Abstract
Because high concentrations of ascorbic acid (AA) are found in the adenohypophysis, we hypothesized that it might have an acute effect on the secretion of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) from the gland, particularly because we have reported that AA rapidly inhibits stimulated LH-releasing hormone (LHRH) release from medial basal hypothalamic explants. Incubation of anterior pituitary halves from adult male rats with graded concentrations of AA for 1 h induced highly significant release of both FSH and LH with a minimal effective concentration of 10−5 M. Release remained on a plateau from 10−5 to 10−2 M. When both AA and an effective concentration of LHRH were incubated together, there was no additive response to LHRH and the response was the same as to either compound alone. The FSH and LH release in response to AA was blocked by incubation with NG-monomethyl-l-arginine (NMMA) (300 μM), a competitive inhibitor of NO synthase. NMMA also inhibited LHRH-induced LH and FSH release and gonadotropin release in the presence of both LHRH and AA, whereas sodium nitroprusside, a releaser of NO, stimulated LH and FSH release. Membrane depolarization caused by incubation in high potassium (K+ = 28 or 56 mM) medium stimulated release of FSH, LH, and AA that was blocked by NMMA. We hypothesize that AA is released with FSH and LH from secretory granules. AA is transported back into gonadotropes by the AA transporter and increases intracellular [Ca2+]-activating NO synthase that evokes exocytosis of gonadotropins and AA by cGMP .
Keywords: anterior pituitary gland, follicle-stimulating hormone, luteinizing hormone, NG-monomethyl-l-arginine, sodium nitroprusside
Ascorbic acid (AA) is a six-carbon ketolactone-synthesized from glucose (1, 2). It is an essential vitamin for humans, primates, guinea pigs, and bats, as they lack l-gulono-γ-lactone oxidase, the last enzyme involved in its synthesis from glucose (1, 3). AA is abundant in many endocrine tissues (4, 5) and plays a pivotal role in the control of adrenal and gonadal steroidogenesis (6). Several tissues in the body accumulate AA and its biologically active oxidized form, dehydroascorbic acid. The anterior pituitary gland, brain, adrenal gland, and gonads have high concentrations of AA (2, 7–10). The concentration of AA in the brain ranges between 1.1 and 1.7 mM and remains constant despite variation in AA ingestion (11, 12). Intracellular AA concentration is much higher than extracellular AA concentration, and changes in neuronal activity were shown to have a profound influence on extracellular AA (13–18). AA is further concentrated in isolated nerve terminals, and synaptic vesicles concentrate AA by an active transport mechanism (19, 20).
Previously, we evaluated the effect of AA on basal and stimulated release of luteinizing hormone (LH)-releasing hormone (LHRH) from medial basal hypothalamic explants produced by high potassium medium and N-methyl-d-aspartic acid (NMDA). Sodium nitroprusside (NP), which spontaneously releases NO-stimulated LHRH release and inhibition of NO release by a competitive inhibitor of NO synthase (NOS), NG-monomethyl-l-arginine (NMMA), inhibited release of LHRH (21). The results demonstrated that AA had no effect on basal release of LHRH, but blocked the release of LHRH induced by the stimulants with a minimal effective concentration of 10−5 M. Therefore, we hypothesized that AA is a cotransmitter released with classical transmitters from synaptic vesicles that acts to reduce chemically the NO formed, thereby providing feed–forward inhibitory control over LHRH release.
Because AA is present in high concentrations in the pituitary, we hypothesized that AA might play a role in the secretion of anterior pituitary (AP) hormones. We initiated our studies by examining its effect on gonadotropin secretion from AP glands incubated in vitro. Anterior pituitary halves (APs) were incubated with graded concentrations of AA to determine its effect on basal LH and follicle-stimulating hormone (FSH) release. The tissues were incubated with AA or together with LHRH to evaluate the effect of AA on pituitary responsiveness to LHRH. In addition, the role of NO in AA-induced LH and FSH release was assessed by studying the influence of a competitive NOS inhibitor, NMMA, and a spontaneous producer of NO, NP. Because membrane depolarization with high potassium stimulates gonadotropin secretion, we also examined its effect on AA release.
Materials and Methods
Adult male Sprague–Dawley rats (Holtzman, Madison, WI; 200–250 g) were housed two per cage under controlled conditions of temperature (23–25°C) and lighting (on from 0500 to 1700 h). The animals had free access to a pellet diet and tap water.
Chemicals.
Sodium ascorbate, NMMA, and NP were purchased from Sigma.
In Vitro Studies.
After acclimatization for 5 or more days in the vivarium, male rats were killed by decapitation.
Incubation of APs.
After removal of the posterior lobe, the anterior pituitary was bisected longitudinally, and each AP was incubated in a tube containing 1 ml of Krebs–Ringer bicarbonate (pH 7.4) buffer (KRB) in an atmosphere of 95% O2/5% CO2 in a Dubnoff shaker (50 cycles per min) for 60 min. After this preincubation, APs were incubated with KRB or KRB containing different concentrations of AA (10−7 to 10−2 M) for 1 h. At the end of 1 h, the medium was aspirated and the tissues were incubated with KRB or KRB + LHRH (4.4 × 10−9 M) or KRB + LHRH + AA for an additional 30 min to study the effect of prior and continued exposure to AA on the responsiveness of the pituitary to LHRH. In the experiment where the effect of NMMA was assessed, the tissues were incubated with KRB, KRB + AA, KRB + NMMA (300 μM), or KRB + NMMA + AA for 1 h. The medium was aspirated and the tissues were incubated with KRB, KRB + LHRH, KRB + NMMA + LHRH, or KRB + NMMA + AA + LHRH for 30 min. After this final incubation, the medium was collected and the media from both the 1-h and the 30-min incubations were stored at −20°C until FSH and LH were determined by RIA using kits supplied by the National Institute of Digestive Diabetes and Kidney Disease (NIDDK). In a separate set of experiments, APs were incubated with 0.5 ml of isotonic medium containing high potassium [K+], and AA that was released into the incubation medium was measured by HPLC. In these experiments, paired incubations, with one half of the pituitary serving as a control and the other half as a treated group, were used for studying the effect of high [K+].
