Abstract
Although prairie vole (Microtus ochrogaster) social behavior is well-characterized in adults, surprisingly little is known about the development of social behavior in voles. Further, the overwhelming majority of studies in prairie voles examine social behavior in a reproductive context. Here, we examine developmental plasticity in affiliation and aggression and their underlying neural correlates. Using sexually naïve males, we characterized interactions with an age-matched, novel, same-sex conspecific in four different age groups that span pre-weaning to adulthood. We found that prosocial behavior decreased and aggression increased as males matured. Additionally, pre-weaning males were more prosocial than nonsocial, whereas post-weaning males were more nonsocial than prosocial. We also examined nonapeptide neural activity in response to a novel conspecific in brain regions important for promoting sociality and aggression using the immediate early gene cFos. Assessment of developmental changes in neural activity showed that vasopressin neurons in the medial bed nucleus of the stria terminalis exhibit functional plasticity, providing a potential functional mechanism that contributes to this change in sociality as prairie voles mature. This behavioral shift corresponds to the transition from a period of allopatric cohabitation with siblings to a period of time when voles disperse and presumably attempt to establish and defend territories. Taken together our data provide a putative mechanism by which brain and behavior prepare for the opportunity to pairbond (characterized by selective affiliation with a partner and aggression toward unfamiliar conspecifics) by undergoing changes away from general affiliation and toward selective aggression, accounting for this important life history event.
Keywords: vasopressin, oxytocin, affiliation, aggression, prairie vole, development
1. Introduction
Phenotypic differences in social behavior, and relevant underlying mechanisms, are most commonly examined on an evolutionary timescale using comparative neuroanatomical studies. Such research has defined distinct neural profiles that underlie differences in closely related species that exhibit monogamous or promiscuous mating phenotypes (Wang, 1995; Young, 1999), highly social or highly territorial phenotypes (Goodson et al., 2012; Wang et al., 2013), and uniparental or biparental phenotypes (Roland and O'Connell, 2015; Bales and Saltzman, 2016). However, variation in social behavior also occurs between or within individuals of the same species. Behavioral phenotypic plasticity can take many forms, including behavioral shifts across the lifespan within individuals.
Life history events are very likely to correspond to important changes in cognition and behavior. For instance, sexually naïve adult prairie voles (Microtus ochrogaster) are commonly considered to be highly social and display non-selective affiliative behavior toward novel conspecifics (relative to other vole species; (Shapiro and Dewsbury, 1990). Interestingly, however, during or just after formation of a pairbond, prairie voles exhibit selective aggression toward unfamiliar conspecifics, and develop selective affiliation toward their familiar partner (Young et al., 2011; Blocker and Ophir, 2016). This shift in behavior is a striking example of a non-seasonal shift in sociality that relates to pairbonding, a poignant moment in the life history of a prairie vole. Similarly, single male prairie voles discriminate between other males but not females at a time when male identity is presumably highly relevant to them (Zheng et al., 2013). However, after males form pairbonds, a time when the identity of other females is likely to be more meaningful, they show clear evidence of social discrimination for unfamiliar females (Blocker and Ophir, 2015).
Development is another often-overlooked timescale on which individuals may exhibit social behavioral plasticity. Although the social needs of an animal vary from birth to adulthood, very few studies have examined developmental shifts in sociality. For example, high amounts of prosocial behavior and low levels of aggression might be most beneficial at young ages when an animal is vulnerable and dependent on parents, siblings, and/or communal members for survival (Shapiro and Insel, 1990; Curley et al., 2009). However, when an organism reaches sexual maturity, the same suite of behaviors might become detrimental, and instead aggression could produce greater fitness enhancing outcomes (i.e., to successfully compete for and obtain territories, mates, and food resources; Nelson et al., 2013). Thus, the valence of social interactions and the subsequent behavioral response is likely to shift as an animal matures in a manner that corresponds with shifts in behavioral priorities associated with greater fitness (Silk, 2007). The social needs and priorities of an animal change throughout development, emphasizing the need for mechanisms enabling behavioral plasticity to appropriately respond to environments they commonly experience or should be biologically prepared to experience (Snell-Rood, 2013).
Prairie voles are an excellent species for examining shifts in social behavior throughout development. In the wild, prairie vole pups are reared in groups comprised of either 1) single-female units with offspring, 2) a male-female breeding pair with multiple generations of offspring, or 3) breeding communal groups comprised of a male-female pair, multiple generations of offspring, and unrelated adults that often serve as alloparents (Getz, 1993; McGuire and Lowell, 1995). Around the time of reproductive maturation (roughly postnatal day [PND] 45), juveniles disperse (McGuire et al., 1993). At dispersal, a minority of prairie voles will remain single (or ‘wander’), whereas a majority of them will adopt a socially monogamous mating tactic known as ‘residency’, in which they establish independent territories, form pairbonds and mate, and ultimately rear offspring biparentally (Getz, 1993; McGuire et al., 1993; Solomon and Jacquot, 2002; Ophir et al., 2008b; McGuire and Getz, 2010).
Prairie vole social behavior has been studied extensively in relation to pairbonding (Young and Wang, 2004; Young et al., 2011; Lieberwirth and Wang, 2016). In contrast, nonsexual affiliation in prairie voles has been surprisingly understudied - particularly at ages prior to adulthood. Preliminary (unpublished) findings from our lab suggest that a shift toward a less social phenotype might occur in sexually naïve males before pairbond formation. In the present study, we assessed whether a developmental shift from a more social phenotype to an increasingly less social and more territorial phenotype exists in the prairie vole outside the context of reproduction. Intuitively, it would be more advantageous for an individual to exhibit greater prosocial behavior and less aggression prior to dispersal given that juveniles benefit from parental care and familial cooperation for survival (Solomon and French, 1997). Similarly, it might be beneficial for prairie voles to become less gregarious as they mature (around the age of sexual maturation and dispersal) in anticipation of shifting toward mating tactics that are closely associated with territoriality and selective affiliation (i.e., residency).
The nonapeptides, vasopressin (VP) and oxytocin (OT), are well known for modulating pairbonding, sexual behavior, nonsexual affiliation, parental care, stress response, and aggression (Young, 2009; Goodson and Thompson, 2010; Neumann et al., 2010). VP and OT are evolutionary conserved peptides produced in distinct neuronal population throughout the basal forebrain and midbrain (Farina-Lipari and Valentino, 1993; Farina Lipari et al., 1995). These distinct cell groups exhibit some overlapping, yet also different, contributions to behavior (Kelly and Goodson, 2014). Among the many VP and OT cell groups, two accessory cell groups are of particular interest for the current study: the VP cell groups of the medial bed nucleus of the stria terminalis (BSTm) and the anterior hypothalamus (AH). VP neurons in the BSTm directly promote gregariousness (a preference for larger groups) and suppress aggression in male finches (Kelly and Goodson, 2013). Furthermore, BSTm VP neurons are responsive to positive social stimuli in songbirds (Goodson et al., 2009), and to copulation, but not aggressive or agonistic interactions, in male mice and chickens (Ho et al., 2010; Xie et al., 2011). Conversely, the VP cell group of the AH has been implicated in promoting aggression. For instance, studies examining VP release or VP receptors in the AH suggest a primary role for AH VP in flank marking and overt aggression (Albers, 2012). Although not a direct assessment of AH VP neurons, selective aggression displayed by pairbonded male prairie voles is closely associated with higher densities of VP receptors and increased levels of VP release within the AH (Gobrogge et al., 2009). Nevertheless, surprisingly few studies have directly examined AH VP neuronal function (Kelly and Goodson, 2014).
Here, we sought to investigate developmental changes in nonsexual affiliative and aggressive behavior in male prairie voles. We examined male interactions with age-matched, novel males at different stages of development ranging from pre-weaning to reproductively mature adulthood (Carter and Getz, 1985; Solomon, 1991; Mateo et al., 1994). Because the BSTm VP and AH VP cell groups play an important role in prosocial and aggressive behaviors, respectively, we also examined immediate early gene (IEG) activity within these cell groups to evaluate nonapeptide neuronal functional changes associated with changes in nonsexual affiliative and aggressive behavior. Our main hypothesis was that males should demonstrate a shift away from a social phenotype toward a more territorial phenotype as they mature, with the main shift likely to occur around sexual maturity and the age of dispersal (i.e., PND45). We also hypothesized that BSTm VP neurons would become less responsive, and that AH VP neurons would become more responsive, to interactions with a novel, same-sex conspecific as prairie vole males reach adulthood. Although we had no a priori predictions about how or if BSTm OT neurons would respond to exposure to a same-sex conspecific over development, we chose to characterize this cell group because OT and VP frequently influence common behaviors and there is an extreme lack of functional data on BSTm OT neurons.
2. Materials and Methods
2.1 Animals
Prairie voles used in this study were obtained from our breeding colony, from breeding pairs that were offspring of wild caught animals we captured in Champagne County, Illinois, USA. All animals were housed in standard polycarbonate rodent cages (29 × 18 × 13cm) lined with Sani-chip bedding and provided nesting material. Animals were kept on a 14L:10D cycle, and were provided with rodent chow (Laboratory Rodent Diet 5001, LabDiet, St. Louis, MO, USA) and water ad libitum. Ambient temperature was maintained at 20±2°C. All procedures were approved by the Institutional Animal Care and Use Committee of Cornell University (2013–0102).
2.2 Experimental design
Our study aimed to assess changes in social behavior, and the underlying neural mechanisms, throughout development of the prairie vole. We tested male prairie voles at four ages to capture ages representative of distinct developmental milestones: PND15 (pre-weaning, dependent on parents for survival), PND30 (post-weaning, independent), PND45 (end of sexual maturation; beginning of dispersal), and PND60 (adulthood; reproductively viable)(Carter and Getz, 1985; Solomon, 1991; Mateo et al., 1994). PND15 subject (and stimulus animals) were housed with their parents and siblings, whereas PND30, PND45, and PND60 subject (and stimulus) animals were housed with a same-sex sibling. Sample sizes for each age group were: PND15 n = 10, PND30 n = 10, PND45 n = 10, and PND60 n = 11.
We characterized nonsexual social behavior in sexually naïve males at each age just mentioned. All tests were video recorded with a camera positioned above the cage for subsequent behavioral scoring (see section 2.3). Stimulus animals received a zip-tie collar the day prior to testing for ease of identification in video analysis. To begin, subjects were transferred into a novel cage containing clean Sani-chip bedding and were allowed to habituate for 30 min. Because this experiment was intended to measure IEG activity (see section 2.4) we included this habituation time to reduce the likelihood of any IEG activity being attributable to the stress of handling and/or investigating a novel environment. Testing did not take place in the subject’s home-cage to avoid setting up a resident-intruder context, which would establish an imbalance of territorial dominance (Insel et al., 1995). Thus, the behavioral interactions took place in a neutral arena, but note that each subject had 30 min to investigate the new cage and the stimulus animal did not. After the habituation phase, a novel same-sex, age- and weight- matched conspecific was placed inside the test cage for 30 min. During the 30 min test period, we assessed affiliative and aggressive behavior (see section 2.3). Next, the stimulus animal was removed from the test cage. Stimulus animals were removed after 30 min to circumvent possible injuries from (potential) sustained aggressive contact. The subject remained in the test cage for an additional 60 minutes. Subjects were perfused precisely 90 min after the introduction of the stimulus animal and brains were extracted to quantify neuronal activation of nonapeptide neurons in response to exposure to the stimulus animal (section 2.4).
