Abstract
Inflammatory bowel disease (IBD) is an inflammatory disorder of the gastrointestinal tract that is caused by multiple factors, including dysfunction of the immune system and genetic and epigenetic alterations. Aberrant epigenetic regulation, especially histone acetylation, was found in biopsies from IBD patients and mouse models of colitis, suggesting that an epigenetic treatment approach may be useful for IBD therapy. Therefore, we investigated the effects of the histone deacetylase (HDAC) inhibitor, suberoylanilide hydroxamic acid (SAHA), in a mouse model of dextran sulfate sodium (DSS)-induced colitis. C57BL/6 mice were treated with 1.5% DSS for 5 days and/or SAHA (25 mg/kg BW/day) for 26 days. Levels of mRNA for the pro-inflammatory cytokines, interleukin (IL)-6 and tumor necrosis factor (TNF)-α, and the chemokines, Ccl2, were examined by qRT-PCR. CD11b, a marker of dendritic cells, macrophages, and monocytes, as well as Ccl2 expression, were examined by immunohistochemistry. IL-6, TNF-α, and Ccl2 gene expression peaked on day 5 in DSS-treated mouse colon, whereas SAHA treatment significantly decreased pro-inflammatory gene expression. Ccl2 protein expression resembled Ccl2 gene expression results. Moreover, localization of CD11b showed that migratory inflammatory cells were dramatically decreased by SAHA treatment compared to DSS-treated mouse colon. Thus, we conclude that the HDAC inhibitor, SAHA, attenuates inflammatory changes in DSS-induced colitis by suppressing local secretion of pro-inflammatory cytokines and chemokines and also by suppressing mobilization and accumulation of inflammatory cells.
Keywords: inflammatory bowel disease, histone deacetylase inhibitor, dextran sodium sulfate, pro-inflammatory cytokines and chemokines
I. Introduction
Inflammatory bowel disease (IBD) is a chronic remitting inflammatory disease of the gastrointestinal tract with two major clinical forms: ulcerative colitis and Crohn’s disease. Although the etiology of IBD is largely unknown, recent studies have suggested that multiple factors play a role, including genetics, epigenetics, diet, environmental factors, and the innate immune system [17]. Among these factors, epigenetics is considered an important factor in IBD onset and pathogenesis. The epigenetic status may be modified by environmental influences [14]. The incidence of IBD, particularly Crohn’s disease, has dramatically increased in industrialized countries, highlighting the putative effect of environmental factors [27]. Interestingly, epigenetic alterations such as differential patterns of histone acetylation are found in both biopsies from IBD patients and mouse models of colitis [28]. These findings suggest that histone acetylation may be a novel target for IBD treatment.
Histone acetylation, a covalent post-transcriptional modification that regulates gene transcription or repression, is catalyzed by histone acetyltransferase and histone deacetylase (HDAC) [7]. HDAC inhibitors are considered powerful epigenetic regulators and immunomodulators. In the clinical setting, HDAC inhibitors have already been introduced for treatment of some cancers, parasitic diseases, and inflammatory diseases including rheumatoid arthritis, asthma, and ischemia-reperfusion injury [10, 11, 23]. The anti-inflammatory properties of HDAC inhibitors are explained by their inhibition of HDAC activity, which may influence transcription factors and inflammatory gene expression profiles [16]. Among HDAC inhibitors, suberoylanilidehydroxamic acid (SAHA) is a potential epigenetic therapeutic agent that suppresses inflammation and peritoneal fibrosis [12, 30]. However, the effect of SAHA in IBD pathogenesis is largely unknown.
Several animal models have been developed to study the pathogenesis of IBD, including chemically induced or genetically targeted models [15]. Among these models, dextran sulfate sodium (DSS) is considered the most suitable model to induce colitis that resembles the histopathology of IBD [19]. During acute colitis, local secretion of pro-inflammatory cytokines and chemokines in colonic mucosa leads to accumulation of migratory macrophages, monocytes, and dendritic cells [1]. Although dendritic cells and macrophages are potential antigen presenting cells (APCs), they may also play important roles in initiation and progression of inflammation [9]. Therefore, we hypothesized that SAHA treatment may reduce local inflammation and secretion of inflammatory cytokines and chemokines, such as tumor necrosis factor (TNF)-α, interleukin (IL)-6, and Ccl2. The reduced secretion of pro-inflammatory cytokines and chemokines may negatively regulate APCs, including dendritic cells and macrophages.
In this study, we investigated the effects of SAHA in a DSS-induced colitis mouse model. Expression levels of mRNA for IL-6, TNF-α, and Ccl2 were measured by quantitative reverse transcriptase (qRT) PCR, and CD11b expression was evaluated as a marker of dendritic cells and macrophages with immunohistochemistry. Our results suggested that SAHA may be a useful therapeutic agent for attenuating the progression of inflammation in DSS-induced colitis by suppressing pro-inflammatory cytokines and chemokines.
II. Materials and Methods
Chemicals
Paraformaldehyde (PFA) was purchased from Merck (Darmstadt, Germany). DSS powder (molecular weight 36,000–50,000) was purchased from MP Biomedicals (Solon, OH, USA), and SAHA was purchased from Cayman Chemicals (Ann Arbor, MI, USA). Bovine serum albumin (BSA), 3-aminopropyl-triethoxysilane, and Brij L23 were from Sigma Chemical Co. (St. Louis, MO, USA), and all other chemicals used in this study were ordered from Wako Pure Chemicals (Osaka, Japan).