Chromatography.
Isocratic analyses were carried out with the Beckman System Gold HPLC pump equipped with a 126 module and diode array detector 168 (Beckman Coulter) operating at 254 nm at a sensitivity of 0.016 absorbance units full scale. The separation was carried out on a μ bondapack Beckman Ultrasphere C-18 column (Beckman Coulter; average particle size, 5 μm, 25 cm × 4.6 mm). The mobile phase was a buffer consisting of 0.1 M sodium dihydrogen phosphate (NaH2 PO4) and 0.2 mM Na2 EDTA adjusted to pH 3.1 with orthophosphoric acid. The buffer was filtered through 0.45 μM membrane filter (Gelman) and degassed before use. The column was maintained at room temperature, and the mobile phase was used at a constant flow rate of 1.0 ml/min.
Preparation of Standard.
A sample buffer consisting of 5 mM each of metaphosphoric acid and Na2 EDTA was prepared in HPLC-grade water (VWR Scientific) and used for preparing AA acid standards and pituitary homogenates. This buffer was previously shown to be stable for 3–4 h (22), and all the estimations were completed within this time. A standard curve for AA was prepared from a stock solution of 1 mg/ml of AA and was found to be linear from 487.5 to 7,800 ng. Standards and incubation medium or homogenates were counted by using a 507 ASE auto sampler, and samples were used at a volume of 30 μl. Each sample (unknown) was passed through syringe filters (Gelman) before being placed in the vial for counting. A standard calibration plot was obtained for AA concentrations (μg/ml) versus peak area (numerical units on the 126 module). Multiple plots for a standard curve were constructed by using freshly prepared samples on different days.
The Pituitary Homogenate.
The pituitary was weighed and placed in 600 μl of sample buffer containing 1 mg/ml of potassium metabisulfite and was then homogenized in a glass homogenizer. The homogenate was thoroughly mixed and centrifuged at 1,000 × g for 10 min at 4°C. The supernatant was decanted and passed through syringe filters before placing in the vials for counting.
Statistics.
Results were analyzed by one-way ANOVA. P < 0.05 was considered significant.
Results
Effect of Graded Concentrations of AA on LH and FSH Release and the Pituitary Responsiveness to LHRH.
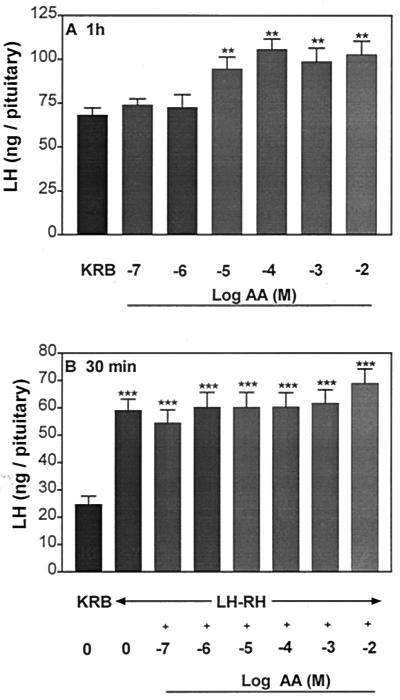
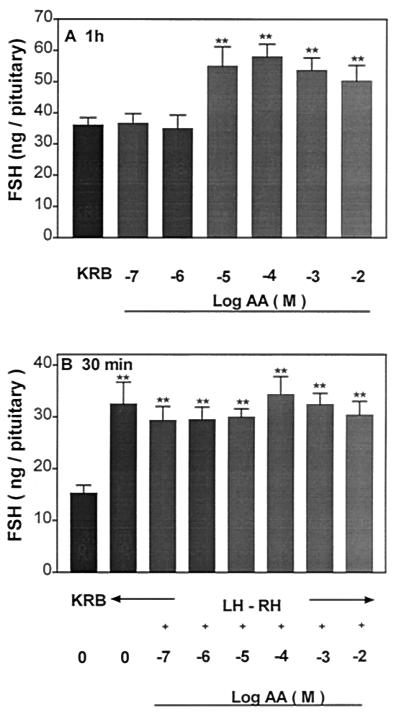
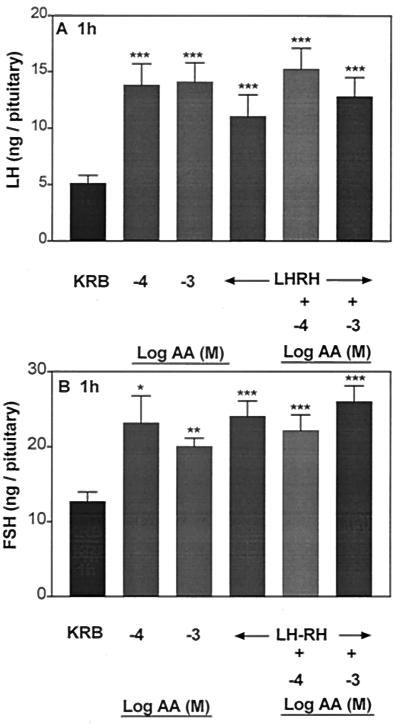
AA highly significantly increased LH (Fig. 1A) and FSH release (Fig. 2A) into the incubation medium after 1 h, and the minimal effective concentration for both gonadotropins was 10−5 M. There was no significant dose–response relationship, and the response remained on a plateau with concentrations of AA ranging from 10−5 to 10−2 M. In another experiment to see if a dose–response curve could be elicited, concentrations of 10−6, 10−5, and 5 × 10−5 M were tested. Again, there was no effect until the 10−5 M concentration was used, and there was no additional effect with 5 × 10−5 M (data not shown). After 1-h incubation with AA, APs were incubated with KRB or LHRH (4.4 × 10−9 M) or LHRH + AA (10−7 to 10−2 M) for 30 min to evaluate the influence of prior and continued exposure to AA on the response to LHRH. The basal rate of release of both hormones was unchanged during this 30-min period. As anticipated, LHRH produced a highly significant increase in LH and FSH release as compared with that of the groups incubated with KRB alone (Figs. 1B and 2B, respectively). However, prior and continued exposure to AA failed to alter the pituitary response to LHRH.
