Abstract
The composition of the intestinal microbiota is related to the health and immune function of the host. Administration of antibiotics affects the composition of the intestinal microbiota. However, the effects of immune function on the composition of the intestinal microbiota are still unclear. In this study, we investigated the lymphocyte composition and determined the relationships between lymphocyte function and the intestinal microbiota following antibiotic treatment in mice. To change the composition of the intestinal microbiota, mice were treated with or without antibiotics. Analysis of intestinal microbiota was performed by metagenomic analysis targeting 16S rRNA. Lymphocyte subsets of splenocytes were measured by flow cytometry. For functional analysis of T cells, splenocytes were stimulated with concanavalin (Con A), and cytokine gene expression was measured by real-time polymerase chain reaction. Firmicutes were predominant in the control group, whereas Bacteroidetes predominated in the antibiotic-treated group, as determined by metagenomic analysis. The diversity of the microbiota decreased in the antibiotic-treated group. Analysis of lymphocyte subsets showed that CD3+ cells decreased, whereas CD19+ cells increased in the antibiotic-treated group. All cytokine genes in splenocytes treated with Con A were downregulated in the antibiotic-treated group; in particular, genes encoding interferon-γ, interleukin (IL)-6, and IL-13 significantly decreased. Taken together, these results revealed that changes in the composition of the intestinal microbiota by antibiotic treatment influenced the population of lymphocytes in splenocytes and affected the immune response.
Keywords: cytokine, immune response, intestinal microbiota, metagenomics analysis, T cell
A wide variety of microbiota is present in the intestinal tract of humans and animals, forming a complex intestinal microbiota. This intestinal microbiota has various physiological activities, including roles in metabolism, maturation of the immune system, and inhibition of pathogenic bacteria, and is closely related to the health of the host [2, 15, 17]. Recent studies on the intestinal microbiota have used molecular biology to target the 16S rRNA gene [20]. In particular, metagenomic analysis of the intestinal microbiota has been conducted via next-generation sequencing, demonstrating that the animal intestinal microbiota is dominated by Firmicutes and Bacteroidetes; the composition ratio varies among individuals [8]. Additionally, the composition of the intestinal microbiota varies according to age, place of residence, ethnicity, dietary habit, and regional environment [1, 6, 28].
The relevance of the composition of the intestinal microbiota in the pathogenesis of diseases is currently being studied [4, 14, 23]. In particular, decreased diversity of the intestinal microbiota has been shown to exacerbate inflammatory diseases [13]. However, the effects of changes in the intestinal microbiota on systemic immunity, immune cell populations, and immune responses remain unclear. In humans, it is reported that the rate of suffering from allergic diseases increases with antibiotic administration to child (2–5 years old) [11]. Changes in intestinal microbiome due to antibiotic treatment in young age are expected to be associated with immune responses in the subsequent growth process. Therefore, in this study, antibiotics were administered to young mice (6 weeks old) in which intestinal microbiome were stable. Then, we performed a metagenomic analysis of the intestinal microbiota and evaluated immune cells and immunological responses in splenocytes using different mouse models, following antibiotic treatment.
MATERIALS AND METHODS
Experimental mice and sampling
Healthy BALB/c mice (6 weeks of age) were used in this study. Mice were housed in sterile cages under constant conditions until the experiment. On the basis of previously reported findings, antibiotic treatment was mixed with three types (penicillin, streptomycin and kanamycin) to widely act on various bacteria to the intestinal microbiota for changing composition [16, 24, 26]. Mice were then divided into two groups with similar body weights. Mice in the antibiotic-treated group were given a regimen of penicillin (1,000 U/ml; Meiji Seika, Tokyo, Japan), kanamycin (1 mg/ml; Meiji Seika), and streptomycin (1 mg/ml; Meiji Seika) was used for the experiment for 1 week with free access to drinking water. Subsequently, the mice were maintained with sterile water and feed during the experimental period. Control mice were also maintained with sterile water and feed during the experiment. Rectal feces, mesenteric lymph nodes (MLN), peyer’s patches (PP) and spleens were collected at 1 week after antibiotic administration. The feces were examined by culturing to determine the colony forming units (CFU), and then subjected to metagenomic analysis. Mononuclear cells were isolated from the spleen, and used for flow cytometry analysis and mitogen stimulation for immune analysis. All experiments were performed according to the Animal Care and Use Committee Control Guidelines for the Ethics of Animal Experiments of Rakuno Gakuen University, and mice were treated according to the Laboratory Animal Control Guidelines of Rakuno Gakuen University (approval numbers: VH21A28 and VH14A3).
Intestinal microbiota analysis
DNA was extracted from fecal samples using a QIAamp DNA Mini kit (Qiagen, Tokyo, Japan). Each library was prepared according to “Illumina 16S Metagenomic Sequencing Library Preparation Guide” with a primer set (27Fmod: 5ʹ- AGR GTT TGA TCM TGG CTC AG-3ʹ and 338R: 5ʹ-TGC TGC CTC CCG TAG GAG T-3ʹ) targeting the V1–V2 region of the 16S rRNA gene. 250 bp paired end sequencing of the amplicon was performed on a MiSeq (Illumina) using MiSeq v2 500 cycle kit. The resulting sequences were analyzed using the QIIME pipeline [10].