Animal experiment
Eight-week-old male C57BL/6 mice were used in the study (Clea Japan, Shizuoka, Japan). Mice were kept in specific pathogen-free conditions with a 12:12 hr light-dark cycle with ad libitum access to food and water. The experimental protocol was approved by the animal ethics review committee of Miyazaki University (2012-502-5), and all experiments were performed in accordance with institutional guidelines. The experimental animals were divided into four groups: control, DSS, DSS+SAHA, and SAHA, and each group consisted of 5–10 mice. To induce colitis, 1.5% DSS was dissolved in drinking water, and the DSS and DSS+SAHA mice received DSS for 5 days ad libitum. On day 5, water with DSS was switched to normal water. The control and SAHA-treated mice received normal water. SAHA powder was dissolved in 5% dimethylsulfoxide (DMSO)/phosphate-buffered saline (PBS) and administered to the SAHA and DSS+SAHA groups by daily intraperitoneal (IP) injection at a concentration of 25 mg/kg body weight from day 1 to day 26 [12]. Control and DSS mice received daily IP injection of 5% DMSO/PBS. Mice were sacrificed on days 5, 12, 19, and 26.
Tissue preparation
Mice were sacrificed by cervical dislocation, and the entire colon was removed from the cecum to the anus. The length and weight of the colon were measured, and then the colon was divided in half longitudinally. The entire length of one half of the tissue was incubated in 4% PFA in PBS overnight at room temperature and embedded in paraffin. The other half of the fresh tissue was embedded in optimal cutting temperature compound for immunohistochemistry or snap frozen and later used for qRT-PCR.
Histological scoring
PFA-fixed colonic tissue was embedded in paraffin and sectioned on a microtome at 5 μm thickness. To evaluate colonic damage, the severity of epithelial damage and inflammation were determined in a blinded manner by microscopic examination of hematoxylin and eosin (HE)-stained tissue. Briefly, the histological score was calculated as the sum of three parameters: surface epithelial loss, crypt destruction, and inflammatory cell infiltration into the mucosa [6, 21].
qRT-PCR
Total RNA from the colon was extracted with Isogen II (Nippon Gene), and first-strand complementary DNA was synthesized from 1 μg of total RNA with oligo (dT)20 primers using the PrimeScript RT reagent kit (Takara, Kusatsu, Japan) according to the manufacturer’s instructions. Transcript expression levels were analyzed by using SYBRPremix Ex Taq IIon Thermal Cycler Dice (Takara) with specific primer pairs after normalization to the expression of GAPDH. Primers sequences are: CCL2 F-5'-AGCAGCAGGTGTCCCAAAGA-3' and R-5'-GTGCTGAAGACCTTAGGGCAGA-3'; TNFα F-5'-GCCCACGTCGTAGCAAACCAC-3' and R-5'-GCAGGGGCTCTTGACGGCAG-3'; IL-6 F-5'-GACTGATGCTGGTGACAACC-3' and R-5'-CCTCCGACTTGTGAAGTGG-3'; GAPDH F-5'-AAATTCAACGGCACAGTCAAG-3' and R-5'-TGGTGGTGAAGACACCAGTAG-3' [1].
Immunohistochemistry
Colonic tissues were sectioned with a cryostat microtome at 5 μm thickness. Frozen sections were postfixed with 4% PFA/PBS for 20 minutes, and the sections were pre-incubated with 500 μg/ml normal goat or donkey IgG in 1% BSA in PBS for 1 hr to block non-specific antibody binding. Subsequently, the tissues were reacted with FITC-conjugated rat anti-mouse CD11b mAb (BD Biosciences) or goat anti-mouse Ccl2 antibodies overnight. After washing with 0.075% Brij L23 in PBS, sections were reacted with specific secondary antibody for 60 minutes. Then, sections were washed with 0.075% Brij L23 in PBS and counterstained with DAPI (Vector Laboratories, Burlingame, CA, USA) for 1 min [3, 29]. Normal rat or goat IgG was used at the same concentration instead of the primary antibodies for each experiment as a negative control.
Statistical analysis
All data are expressed as the mean ± SD. Differences between experimental groups were assessed by Student’s t-test. A p value < 0.05 was considered statistically significant. All analyses were performed with the Statistical Package for Social Sciences (SPSS version 20, Chicago, IL, USA).
III. Results
SAHA prevents shortening of the colonic length during DSS-induced colitis
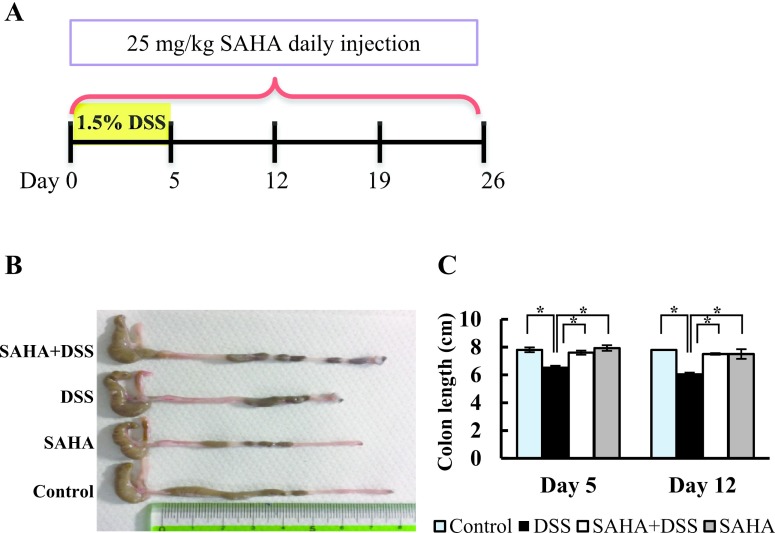
A schematic representation of the animal model is shown in Fig. 1A. The severity of colonic inflammation is strongly correlated with colon length. Therefore, we measured the length of the entire colon in all mice (Fig. 1B). On day 5, the mean colon length of DSS-treated mice was significantly shorter than that of the control, DSS+SAHA, and SAHA-treated groups. This tendency remained on days 12 (Fig. 1C) and 19, whereas the colonic length became similar on day 26, indicating that the mice had recovered from the colitis (data not shown).