Figure 1.
Effect of graded concentrations of ascorbic acid (AA) (10−7 to 10−2 M) on basal LH release from anterior pituitary halves (APs) (A) and the pituitary response to LHRH (4.4 × 10 −9 M) (B). The results are the mean ± SEM. Number of tissues per group was 10–14 in this and subsequent experiments. **, P < 0.01; ***, P < 0.001 vs. control group incubated with KRB.
Figure 2.
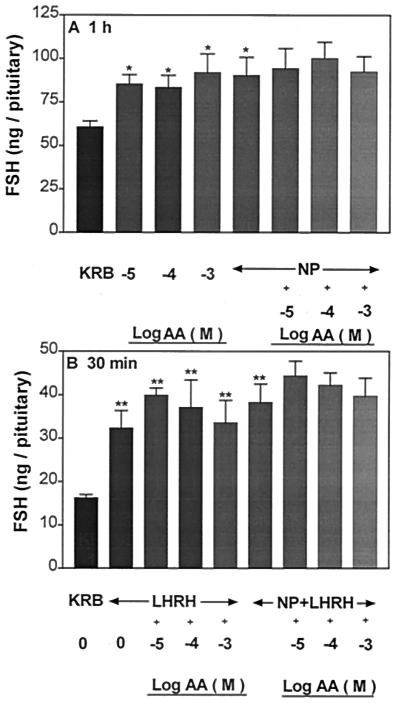
Effect of graded concentrations of AA (10−7 to 10−2 M) on FSH release from APs (A) and the pituitary response to LHRH (4.4 × 10 −9 M) (B). **, P < 0.01 vs. control group incubated with KRB.
Duration of the Effect of AA on LH and FSH Release and the Pituitary Response to LHRH.
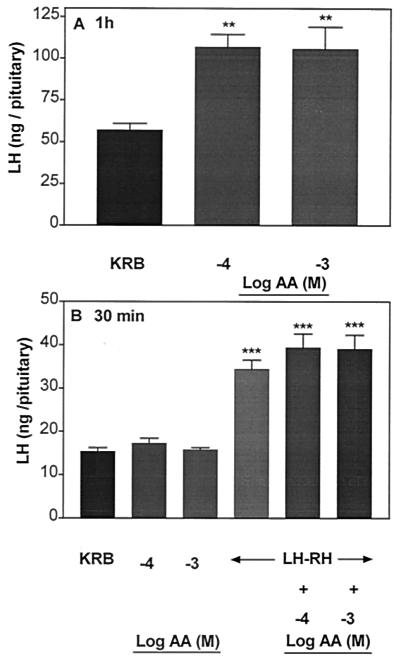
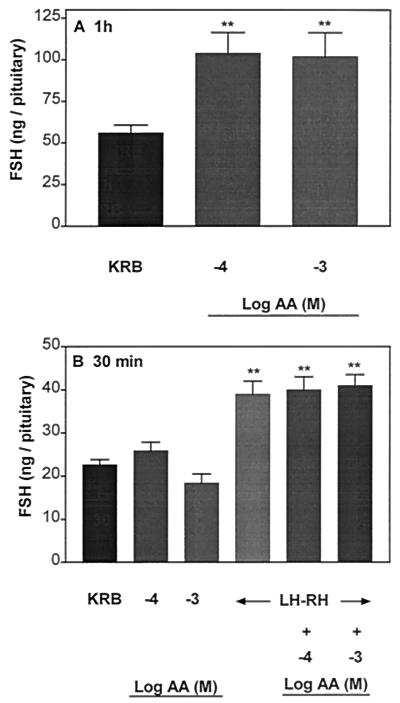
Incubation of APs with AA (10−4 and 10−3 M) for 1 h increased LH (Fig. 3A) and FSH release (Fig. 4A) significantly to the same extent as in the above experiment (Figs. 1A and 2A, respectively), but this increase was not sustained during the subsequent 30-min incubation with AA (Figs. 3B and 4B, respectively). During this incubation period, the pituitary response to LHRH was not altered by AA.
Figure 3.
Effect of AA on LH release after 1 h of incubation (A) followed by incubation with AA for another 30 min on the responsiveness of the pituitary to LHRH (B). **, P < 0.01; ***, P < 0.001 vs. KRB.