For determination of CFU in fecal samples, fecal samples were diluted with a buffer that consisted of 0.45 g KH2PO4 (Kishida Chemical, Osaka, Japan), 1.68 g Na2HPO4•12H2O (Kanto Chemical, Tokyo, Japan), 0.05 g ʟ-cysteine HCl•H2O, 0.05 g Tween® 80, and 0.1 g agar in 100 ml distilled water. Samples were subjected to serial 10-fold (w/v) dilutions with dilution buffer and vortexed. In order to calculate the total number of bacteria, 50 µl of the diluted sample was applied to GAM agar medium (Nissui Pharmaceutical, Tokyo, Japan) and cultured for 48 hr under anaerobic environment. Finally, the number of colonies was counted, and the log CFU/g was calculated.
Flow cytometry analysis
To monitor lymphocyte subsets in splenocytes from each group of mice, the separated cells were incubated with rat anti-mouse CD4-fluorescein isothiocyanate (FITC) monoclonal antibodies and rat anti-mouse CD8a/Lyt-2-phycoerythrin (PE), hamster anti-mouse CD3ε-PE or FITC-conjugated rat anti-mouse CD19 antibodies (Beckman Coulter, Brea, CA, U.S.A.) for 30 min at room temperature (23 ± 3°C). The cells were then washed with phosphate-buffered saline (PBS) twice, treated with 0.5% formalin-PBS, and used for flow cytometry analysis (Beckman Coulter).
Cytokine gene expression
Changes in cytokine gene expression (IL-4, IL-6, IL-10, IL-12, IL-13, IL-17, IFN-r, TGF-b) in Mesenteric lymph nodes (MLN) and Peyer’s patches (PP) of lymphoid organs were examined in the mice groups with different intestinal microbiota. Splenocytes (2 × 105) were stimulated with concanavalin A (Con A; 3 µg/ml) for 5 hr to analyze the cytokine response of T cells. Lymphocytes were isolated from MLN and PP from each mice group. RNA was extracted from the collected cells using an RNeasy Mini kit (Qiagen, Japan), and cDNA was then synthesized using a First Standard cDNA Synthesis kit (Roche, Basel, Switzerland). Quantitative PCR (qPCR) was carried out using a Light Cycler 2.0 (Roche), and the genes were detected with a QuantiTect SYBR Green Kit (Qiagen, Hilden, Germany). The amplification conditions consisted of 45 cycles of 94°C for 15 sec, 60°C for 30 sec, and 72°C for 15 sec. The following primers were used for quantification of cytokine gene expression: interferon (IFN)-γ, sense 5′-TGAAAGCCTAGAAAGTCTGAATAA-3′, antisense 5′-GTTGTTGCTGATGGCCTGAT-3′, interleukin (IL)-6, sense 5′-CAGAGGATACCACTCCCAACA-3′, antisense 5′-TGAATTGCCATTGCACAACT-3′, IL-13, sense 5′-CCAGGGCTACACAGAAGTGC-3′, transforming growth factor (TGF)-β, sense 5′-AGCCCTGTATTCCGTCTCCT-3′, antisense 5′-CAATTCCTGGCGTTACCTTG-3′ and glyceraldehyde 3-phosphate dehydrogenase (GAPDH), sense 5′-CGTGAGTGGAGTCATACTGAT-3′, antisense 5′-AACGGATTTGGCCGTATTG-3′. IL-4, IL-12, IL-17 and IL-10 primers in RT2 qPCR Primer Assays were purchased from Qiagen (Germany).
Statistical analysis
Statistical analysis was performed using SPSS version 11.5 (SPSS Inc., Chicago, IL, U.S.A.). Significant differences in relative abundance and colonies counts in flora between the antibiotic-treated and control groups were calculated using Student’s t-test. For the principal component analysis (PCA) of intestinal microbiota, R software (ver. 3.4.0) was used.