Fig. 1.
Animal experimental model and colon length measurement. A. Schematic diagram of DSS-induced colitis in mouse. The DSS and DSS+SAHA groups received 1.5% DSS in drinking water for 5 days, followed by a switch to normal drinking water. The SAHA and DSS+SAHA groups were treated with daily intraperitoneal injection of SAHA at a dose of 25 mg/kg. B. Macrophotography of the mouse colon on day 12. C. Colon length on days 5 and 12 in all experimental groups. Asterisks indicate statistically significant differences (*p < 0.05). Data represent the mean ± SD of 6–9 mice.
SAHA attenuates the progression of inflammation
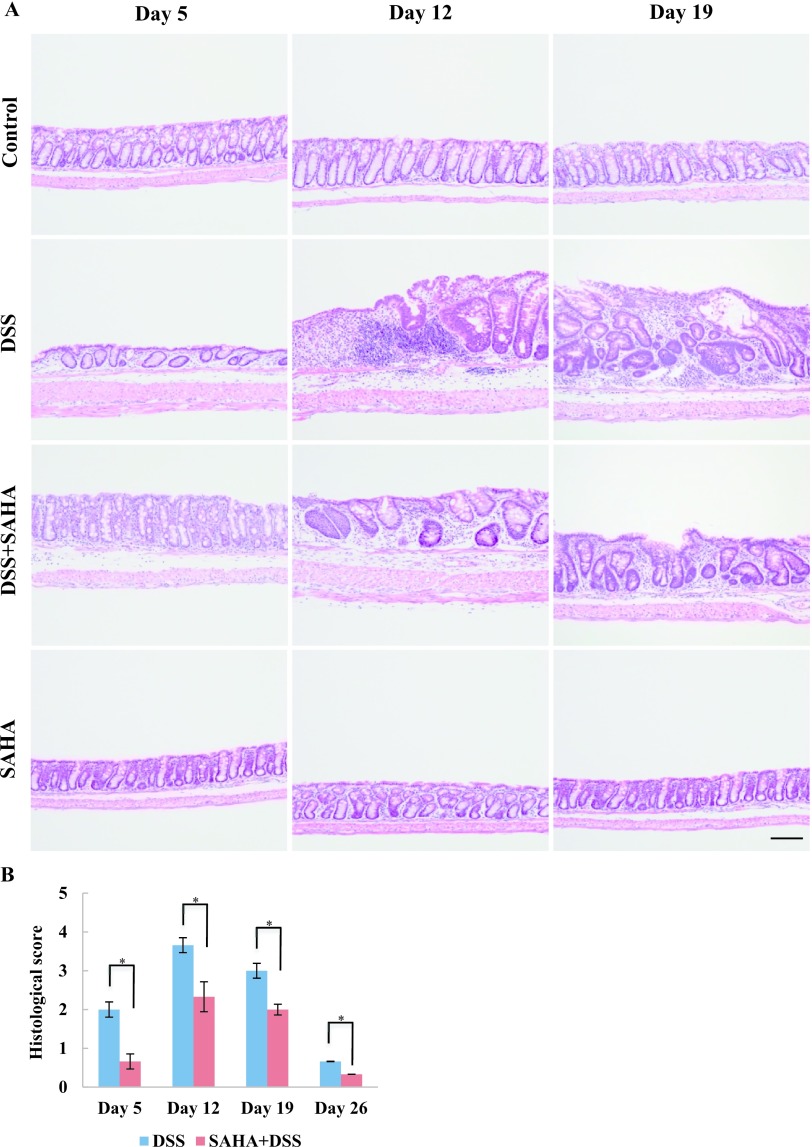
Histopathological evaluation is a generally accepted method for reliable assessment of inflammatory changes [8]. Based on histopathological evaluation, acute inflammation, including shortening and loss of crypts and infiltration of inflammatory cells in the lamina propria, was observed in DSS-treated mouse colon on day 5 (Fig. 2A). However, the surface epithelium was intact due to only mild inflammation on day 5. The most severe damage, including crypt abscesses, loss of surface epithelium, and infiltration of inflammatory cells into the lamina propria and submucosa, was found in DSS-treated mouse colon on day 12. Although extensive damage was still found in DSS-treated mouse colon on day 19, we began to see improved histological changes at this time, including re-epithelization and hyperplastic epithelium. Surprisingly, DSS+SAHA-treated mouse colon revealed only mild damage on all days compared to DSS-treated mouse colon. In fact, only the basal one-third of the crypt was lost, and moderate infiltration of inflammatory cells and an intact surface epithelium were found in DSS+SAHA-treated mouse colon on day 12. Minor histological changes were observed in DSS+SAHA-treated mouse colon on day 26 (data not shown). As shown with HE staining, the control and SAHA-treated mouse colon showed no histopathological changes (Fig. 2A). In DSS+SAHA-treated mouse colon, a significantly lower histological score was found on all sampling days compared to DSS-treated mouse colon (Fig. 2B).
Fig. 2.