Figure 4.
Effect of AA on FSH release after 1 h of incubation (A) followed by incubation with AA for another 30 min on the responsiveness of the pituitary to LHRH (B). **, P < 0.01 vs. KRB.
Effect of AA or Combinations of AA + LHRH on LH and FSH Release After an Hour of Incubation.
Because LHRH had been added in the first experiment after a 1-h incubation with AA, in this experiment we added both together to determine whether simultaneous addition of both AA and LHRH might result in an effect of AA on LHRH responsiveness. Performed in this way, AA alone (10−4 and 10−3M) induced a much greater effect on hormone release than it did in the prior experiments, increasing LH release 2.5-fold (Fig. 5A) and FSH release nearly 2-fold (Fig. 5B), as opposed to the earlier results, in which release increased about 40% (Fig. 1). However, as in the first experiment, when LHRH was added after prior incubation with AA for 1 h, and when AA and LHRH were incubated together for 1 h, the stimulation of both FSH and LH by AA + LHRH was not altered.
Figure 5.
Influence of AA or combination of AA + LHRH after 1 h of incubation on LH and FSH release (A and B, respectively). *, P < 0.05; **, P < 0.01; ***, P < 0.001 vs. KRB.
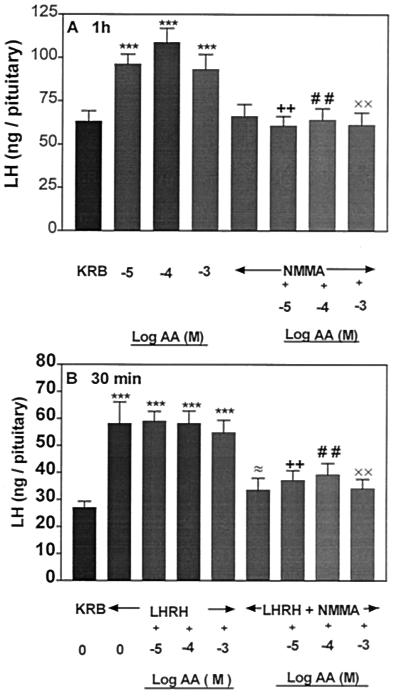
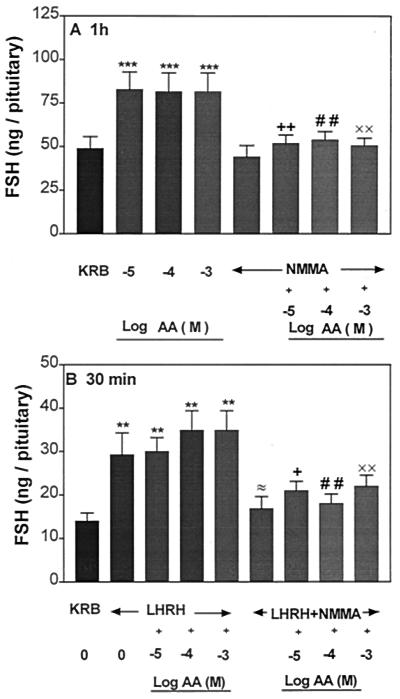
Effect of NMMA on AA-Induced LH and FSH Release and the Pituitary Response to LHRH.
To determine whether NO mediates the gonadotropin-releasing action of AA, the APs were incubated with an inhibitor of NOS, NMMA (300 μM). NMMA had no effect on basal LH and FSH release (Figs. 6A and 7A, respectively); however, AA-induced LH and FSH release was completely blocked in the presence of NMMA. As before, AA had no effect on LHRH-induced LH and FSH release in the subsequent 30-min incubation (Figs. 6B and 7B, respectively). NMMA significantly suppressed LHRH-stimulated LH and FSH. AA as before did not alter the FSH and LH release induced by LHRH but NMMA blocked LH and FSH release in the presence of AA and LHRH (Figs. 6B and 7B).
Figure 6.
Influence of AA or NMMA or AA + NMMA on basal LH release (A) and the responsiveness of the pituitary to LHRH (B). ***, P < 0.001 vs. KRB; + +, P < 0.01 vs. the group treated with AA (10−5 M); # #, P < 0.01 vs. AA (10−4 M); ××, P < 0.01 vs. AA (10−3 M); ≈, P < 0.05 vs. the group treated with LHRH alone.
Figure 7.
Influence of AA or NMMA or AA + NMMA on FSH release (A) and the responsiveness of the pituitary to LHRH (B). **, P < 0.01, ***, P < 0.001 vs. KRB; +, P < 0.05; ++, P < 0.01 vs. the group treated with AA (10−5 M); # #, P < 0.01 vs. AA (10−4 M); ××, P < 0.01 vs. AA (10−3 M); ≈, P < 0.05 vs. the group treated with LHRH alone.
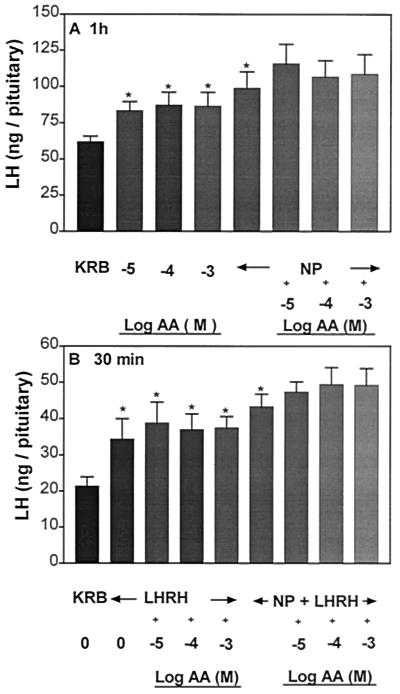
Effect of NP on AA-Induced LH and FSH Release and the Pituitary Response to LHRH.
