminergic neurons. J Neurosci. 2010;30:218–229. doi: 10.1523/JNEUROSCI.3884-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- España RA, Baldo BA, Kelley AE, Berridge CW. Wake-promoting and sleep-suppressing actions of hypocretin (orexin): basal forebrain sites of action. Neuroscience. 2001;106:699–715. doi: 10.1016/s0306-4522(01)00319-0. [DOI] [PubMed] [Google Scholar]
- España RA, Melchior JR, Roberts DCS, Jones SR. Hypocretin 1/orexin A in the ventral tegmental area enhances dopamine responses to cocaine and promotes cocaine self-administration. Psychopharmacology. 2011:1–12. doi: 10.1007/s00213-010-2048-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- España RA, Oleson EB, Locke JL, Brookshire BR, Roberts DCS, Jones SR. The hypocretin-orexin system regulates cocaine self-administration via actions on the mesolimbic dopamine system. Eur J Neurosci. 2010;31:336–348. doi: 10.1111/j.1460-9568.2009.07065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- España RA, Roberts DCS, Jones SR. Short-acting cocaine and long-acting GBR-12909 both elicit rapid dopamine uptake inhibition following intravenous delivery. Neuroscience. 2008;155:250–257. doi: 10.1016/j.neuroscience.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadel J, Deutch AY. Anatomical substrates of orexin-dopamine interactions: lateral hypothalamic projections to the ventral tegmental area. Neuroscience. 2002;111:379–387. doi: 10.1016/s0306-4522(02)00017-9. [DOI] [PubMed] [Google Scholar]
- Federici M, Latagliata EC, Ledonne A, Rizzo FR, Feligioni M, Sulzer D, Dunn M, Sames D, Gu H, Nistico R, Puglisi-Allegra S, Mercuri NB. Paradoxical abatement of striatal dopaminergic transmission by cocaine and methylphenidate. The Journal of biological chemistry. 2014;289:264–274. doi: 10.1074/jbc.M113.495499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotter AL, Webber AL, Coleman PJ, Renger JJ, Winrow CJ. International Union of Basic and Clinical Pharmacology. LXXXVI. Orexin receptor function, nomenclature and pharmacology. Pharmacol Rev. 2012;64:389–420. doi: 10.1124/pr.111.005546. [DOI] [PubMed] [Google Scholar]
- Hagan JJ, Leslie RA, Patel S, Evans ML, Wattam TA, Holmes S, Benham CD, Taylor SG, Routledge C, Hemmati P, Munton RP, Ashmeade TE, Shah AS, Hatcher JP, Hatcher PD, Jones DN, Smith MI, Piper DC, Hunter AJ, Porter RA, Upton N. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc Natl Acad Sci U S A. 1999;96:10911–10916. doi: 10.1073/pnas.96.19.10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Randall-Thompson JF, Aston-Jones G. Lateral hypothalamic orexin neurons are critically involved in learning to associate an environment with morphine reward. Behav Brain Res. 2007;183:43–51. doi: 10.1016/j.bbr.2007.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander JA, Pham D, Fowler CD, Kenny PJ. Hypocretin-1 receptors regulate the reinforcing and reward-enhancing effects of cocaine: pharmacological and behavioral genetics evidence. Front Behav Neurosci. 2012;6:47. doi: 10.3389/fnbeh.2012.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheson DM, Quarta D, Halbout B, Rigal A, Valerio E, Heidbreder C. Orexin-1 receptor antagonist SB-334867 reduces the acquisition and expression of cocaine-conditioned reinforcement and the expression of amphetamine-conditioned reward. Behavioural pharmacology. 2011;22:173–181. doi: 10.1097/FBP.0b013e328343d761. [DOI] [PubMed] [Google Scholar]
- Johnson LA, Furman CA, Zhang M, Guptaroy B, Gnegy ME. Rapid delivery of the dopamine transporter to the plasmalemmal membrane upon amphetamine stimulation. Neuropharmacology. 2005;49:750–758. doi: 10.1016/j.neuropharm.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Korotkova TM, Eriksson KS, Haas HL, Brown RE. Selective excitation of GABAergic neurons in the substantia nigra of the rat by orexin/hypocretin in vitro. Regul Pept. 2002;104:83–89. doi: 10.1016/s0167-0115(01)00323-8. [DOI] [PubMed] [Google Scholar]