RESULTS
Analysis of intestinal microflora by metagenomic analysis
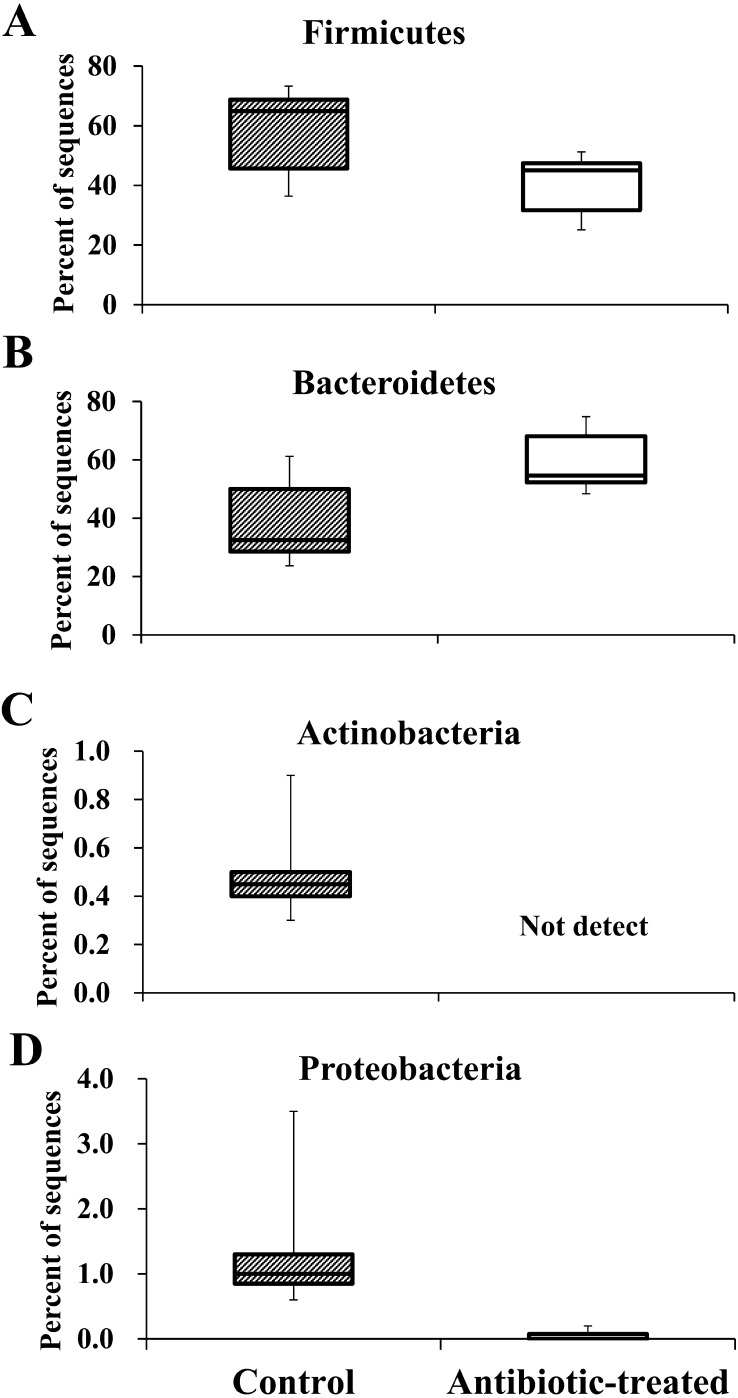
The number of leads of classifiable 16S rRNA genes was 14,229 ± 2,634 (mean ± standard error [SE]), as determined by metagenome analysis. At the phylum level, Firmicutes (relative abundance [RA]: 58.1 ± 14.4%) was predominant in the control group, whereas in the antibiotic-treated group, Firmicutes (RA: 40.3 ± 10.1%) decreased, and Bacteroidetes (RA: 59.4 ± 10.2%) were predominant (Fig. 1A and 1B). Actinobacteria and Proteobacteria were detected in the control group (Actinobacteria RA: 0.5 ± 0.2%, Proteobacteria RA: 1.4 ± 1.0%). In the antibiotic-treated group, Proteobacteria was present at very low abundance (RA: 0.1 ± 0.1%), and Actinobacteria was not detected (Fig. 1C and 1D).
Fig. 1.
The relative abundances of intestinal flora determined by metagenomic analysis. The relative abundances of Firmicutes (A), Bacteroidetes (B), Actinobacteria (C), and Proteobacteria (D) in the intestinal microbiome of mice are shown (n=6).