2.3 Behavioral quantification
Behavior was scored using Behavioral Observation Research Interactive Software (BORIS; (Friard and Gamba, 2016). The first 10 min of interactions with the stimulus animal were scored because this time is the most representative window for behavior that relates to neural activity. In addition, the most dynamic behaviors were exhibited during the initial 5–10 min of subject-stimulus interaction. Behaviors measured included: prosocial contact (any positive physical contact that included passive bodily contact, allogrooming, play, or huddling), allogrooming (subject grooming the stimulus), play (pinning, pouncing, and wrestling that ended in positive physical contact), huddling (an immobile state when subject and stimulus were shoulder to shoulder or one laying on top of the other), and aggression (attacks, chases, lunges, bites, and defensive/offensive upright posture). For prosocial contact, allogrooming, huddling, and aggression, we quantified behavior as the duration of 10 min test time spent engaging in that behavior. Because play behavior occurred very infrequently and was generally brief, we only analyzed the number of play events exhibited by subjects. Lastly, we analyzed the duration of test time spent exhibiting nonsocial behavior; this was calculated as the total duration of aggression and prosocial contact (thus, all contact and overtly directed behavior regardless of valence) subtracted from the total 10 min test time. Nonsocial behavior was by and large comprised of immobile behavior with substantial distance between the subject and stimulus animal.
2.4 Histology and immunocytochemistry
Neuronal activation of VP and OT cells was quantified using the IEG cFos (assessed via its immunocytochemically labeled protein, Fos). Fos functions by rapidly altering gene expression, either positively or negatively, in response to cell surface signals (Hoffman et al., 1993). Fos is rapidly induced in neurons, with its protein product reaching a maximum 60–90 min after stimulation. Quantification of Fos co-expressed in cells positively labeled for OT and VP is indicative of VP or OT neuronal activation.
To visualize VP, OT, and Fos, subjects were sacrificed by isoflurane overdose and transcardially perfused with 0.1M phosphate buffered saline (PBS) followed by 4% paraformaldehyde dissolved in 0.1M borate buffer (pH 9.5). Brains were extracted, post-fixed overnight in 4% paraformaldehyde dissolved in 0.1M borate buffer (pH 9.5) before cryoprotection in 30% sucrose dissolved in PBS for 48 h. Tissue was sectioned into three 40μm series. One series of tissue was immunofluorescently stained for VP, OT, and Fos. Tissue was rinsed 5× for 10 min in 0.1M PBS (pH 7.4), incubated for 1 h in block (PBS + 10% normal donkey serum + 0.03% Triton-X-100), and then incubated for approximately 48 h in primary antibodies diluted in PBS containing 5% normal donkey serum + 0.03% Triton-X-100. Primary antibodies used were guinea pig anti-VP (1:1000; Peninsula Laboratories, San Carlos, CA), mouse anti-OT (3:1000; Millipore, Billerica, MA), and rabbit anti-Fos (5:1000; Santa Cruz Biotechnology, Santa Cruz, CA). Antibodies were previously validated with preadsorption controls to verify specificity (Kelly et al., 2017). The primary incubation was followed by two 30 min rinses in PBS. Tissue was incubated for 1 h in a biotinylated donkey anti-guinea pig secondary (8:1000; Jackson Immunoresearch, West Grove, PA), rinsed twice for 15 min in PBS, and incubated for 2 h at room temperature in streptavidin conjugated to Alexa Fluor 488 (3:1000), donkey anti-mouse secondary conjugated to Alexa Fluor 680 (6:1000), and donkey anti-rabbit secondary conjugated to Alexa Fluor 594 (5:1000). All secondary antibodies were diluted in PBS containing 5% normal donkey serum + 0.03% Trion-X-100. Alexa Fluor conjugates were obtained from ThermoFisher Scientific (Waltham, MA). Following two 30 min rinses in PBS, sections were mounted on microscope slides and cover-slipped with Prolong Gold antifade containing a DAPI nuclear stain (ThermoFisher Scientific).
2.5 Neural quantification
To perform cell counts, images were obtained using a Zeiss AxioImager II microscope outfitted with an AxioCam MRm, z-drive, and an Apotome optical dissector (Carl Zeiss Inc., Gottingen, Germany). Flattened z-stack images were used by observers blind to age of the subject to conduct cell counts in Photoshop CS6 (Adobe Systems, San Jose, CA) and Image J (National Institutes of Health, Bethesda, MD) as previously described (Goodson and Wang, 2006; Kelly et al., 2017).
To account for individual differences in nonapeptide anatomy (e.g., VP-ir and OT-ir cell numbers), we examined the percentage of VP and OT cells that were doubled labeled for Fos (hereafter referred to as VP-Fos or OT-Fos colocalization). VP-Fos colocalization was quantified in the BSTm and AH, and OT-Fos colocalization was quantified in the BSTm. VP-Fos and OT-Fos colocalization was quantified at two levels (see Fig. 1 for a diagram of cell group location that correspond roughly to the Allen Mouse Brain Atlas: P53 and P55 for the BSTm; P60 and P62 for the AH). There were no significant differences between rostral and caudal levels, and thus a combined measurement of both levels was used for analysis. Data are reported as the percentage of VP or OT cells that were co-expressed with Fos (%VP-Fos or %OT-Fos colocalization).
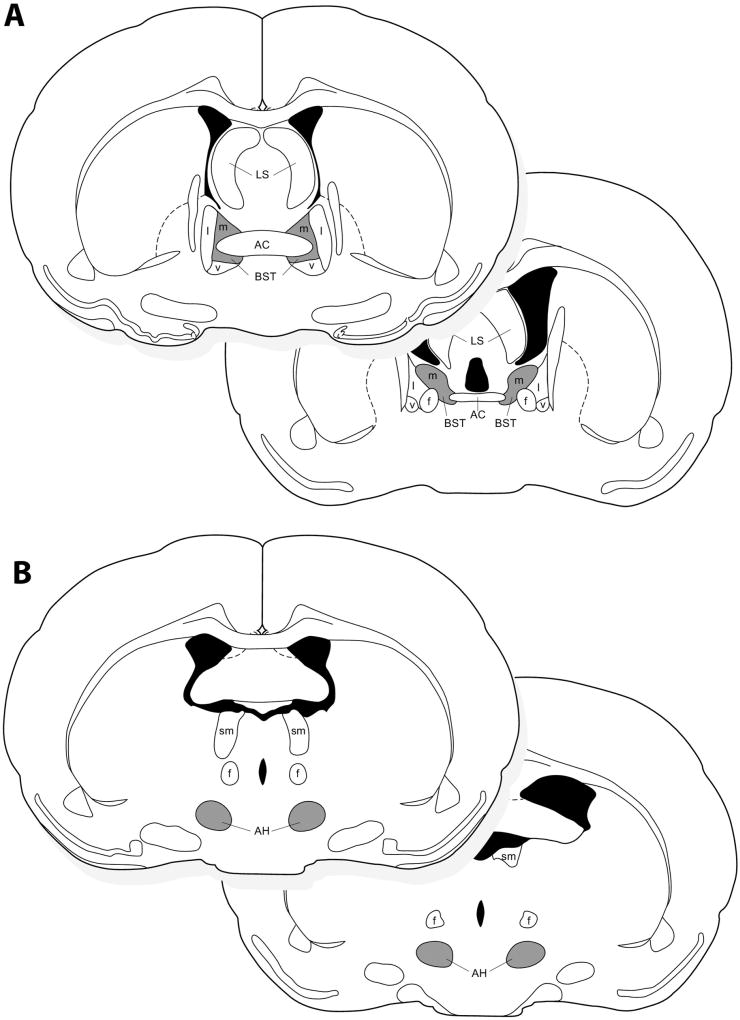
Fig. 1.
A diagram representing the location of VP-ir and OT-ir neurons quantified at rostral and caudal levels in (A) the medial bed nucleus of the stria terminalis (BSTm), and (B) VP-ir neurons in the anterior hypothalamus (AH). Regions where cells were quantified are shaded in gray. AC, anterior commissure; f, fornix; LS, lateral septum; l, lateral portion of the BST; m, medial portion of the BST; v, ventral portion of the BST; sm, stria medullaris.
2.6 Statistical analysis
Behavioral and neural (VP-Fos and OT-Fos colocalization) data were not normally distributed and were therefore analyzed using Kruskal-Wallis H tests (Sections 3.1, 3.2, and 3.4). Dunn’s nonparametric comparisons were used for posthoc analyses, and corrections for multiple comparisons were conducted using Benjamini-Hochberg’s correction for false discovery rate (Benjamini and Hochberg, 1995). For behavioral comparisons within groups, Friedman’s test was utilized, and separate paired Wilcoxon-signed ranks tests were run to determine posthoc comparisons. Multiple comparisons were corrected with Benjamini-Hochberg’s correction (Section 3.3). Cohen’s d effect sizes are provided for all Posthoc comparisons. General linear models (GLMs) were used to determine if age and neural activity interact to predict behavior (Section 3.5). Residuals generated from GLM analyses were checked for normality. Analyses examining prosocial contact were normally distributed; analyses examining huddling and aggression were normally distributed after a log transformation of the dependent variable. Linear regressions were used for examining the relationship between behavioral and neural data. Analyses of prosocial contact, aggression, and nonsocial behavior yielded normally distributed residuals; analyses examining huddling were normally distributed after a log transformation of the dependent variable (Section 3.5). All data were analyzed in SPSS 24 (IBM Analytics, USA).
3. Results
3.1 Changes in affiliative behavior throughout development
To determine whether affiliative behavior changes throughout development, we examined interactions with a novel, same-sex conspecific. We quantified a general measure of prosocial contact behavior, and also analyzed distinct behaviors that were included in the general prosocial contact measurement to examine more nuanced social behavior.