Incubation of APs with NP (300 μM) significantly stimulated LH and FSH release (Figs. 8A and 9A, respectively). AA (10−5 to 10−3 M) stimulated LH and FSH release as before. A simultaneous exposure of APs to NP and AA maintained, but did not further augment, the increased release of LH and FSH induced by either AA or NP. Incubation of APs with LHRH (4.4 × 10−9 M) evoked a significant increase in LH and FSH release as before (Figs. 8B and 9B, respectively). Prior and continued exposure to AA or NP + AA released LH and FSH in amounts comparable to that of the group incubated with LHRH alone.
Figure 8.
Influence of AA or NP or AA + NP on basal LH (A) and the responsiveness of the pituitary to LHRH (B). *, P < 0.05 vs. KRB.
Figure 9.
Influence of AA or NP or AA + NP on FSH release (A) and the responsiveness of the pituitary to LHRH (B). *, P < 0.05; **, P < 0.01 vs. KRB.
Effect of High [K+] on AA Release and Content.
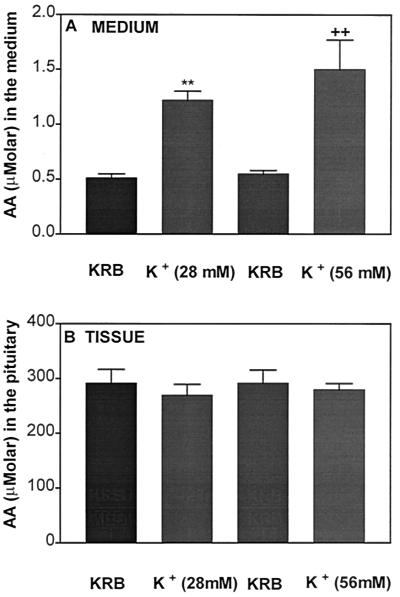
AA is present in high concentrations in the pituitary. Therefore, it was of interest to observe whether or not cell membrane depolarization by potassium was able to evoke a release of AA from the pituitary. The anterior pituitaries were incubated with two different concentrations of [K+] for 1 h in paired incubations, with one half serving as the control and the other half serving as a treated group. As anticipated, high [K+] at either 28 or 56 mM released significantly greater AA into the incubation medium as compared with the corresponding control groups but there was no dose–response relationship (Fig. 10A). The pituitary content of AA was not altered by the high concentrations of [K+] (Fig. 10B). Comparison of AA release and content revealed a much greater AA concentration in the tissue than the amount of AA released into the incubation medium, indicating that only a tiny fraction of AA in the tissue remained in the medium at the end of the incubation.
Figure 10.
Effect of high [K+] (28 and 56 mM) on AA content in the medium (A) or tissue (B) after 1 h of incubation. One half of the pituitary was used as a control and the other half was used for incubation with high [K+]. **, P < 0.01; ++, P < 0.01 vs. the corresponding control group.
Effect of High [K+] on LH and FSH Release.
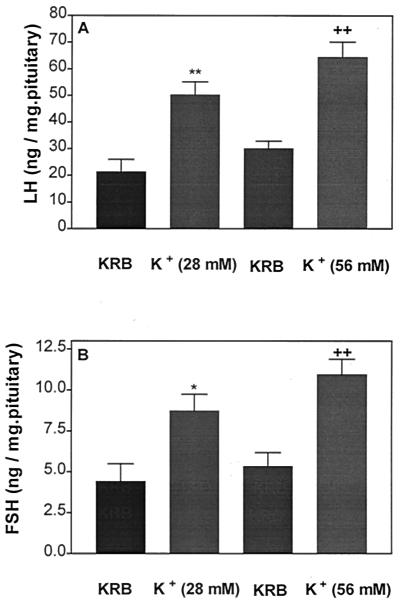
Incubation of APs with the same concentrations of high [K+] as in the previous experiment resulted in a greater release of both LH and FSH. These effects were not dose-related, as was the case with the increase of AA in the medium (Fig. 11 A and B, respectively).
Figure 11.
Effect of high [K+] on LH (A) and FSH (B) release after 1 h of incubation. *, P < 0.05; **, P < 0.01; ++, P < 0.01 vs. the corresponding control group.
Effect of High [K+] or High [K+] Plus NMMA on AA Release.
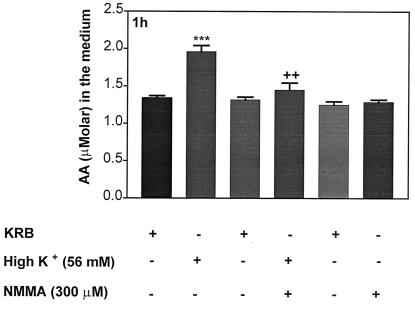
To determine whether AA release was mediated by NOS, APs were incubated with high [K+] (56 mM) for 1 h, resulting in a greater release of AA than that from control APs (Fig. 12), as reported above. Incubation with high [K+] plus NMMA (300 μM) resulted in a total blockade of [K+]-induced AA release, whereas NMMA alone failed to alter release of AA.
Figure 12.