- Korotkova TM, Sergeeva OA, Eriksson KS, Haas HL, Brown RE. Excitation of ventral tegmental area dopaminergic and nondopaminergic neurons by orexins/hypocretins. J Neurosci. 2003;23:7–11. doi: 10.1523/JNEUROSCI.23-01-00007.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebold TP, Bonaventure P, Shireman BT. Selective orexin receptor antagonists. Bioorganic & medicinal chemistry letters. 2013;23:4761–4769. doi: 10.1016/j.bmcl.2013.06.057. [DOI] [PubMed] [Google Scholar]
- Levy KA, Brodnik ZD, Shaw JK, Perrey DA, Zhang Y, España RA. Hypocretin receptor 1 blockade produces bimodal modulation of cocaine-associated mesolimbic dopamine signaling. Psychopharmacology (Berl) 2017 doi: 10.1007/s00213-017-4673-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Smith RJ, Aston-Jones G. Interactions between VTA orexin and glutamate in cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2013;226:687–698. doi: 10.1007/s00213-012-2681-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Smith RJ, Moorman DE, Sartor GC, Aston-Jones G. Multiple roles for orexin/hypocretin in addiction. Prog Brain Res. 2012;198:79–121. doi: 10.1016/B978-0-444-59489-1.00007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- McElhinny CJ, Jr, Lewin AH, Mascarella SW, Runyon S, Brieaddy L, Carroll FI. Hydrolytic instability of the important orexin 1 receptor antagonist SB-334867: possible confounding effects on in vivo and in vitro studies. Bioorganic & medicinal chemistry letters. 2012;22:6661–6664. doi: 10.1016/j.bmcl.2012.08.109. [DOI] [PubMed] [Google Scholar]
- Moorman DE, Aston-Jones G. Orexin/hypocretin modulates response of ventral tegmental dopamine neurons to prefrontal activation: diurnal influences. J Neurosci. 2010;30:15585–15599. doi: 10.1523/JNEUROSCI.2871-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen OV, Larsen MB, Prasad BM, Amara SG. Genetic complementation screen identifies a mitogen-activated protein kinase phosphatase, MKP3, as a regulator of dopamine transporter trafficking. Molecular biology of the cell. 2008;19:2818–2829. doi: 10.1091/mbc.E07-09-0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschamp JW, Hollander JA, Thompson JL, Voren G, Hassinger LC, Onvani S, Kamenecka TM, Borgland SL, Kenny PJ, Carlezon WA., Jr Hypocretin (orexin) facilitates reward by attenuating the antireward effects of its cotransmitter dynorphin in ventral tegmental area. Proc Natl Acad Sci U S A. 2014;111:E1648–1655. doi: 10.1073/pnas.1315542111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Nagumo Y, Hashimoto S, Narita M, Khotib J, Miyatake M, Sakurai T, Yanagisawa M, Nakamachi T, Shioda S, Suzuki T. Direct involvement of orexinergic systems in the activation of the mesolimbic dopamine pathway and related behaviors induced by morphine. J Neurosci. 2006;26:398–405. doi: 10.1523/JNEUROSCI.2761-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owesson-White CA, Ariansen J, Stuber GD, Cleaveland NA, Cheer JF, Wightman RM, Carelli RM. Neural encoding of cocaine-seeking behavior is coincident with phasic dopamine release in the accumbens core and shell. The European journal of neuroscience. 2009;30:1117–1127. doi: 10.1111/j.1460-9568.2009.06916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozcan M, Ayar A, Serhatlioglu I, Alcin E, Sahin Z, Kelestimur H. Orexins activates protein kinase C-mediated Ca(2+) signaling in isolated rat primary sensory neurons. Physiological research / Academia Scientiarum Bohemoslovaca. 2010;59:255–262. doi: 10.33549/physiolres.931739. [DOI] [PubMed] [Google Scholar]
- Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM. Subsecond dopamine release promotes cocaine seeking. Nature. 2003;422:614–618. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Quick EA, Reeder DC, Morgan ZR, Kalivas PW. Calcium-mediated second messengers modulate the expression of behavioral sensitization to cocaine. The Journal of pharmacology and experimental therapeutics. 1998;286:1171–1176. [PubMed] [Google Scholar]