Histopathological changes and histological scoring of DSS-induced colitis. A. Paraffin-embedded sections of mouse colon were used for HE staining. Representative photomicrographs of control, DSS, DSS+SAHA, and SAHA-treated mouse colon on days 5, 12, and 19 are shown. Original magnification 200×, Bar = 100 μm. B. Histological scoring of DSS and DSS+SAHA-treated mice on days 5, 12, 19 and 26. Surface epithelial loss, crypt destruction, and inflammatory cell infiltration were evaluated for histological scoring. Asterisks indicate statistically significant differences (*p < 0.05).
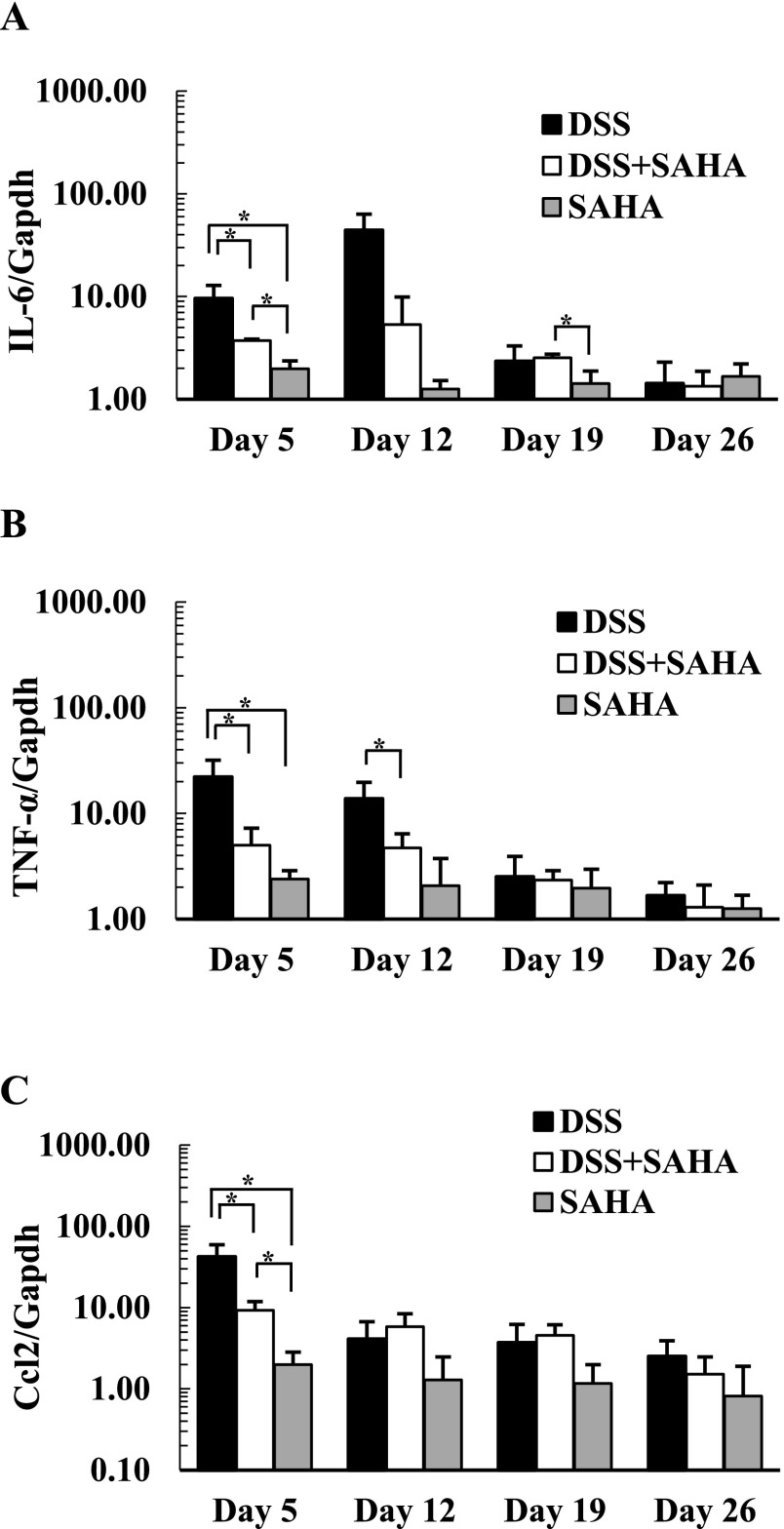
SAHA diminishes local expression of pro-inflammatory genes
During acute colitis, the local secretion of pro-inflammatory cytokines and chemokines increases, and inflammatory cells such as macrophages, dendritic cells, monocytes, and eosinophils accumulate in colonic mucosa [1, 13]. The expression levels of transcripts for pro-inflammatory cytokines and chemokines, including IL-6, TNF-α, and Ccl2, were examined in control, DSS, DSS+SAHA, and SAHA-treated mouse colon. Highest expression levels of IL-6 and TNF-α were found in DSS-treated mouse colon on days 5 and 12, whereas significantly lower expression was found in DSS+SAHA-treated mouse colon (Fig. 3A, B). After the acute phase of inflammation, we found no significant differences in the expression of IL-6 or TNF-α on days 19 or 26. The expression level of Ccl2 was dramatically increased in DSS-treated mouse colon on day 5 and then decreased continually at later time points (Fig. 3C). Importantly, DSS+SAHA-treated mouse colon showed significantly lower expression of Ccl2 on day 5 compared to DSS-treated mouse colon.
Fig. 3.