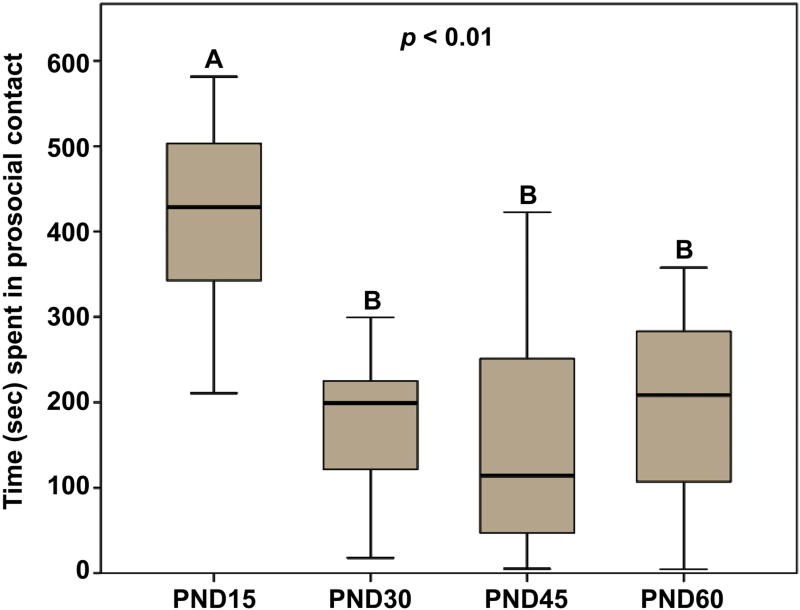
A Kruskal-Wallis H test showed a statistically significant difference between the different age groups in the duration of time that males spent in general prosocial contact with a novel, same-sex conspecific (x2(3) = 17.72; p < 0.01; Fig. 2). Posthoc analyses revealed that PND15 males spent more time in prosocial contact with a novel, same-sex conspecific compared to PND30 (p < 0.01; d = 2.59), PND45 (p < 0.01; d = 2.09), and PND60 (p < 0.01; d = 2.01) males. Interestingly, prosocial contact behavior did not differ between PND30, PND45, and PND60 males (all p > 0.52), suggesting that male prairie voles become less affiliative during a developmental window of PND15 and PND30.
Fig. 2.
Duration of test time (sec) spent in prosocial contact with a novel, same-sex conspecific. Pre-weaning (PND15) males spent significantly more time in prosocial contact than older males (PND30-PND60). Box plots show the median (black line), 75th and 25th percentiles (box), and 95% confidence interval (whiskers). Letters above graphs (A, B) indicate statistical similarity.
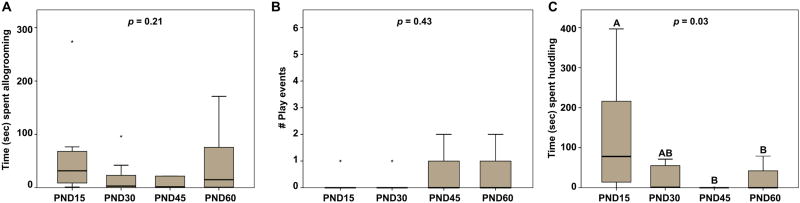
Upon examination of specific prosocial behaviors that were included in the “prosocial contact behavior” measurement, we found that analysis of allogrooming behavior did not yield a significant result (x2(3) = 4.58; p = 0.21; Fig. 3A), suggesting that males do not exhibit differences throughout development in the time spent allogrooming a novel male. Similarly, we observed no significant difference between age groups for play behavior (x2(3) = 2.76; p = 0.43; Fig. 3B).
Fig. 3.
Deconstruction of prosocial behavior. (A) Test time (sec) spent allogrooming a same-sex, novel conspecific and (B) the number of play events exhibited did not differ between the age groups. (C) Pre-weaning (PND15) males spent significantly more time huddling with the stimulus male than PND45 and PND60, but not PND30, males. Box plots show the median (black line), 75th and 25th percentiles (box), and 95% confidence interval (whiskers). * represents extreme outliers. Letters above graphs (A, B) indicate statistical similarity.
Our analyses revealed a statistically significant difference between the age groups in the duration males spent huddling with a novel, same-sex conspecific (x2(3) = 8.78; p = 0.03; Fig. 3C). Posthoc analyses showed that huddling behavior did not significantly differ between PND15 and PND30 males (p = 0.07; d = 1.04). However, PND15 males spent significantly more time huddling than PND45 (p < 0.01; d = 0.92) and PND60 (p = 0.03; d = 0.86) males. Huddling behavior did not differ between PND30, PND45, and PND60 animals (all p > 0.32). These results suggest that huddling behavior decreases during a larger developmental window (between PND15 and PND45) than general prosocial contact behavior (between PND15 and PND30, reported just above).
3.2 Changes in aggressive and nonsocial behavior throughout development
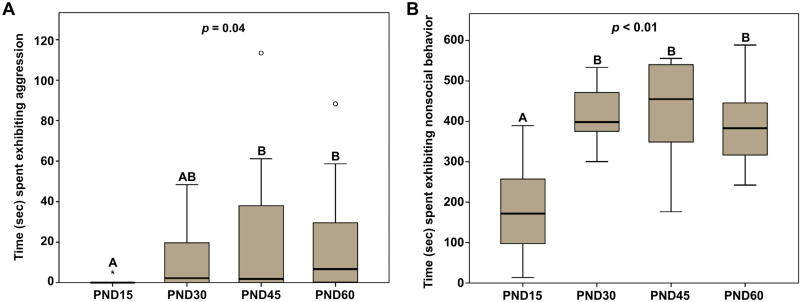
In addition to examination of affiliative behaviors, we also measured how aggressive behavior changes throughout development, and hypothesized that aggression would increase as males reach sexual maturity (PND45). Indeed, we observed a statistically significant difference between the age groups in the duration that males spent being aggressive with a novel same-sex conspecific (x2(3) = 8.59; p = 0.04; Fig. 4A). Posthoc analyses revealed that PND15 and PND30 males did not differ in the amount of time they spent being aggressive (p = 0.08; d = 0.90). However, PND15 males engaged in significantly less aggression compared to PND45 (p = 0.02; d = 0.83) and PND60 (p < 0.01; d = 0.98) males. Aggressive behavior did not significantly differ between PND30, PND45, and PND60 males (all p > 0.34). Like huddling behavior, these results suggest that aggression increased during a developmental window between PND15 and PND45.
Fig. 4.
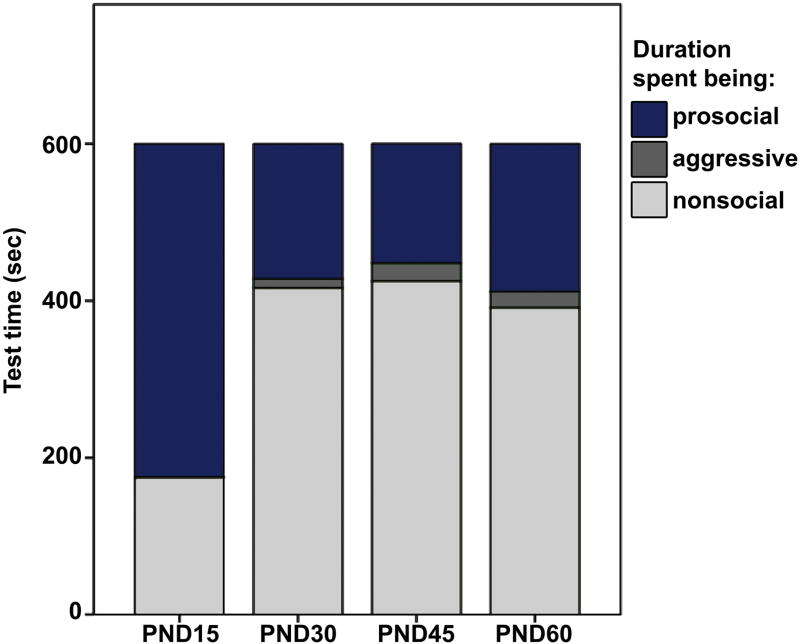
(A) Duration of test time (sec) spent exhibiting aggressive behavior with a novel, same-sex conspecific. Pre-weaning (PND15) males spent significantly less time being aggressive than PND45 and PND60, but not PND30, males. (B) Duration of test time (sec) exhibiting nonsocial behavior. Pre-weaning (PND15) males spent significantly less time being nonsocial compared to older males. Box plots show the median (black line), 75th and 25th percentiles (box), and 95% confidence interval (whiskers). ° represents outliers. * represents extreme outliers. Letters above graphs (A, B) indicate statistical similarity.
Given that prairie voles do not always interact with conspecifics or engage in some form of social behavior (positive or negative), we also analyzed the time subjects spent exhibiting no social behavior (i.e., nonsocial behavior). Analyses revealed a statistically significant difference between the different age groups in the duration of time that males exhibited nonsocial behavior (x2(3) = 17.28; p < 0.01; Fig. 4B). Posthoc analyses revealed that PND15 males spent less time being nonsocial compared to PND30 (p < 0.01; d = 2.46), PND45 (p < 0.01; d = 2.05), and PND60 (p < 0.01; d = 1.94) males. We found that nonsocial behavior did not differ between PND30, PND45, and PND60 males (all p > 0.40), suggesting that during a developmental window of PND15 and PND30, male prairie voles begin spending less time interacting with novel, same-sex conspecifics.
3.3 Characterization of behavioral profiles throughout development
We compared the duration of test time spent exhibiting prosocial contact, aggressive behavior, and nonsocial behavior to examine a more complete characterization of all behavior exhibited during the first 10 min of interaction with a novel, same-sex conspecific, within each age group (Fig. 5). For PND15 males, a Friedman’s test revealed a significant difference in the durations of different types of behavior (prosocial, aggressive, and nonsocial) that were exhibited (x2(2) = 18.20; p < 0.01). Posthoc analysis with Wilcoxon signed-rank tests showed that PND15 males spent significantly more time exhibiting prosocial behavior than nonsocial behavior (Z = −2.29; p = 0.02; d = 2.09), more time being prosocial than aggressive (Z = −2.80; p < 0.01; d = 5.03), and more time being nonsocial than aggressive (Z = −2.80; p < 0.01; d = 2.05).
Fig. 5.
Characterization of behavior (Prosocial, dark blue; Aggressive, dark gray; Nonsocial, light gray) during 10 min exposure to a novel, same-sex conspecific. Males of all ages spent significantly more time engaging in prosocial than aggressive behavior. However, only PND15 males spent significantly more time being prosocial than nonsocial. PND30, PND45, and PND60 males spent significantly more time being nonsocial than prosocial.
Conversely, analyses for older subjects revealed that PND30 and older males allocate their time to different behavioral types in a manner that is distinct from PND15 males. Friedman’s analyses revealed main effects for PND30 (x2(2) = 18.20; p < 0.01), PND45 (x2(2) = 12.80; p < 0.01), and PND60 (x2(2) = 14.73; p < 0.01). Unlike PND15 males, posthoc analyses showed that older males spent significantly less time exhibiting prosocial behavior than nonsocial behavior (PND30, Z = −2.80, p < 0.01, d = 3.50; PND45, Z = −2.29, p = 0.02, d = 2.05; PND60, Z = −2.49, p = 0.01, d = 1.86). However, consistent with PND15 males, older males spent more time being prosocial than aggressive (PND30, Z = −2.70, p < 0.01, d = 3.16; PND45, Z = −2.19, p = 0.03, d = 1.25; PND60, Z = −2.67, p < 0.01, d = 1.98), and more time being nonsocial than aggressive (PND30, Z = −2.80, p < 0.01, d = 7.95; PND45, Z = −2.80, p < 0.01, d = 4.38; PND60, Z = −2.93, p < 0.01, d = 4.92).