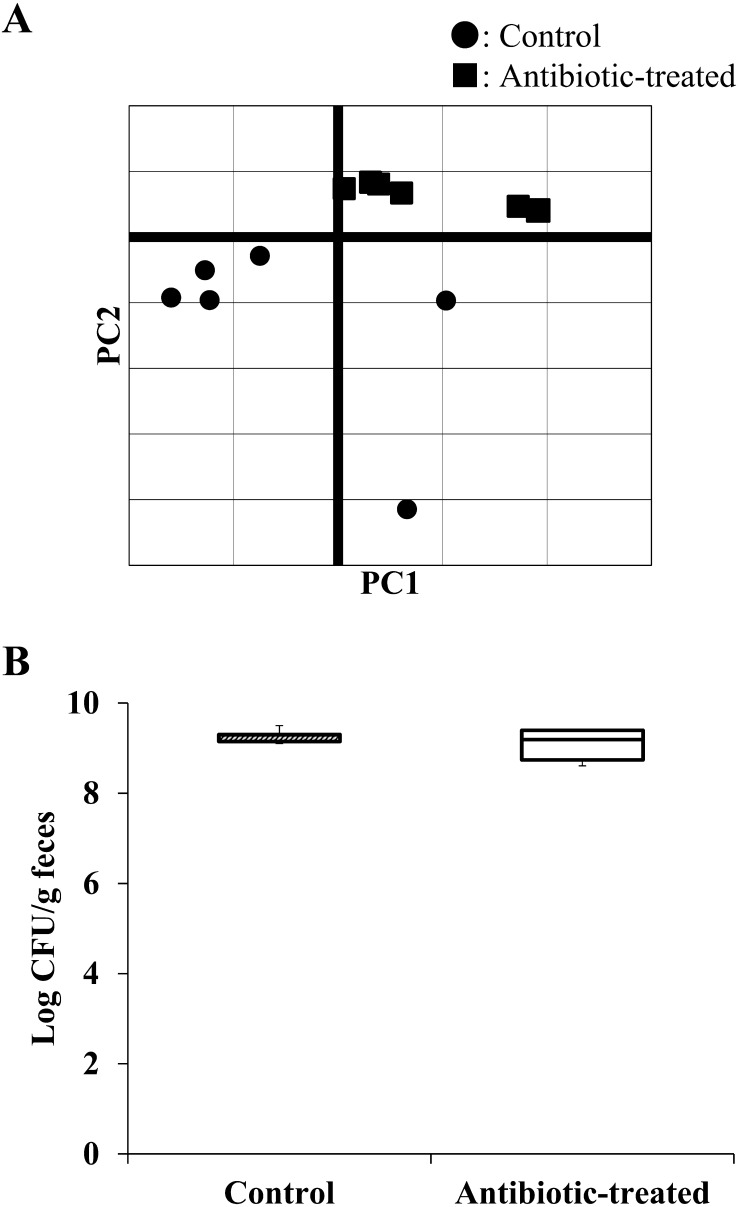
At the family level, fewer detectable strains were found in the antibiotic-treated group than in the control group (Table 1). PCA of the metagenomic analysis between both mouse groups showed similarities in each group, although the two groups formed separate clusters (Fig. 2A). In addition, the total number of bacteria identified by the culture method did not differ between groups (control group: 9.3 ± 0.1 log CFU/g; antibiotic-treated group: 9.1 ± 0.3 log CFU/g; Fig. 2B).
Table 1. Relative abundances of intestinal flora by metagenomic analysis.
| Phylum | Family | Control group |
Antibiotic-treated group |
||
|---|---|---|---|---|---|
| Average (%) | SE | Average (%) | SE | ||
| Firmicutes | Lactobacillaceae | 15.30 | 7.56 | 12.80 | 0 |
| Streptococcaceae | 0.82 | 0.61 | 0 | 0 | |
| Turicibacteraceae | 0.12 | 0.22 | 0.10 | 0.12 | |
| Clostidiales | 22.92 | 11.63 | 13.62 | 6.70 | |
| Clostridiaceae | 0.72 | 1.12 | 0.18 | 0.09 | |
| Dehalobacteriaceae | 0.05 | 0.05 | 0.07 | 0.07 | |
| Lachnospiraceae | 10.60 | 5.51 | 5.82 | 1.78 | |
| Ruminococcaceae | 5.60 | 2.26 | 6.90 | 4.51 | |
| Mogibacteriaceae | 0.30 | 0.06 | 0.10 | 0.08 | |
| Erysipelotrichaceae | 1.20 | 0.85 | 0.37 | 0.20 | |
| Bacteroidetes | Bacteroidaceae | 6.57 | 3.79 | 18.67 | 7.75 |
| Prevotellaceae | 1.70 | 1.24 | 9.77 | 2.90 | |
| Rikenellaceae | 3.63 | 1.42 | 4.92 | 3.24 | |
| Bacteroidales S24-7 | 26.82 | 9.24 | 26.00 | 6.64 | |
| Odoribacteraceae | 0.10 | 0.06 | 0 | 0 | |
| Proteobacteria | Alcaligenaceae | 0.33 | 0.20 | 0 | 0 |
| Desulfovibrionaceae | 0.62 | 0.35 | 0 | 0 | |
| Enterobacteriaceae | 0.45 | 1.01 | 0 | 0 | |
| Actinobacteria | Coriobacteriaceae | 0.48 | 0.19 | 0 | 0 |
Data show averages and standard errors (SEs) of relative abundances in each group of mice.
Fig. 2.
Principal component analysis (PCA) by metagenomic analysis and CFU in feces of each group. Analysis results by PCA showed the composition of the intestinal microbiota of each mouse as the first principal component (PC 1) on the horizontal axis and the second principal component (PC 2) on the vertical axis (A). The total number of fecal samples in each group was measured by the anaerobic culture method using GAM medium. The graph shows the log CFU/g of each group (B) (n=6).