The relative expression of IL-6, TNF-α, and Ccl2 in mouse colitis. The expression level was measured with quantitative reverse transcriptase-PCR. Relative expression of IL-6 (A), TNF-α (B), and Ccl2 (C) were evaluated in control, DSS, DSS+SAHA, and SAHA-treated mouse colon. The expression level of IL-6, TNF-α, and Ccl2 represents the fold increase compared to expression in control mouse colon. Data are the mean ± SD from at least three mice. Asterisks indicate statistically significant differences (*p < 0.05).
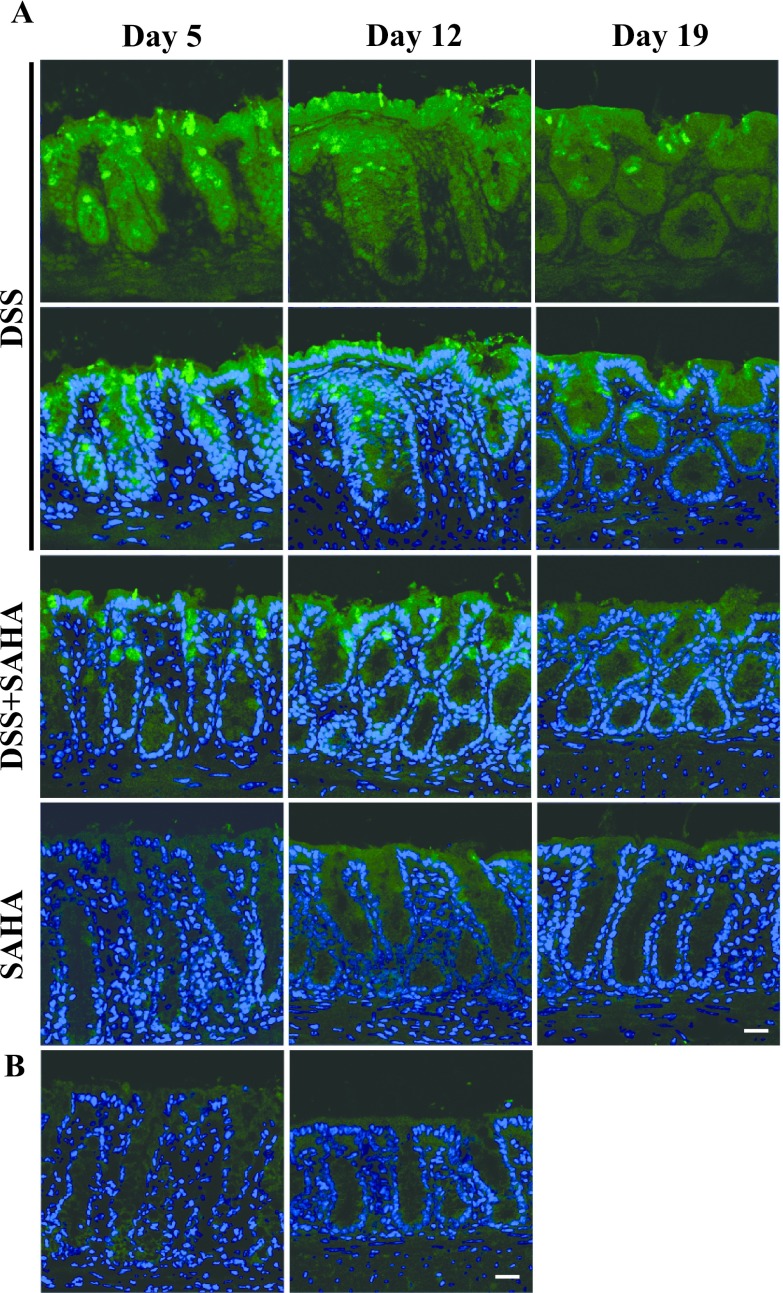
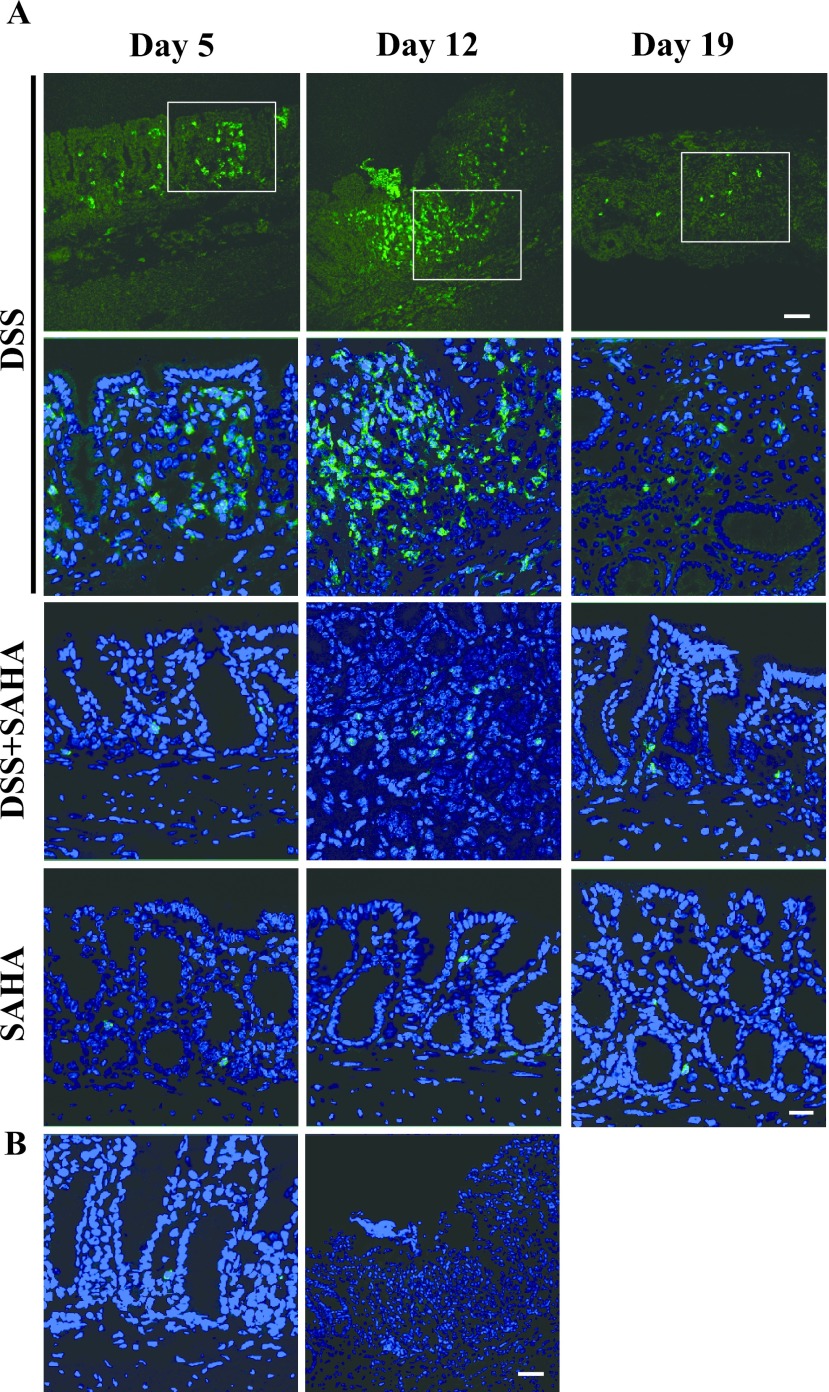
SAHA decreases the local secretion of Ccl2 in DSS-induced colitis
To confirm the qRT-PCR results, we performed immunohistochemistry to determine Ccl2 protein expression in colonic mucosa (Fig. 4A). In DSS-treated mouse colon on day 5, a strong fluorescent signal for Ccl2 was found throughout the colon epithelium, and the signal was especially strong in goblet cells. Although Ccl2 expression remained in DSS-treated mouse colon on days 12 and 19, the staining intensity was decreased significantly, and only a few goblet cells were positive for Ccl2. Consistent with Ccl2 gene expression (Fig. 3C), DSS+SAHA-treated mouse colon showed a dramatic decrease in Ccl2 expression on all days compared to DSS-treated mouse colon. SAHA-only and vehicle-only control mouse colons were negative for Ccl2 expression. The control section reacted with normal goat IgG was negative (Fig. 4B).
Fig. 4.
Immunohistochemical localization of Ccl2 in mouse colitis. A. Fresh frozen tissues were cryo-sectioned and mounted on silane-coated slide glasses. Immunohistochemical localization of Ccl2 in DSS, DSS+SAHA, and SAHA-treated mouse colon on days 5, 12, and 19. B. Ccl2 expression in control mouse colon. A negative control section of DSS-treated mouse colon on day 5 is shown in the right panel. Original magnification 200×, Bar = 20 μm.
SAHA attenuates the accumulation of CD11b-positive cells in DSS-induced colitis