3.4 Nonapeptide neuronal responses to interactions with a novel, same-sex conspecific mirror patterns of behavioral changes throughout development
We compared VP and OT neural activity between the age groups in two brain regions known to play important roles in affiliative and aggressive behaviors (the BSTm and AH, respectively). Although the scope of this paper is to examine neuronal function, it is worth noting that Kruskal-Wallis H tests analyzing the total number of VP-ir and OT-ir neurons in the BSTm and AH yielded no significant differences between the ages (BSTm VP-ir, x2(3) = 3.78, p = 0.29; BSTm OT-ir, x2(3) = 5.95, p = 0.11; AH VP-ir, x2(3) = 1.49, p = 0.68). Table 1 shows the mean number of peptidergic immunoreactive neurons in each cell group for all ages.
Table 1.
Vasopressin and oxytocin immunoreactive(-ir) cell numbers.
| #Peptide-ir Cells | |||||
|---|---|---|---|---|---|
| Brain Region | Peptide | PND15 | PND30 | PND45 | PND60 |
| AH | Vasopressin | 29.60 ± 5.12 | 29.80 ± 5.36 | 28.00 ± 4.72 | 24.18 ± 3.98 |
| BSTm | Vasopressin | 13.10 ± 1.20 | 14.10 ± 2.51 | 10.90 ± 1.91 | 11.18 ± 1.44 |
| BSTm | Oxytocin | 11.40 ± 1.66 | 8.20 ± 1.48 | 9.20 ± 2.04 | 8.09 ± 1.39 |
Results are expressed as the mean ± SEM. The total number of peptidergic cells within a cell group did not differ between age groups. BSTm, medial bed nucleus of the stria terminalis; AH, anterior hypothalamus.
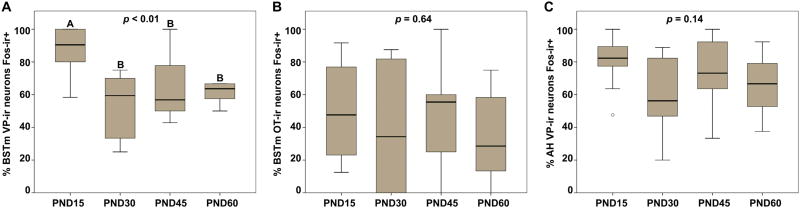
A Kruskal-Wallis H test revealed a main effect of age on BSTm VP neural activity (x2(3) = 12.39; p < 0.01; Fig. 6A), and posthoc analyses revealed that PND15 males exhibited more VP-Fos colocalization in response to a novel male compared to PND30 (p < 0.01; d = 1.99), PND45 (p < 0.01; d = 1.33), and PND60 (p = 0.01; d = 1.44) males. However, VP-Fos colocalization did not differ between PND30, PND45, and PND60 males (all p > 0.37). Fig. 7 shows representative photomicrographs of BSTm VP-ir neurons Fos-ir+ in PND15 and PND60 males. These findings suggest that BSTm VP neurons in males become less responsive to novel same-sex conspecifics during a developmental window between PND15 and PND30. Interestingly, this finding mirrors the pattern observed for general prosocial contact behavior, suggesting a possible role for the involvement of BSTm VP neurons in promoting affiliative behavior.
Fig. 6.
Percentage of nonapeptide neurons expressing Fos in response to a novel, same-sex conspecific. (A) The percentage of BSTm VP-ir neurons that exhibit Fos-ir nuclei was significantly greater in PND15 males compared to older males. The percentage of (B) BSTm OT-ir and (C) AH VP-ir neurons colocalized with Fos-ir did not differ between age groups. Box plots show the median (black line), 75th and 25th percentiles (box), and 95% confidence interval (whiskers). ° represents outliers. Letters above graphs (A, B) indicate statistical similarity.
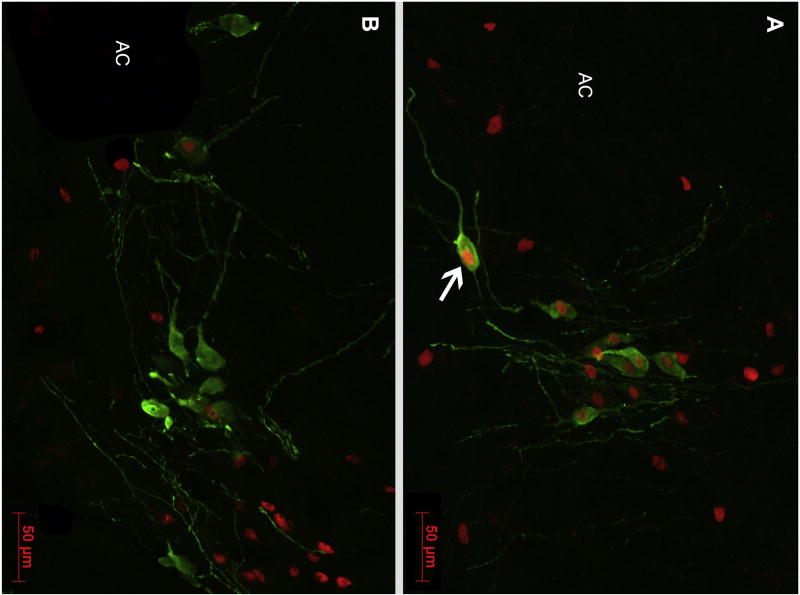
Fig. 7.
Representative immunocytochemical staining of VP (green; Alexa Fluor 488) and Fos (red; Alexa Fluor 594) in the BSTm of (A) a PND15 male subject and (B) a PND60 male subject. The arrow in Panel A indicates an example of a VP-ir neuron colocalized with Fos-ir. AC, anterior commissure. Scale bar = 50 µm.
Although BSTm VP neural activity in response to a novel, same-sex conspecific differed across development, we found that BSTm OT-Fos colocalization did not differ between the age groups (x2(3) = 1.67; p = 0.64; Fig. 6B). This finding suggests that BSTm OT neurons are either not responsive to interactions with a novel, same-sex conspecific or that BSTm OT neurons exhibit consistent nonsexual social functional responses and these responses do not change throughout development.
Similarly, although we predicted that AH VP neural activity would increase alongside increases in aggression throughout development, we observed no significant difference in AH VP-Fos colocalization between the age groups (x2(3) = 5.46; p = 0.14; Fig. 6C). Again, this suggests that AH VP neurons are either non-responsive to interactions with another male or that AH VP neurons exhibit consistent functional profiles throughout development.
3.5 Neurochemical signatures of behavior
Next, we examined whether neural activity predicts behavior differentially across age groups. We used GLMs to determine if behavior is predicted by both neural activity and age. Surprisingly, we found no significant interaction of BSTm VP-Fos colocalization and age for prosocial contact behavior (F(3,33) = 0.85; p = 0.48), huddling behavior (F(3,33) = 0.55; p = 0.66), aggression (F(3,33) = 0.22; p = 0.88), or nonsocial behavior (F(3,33) = 1.15; p = 0.34). Similarly, we found no significant interaction of BST OT-Fos colocalization and age for prosocial contact behavior (F(3,33) = 0.39; p = 0.76), huddling behavior (F(3,33) = 0.41; p = 0.75), aggression (F(3,33) = 0.48; p = 0.70), or nonsocial behavior (F(3,33) = 0.59; p = 0.62). Lastly, we found no significant interaction of AH VP-Fos colocalization and age for prosocial contact behavior (F(3,33) = 0.15; p = 0.93), huddling behavior (F(3,33) = 0.33; p = 0.81), aggression (F(3,33) = 0.69; p = 0.57), or nonsocial behavior (F(3,33) = 0.11; p = 0.95). Although the results discussed above suggest that BSTm VP neurons become less responsive to a novel, same-sex conspecific post-weaning, these findings suggest that the relationship between neural activity and behavior do not exhibit differences across development.
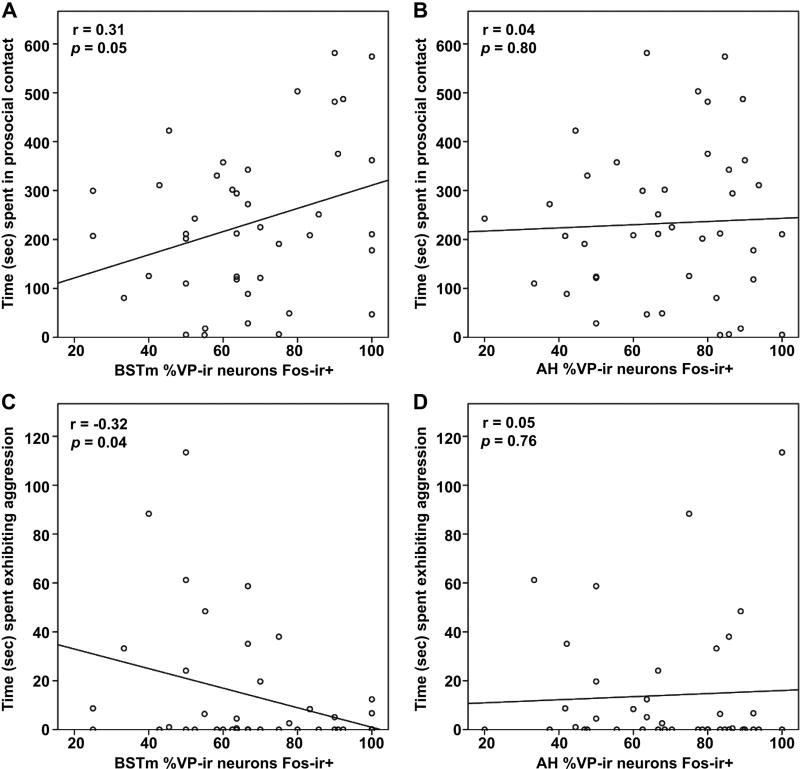
Because of the absence of differences just reported, we collapsed the age groups, thereby increasing statistical power, and conducted linear regressions to determine the relationships between neural activity and behavior. Analyses revealed a significant positive correlation for BSTm VP neural activity and prosocial contact behavior (Pearson’s correlation: r = 0.31; p = 0.05; Fig. 8A), but no relationship for huddling (r = 0.16; p = 0.33). Interestingly, BSTm VP neural activity negatively correlated with aggression (r = −0.32; p = 0.04; Fig. 8C). We observed no significant relationship between BSTm VP neural activity and nonsocial behavior (r = 0.28; p = 0.08).
Fig. 8.
Regression of neural activity and behavior. (A) Test time spent (sec) in prosocial contact positively correlates with the percentage of BSTm VP-ir neurons colocalized with Fos-ir, and (C) time spent exhibiting aggression negatively correlates with the percentage of BSTm VP-ir neurons colocalized with Fos-ir. However, (B) prosocial contact and (D) aggressive behavior do not correlate with AH VP-ir neural activity.
Regression analyses showed that BSTm OT neural activity does not significantly predict prosocial contact (r = −0.10; p = 0.55), huddling (r = −0.12; p = 0.47), aggression (r = −0.09; p = 0.58), or nonsocial behavior (r = 0.12; p = 0.45). Similarly, AH VP neural activity does not significantly predict prosocial contact (r = 0.04; p = 0.80; Fig. 8B), huddling (r = −0.01; p = 0.95), aggression (r = 0.05; p = 0.76; Fig. 8D), or nonsocial behavior (r = 0.05; p = 0.74).