- Prince CD, Rau AR, Yorgason JT, España RA. Hypocretin/Orexin regulation of dopamine signaling and cocaine self-administration is mediated predominantly by hypocretin receptor 1. ACS chemical neuroscience. 2015;6:138–146. doi: 10.1021/cn500246j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarta D, Valerio E, Hutcheson DM, Hedou G, Heidbreder C. The orexin-1 receptor antagonist SB-334867 reduces amphetamine-evoked dopamine outflow in the shell of the nucleus accumbens and decreases the expression of amphetamine sensitization. Neurochem Int. 2010;56:11–15. doi: 10.1016/j.neuint.2009.08.012. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DCS. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Schneider ER, Rada P, Darby RD, Leibowitz SF, Hoebel BG. Orexigenic peptides and alcohol intake: differential effects of orexin, galanin, and ghrelin. Alcoholism, clinical and experimental research. 2007;31:1858–1865. doi: 10.1111/j.1530-0277.2007.00510.x. [DOI] [PubMed] [Google Scholar]
- Selbach O, Bohla C, Barbara A, Doreulee N, Eriksson KS, Sergeeva OA, Haas HL. Orexins/hypocretins control bistability of hippocampal long-term synaptic plasticity through co-activation of multiple kinases. Acta physiologica (Oxford, England) 2010;198:277–285. doi: 10.1111/j.1748-1716.2009.02021.x. [DOI] [PubMed] [Google Scholar]
- Sharf R, Guarnieri DJ, Taylor JR, DiLeone RJ. Orexin mediates morphine place preference, but not morphine-induced hyperactivity or sensitization. Brain Res. 2010;1317:24–32. doi: 10.1016/j.brainres.2009.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw JK, Ferris MJ, Locke JL, Brodnik ZD, Jones SR, España RA. Hypocretin/orexin knock-out mice display disrupted behavioral dopamine responses to cocaine Addict Biol. 2016 doi: 10.1111/adb.12432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, Tahsili-Fahadan P, Aston-Jones G. Orexin/hypocretin is necessary for context-driven cocaine-seeking. Neuropharmacology. 2010;58:179–184. doi: 10.1016/j.neuropharm.2009.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed EC, Grima LL, Magill PJ, Bogacz R, Brown P, Walton ME. Action initiation shapes mesolimbic dopamine encoding of future rewards. Nature neuroscience. 2016;19:34–36. doi: 10.1038/nn.4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanguy Y, Biferi MG, Besse A, Astord S, Cohen-Tannoudji M, Marais T, Barkats M. Systemic AAVrh10 provides higher transgene expression than AAV9 in the brain and the spinal cord of neonatal mice. Frontiers in molecular neuroscience. 2015;8:36. doi: 10.3389/fnmol.2015.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung LW, Lu GL, Lee YH, Yu L, Lee HJ, Leishman E, Bradshaw H, Hwang LL, Hung MS, Mackie K, Zimmer A, Chiou LC. Orexins contribute to restraint stress-induced cocaine relapse by endocannabinoid-mediated disinhibition of dopaminergic neurons. Nature communications. 2016;7:12199. doi: 10.1038/ncomms12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukairo OT, Ramanujapuram S, Surratt CK. Fluctuation of the dopamine uptake inhibition potency of cocaine, but not amphetamine, at mammalian cells expressing the dopamine transporter. Brain research. 2007;1131:68–76. doi: 10.1016/j.brainres.2006.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venton BJ, Seipel AT, Phillips PE, Wetsel WC, Gitler D, Greengard P, Augustine GJ, Wightman RM. Cocaine increases dopamine release by mobilization of a synapsin-dependent reserve pool. J Neurosci. 2006;26:3206–3209. doi: 10.1523/JNEUROSCI.4901-04.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Sheen W, Morales M. Glutamatergic neurons are present in the rat ventral tegmental area. Eur J Neurosci. 2007;25:106–118. doi: 10.1111/j.1460-9568.2006.05263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Coulouris G, Zaretskaya I, Cutcutache I, Rozen S, Madden TL. Primer-BLAST: a tool to design target-specific primers for polymerase chain reaction. BMC bioinformatics. 2012;13:134. doi: 10.1186/1471-2105-13-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorgason JT, España RA, Jones SR. Demon Voltammetry and Analysis software: Analysis of cocaine-induced alterations in dopamine signaling using multiple kinetic measures. J Neurosci Methods. 2011 doi: 10.1016/j.jneumeth.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahniser NR, Sorkin A. Trafficking of dopamine transporters in psychostimulant actions. Seminars in cell & developmental biology. 2009;20:411–417. doi: 10.1016/j.semcdb.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.