To investigate the accumulation of migratory cells, we performed immunohistochemistry using CD11b, which is expressed in dendritic cells, macrophages, monocytes, and eosinophils. On day 5, DSS-treated mouse colon showed accumulation of CD11b-positive cells in colonic mucosa (Fig. 5A). Interestingly, DAPI staining revealed intact crypt morphology on day 5, suggesting that the inflammatory process has just initiated with migrating inflammatory cells. Surprisingly, CD11b-positive cells were significantly increased at inflammatory sites in DSS-treated mouse colon on day 12. Crypt morphology was totally destroyed due to active inflammation and accumulation of inflammatory cells. Moreover, CD11b-positive cells were localized not only in the mucosa, but also in the submucosa and smooth muscle layer, indicating that the most severe inflammation had occurred. In DSS-treated mouse colon, crypt recovery was observed on day 19 with decreased numbers of CD11b-positive cells. On day 5, DSS+SAHA-treated mice had no obvious damage in the colonic crypt, and only a few CD11b-positive cells were detected. In DSS+SAHA-treated mouse colon on day 12, the number of CD11b-positive cells was dramatically decreased, and minor damage in the colonic crypt was observed. As expected, SAHA-only treated mouse colon on all days showed only a few positive cells, similar to vehicle only-treated control mouse colon (Fig. 5B). The control section reacted with normal rat IgG at the same concentration was negative (Fig. 5B).
Fig. 5.
Immunohistochemical localization of CD11b in mouse colitis. A. Fresh frozen tissues were cryo-sectioned and mounted on silane-coated slide glasses. Immunohistochemical localization of CD11b in DSS, DSS+SAHA, and SAHA-treated mouse colon on days 5, 12, and 19. B. CD11b expression in control mouse colon. A negative control section of DSS-treated mouse colon on day 12 is shown in the right panel. Original magnification 200×, Bar = 50 μm in low-magnification photomicrographs and 20 μm in high-magnification photomicrographs.
IV. Discussion
The major finding of this study was that the HDAC inhibitor, SAHA, attenuated inflammatory changes in DSS-induced colitis by suppressing local secretion of pro-inflammatory cytokines and chemokines. Moreover, SAHA suppressed mobilization and accumulation of inflammatory cells such as macrophages, dendritic cells, monocytes, and eosinophils. These results indicate that HDAC inhibitors, in particular SAHA, may be important and useful for suppressing the inflammation of IBD.