Overall, nonapeptide-Fos colocalization was relatively high (in general between 50% to 80%, see Fig 6). However the high levels of colocalization are typical for relatively small accessory nonapeptide cell groups (e.g., Goodson et al., 2009; Ho et al., 2010).
These results indicate that BSTm VP neurons may actively promote nonsexual affiliation, while concomitantly suppressing aggression in male prairie voles. Furthermore, although AH VP has been implicated in promoting aggression, our results do not provide any evidence that AH VP neurons are involved in aggression in sexually naïve male prairie voles under interactions with novel, same-sex conspecifics.
4. Discussion
The aim of this study was to characterize changes in social behavior throughout development. We hypothesized that a shift from a more social phenotype to a less social and more territorial phenotype would occur around sexual maturity and the age of dispersal (PND45). Our results demonstrated that general prosocial contact behavior decreases during a developmental time window between PND15 and PND30, whereas huddling decreases and aggression increases during a developmental period between PND15 and PND45. Overall, animals of all ages spent more time being prosocial than being aggressive. However, although PND15 animals spent more time being prosocial than nonsocial, a developmental behavioral shift occurred such that males exhibited significantly more nonsocial than prosocial behavior by PND30. As behavior changes throughout development, so in turn might the neural mechanisms underlying behavior. To address the hypothesis that neural correlates relate to developmental changes in behavior, we examined IEG activity in VP and OT neurons in brain regions important for modulating sociality and aggression (e.g., BSTm and AH). Our results revealed that BSTm VP neural activity decreased in a developmental window of PND15 and PND30, in the same way that prosocial contact decreased. Furthermore, regardless of age, BSTm VP neural activity positively related to prosocial contact behavior and negatively related to aggression, suggesting an important role for this cell group in modulating prosociality in sexually naïve male prairie voles. Further studies that directly assess this cell group are necessary to determine its direct contribution to social behavior. Interestingly, our data did not support the prediction that AH VP neural activity would relate to aggressive behavior.
4.1 Putative function of developmental plasticity in behavior
Exhibiting behavioral plasticity allows animals to appropriately respond and adapt to dynamic environments (Goodson and Kabelik, 2009; Taff and Vitousek, 2016). Developmental behavioral plasticity, in particular, is extremely adaptive because it can enable a given species or population to match changing environments they commonly experience or should be prepared to experience based on species-level life history that has shaped the evolution of that species (Snell-Rood, 2013). For example, decreasing prosocial behavior and increasing aggression over the course of early development in prairie voles corresponds to the transition from a period of allopatric cohabitation with siblings to a period of time when voles disperse and presumably attempt to establish and defend territories (McGuire et al., 2013). When prairie voles disperse, they adopt one of two mating tactics. They will either remain single, traverse large areas of space, refrain from being territorial, and presumably attempt to mate multiply, a tactic known as ‘wandering.’ Alternatively, they might establish a territory, acquire a mate and form a pairbond, and engage in socially monogamous behavior, a tactic referred to as ‘residency.’ Although there is some debate as to which tactic is associated with greater fitness (see Solomon and Jacquot, 2002), much of the available evidence suggests that residents benefit from a selective advantage (Ophir et al., 2008b; Ophir et al., 2008c; Blocker and Ophir, 2016). The results from the current study indicate that developing prairie voles demonstrate a behavioral change from being highly prosocial to less prosocial and increasingly aggressive with strangers. These results provide further evidence that the evolved life history of prairie voles is consistent with the idea that prairie voles benefit from adopting residency. Our results showing that BST VP functional responses parallel this life history shift provide a potential functional mechanism that contributes to this change in sociality as prairie voles mature.
4.2 How social is the highly social prairie vole?
Prairie voles are widely recognized as a highly social species (McGraw and Young, 2010; Carter and Porges, 2013). Arguably the most commonly studied form of prosocial behavior in prairie voles is pairbonding (Young et al., 2008; McGraw and Young, 2010; Gobrogge and Wang, 2016; Ophir, 2017), closely followed by parent-infant bonds (Perkeybile et al., 2013; Prounis et al., 2015; Bales and Saltzman, 2016; Numan and Young, 2016). An important distinction to be made is that this high sociality in prairie voles occurs before mating and bonding (Getz, 1993; Young, 2009; Cushing, 2016; Gobrogge and Wang, 2016). After bonding, males and females may show high affiliation toward their partner and offspring, but become selectively aggressive to other conspecifics (Winslow et al., 1993; Aragona et al., 2006; Gobrogge et al., 2007; Gobrogge et al., 2009). This point stresses the importance of social context and warrants caution when using terminology to broadly define the social phenotype of a species (Goodson, 2013; Kelly and Ophir, 2015).
Nevertheless, few studies have examined prairie vole social bonds, and the development of sociality, outside of a reproductive context (i.e., bonds excluding mating, pairbonds, parent-infant relations). In the present study, we found that prosocial behavior with an age-matched, novel, same-sex conspecific, outside of a reproductive context, decreased as prairie vole males matured from pre-weaning to adulthood. Specifically, we found that prosocial contact decreased during a developmental window between PND15 and PND30. This suggests that prairie voles demonstrate the highest degree of prosociality when they are at an age where communal living is most likely. The period that encompasses weaning (PND 21) and pre-dates sexual maturity (a time that is relatively equivalent to adolescence (Mateo et al., 1994) appears to capture the time when the degree of prosociality declines and an increased predisposition to selective aggression develops.
It is very important to note that when all behavior during the test was divided into prosocial, antisocial (aggressive), and nonsocial categories, we found that males of all ages spent significantly more time being prosocial than antisocial. It is therefore important to acknowledge that the decline in prosociality is a relative measure and that these animals continued to act in a way that could be viewed as prosocial. However, upon examination of nonsocial behavior, we found that only PND15 males spent significantly more time being prosocial than nonsocial, whereas PND30, PND45, and PND60 males spent significantly less time being prosocial than nonsocial. Thus, outside of a reproductive context, post-weaning aged male prairie voles did not exhibit a behavioral phenotype that is consistent with being described as “highly social” when interacting with a novel, same-sex conspecific. Instead, our data suggest that although post-weaning male prairie voles are more prosocial than they are aggressive, they also spend substantially more time being nonsocial than prosocial.
4.3 Factors driving prosocial behavior: Insights from development
There are numerous factors that might explain our finding that PND15 males are more prosocial than PND30, PND45, and PND60 males. These factors include stress-buffering (Kikusui et al., 2004; Curley et al., 2009; Dettmer et al., 2016), capacity for social recognition (however, see Porter, 1988), having lower levels of steroid hormones associated with being pre-pubertal (Soma, 2006; Fuxjager et al., 2011; Vetter-O'Hagen and Spear, 2012; Cushing, 2016), maximizing metabolic efficiency (Harshaw et al., 2014), and thermoregulatory heat-seeking (Alberts, 2007). It is worth noting that our measure of prosocial contact included several prosocial behaviors, including huddling, play, allogrooming, and nonaggressive/passive bodily contact. When these behaviors were analyzed separately, only huddling yielded significant age differences.
A plausible alternative explanation for our result demonstrating PND15 pups were more prosocial could be that these younger animals were attempting to thermoregulate, and simply had the appearance of greater prosocial behavior. Many aspects of thermoregulatory ability (e.g., poikilothermia, homoiothermy, muscle shivering, adrenomedullary thermoregulation) reach adult levels by 3 weeks of development (Lagerspetz, 1966). Thus, the PND15 subjects in our study presumably did not have fully developed thermoregulatory abilities compared to older age groups. Indeed, in the first 2–3 weeks of life, huddling among rodent pups is driven by thermal cues (Alberts, 2007) and huddling could reflect thermoregulation rather than general prosocial contact. The increased aggressive behavior observed in older animals could also be interpreted as a consequence of a reduced need to huddle, which would effectively liberate animals to engage in other behaviors – in this case aggression. Interestingly, however, huddling behavior did not significantly differ between PND15 and PND30 males. Yet, PND30 males should have fully developed thermoregulatory abilities. This suggests that thermoregulation is likely not the sole reason for higher levels of prosocial behavior in the PND15 males, although we cannot rule this factor out completely. In addition to the thermoregulatory benefits of huddling, huddling also increases growth rates and reduces nutritive energy requirements through increased metabolic efficiency (Harshaw et al., 2014). Importantly, prairie voles are undergoing substantial developmental changes between PND15 and PND45. Thus, it is possible that the relatively high levels of huddling in pre-weaning aged prairie voles might be better viewed through a physiological regulation lens rather than a purely prosocial lens. The inference from this interpretation of the data would be that increased BSTm VP neural activity of PND15 males reflects a response to cold-stress and/or difficulties thermoregulating. However, BSTm VP neural activity has not been implicated in thermoregulation.
4.4 Antisocial and nonsocial behavior in the prairie vole
Prairie voles are not commonly used for the direct study of aggressive behavior because they exhibit relatively low levels of aggression compared to other species (e.g., hamsters; Young et al., 2011; Albers, 2012; Takahashi and Miczek, 2014). However, research that has examined aggression in prairie voles has largely been in a reproductive context (Winslow et al., 1993; Aragona et al., 2006; Ophir et al., 2008a; Gobrogge and Wang, 2011; Blondel and Phelps, 2016). The most commonly studied form of aggression in prairie voles is that of mating-induced aggression. After pairbonding, males exhibit selective affiliation toward their female partner and selective aggression toward novel males and females (Winslow et al., 1993; Aragona et al., 2006; Gobrogge et al., 2007; Gobrogge et al., 2009). Similarly, female prairie voles exhibit increased aggressive behavior and decreased affiliative behavior to female conspecifics following cohabitation with a male and during pregnancy (Bowler et al., 2002).
Surprisingly few studies have examined aggression in prairie voles outside of a reproductive context. One such study that has, found that sexually naïve adult males are significantly more aggressive with a novel, same-sex conspecific compared to females (Bales and Carter, 2003). The extent to which aggression naturally changes over development, however, remains unclear. In the present study, we found that aggressive behavior with an unfamiliar same-sex conspecific increased as males matured. Specifically, we found that aggression increased during a developmental window between PND15 and PND45, although PND15 and PND30 males did not statistically differ in the time spent exhibiting aggression. Nevertheless, only a single PND15 subject exhibited aggressive behavior, whereas half of the PND30 subjects exhibited aggression. This trend for a general increase in aggression as males mature continued with 60% of PND45 subjects and 72% of PND60 subjects exhibiting aggression. Interestingly, the age group that exhibited the most variation in aggression was PND45 – the age group that best represents the transition to sexual maturation. This variation, combined with our finding that aggression significantly increased between PND15 and PND45, strongly suggests that shifts in aggressive phenotype across development are tied to hormonal changes (i.e., increases in sex steroids) associated with puberty and sexual maturation. This highlights the importance of also considering a development perspective when examining plasticity in social behavioral phenotype.