Effect of high [K+] (56 mM) or combination of [K+] + NMMA on AA in the medium. ***, P < 0.001 vs. KRB; ++, P < 0.01 vs. [K+].
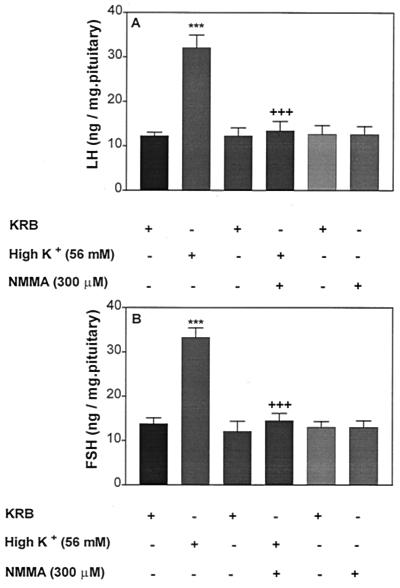
Effect of High [K+] or Combinations of High [K+] Plus NMMA on LH and FSH Release.
Incubation of pituitaries with high [K+]-induced a significant increase in both LH and FSH release (Fig. 13 A and B, respectively). A combination of high [K+] + NMMA (300 μM) produced a total blockade of high [K+]-induced LH and FSH release. LH and FSH release was not altered by NMMA alone.
Figure 13.
Effect of high [K+] or combination of [K+] + NMMA on LH (A) and FSH (B) release. ***, P < 0.001 vs. KRB; +++, P < 0.001 vs. [K+].
Discussion
Our results show a clear FSH- and LH-releasing activity of AA at concentrations from 10−5 M to 10−2 M, but without a dose–response relationship. The effect was highly significant for both hormones, and increases could range from 40% to >100% of basal release. The effect was present when incubation was carried out for 1 h; however, incubating for an additional 30 min with AA had no effect. Therefore, this response is limited in time. When AA was incubated together with LHRH at a dose that gave approximately equal stimulation to that obtained with AA for both FSH and LH release, there was no effect on the FSH- and LH-releasing activity of LHRH if the tissue had been previously incubated for 1 h with AA. In other words, in the subsequent 30 min of AA by itself had no effect; however, when the two were incubated together for 30 min at a time when AA was effective to release gonadotropins, there was still no additive effect of the separate stimuli. The FSH and LH release remained at the same level as it was with AA or LHRH alone. Because this increase is not maximal in this type of incubation system, this is a puzzling result and indicates that, for some reason, there is no additive effect of these two dissimilar stimuli. A transient stimulation of LH and FSH release from superfused male hamster anterior pituitary glands was reported earlier, but the effective concentration of AA 10−3 M or higher (23) and 100-fold greater than that found in the present experiments.
NMMA, an inhibitor of NOS, completely blocked AA-induced LH and FSH release. As reported (24), the response to LHRH was also blocked. When both AA and LHRH were present, the response to both was inhibited by NMMA. As reported earlier (24), NP that releases NO spontaneously released both FSH and LH; however, it failed to modify AA or LHRH-stimulated LH and FSH release. We expected that adding NP together with AA or LHRH would produce an additive effect because the concentrations of LHRH and AA did not produce maximal release of FSH or LH. However, taken together, our experiments indicate that both AA- and LHRH-induced gonadotropin release is mediated by NO.
NP-stimulated LH and FSH release was shown to depend on the presence of calcium (24, 25). Therefore, we hypothesized that AA increased intracellular [Ca2+], which combined with calmodulin to activate the NOS that released NO. NO enhanced the release of LH and FSH by activation of guanylyl cyclase and consequent generation of cGMP that activated protein kinase C, leading to exocytosis of FSH and LH secretory granules. Interestingly, in a recent study, incubation of isolated umbilical vein endothelial cells with 0.1 to 100 μM AA increased the production of NO, as measured by its coproduct citrulline, and also increased cGMP (26). Furthermore, it has been documented that NO stimulates cGMP production in the pituitary (27). Consequently, we believe that AA acts as indicated above by the NO–cGMP pathway to stimulate gonadotropin secretion.
In contrast to the stimulatory effect of AA on gonadotropin secretion from APs, AA had no effect on basal LHRH release from medial basal hypothalamic explants but significantly lowered NP or excitatory amino acid NMDA-stimulated LHRH release. NP or NMDA-induced LHRH release is mediated by NO, and AA acts as an inhibitory transmitter in the hypothalamus to inhibit stimulated LHRH release by scavenging NO (21). Our current data suggest that AA has opposite effects at the hypothalamic and pituitary levels, inhibiting LHRH release in the medial basal hypothalamus and inducing gonadotropin release from the anterior pituitary.
The AA concentration measured in the AP in the present study was 300 μM, an extremely high value that is in agreement with an earlier study (7) and is greater than the concentration found in the hypothalamus (21). Incubation of APs with medium containing high [K+] resulted in increased release of AA into the medium by a factor of 3 without altering tissue concentration, but the release was not dose-dependent. Comparison of pituitary AA content and release revealed that the AP content of AA was significantly greater (200-fold) than that in the incubation medium, illustrating that a very small percentage of the amount present in the tissue was released into the incubation medium. However, AA may have been reduced during the incubation period to dehydroascorbic acid that was immeasurable by our assay, thereby reducing the apparent release of AA.