Cytokine gene expressions in MLN and PP
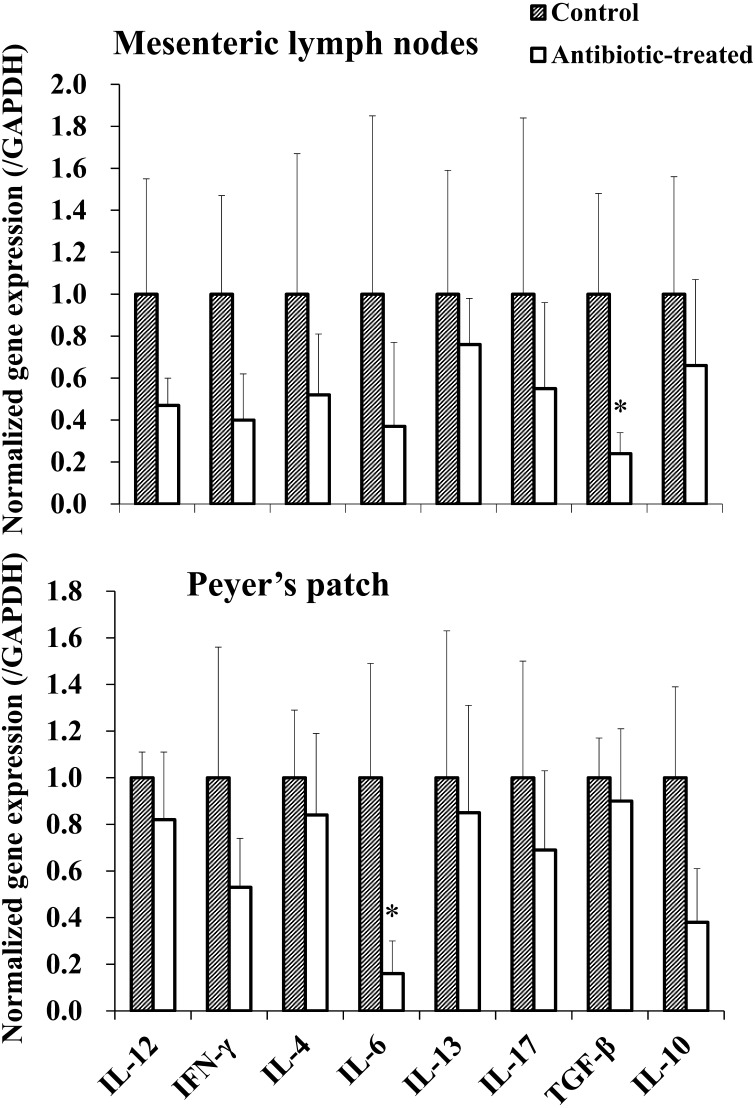
Changes in 8 kinds of cytokine gene expression in MLN and PP near the intestinal tract were examined in mice groups with different intestinal microbiome. As a result, all measured cytokine gene expression decreased. In particular, TGF-β expression in MLN and IL-6 expression in PP were significantly decreased in the antibiotic treated group (Fig. 3; P<0.05).
Fig. 3.
Cytokine gene expression of MLN and PP. The cytokine gene expression of MLN and PP in mice group with different intestinal microbiome was measured by real-time PCR. The expression of each cytokine gene was normalized to GAPDH gene expression, and the values in the control group were set as 1 (n=4). * P<0.05.
Lymphocyte subsets in splenocytes
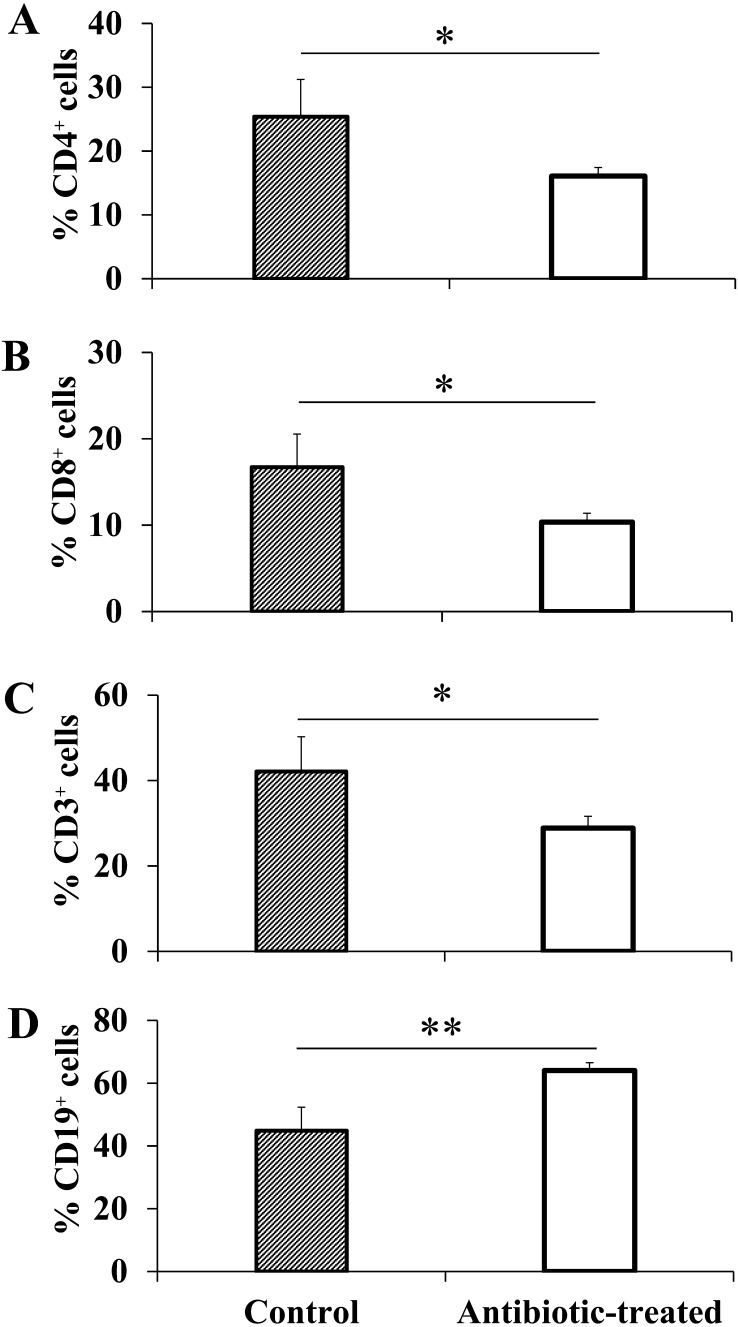
In a comparison of lymphocyte subsets, a significant decrease in CD3+ cells was observed in the antibiotic-treated group (control group: 42.1%, antibiotic-treated group: 28.9%; Fig. 4C; P<0.05). Compared with the control group, the antibiotic-treated group showed significant decreases in CD4+ cells (control group: 25.4%, antibiotic-treated group: 16.1%) and CD8+ cells (control group: 16.7%, antibiotic-treated group: 10.4%) compared with the control group (Fig. 4A and 4B; P<0.05). In antibiotic-treated group, the ratio of CD4+ and CD8+ positive cells in CD3+ cells decreased about 5% from the