4.5 Neurochemical profiles of behavior
In addition to characterizing developmental plasticity in social behavior, we were interested in determining whether the nonapeptide system exhibits functional plasticity across development in relation to changes in social behavior. To this end, we characterized IEG responses of VP and OT neurons during interactions with a novel, same-sex conspecific. We focused on two accessory nonapeptide cell groups in the brain that are particularly well known for modulating affiliation and aggression – the BSTm and the AH – because our study specifically examined prosocial and aggressive behavior. Moreover we had a priori reasons to expect that VP in these structures would be functionally related to either social behavior (in the BSTm) or aggression (in the AH). In the present study, we found that BSTm VP-Fos colocalization positively correlated with prosocial contact behavior and negatively correlated with aggression. Our results are consistent with previous work. For example, studies in numerous species have implicated BSTm VP neurons as contributors to prosocial behavior. IEG studies have shown that BSTm VP neurons express greater levels of VP-Fos colocalization in response to positive, social stimuli in birds (Goodson et al., 2009). BSTm VP neurons are also activated after copulation, but not aggressive or agonistic interactions, in male mice and chickens (Ho et al., 2010; Xie et al., 2011). Furthermore, male mice selected for a low-aggression phenotype exhibit significantly more BSTm VP neurons than males selected for high aggression (Compaan et al., 1993). Direct assessment of BSTm VP neurons utilizing antisense oligonucleotides demonstrate that BSTm VP neurons in male zebra finches promote gregariousness, while concomitantly suppressing aggression (Kelly et al., 2011; Kelly and Goodson, 2013). Finally, microdialysis studies in rats have shown that VP release within the BST negatively correlates with intermale aggression in a resident-intruder test (Veenema et al., 2010). Together, these findings suggest a strong evolutionary conservation of BSTm VP social function across taxa.
To our knowledge, extremely few studies have examined developmental functional plasticity of nonapeptide neurons in relation to social behavior, and we are not aware of any studies that have specifically examined differences in BSTm VP neural responses across development. We observed that the number of VP-ir neurons in the BSTm do not significantly differ between the age groups, suggesting that this accessory cell group is fully developed by PND15 (consistent with previous findings, Kelly and Ophir unpub. obs.). However, we also found that a greater percentage of BSTm VP neurons were co-expressed with Fos in response to exposure to a novel, age-matched, same-sex conspecific in PND15 males compared to all older age groups. This finding directly mirrors the behavioral results for prosocial contact behavior. The most prosocial and one of the least aggressive ages (PND15) also exhibited the greatest BSTm VP neural activity, suggesting that BSTm VP neurons are involved in facilitating prosociality and inhibiting aggression. This interpretation is supported by the regression analyses that revealed that BSTm VP neural activity (for all ages) positively correlates with prosocial contact and negatively correlates with aggressive behavior. In other words, the functional role of this cell group in modulating aggression and affiliation appears to be consistent throughout development. However, this cell group was most responsive in this role to interactions with a same-sex conspecific before weaning. Although BSTm VP neurons may consistently be involved in promoting prosocial behavior and concomitantly suppressing aggression throughout development, the degree of this modulation appears to decrease as male voles mature. This age difference in the degree of BSTm VP neural response could reflect changes in the social needs of male voles as they mature.
Much like BST VP-ir neurons, accessory extrahypothalamic VP-ir neurons in the AH are present in amphibians, reptiles, birds, and rodents (Moore and Lowry, 1998). The majority of studies that investigate the role of AH VP have examined VP receptors (vasopressin receptor 1a, V1aR), and surprisingly few studies have examined AH VP neuronal function (Kelly and Goodson, 2014). The prevailing literature is somewhat inconsistent and it appears that there are sex- and context-specific effects of AH VP on aggression (Ferris and Potegal, 1988; Ferris et al., 1989; David et al., 2004; Melloni and Ricci, 2009; Gutzler et al., 2010; Albers, 2012). Although few studies on VP-mediated aggression have been conducted in prairie voles, (Gobrogge et al., 2009) elegantly demonstrated that selective aggression displayed by pairbonded male prairie voles is associated with higher densities of VP receptors and increased levels of VP release within the AH, and that AH VP is both necessary and sufficient for the regulation of selective aggression associated with pairbonding. For these reasons, it was somewhat surprising that we did not find a relationship between changes in aggression over development and AH VP activation. Although activation of V1aRs in the AH clearly facilitates aggression under some contexts, it remains unclear if the endogenous source of VP originates within or outside of the AH. The apparent inconsistency between our data (i.e., lack of relationship between AH VP activation and aggression) and other studies demonstrating a link between AH VP and aggression could be resolved if the endogenous source of VP activates AH receptors in a distal paracrine fashion (Landgraf and Neumann, 2004; Ludwig and Leng, 2006).
Despite it being relatively less studied, we also examined OT neural activity in the BSTm. Interestingly, unlike the deep evolutionary roots of VP in the BSTm, BST OT neurons (or the non-mammalian homologue, mesotocin neurons) are only found in amphibians and mammals (Thepen et al., 1987; Takei et al., 2016). Thus, there have been fewer opportunities across taxa for probing the functions of the BST OT neurons. The few studies that have specifically examined BSTm OT neuronal function suggest a potential involvement of this cell group in stress caused by social defeat (Steinman et al., 2016) and parental separation (Kelly et al., 2017). We found no age differences in BSTm OT neural activity in response to interactions with a novel, same-sex conspecific. This suggests that there simply may not be functional differences throughout development, or that BSTm OT neurons are not responsive to interactions with a novel male. Regardless of age, we found no significant relationships between BSTm OT neural activity and any type of behavior examined. Therefore, our data suggest that OT neurons of the BSTm are not responsive to same-sex social interactions (positive or negative) in sexually naïve male prairie voles.
5. Conclusions
We demonstrated that affiliation, aggression, and nonapeptide neuronal function change throughout development, providing novel evidence for behavioral and functional neural plasticity on a developmental timescale. Our results indicated that developing prairie voles demonstrate a behavioral change from being highly prosocial to less prosocial, and increasingly aggressive with novel, same-sex conspecifics. This behavioral shift corresponds to the transition from a period of allopatric cohabitation with siblings to a period of time when voles disperse and presumably attempt to establish and defend territories. Our results show that BSTm VP functional responses parallel this life history shift, and provide a potential functional mechanism that contributes to this change in sociality as prairie voles mature. Although the relationship between BSTm VP activity and behavior is consistent throughout development, the VP neurons of the BSTm are most responsive to interactions with a same-sex conspecific in pre-weaning males. Perhaps non-coincidentally, this is the age group that also exhibited the highest levels of prosocial behavior. These results suggest that a direct behavioral function of the BSTm VP neuronal population is to promote prosocial behavior and suppress aggressive behavior, a function that does not change over development. However, our data also suggest that the degree and strength of this function may exhibit plasticity such that this cell group becomes decreasingly responsive and less active during nonsexual, social interactions as males mature. Furthermore, pre-weaning males were more prosocial than nonsocial, but post-weaning males were significantly more nonsocial than prosocial. This calls into question the use of the label “highly social” for prairie voles. Nevertheless, males exhibited relatively low levels of aggressive behavior, speaking to the relative prosociality of this species. Overall, our data suggest that social context is extremely influential in the amount of prosocial behavior that male prairie voles exhibit. Together, these findings contribute to a growing body of work characterizing social behavior and the underlying nonapeptide mechanisms in prairie voles, and add previously unknown findings from a developmental perspective in an underappreciated social context.
Highlights.
Little is known about the development of social behavior in prairie voles.
Few studies examine non-reproductive sociality in prairie voles.
Males become less prosocial and more aggressive as they mature.
This behavioral shift corresponds to an important life history transition.
BSTm VP functional plasticity relates to developmental plasticity in behavior.
Acknowledgments
The authors acknowledge the support from the National Institutes of Health (Eunice Kennedy Shriver National Institute of Child Health and Human Development HD081959 to AMK and HD079573 to AGO).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albers HE. The regulation of social recognition, social communication and aggression: vasopressin in the social behavior neural network. Horm. Behav. 2012;61:283–292. doi: 10.1016/j.yhbeh.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Alberts JR. Huddling by rat pups: ontogeny of individual and group behavior. Dev Psychobiol. 2007;49:22–32. doi: 10.1002/dev.20190. [DOI] [PubMed] [Google Scholar]
- Aragona BJ, Liu Y, Yu YJ, Curtis JT, Detwiler JM, Insel TR, Wang Z. Nucleus accumbens dopamine differentially mediates the formation and maintenance of monogamous pair bonds. Nat. Neurosci. 2006;9:133–139. doi: 10.1038/nn1613. [DOI] [PubMed] [Google Scholar]
- Bales KL, Carter CS. Sex differences and developmental effects of oxytocin on aggression and social behavior in prairie voles (Microtus ochrogaster) Horm. Behav. 2003;44:178–184. doi: 10.1016/s0018-506x(03)00154-5. [DOI] [PubMed] [Google Scholar]
- Bales KL, Saltzman W. Fathering in rodents: Neurobiological substrates and consequences for offspring. Horm. Behav. 2016;77:249–259. doi: 10.1016/j.yhbeh.2015.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. Roy. Stat. Soc. Ser. B. (Stat. Method.) 1995;57:289–300. [Google Scholar]
- Bergeron P, Careau V, Humphries MM, Reale D, Speakman JR, Garant D. The energetic and oxidative costs of reproduction in a free-ranging rodent. Funct. Ecol. 2011;25:1063–1071. [Google Scholar]