Although histopathological damage was minor on day 5, the peak of inflammatory gene expression as seen with qRT-PCR was found in DSS-treated mouse colon. In contrast, SAHA treatment dramatically decreased pro-inflammatory gene expression in DSS-treated colon. These findings demonstrate that SAHA has immunomodulatory effects on the innate immune system, including suppression of local secretion of pro-inflammatory cytokines and chemokines. Other reports also support our findings, such as HDAC inhibitors, trichostatin A and ITF2357 significantly attenuate the expression of pro-inflammatory cytokines and chemokines both in vivo and in vitro [4, 22]. Moreover, our results demonstrate that both gene and protein expression of Ccl2 were suppressed by SAHA. In agreement with other reports, Ccl2 expression was observed in colonic epithelial cells, especially in goblet cells [2, 5]. Goblet cells produce not only mucin, but also pro-inflammatory cytokines and chemokines during stress conditions [26]. Recent reports show that alteration of histone modification, such as acetylation and methylation, in colonic epithelial cells is important for onset and progression of colitis [24, 25, 28]. Therefore, based on IBD pathogenesis, an epigenetic targeted approach using the HDAC inhibitor, SAHA, may be effective for control of local inflammation.
In this study, the most severe histopathological damage as well as the accumulation of APCs including dendritic cells, macrophages, monocytes, and eosinophils were found in DSS-treated mouse colon on day 12. Surprisingly, fewer migratory cells were seen in SAHA-treated mouse colon on day 12. These results suggest that APCs are negatively regulated by decreased secretion of cytokines and chemokines in colonic mucosa. Many studies also reported that HDAC inhibitors such as MS-275 affect the differentiation and functional activity of dendritic cells and decrease the secretion of IL-6 and TNF-α [9, 20]. Therefore, SAHA treatment decreases the mobilization and accumulation of inflammatory cells in colonic mucosa, and may have a dramatic protective effect against inflammation in DSS-induced colitis.
In the clinical setting, HDAC inhibitors are mainly used for anticancer treatment based on their potential effects including cell cycle inhibition, induction of apoptosis, and anti-angiogenesis effects [18]. The potential anti-inflammatory effects are likely important in other diseases, such as rheumatoid arthritis, peritoneal fibrosis, and asthma [12, 23, 30]. The DSS-induced colitis model most resembles the histopathological changes seen in human IBD, and SAHA may have protective effects by suppressing the innate immune system.
In conclusion, the present study demonstrated that SAHA attenuates inflammatory changes in DSS-induced colitis by suppressing pro-inflammatory cytokines and chemokines as well as accumulation of active inflammatory cells. SAHA may be a useful therapeutic agent for IBD. However, detailed investigations are necessary to reveal the molecular mechanisms of the effects of SAHA in IBD pathogenesis.
V. Conflicts of Interest
The authors declare that there are no conflicts of interest.
VI. Acknowledgments
This study was supported in part by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (No. 16K08471 to Y. Hishikawa).
VII. References
- 1.Arimura K., Takagi H., Uto T., Fukaya T., Nakamura T., Choijookhuu N., Hishikawa Y., Yamashita Y. and Sato K. (2017) Crucial role of plasmacytoid dendritic cells in the development of acute colitis through the regulation of intestinal inflammation. Mucosal Immunol. 10; 957–970. [DOI] [PubMed] [Google Scholar]
- 2.Banks C., Bateman A., Payne R., Johnson P. and Sheron N. (2003) Chemokine expression in IBD. Mucosal chemokine expression is unselectively increased in both ulcerative colitis and Crohn’s disease. J. Pathol. 199; 28–35. [DOI] [PubMed] [Google Scholar]
- 3.Batmunkh B., Choijookhuu N., Srisowanna N., Byambatsogt U., Synn Oo P., Noor Ali M., Yamaguchi Y. and Hishikawa Y. (2017) Estrogen accelerates cell proliferation through estrogen receptor alpha during rat liver regeneration after partial hepatectomy. Acta Histochem. Cytochem. 50; 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bode K. A., Schroder K., Hume D. A., Ravasi T., Heeg K., Sweet M. J. and Dalpke A. H. (2007) Histone deacetylase inhibitors decrease Toll-like receptor-mediated activation of proinflammatory gene expression by impairing transcription factor recruitment. Immunology 122; 596–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chun E., Lavoie S., Michaud M., Gallini C. A., Kim J., Soucy G., Odze R., Glickman J. N. and Garrett W. S. (2015) CCL2 promotes colorectal carcinogenesis by enhancing polymorphonuclear myeloid-derived suppressor cell population and function. Cell Rep. 12; 244–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper H. S., Murthy S. N., Shah R. S. and Sedergran D. J. (1993) Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab. Invest. 69; 238–249. [PubMed] [Google Scholar]
- 7.Eberharter A. and Becker P. B. (2002) Histone acetylation: a switch between repressive and permissive chromatin. Second in review series on chromatin dynamics. EMBO Rep. 3; 224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erben U., Loddenkemper C., Doerfel K., Spieckermann S., Haller D., Heimesaat M. M., Zeitz M., Siegmund B. and Kuhl A. A. (2014) A guide to histomorphological evaluation of intestinal inflammation in mouse models. Int. J. Clin. Exp. Pathol. 7; 4557–4576. [PMC free article] [PubMed] [Google Scholar]