control mice level (CD3+ CD4+: CN 60.0 ± 2.4, ABT 55.8 ± 2.0 (Ave. ± SE), CD3+ CD8+: CN 40.0 ± 4.3, ABT 35.3 ± 0.5). The population of CD19+ cells was significantly higher in the antibiotic-treated group, and the ratio was higher than the CD3+ cell ratio exceeding 60% on average (control group: 44.9%, antibiotic-treated group: 64.1%; Fig. 4D; P<0.01).
Fig. 4.
Lymphocyte subsets of splenocytes. Spleen cells of each group were analyzed by flow cytometry. The results showed the rates of CD4-positive cells (A), CD8-positive cells (B), CD3-positive cells (C), and CD19-positive cells (D) (n=4). * P<0.05, **P<0.01.
Cytokine response to Con A stimulation in splenocytes
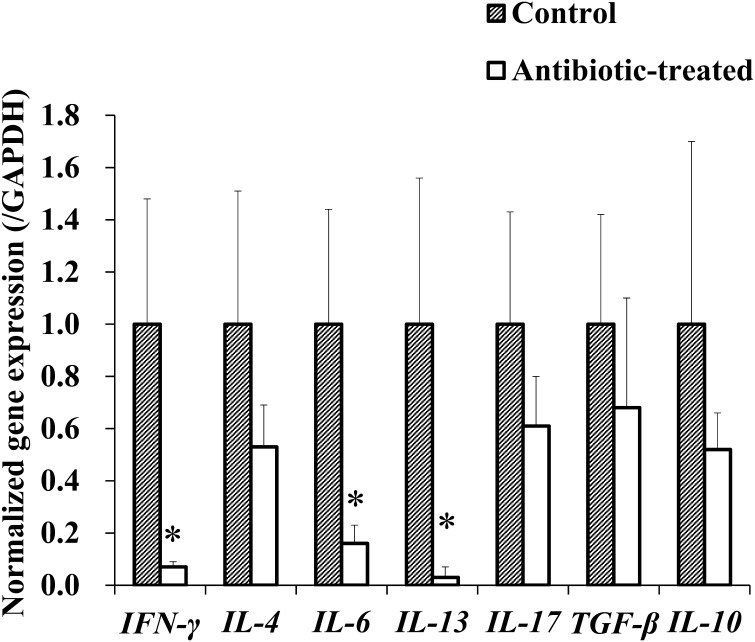
In the cytokine response to Con A stimulation of splenocytes, the antibiotic-treated group showed decreased expression of all measured cytokine genes compared with those in the control group. Notably, the expression of cytokine genes in the antibiotic-treated group was reduced to approximately one-tenth of that in the control group (IFN-γ: 1 to 0.07, IL-6: 1 to 0.16, IL-13: 1 to 0.03; Fig. 5; P<0.05).
Fig. 5.
Mitogen response in splenocytes. Spleen cells (2 × 105) were stimulated with Con A (3 µg/ml) for 5 hr. Thereafter, cytokine gene expression was measured by real-time PCR. The expression of each cytokine gene was normalized to GAPDH gene expression, and the values in the control group were set as 1 (n=4). * P<0.05.