- Blocker TD, Ophir AG. Social recognition in paired but not single male prairie voles. Anim Behav. 2015;108:1–8. doi: 10.1016/j.anbehav.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blocker TD, Ophir AG. A preference to bond? Male prairie voles form pair bonds even in the presence of multiple receptive females. Anim Behav. 2016;122:89–97. doi: 10.1016/j.anbehav.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blondel DV, Phelps SM. Effects of acute corticosterone treatment on male prairie voles (Microtus ochrogaster): Territorial aggression does not accompany induced social preference. J Comp Psychol. 2016;130:400–406. doi: 10.1037/com0000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowler CM, Cushing BS, Carter CS. Social factors regulate female-female aggression and affiliation in prairie voles. Physiol. Behav. 2002;76:559–566. doi: 10.1016/s0031-9384(02)00755-2. [DOI] [PubMed] [Google Scholar]
- Carter CS, Getz LL. Social and hormonal determinants of reproductive patterns in the prairie vole. Neurobiology. 1985:18–36. [Google Scholar]
- Carter CS, Porges SW. The biochemistry of love: an oxytocin hypothesis. EMBO reports. 2013;14:12–16. doi: 10.1038/embor.2012.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compaan JC, Buijs RM, Pool CW, De Ruiter AJ, Koolhaas JM. Differential lateral septal vasopressin innervation in aggressive and nonaggressive male mice. Brain Res. Bull. 1993;30:1–6. doi: 10.1016/0361-9230(93)90032-7. [DOI] [PubMed] [Google Scholar]
- Curley JP, Jordan ER, Swaney WT, Izraelit A, Kammel S, Champagne FA. The meaning of weaning: influence of the weaning period on behavioral development in mice. Dev Neurosci. 2009;31:318–331. doi: 10.1159/000216543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushing BS. Estrogen Receptor Alpha Distribution and Expression in the Social Neural Network of Monogamous and Polygynous Peromyscus. PLoS One. 2016;11:e0150373. doi: 10.1371/journal.pone.0150373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushing BS, Wynne-Edwards KE. Estrogen receptor-alpha distribution in male rodents is associated with social organization. J. Comp. Neurol. 2006;494:595–605. doi: 10.1002/cne.20826. [DOI] [PubMed] [Google Scholar]
- David JT, Cervantes MC, Trosky KA, Salinas JA, Delville Y. A neural network underlying individual differences in emotion and aggression in male golden hamsters. Neuroscience. 2004;126:567–578. doi: 10.1016/j.neuroscience.2004.04.031. [DOI] [PubMed] [Google Scholar]
- Dettmer AM, Kaburu SS, Simpson EA, Paukner A, Sclafani V, Byers KL, Murphy AM, Miller M, Marquez N, Miller GM, Suomi SJ, Ferrari PF. Neonatal face-to-face interactions promote later social behaviour in infant rhesus monkeys. Nat Commun. 2016;7:11940. doi: 10.1038/ncomms11940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumais KM, Alonso AG, Immormino MA, Bredewold R, Veenema AH. Involvement of the oxytocin system in the bed nucleus of the stria terminalis in the sex-specific regulation of social recognition. Psychoneuroendocrinol. 2016;64:79–88. doi: 10.1016/j.psyneuen.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebensperger LA. A review of the evolutionary causes of rodent group-living. Acta Theriologica. 2001;46:115–144. [Google Scholar]
- Farina Lipari E, Valentino B, Lipari D. Immunohistochemical research on oxytocin in the hypothalamic accessory nuclei. Ital J Anat Embryol. 1995;100:189–193. [PubMed] [Google Scholar]
- Farina-Lipari E, Valentino B. Immunohistochemical research on vasopressin in the accessory hypothalamic nuclei. Ital J Anat Embryol. 1993;98:207–214. [PubMed] [Google Scholar]
- Ferris CF, Axelson JF, Martin AM, Roberge LF. Vasopressin immunoreactivity in the anterior hypothalamus is altered during the establishment of dominant/subordinate relationships between hamsters. Neuroscience. 1989;29:675–683. doi: 10.1016/0306-4522(89)90140-1. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Potegal M. Vasopressin receptor blockade in the anterior hypothalamus suppresses aggression in hamsters. Physiol. Behav. 1988;44:235–239. doi: 10.1016/0031-9384(88)90144-8. [DOI] [PubMed] [Google Scholar]
- French JA, Mustoe AC, Cavanaugh J, Birnie AK. The influence of androgenic steroid hormones on female aggression in 'atypical' mammals. Philos Trans R Soc Lond B Biol Sci. 2013;368:20130084. doi: 10.1098/rstb.2013.0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friard O, Gamba M. BORIS: A free versatile open-source event-logging software for video/audio coding and live observations. Methods in Ecology and Evolution. 2016;7:1325–1330. [Google Scholar]
- Fuxjager MJ, Montgomery JL, Marler CA. Species differences in the winner effect disappear in response to post-victory testosterone manipulations. Proc. Biol. Sci. 2011;278:3497–3503. doi: 10.1098/rspb.2011.0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getz LL, McGuire B, Pizzuto T, Hofmann JE, Frase B. Social organization of the prairie vole (Microtus ochrogaster) J. Mammal. 1993;74:44–58. [Google Scholar]
- Gobrogge K, Wang Z. The ties that bond: neurochemistry of attachment in voles. Curr. Opin. Neurobiol. 2016;38:80–88. doi: 10.1016/j.conb.2016.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobrogge KL, Liu Y, Jia X, Wang Z. Anterior hypothalamic neural activation and neurochemical associations with aggression in pair-bonded male prairie voles. J. Comp. Neurol. 2007;502:1109–1122. doi: 10.1002/cne.21364. [DOI] [PubMed] [Google Scholar]
- Gobrogge KL, Liu Y, Young LJ, Wang Z. Anterior hypothalamic vasopressin regulates pair-bonding and drug-induced aggression in a monogamous rodent. Proc. Natl. Acad. Sci. USA. 2009;106:19144–19149. doi: 10.1073/pnas.0908620106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobrogge KL, Wang ZW. Genetics of aggression in voles. Adv Genet. 2011;75:121–150. doi: 10.1016/B978-0-12-380858-5.00003-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL. Deconstructing sociality, social evolution and relevant nonapeptide functions. Psychoneuroendocrinol. 2013;38:465–478. doi: 10.1016/j.psyneuen.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Kabelik D. Dynamic limbic networks and social diversity in vertebrates: From neural context to neuromodulatory patterning. Front. Neuroendocrinol. 2009;30:429–441. doi: 10.1016/j.yfrne.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Kelly AM, Kingsbury MA. Evolving nonapeptide mechanisms of gregariousness and social diversity in birds. Horm. Behav. 2012;61:239–250. doi: 10.1016/j.yhbeh.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Rinaldi J, Kelly AM. Vasotocin neurons in the bed nucleus of the stria terminalis preferentially process social information and exhibit properties that dichotomize courting and non-courting phenotypes. Horm. Behav. 2009;55:197–202. doi: 10.1016/j.yhbeh.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Thompson RR. Nonapeptide mechanisms of social cognition, behavior and species-specific social systems. Curr. Opin. Neurobiol. 2010;20:784–794. doi: 10.1016/j.conb.2010.08.020. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Wang Y. Valence-sensitive neurons exhibit divergent functional profiles in gregarious and asocial species. Proc. Natl. Acad. Sci. USA. 2006;103:17013–17017. doi: 10.1073/pnas.0606278103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutzler SJ, Karom M, Erwin WD, Albers HE. Seasonal regulation of social communication by photoperiod and testosterone: Effects of arginine-vasopressin, serotonin and galanin in the medial preoptic area-anterior hypothalamus. Behav. Brain Res. 2010 doi: 10.1016/j.bbr.2010.07.042. [DOI] [PubMed] [Google Scholar]
- Harshaw C, Culligan JJ, Alberts JR. Sex differences in thermogenesis structure behavior and contact within huddles of infant mice. PLoS One. 2014;9:e87405. doi: 10.1371/journal.pone.0087405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho JM, Murray JH, Demas GE, Goodson JL. Vasopressin cell groups exhibit strongly divergent responses to copulation and male-male interactions in mice. Horm. Behav. 2010;58:368–377. doi: 10.1016/j.yhbeh.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman GE, Smith MS, Verbalis JG. c-Fos and related immediate early gene products as markers of activity in neuroendocrine systems. Front. Neuroendocrinol. 1993;14:173–213. doi: 10.1006/frne.1993.1006. [DOI] [PubMed] [Google Scholar]
- Insel TR, Preston S, Winslow JT. Mating in the monogamous male: behavioral consequences. Physiol. Behav. 1995;57:615–627. doi: 10.1016/0031-9384(94)00362-9. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Goodson JL. Functional significance of a phylogenetically widespread sexual dimorphism in vasotocin/vasopressin production. Horm. Behav. 2013;64:840–846. doi: 10.1016/j.yhbeh.2013.09.006. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Goodson JL. Social functions of individual vasopressin-oxytocin cell groups in vertebrates: What do we really know? Front. Neuroendocrinol. 2014;35:512–529. doi: 10.1016/j.yfrne.2014.04.005. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Hiura LC, Saunders AG, Ophir AG. Oxytocin neurons exhibit extensive functional plasticity due to offspring age in mothers and fathers. Integr. Comp. Biol. 2017;57:603–618. doi: 10.1093/icb/icx036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AM, Kingsbury MA, Hoffbuhr K, Schrock SE, Waxman B, Kabelik D, Thompson RR, Goodson JL. Vasotocin neurons and septal V1a-like receptors potently modulate songbird flocking and responses to novelty. Horm. Behav. 2011;60:12–21. doi: 10.1016/j.yhbeh.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AM, Ophir AG. Compared to what: What can we say about nonapeptide function and social behavior without a frame of reference? Curr. Opin. Behav. Sci. 2015;6:97–103. doi: 10.1016/j.cobeha.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenkel WM, Paredes J, Yee JR, Pournajafi-Nazarloo H, Bales KL, Carter CS. Neuroendocrine and behavioural responses to exposure to an infant in male prairie voles. J. Neuroendocrinol. 2012;24:874–886. doi: 10.1111/j.1365-2826.2012.02301.x. [DOI] [PubMed] [Google Scholar]
- Kikusui T, Takeuchi Y, Mori Y. Early weaning induces anxiety and aggression in adult mice. Physiol. Behav. 2004;81:37–42. doi: 10.1016/j.physbeh.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Lagerspetz KYH. Postnatal development of thermoregulation in laboratory mice. Helgol. Mar. Res. 1966;14:559–571. [Google Scholar]
- Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Front. Neuroendocrinol. 2004;25:150–176. doi: 10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Lieberwirth C, Wang Y, Jia X, Liu Y, Wang Z. Fatherhood reduces the survival of adult-generated cells and affects various types of behavior in the prairie vole (Microtus ochrogaster) Eur J Neurosci. 2013;38:3345–3355. doi: 10.1111/ejn.12323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberwirth C, Wang Z. The neurobiology of pair bond formation, bond disruption, and social buffering. Curr. Opin. Neurobiol. 2016;40:8–13. doi: 10.1016/j.conb.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvin Y, Turner CA, Rios MB, Maras PM, Chaudhury S, Baker MR, Blandino P, Jr, Watson SJ, Jr, Akil H, McEwen B. Fibroblast growth factor 2 alters the oxytocin receptor in a developmental model of anxiety-like behavior in male rat pups. Horm. Behav. 2016;86:64–70. doi: 10.1016/j.yhbeh.2016.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonstein JS, De Vries GJ. Influence of gonadal hormones on the development of parental behavior in adult virgin prairie voles (Microtus ochrogaster) Behav. Brain Res. 2000;114:79–87. doi: 10.1016/s0166-4328(00)00192-3. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Leng G. Dendritic peptide release and peptide-dependent behaviours. Nat. Rev. Neurosci. 2006;7:126–136. doi: 10.1038/nrn1845. [DOI] [PubMed] [Google Scholar]