- 9.Frikeche J., Peric Z., Brissot E., Gregoire M., Gaugler B. and Mohty M. (2012) Impact of HDAC inhibitors on dendritic cell functions. Exp. Hematol. 40; 783–791. [DOI] [PubMed] [Google Scholar]
- 10.Grabiec A. M., Krausz S., de Jager W., Burakowski T., Groot D., Sanders M. E., Prakken B. J., Maslinski W., Eldering E., Tak P. P. and Reedquist K. A. (2010) Histone deacetylase inhibitors suppress inflammatory activation of rheumatoid arthritis patient synovial macrophages and tissue. J. Immunol. 184; 2718–2728. [DOI] [PubMed] [Google Scholar]
- 11.Granger A., Abdullah I., Huebner F., Stout A., Wang T., Huebner T., Epstein J. A. and Gruber P. J. (2008) Histone deacetylase inhibition reduces myocardial ischemia-reperfusion injury in mice. FASEB J. 22; 3549–3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Io K., Nishino T., Obata Y., Kitamura M., Koji T. and Kohno S. (2015) SAHA suppresses peritoneal fibrosis in mice. Perit. Dial. Int. 35; 246–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawano Y., Nakae J., Watanabe N., Kikuchi T., Tateya S., Tamori Y., Kaneko M., Abe T., Onodera M. and Itoh H. (2016) Colonic pro-inflammatory macrophages cause insulin resistance in an intestinal Ccl2/Ccr2-dependent manner. Cell Metab. 24; 295–310. [DOI] [PubMed] [Google Scholar]
- 14.Khor B., Gardet A. and Xavier R. J. (2011) Genetics and pathogenesis of inflammatory bowel disease. Nature 474; 307–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kiesler P., Fuss I. J. and Strober W. (2015) Experimental models of inflammatory bowel diseases. Cell. Mol. Gastroenterol. Hepatol. 1; 154–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kwon H. S., Lim H. W., Wu J., Schnolzer M., Verdin E. and Ott M. (2012) Three novel acetylation sites in the Foxp3 transcription factor regulate the suppressive activity of regulatory T cells. J. Immunol. 188; 2712–2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loddo I. and Romano C. (2015) Inflammatory bowel disease: genetics, epigenetics, and pathogenesis. Front. Immunol. 6; 551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marks P. A. and Jiang X. (2005) Histone deacetylase inhibitors in programmed cell death and cancer therapy. Cell Cycle 4; 549–551. [DOI] [PubMed] [Google Scholar]
- 19.Melgar S., Karlsson A. and Michaelsson E. (2005) Acute colitis induced by dextran sulfate sodium progresses to chronicity in C57BL/6 but not in BALB/c mice: correlation between symptoms and inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 288; G1328–1338. [DOI] [PubMed] [Google Scholar]
- 20.Nencioni A., Beck J., Werth D., Grunebach F., Patrone F., Ballestrero A. and Brossart P. (2007) Histone deacetylase inhibitors affect dendritic cell differentiation and immunogenicity. Clin. Cancer Res. 13; 3933–3941. [DOI] [PubMed] [Google Scholar]
- 21.Okayasu I., Hatakeyama S., Yamada M., Ohkusa T., Inagaki Y. and Nakaya R. (1990) A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology 98; 694–702. [DOI] [PubMed] [Google Scholar]
- 22.Reddy P., Sun Y., Toubai T., Duran-Struuck R., Clouthier S. G., Weisiger E., Maeda Y., Tawara I., Krijanovski O., Gatza E., Liu C., Malter C., Mascagni P., Dinarello C. A. and Ferrara J. L. (2008) Histone deacetylase inhibition modulates indoleamine 2,3-dioxygenase-dependent DC functions and regulates experimental graft-versus-host disease in mice. J. Clin. Invest. 118; 2562–2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Royce S. G. and Karagiannis T. C. (2012) Histone deacetylases and their role in asthma. J. Asthma. 49; 121–128. [DOI] [PubMed] [Google Scholar]
- 24.Stylianou E. (2013) Epigenetics: the fine-tuner in inflammatory bowel disease? Curr. Opin. Gastroenterol. 29; 370–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takeshima H., Ikegami D., Wakabayashi M., Niwa T., Kim Y. J. and Ushijima T. (2012) Induction of aberrant trimethylation of histone H3 lysine 27 by inflammation in mouse colonic epithelial cells. Carcinogenesis 33; 2384–2390. [DOI] [PubMed] [Google Scholar]
- 26.Tanabe T. and Rubin B. K. (2016) Airway goblet cells secrete pro-inflammatory cytokines, chemokines, and growth factors. Chest 149; 714–720. [DOI] [PubMed] [Google Scholar]
- 27.Thia K. T., Loftus E. V. Jr., Sandborn W. J. and Yang S. K. (2008) An update on the epidemiology of inflammatory bowel disease in Asia. Am. J. Gastroenterol. 103; 3167–3182. [DOI] [PubMed] [Google Scholar]
- 28.Tsaprouni L. G., Ito K., Powell J. J., Adcock I. M. and Punchard N. (2011) Differential patterns of histone acetylation in inflammatory bowel diseases. J. Inflamm. (Lond). 8; 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsuchiya K., Ikeda T., Batmunkh B., Choijookhuu N., Ishizaki H., Hotokezaka M., Hishikawa Y. and Nanashima A. (2017) Frequency of CD4+CD161+ T cell and interleukin-10 expression in inflammatory bowel diseases. Acta Histochem. Cytochem. 50; 21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Z., Chen C., Finger S. N., Kwajah S., Jung M., Schwarz H., Swanson N., Lareu F. F. and Raghunath M. (2009) Suberoylanilide hydroxamic acid: a potential epigenetic therapeutic agent for lung fibrosis? Eur. Respir. J. 34; 145–155. [DOI] [PubMed] [Google Scholar]