DISCUSSION
The intestinal flora is strongly related to the immune function of the host [13, 19]. In the present study, we investigated a mouse model in which the intestinal microbiota was changed by combined antibiotic treatment. The composition of intestinal microbiota of these mice was revealed by metagenomic analysis, which confirmed changes in dominant bacteria at the phylum level and changes in clusters. In the untreated control group, Firmicutes was predominant, whereas Bacteroidetes became dominant in the antibiotic-treated group. At the family level, the diversity of intestinal flora was reduced by antibiotic treatment. PCA showed that different clusters were formed for each group and that the microflora organization differed. Analysis of the total number of bacteria, as determined by the culture method, revealed that there were no differences between groups. Thus, our findings demonstrated that antibiotic treatment resulted in a compositional change in the intestinal microbiota accompanied by a decrease in diversity without affecting the total intestinal bacterial count.
The prevalence of allergic diseases is related to the composition of the intestinal flora [7, 22, 27]. In addition, decreased diversity in the intestinal microbiota is correlated with the development of allergic diseases [21, 25]. In this present study, there was a significant decrease in CD3+ cells and a significant increase in CD19+ cells in mice treated with antibiotics. The results showed that T cells (CD3+, CD4+ and CD8+ cells) in splenocytes decreased when the diversity of the bacterial species was reduced, e.g., when Bacteroidetes became dominant in the intestinal microbiota following antibiotic treatment. Cytokine gene expression in MLN and PP of lymphoid organs near the intestinal tract was decreased in mice group with different intestinal flora. This result is presumed to be the result of some influence of the change in the composition of the intestinal flora caused by antibiotic administration. Emekciu et al., also reports that antibiotic administration affects lymphocyte subsets [9], but the results are somewhat different from this study. Perhaps it may be due to different species of mouse used and different antibiotic types between the studies. In addition, the mechanism of lymphocyte subsets variation due to changes in intestinal flora is not clarified only by the results of this study. However, the cytokine expression involved in lymphocyte stimulation, including B cell, in the lymph organ (MLN and PP) was changed by the alteration of the intestinal flora composition in this study (Fig. 3). T cells play an important role in immune responses and contribute to inflammatory and allergic diseases [12, 18]. Here, we investigated the response ability of T cells using Con A-stimulated splenocytes from both groups of mice. Interestingly, mice in the antibiotic-treated group showed downregulation of cytokine genes. In particular, IFN-γ, IL-6 and IL-13 transcript levels were significantly reduced. It has been reported that the proportion of Fumicutes and Bacteroidetes in the intestinal bacterial flora is related to inflammatory diseases and allergic diseases. In patients with inflammatory bowel disease and colorectal cancer, a decrease in Firmicutes and an increase in Bactoroidetes have been reported [3]. It is known that IL-6 and IFN-γ cytokines are highly expressed in patients with inflammatory diseases, which reflects the outcome of disease progression. The data of our study may be different from phenomena occurring in the mice treated with antibiotics. However, it is suggested that such intestinal bacterial flora influences cytokine expression of lymphocytes (MLN and PP), and the composition of intestinal bacteria may have some relation to immune system.
In milk allergy patients, it is reported that the composition ratio of intestinal Firmicutes is poor [5]. These findings and this result suggest that the composition ratio of Firmicutes and Bacteoridetes influences the immune response. In our study, the decrease in Firmicutes and the increase in Bacteroidetes confirmed in the antibiotic treatment group were accompanied by a decrease in cytokine expression in the intestinal lymphoid organs. Moreover, the decrease in the cytokine response of T cells may influence the immune response, implying that the compositional balance of the intestinal microbiota is an important factor mediating the immune response of the individual host. It is presumed that the composition ratio of Firmicutes and Bacteoridetes affect spleen cells including the intestinal immune system. Therefore, it is necessary to compare composition ratios in diseased individuals and investigate their relationship.
In summary, our findings showed that changes in the intestinal microflora affected the function of immune cells. In particular, we focused on diseases other than those in the intestinal tract because we observed the influence of antibiotics on the immune response in splenocytes. These results provide important insights into studies on the relationship between intestinal bacterial flora and immunoreactions in mice. However, we did not clarify whether these changes were associated with immune responses related to disease; therefore, further studies are needed to investigate the impact of antibiotics on inflammatory diseases.
Acknowledgments
This study was supported by the Japan Society for the Promotion of Science’s Grants-in-Aid for Scientific Research, JSPS KAKENHI grant number 26292160. We acknowledge the NGS core facility of the Genome Information Research Center at the Research Institute for Microbial Diseases of Osaka University. We also thank M. Nagahara, D.V.M for technical assistance.