- Mateo JM, Holmes WG, Bell AM, Turner M. Sexual maturation in male prairie voles: effects of the social environment. Physiol. Behav. 1994;56:299–304. doi: 10.1016/0031-9384(94)90198-8. [DOI] [PubMed] [Google Scholar]
- McGraw LA, Young LJ. The prairie vole: an emerging model organism for understanding the social brain. Trends Neurosci. 2010;33:103–109. doi: 10.1016/j.tins.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire B, Getz LL. Alternative male reproductive tactics in a natural population of prairie voles (Microtus ochrogaster) Acta Theriologica. 2010;55:261–270. [Google Scholar]
- McGuire B, Getz LL, Bemis WE. Social dynamics and dispersal in free-living prairie voles (Microtus ochrogaster) J. Mammal. 2013;94:40–49. [Google Scholar]
- McGuire B, Getz LL, Hofmann JE, Pizzuto T, Frase B. Natal dispersal and philopatry in prairie voles (Microtus ochrogaster) in relation to population density, season, and natal social environment. Behav. Ecol. Sociobiol. 1993;32:293–302. [Google Scholar]
- McGuire B, Lowell LL. Communal Nesting in Prairie Voles (Microtus-Ochrogaster) - an Evaluation of Costs and Benefits Based on Patterns of Dispersal and Settlement. Can J Zool. 1995;73:383–391. [Google Scholar]
- Melloni RHJ, Ricci LA. Adolescent exposure to anabolic/androgenic steroids and the neurobiology of offensive aggression: A hypothalamic neural model based on findings in pubertal Syrian hamsters. Horm. Behav. 2009;58:177–191. doi: 10.1016/j.yhbeh.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Moaddab M, Dabrowska J. Oxytocin receptor neurotransmission in the dorsolateral bed nucleus of the stria terminalis facilitates the acquisition of cued fear in the fear-potentiated startle paradigm in rats. Neuropharmacology. 2017;121:130–139. doi: 10.1016/j.neuropharm.2017.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore FL, Lowry CA. Comparative neuroanatomy of vasotocin and vasopressin in amphibians and other vertebrates. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 1998;119:251–260. doi: 10.1016/s0742-8413(98)00014-0. [DOI] [PubMed] [Google Scholar]
- Nelson AC, Colson KE, Harmon S, Potts WK. Rapid adaptation to mammalian sociality via sexually selected traits. BMC Evol Biol. 2013;13:81. doi: 10.1186/1471-2148-13-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann ID, Veenema AH, Beiderbeck DI. Aggression and anxiety: social context and neurobiological links. Front. Behav. Neurosci. 2010;4:12. doi: 10.3389/fnbeh.2010.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numan M, Young LJ. Neural mechanisms of mother-infant bonding and pair bonding: Similarities, differences, and broader implications. Horm. Behav. 2016;77:98–112. doi: 10.1016/j.yhbeh.2015.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ophir AG. Navigating Monogamy: Nonapeptide Sensitivity in a Memory Neural Circuit May Shape Social Behavior and Mating Decisions. Front Neurosci. 2017;11:397. doi: 10.3389/fnins.2017.00397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ophir AG, Crino OL, Wilkerson QC, Wolff JO, Phelps SM. Female-directed aggression predicts paternal behavior, but female prairie voles prefer affiliative males to paternal males. Brain Behav. Evol. 2008a;71:32–40. doi: 10.1159/000108609. [DOI] [PubMed] [Google Scholar]
- Ophir AG, Phelps SM, Sorin AB, Wolff JO. Social but not genetic monogamy is associated with higher reproductive success in prairie voles. Anim. Behav. 2008b;75:1143–1154. [Google Scholar]
- Ophir AG, Wolff JO, Phelps SM. Variation in neural V1aR predicts sexual fidelity and space use among male prairie voles in semi-natural settings. Proc. Natl. Acad. Sci. USA. 2008c;105:1249–1254. doi: 10.1073/pnas.0709116105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkeybile AM, Bales KL. Early rearing experience is related to altered aggression and vasopressin production following chronic social isolation in the prairie vole. Behav. Brain Res. 2015;283:37–46. doi: 10.1016/j.bbr.2015.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkeybile AM, Griffin LL, Bales KL. Natural variation in early parental care correlates with social behaviors in adolescent prairie voles (Microtus ochrogaster) Front. Behav. Neurosci. 2013;7:21. doi: 10.3389/fnbeh.2013.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter RH. The Ontogeny of Sibling Recognition in Rodents - Superfamily Muroidea. Behav. Genet. 1988;18:483–494. doi: 10.1007/BF01065516. [DOI] [PubMed] [Google Scholar]
- Prounis GS, Foley L, Rehman A, Ophir AG. Perinatal and juvenile social environments interact to shape cognitive behaviour and neural phenotype in prairie voles. Proc. Biol. Sci. 2015;282 doi: 10.1098/rspb.2015.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland AB, O'Connell LA. Poison frogs as a model system for studying the neurobiology of parental care. Curr. Opin. Behav. Sci. 2015;6:76–81. [Google Scholar]
- Sanna F, Bratzu J, Argiolas A, Melis MR. Oxytocin induces penile erection and yawning when injected into the bed nucleus of the stria terminalis: Involvement of glutamic acid, dopamine, and nitric oxide. Horm. Behav. 2017;96:52–61. doi: 10.1016/j.yhbeh.2017.09.004. [DOI] [PubMed] [Google Scholar]
- Scotti MA, Carlton ED, Demas GE, Grippo AJ. Social isolation disrupts innate immune responses in both male and female prairie voles and enhances agonistic behavior in female prairie voles (Microtus ochrogaster) Horm. Behav. 2015;70:7–13. doi: 10.1016/j.yhbeh.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro LE, Dewsbury DA. Differences in affiliative behavior, pair bonding, and vaginal cytology in two species of vole (Microtus ochrogaster and M. montanus) J Comp Psychol. 1990;104:268–274. doi: 10.1037/0735-7036.104.3.268. [DOI] [PubMed] [Google Scholar]
- Shapiro LE, Insel TR. Infant's response to social separation reflects adult differences in affiliative behavior: a comparative developmental study in prairie and montane voles. Dev Psychobiol. 1990;23:375–393. doi: 10.1002/dev.420230502. [DOI] [PubMed] [Google Scholar]
- Silk JB. The adaptive value of sociality in mammalian groups. Philos Trans R Soc Lond B Biol Sci. 2007;362:539–559. doi: 10.1098/rstb.2006.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell-Rood EC. An overview of the evolutionary causes and consequences of behavioural plasticity. Anim. Behav. 2013;85:1004–1011. [Google Scholar]
- Solomon NG. Current indirect fitness benefits associated with philopatry in juvenile prairie voles. Behav. Ecol. Sociobiol. 1991;29:277–282. [Google Scholar]
- Solomon NG, French JA. Cooperative breeding in mammals. Cambridge University Press; New York: 1997. [Google Scholar]
- Solomon NG, Jacquot JJ. Characteristics of resident and wandering prairie voles, Microtus ochrogaster. Canadian Journal of Zoology. 2002;80:951–955. [Google Scholar]
- Soma KK. Testosterone and aggression: Berthold, birds and beyond. J Neuroendocrinol. 2006;18:543–551. doi: 10.1111/j.1365-2826.2006.01440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speakman JR. The physiological costs of reproduction in small mammals. Philos Trans R Soc Lond B Biol Sci. 2008;363:375–398. doi: 10.1098/rstb.2007.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman MQ, Duque-Wilckens N, Greenberg GD, Hao R, Campi KL, Laredo SA, Laman-Maharg A, Manning CE, Doig IE, Lopez EM, Walch K, Bales KL, Trainor BC. Sex-Specific Effects of Stress on Oxytocin Neurons Correspond With Responses to Intranasal Oxytocin. Biol Psychiatry. 2016;80:406–414. doi: 10.1016/j.biopsych.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taff CC, Vitousek MN. Endocrine Flexibility: Optimizing Phenotypes in a Dynamic World? Trends Ecol Evol. 2016;31:476–488. doi: 10.1016/j.tree.2016.03.005. [DOI] [PubMed] [Google Scholar]
- Takahashi A, Miczek KA. Neurogenetics of aggressive behavior: studies in rodents. Curr Top Behav Neurosci. 2014;17:3–44. doi: 10.1007/7854_2013_263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei Y, Ando H, Tsutsui K. Non-mammalian oxytocin family peptides. Handbook of Hormones: Comparative Endocrinology for Basic and Clinical Research Elsevier Inc. 2016:50–54. [Google Scholar]
- Thepen T, Voorn P, Stoll CJ, Sluiter AA, Pool CW, Lohman AH. Mesotocin and vasotocin in the brain of the lizard Gekko gecko. An immunocytochemical study. Cell Tissue Res. 1987;250:649–656. doi: 10.1007/BF00218959. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Beiderbeck DI, Lukas M, Neumann ID. Distinct correlations of vasopressin release within the lateral septum and the bed nucleus of the stria terminalis with the display of intermale aggression. Horm. Behav. 2010;58:273–281. doi: 10.1016/j.yhbeh.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Vetter-O'Hagen CS, Spear LP. Hormonal and physical markers of puberty and their relationship to adolescent-typical novelty-directed behavior. Dev Psychobiol. 2012;54:523–535. doi: 10.1002/dev.20610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Xu L, Pan Y, Wang Z, Zhang Z. Species differences in the immunoreactive expression of oxytocin, vasopressin, tyrosine hydroxylase and estrogen receptor alpha in the brain of Mongolian gerbils (Meriones unguiculatus) and Chinese striped hamsters (Cricetulus barabensis) PLoS One. 2013;8:e65807. doi: 10.1371/journal.pone.0065807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z. Species differences in the vasopressin-immunoreactive pathways in the bed nucleus of the stria terminalis and medial amygdaloid nucleus in prairie voles (Microtus ochrogaster) and meadow voles (Microtus pennsylvanicus) Behav. Neurosci. 1995;109:305–311. doi: 10.1037//0735-7044.109.2.305. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature. 1993;365:545–548. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- Xie J, Kuenzel WJ, Sharp PJ, Jurkevich A. Appetitive and consummatory sexual and agonistic behaviour elicits FOS expression in aromatase and vasotocin neurones within the preoptic area and bed nucleus of the stria terminalis of male domestic chickens. J. Neuroendocrinol. 2011;23:232–243. doi: 10.1111/j.1365-2826.2011.02108.x. [DOI] [PubMed] [Google Scholar]
- Young KA, Gobrogge KL, Liu Y, Wang Z. The neurobiology of pair bonding: insights from a socially monogamous rodent. Front. Neuroendocrinol. 2011;32:53–69. doi: 10.1016/j.yfrne.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KA, Liu Y, Wang Z. The neurobiology of social attachment: A comparative approach to behavioral, neuroanatomical, and neurochemical studies. Comparative biochemistry and physiology. Toxicology & pharmacology : CBP. 2008;148:401–410. doi: 10.1016/j.cbpc.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ. Frank A. Beach Award. Oxytocin and vasopressin receptors and species-typical social behaviors. Horm. Behav. 1999;36:212–221. doi: 10.1006/hbeh.1999.1548. [DOI] [PubMed] [Google Scholar]
- Young LJ. The neuroendocrinology of the social brain. Front. Neuroendocrinol. 2009;30:425–428. doi: 10.1016/j.yfrne.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Young LJ, Wang Z. The neurobiology of pair bonding. Nat. Neurosci. 2004;7:1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- Zheng DJ, Foley L, Rehman A, Ophir AG. Social recognition is context dependent in single male prairie voles. Anim. Behav. 2013;86 doi: 10.1016/j.anbehav.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]