Increased AA release into the incubation medium was paralleled by increased release of LH and FSH by both doses of high [K+], which is consistent with earlier reports documenting depolarization-induced LH and FSH release (28, 29). In the case of both gonadotropins, only 2–3% of the gonadotropin content was released. Collectively, these data emphasize that depolarizing stimuli that induce the release of neurotransmitters and pituitary hormones also release AA from the pituitary. A similar conclusion regarding depolarization-induced AA release was drawn from earlier studies using synaptosomes from different regions of the brain (31, 32). In our experiments with medial basal hypothalami, exclusion of [Ca2+] from the medium containing high [K+] significantly attenuated AA release, illustrating that high [K+]-induced AA release is a [Ca2+]-dependent phenomenon (21). The role of NO in high [K+]-induced AA, LH, and FSH release was ascertained by combined incubation with high [K+] plus NMMA, a competitive inhibitor of NOS. There was a total blockade of high [K+]-induced AA, LH and FSH release, illustrating that the release of all these substances is mediated by NO.
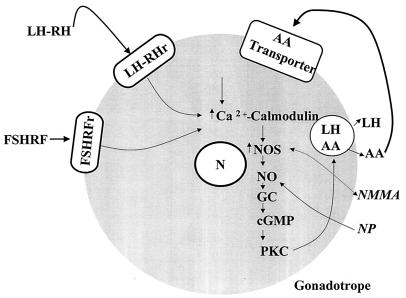
NO is formed from l-arginine by the enzyme NOS (33–37). There are two forms of NOS, a calcium-independent inducible form induced by bacterial lipopolysaccharide and cytokines, such as IL-1, and two calcium-dependent constitutive forms found in vascular endothelial cells and neurons (33, 35–37). Both forms of NOS require l-arginine as the substrate and are inhibited by l-arginine analogues such as NMMA (35, 36). The presence of neural NOS and its mRNA in folliculostellate cells and LH gonadotrophs of the pituitary has been reported (38). Our hypothesis for the action of AA to stimulate LH and FSH release is presented in Fig. 14. We presume that AA is stored in the secretory granules that also contain LH and FSH and is coreleased with FSH and LH by exocytosis of gonadotropin-containing secretory granules. Once AA is outside the cell membrane, it is transported by means of an AA transporter into the cells (39). Once inside the cell, it induces an increase in intracellular [Ca2+] that combines with calmodulin and activates NOS, which, in turn, releases NO. The released NO activates soluble guanylyl cyclase, which converts GTP to cGMP. cGMP releases intracellular [Ca2+] (40) and activates protein kinase C, which induces exocytosis of AA from LH and FSH secretory granules. We hypothesize that AA is a vitaminergic transmitter that activates the release of both FSH and LH from the anterior pituitary gland.
Figure 14.
A schematic diagram of the hypothesized mechanism of action of AA as a vitaminergic neurotansmitter. LHRHr, LHRH receptor. FSHRFr, FSH-releasing factor receptor. GC, Guanylyl cyclase. PKC, Protein kinase C.
Acknowledgments
We thank Judy Scott for her excellent secretarial assistance. This work was supported by National Institutes of Health Grant MH51853.
Abbreviations
- AA
ascorbic acid
- FSH
follicle-stimulating hormone
- LH
luteinizing hormone
- LHRH
LH-releasing hormone
- APs
anterior pituitary halves
- NMMA
NG-monomethyl-l-arginine
- NOS
NO synthase
- NP
nitroprusside
- KRB
Krebs–Ringer bicarbonate (pH 7.4) buffer
References
- 1.Grollman A P, Lehninger A L. Arch Biochem Biophys. 1957;69:458–467. doi: 10.1016/0003-9861(57)90510-6. [DOI] [PubMed] [Google Scholar]
- 2.Chatterjee I B. World Rev Nutr Diet. 1978;30:69–87. doi: 10.1159/000401236. [DOI] [PubMed] [Google Scholar]
- 3.Nishikimi M, Yagi K. Am J Clin Nutr. 1991;54,Suppl.:1203S–1208S. doi: 10.1093/ajcn/54.6.1203s. [DOI] [PubMed] [Google Scholar]
- 4.Chinoy N J, Rao M V, Seethalakshmi L. Indian J Exp Biol. 1979;17:1171–1175. [PubMed] [Google Scholar]
- 5.Chinoy N J. Comp Biochem Physiol. 1978;42A:945–952. doi: 10.1016/0300-9629(72)90400-8. [DOI] [PubMed] [Google Scholar]
- 6.Datta S, Sanyal S. Indian J Exp Biol. 1978;16:166–169. [PubMed] [Google Scholar]
- 7.Das P C, Das K P, Bagchi K, Dey C D. Life Sci. 1993;52:1493–1498. doi: 10.1016/0024-3205(93)90111-f. [DOI] [PubMed] [Google Scholar]
- 8.Lutwak–Mann C. J Agric Sci. 1954;44:477–480. [Google Scholar]
- 9.Hornig D. Ann NY Acad Sci. 1975;258:103–118. doi: 10.1111/j.1749-6632.1975.tb29271.x. [DOI] [PubMed] [Google Scholar]
- 10.Schreiber M, Trojan S. Physiol Res. 1991;40:413–418. [PubMed] [Google Scholar]
- 11.Schenk J O, Miller E, Gaddis R, Adams R N. Brain Res. 1982;253:353–356. doi: 10.1016/0006-8993(82)90709-0. [DOI] [PubMed] [Google Scholar]
- 12.Spector R, Lorenzo A V. Am J Physiol. 1973;225:757–763. doi: 10.1152/ajplegacy.1973.225.4.757. [DOI] [PubMed] [Google Scholar]
- 13.Schenko J O, Miller E, Gaddis R, Adams R N. Brain Res. 1982;253:353–356. doi: 10.1016/0006-8993(82)90709-0. [DOI] [PubMed] [Google Scholar]
- 14.Rice M E, Cammack J. Neurosci Lett. 1991;132:141–145. doi: 10.1016/0304-3940(91)90287-4. [DOI] [PubMed] [Google Scholar]
- 15.Rice M E, Nicholson C. Am J Physiol. 1973;225:757–763. [Google Scholar]
- 16.Boutelle M G, Svesson L, Fillenz M. Neuroscience. 1989;30:11–17. doi: 10.1016/0306-4522(89)90349-7. [DOI] [PubMed] [Google Scholar]
- 17.Clemens J A, Phebus L A. Life Sci. 1984;35:671–677. doi: 10.1016/0024-3205(84)90262-5. [DOI] [PubMed] [Google Scholar]
- 18.Grunewald R A, O'Neill R D, Fillenz M, Albery W J. Neurochem Int. 1983;5:773–778. doi: 10.1016/0197-0186(83)90103-1. [DOI] [PubMed] [Google Scholar]
- 19.Hadjiconstantinou M, Neff N H. Neuropharmacology. 1983;22:939–943. doi: 10.1016/0028-3908(83)90209-5. [DOI] [PubMed] [Google Scholar]
- 20.Thorn N A, Christensen B L, Jeppenson C, Nielsen F S. Acta Physiol Scand. 1985;124:87–92. doi: 10.1111/j.1748-1716.1985.tb07635.x. [DOI] [PubMed] [Google Scholar]
- 21.Karanth S, Yu W H, Walczewska A, Mastronardi C, McCann S M. Proc Natl Acad Sci USA. 2000;97:1891–1896. doi: 10.1073/pnas.97.4.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harapanhalli R S, Howell R W, Rao D V. J Chromatogr. 1993;614:233–243. doi: 10.1016/0378-4347(93)80314-t. [DOI] [PubMed] [Google Scholar]
- 23.Wun W S A, Berkowitz A S, Preslock J P. Chin J Physiol. 1994;37:129–132. [PubMed] [Google Scholar]
- 24.Yu W H, Walczewska A, Karanth S, McCann S M. Endocrinology. 1997;138:5055–5058. doi: 10.1210/endo.138.11.5649. [DOI] [PubMed] [Google Scholar]
- 25.Pinilla L, Gonzalez D, Tena-Sempere M, Aguilar E. Neuroendocrinology. 1998;68:180–186. doi: 10.1159/000054364. [DOI] [PubMed] [Google Scholar]
- 26.Heller R, Munscher-Paulig F, Grabner R, Till U. J Biol Chem. 1999;274:8254–8260. doi: 10.1074/jbc.274.12.8254. [DOI] [PubMed] [Google Scholar]
- 27.Duvilanski B H, Zambruno C, Lasaga M, Pisera D, Seilicovich A. J Neuroendocrinol. 1996;8:909–913. doi: 10.1111/j.1365-2826.1996.tb00820.x. [DOI] [PubMed] [Google Scholar]
- 28.Wakabayashi K, Kamberi I A, McCann S M. Endocrinology. 1969;85:1046–1056. doi: 10.1210/endo-85-6-1046. [DOI] [PubMed] [Google Scholar]
- 29.Turgeon J L, Waring D W. Am J Physiol. 1983;244:E170–E176. doi: 10.1152/ajpendo.1983.244.2.E170. [DOI] [PubMed] [Google Scholar]
- 30.Milby K H, Mefford I N, Chey W, Adams R N. Brain Res Bull. 1981;7:237–242. doi: 10.1016/0361-9230(81)90013-7. [DOI] [PubMed] [Google Scholar]
- 31.Grunewald R A, Fillenz M. Neurochem Int. 1984;6:491–500. doi: 10.1016/0197-0186(84)90120-7. [DOI] [PubMed] [Google Scholar]
- 32.Miele M, Boutelle M G, Fillenz M. Neuroscience. 1994;62:87–91. doi: 10.1016/0306-4522(94)90316-6. [DOI] [PubMed] [Google Scholar]
- 33.Vincent S R, Kimura H. Neuroscience. 1992;46:755–784. doi: 10.1016/0306-4522(92)90184-4. [DOI] [PubMed] [Google Scholar]
- 34.Moncada S, Palmer R M, Higgs E A. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 35.Knowles R G, Palacios M, Palmer R M, Moncada S. Proc Natl Acad Sci USA. 1989;86:5159–5162. doi: 10.1073/pnas.86.13.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Snyder S H, Bredt B T. Sci Am. 1992;266:68–71. doi: 10.1038/scientificamerican0592-68. , 74–77. [DOI] [PubMed] [Google Scholar]
- 37.Vincent S R, Hope B T. Trends Neurosci. 1992;15:108–113. doi: 10.1016/0166-2236(92)90021-y. [DOI] [PubMed] [Google Scholar]
- 38.Ceccatelli S, Hulting A L, Zhang X, Gustafsson L, Villar M S, Hokfelt T. Proc Natl Acad Sci USA. 1993;90:11292–11296. doi: 10.1073/pnas.90.23.11292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Washko P, Rotrosen D, Levine M. J Biol Chem. 1989;264:18996–19002. [PubMed] [Google Scholar]
- 40.Xu X, Star R A, Tortorici G, Muallem S. J Biol Chem. 1994;269:12645–12653. [PubMed] [Google Scholar]