REFERENCES
- 1.Arumugam M., Raes J., Pelletier E., Le Paslier D., Yamada T., Mende D. R., Fernandes G. R., Tap J., Bruls T., Batto J. M., Bertalan M., Borruel N., Casellas F., Fernandez L., Gautier L., Hansen T., Hattori M., Hayashi T., Kleerebezem M., Kurokawa K., Leclerc M., Levenez F., Manichanh C., Nielsen H. B., Nielsen T., Pons N., Poulain J., Qin J., Sicheritz-Ponten T., Tims S., Torrents D., Ugarte E., Zoetendal E. G., Wang J., Guarner F., Pedersen O., de Vos W. M., Brunak S., Doré J., Antolín M., Artiguenave F., Blottiere H. M., Almeida M., Brechot C., Cara C., Chervaux C., Cultrone A., Delorme C., Denariaz G., Dervyn R., Foerstner K. U., Friss C., van de Guchte M., Guedon E., Haimet F., Huber W., van Hylckama-Vlieg J., Jamet A., Juste C., Kaci G., Knol J., Lakhdari O., Layec S., Le Roux K., Maguin E., Mérieux A., Melo Minardi R., M’rini C., Muller J., Oozeer R., Parkhill J., Renault P., Rescigno M., Sanchez N., Sunagawa S., Torrejon A., Turner K., Vandemeulebrouck G., Varela E., Winogradsky Y., Zeller G., Weissenbach J., Ehrlich S. D., Bork P., MetaHIT Consortium. 2011. Enterotypes of the human gut microbiome. Nature 473: 174–180. doi: 10.1038/nature09944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balzola F., Bernstein C., Ho G. T., Lees C.2010. A human gut microbial gene catalogue established by metagenomic sequencing--Commentary. Inflamm. Bowel Dis. Monitor. 11: 28. [Google Scholar]
- 3.Bamola V. D., Ghosh A., Kapardar R. K., Lal B., Cheema S., Sarma P., Chaudhry R.2017. Gut microbial diversity in health and disease: experience of healthy Indian subjects, and colon carcinoma and inflammatory bowel disease patients. Microb. Ecol. Health Dis. 28: 1322447. doi: 10.1080/16512235.2017.1322447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Björkstén B., Sepp E., Julge K., Voor T., Mikelsaar M.2001. Allergy development and the intestinal microflora during the first year of life. J. Allergy Clin. Immunol. 108: 516–520. doi: 10.1067/mai.2001.118130 [DOI] [PubMed] [Google Scholar]
- 5.Bunyavanich S., Shen N., Grishin A., Wood R., Burks W., Dawson P., Jones S. M., Leung D. Y. M., Sampson H., Sicherer S., Clemente J. C.2016. Early-life gut microbiome composition and milk allergy resolution. J. Allergy Clin. Immunol. 138: 1122–1130. doi: 10.1016/j.jaci.2016.03.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Filippo C., Cavalieri D., Di Paola M., Ramazzotti M., Poullet J. B., Massart S., Collini S., Pieraccini G., Lionetti P.2010. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. U.S.A. 107: 14691–14696. doi: 10.1073/pnas.1005963107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diesner S. C., Bergmayr C., Pfitzner B., Assmann V., Krishnamurthy D., Starkl P., Endesfelder D., Rothballer M., Welzl G., Rattei T., Eiwegger T., Szépfalusi Z., Fehrenbach H., Jensen-Jarolim E., Hartmann A., Pali-Schöll I., Untersmayr E.2016. A distinct microbiota composition is associated with protection from food allergy in an oral mouse immunization model. Clin. Immunol. 173: 10–18. doi: 10.1016/j.clim.2016.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eckburg P. B., Bik E. M., Bernstein C. N., Purdom E., Dethlefsen L., Sargent M., Gill S. R., Nelson K. E., Relman D. A.2005. Diversity of the human intestinal microbial flora. Science 308: 1635–1638. doi: 10.1126/science.1110591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ekmekciu I., von Klitzing E., Fiebiger U., Escher U., Neumann C., Bacher P., Scheffold A., Kühl A. A., Bereswill S., Heimesaat M. M.2017. Immune responses to broad-spectrum antibiotic treatment and fecal microbiota transplantation in mice. Front. Immunol. 8: 397. doi: 10.3389/fimmu.2017.00397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frank D. N., St Amand A. L., Feldman R. A., Boedeker E. C., Harpaz N., Pace N. R.2007. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl. Acad. Sci. U.S.A. 104: 13780–13785. doi: 10.1073/pnas.0706625104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanada K., Yang L., Narita M., Saito H., Ohya Y.2017. Influence of antibiotic use in early childhood on asthma and allergic diseases at age 5. Ann. Allergy Asthma Immunol. 119: 54–58. doi: 10.1016/j.anai.2017.05.013 [DOI] [PubMed] [Google Scholar]
- 12.Harada Y., Tanaka S., Motomura Y., Harada Y., Ohno S., Ohno S., Yanagi Y., Inoue H., Kubo M.2012. The 3′ enhancer CNS2 is a critical regulator of interleukin-4-mediated humoral immunity in follicular helper T cells. Immunity 36: 188–200. doi: 10.1016/j.immuni.2012.02.002 [DOI] [PubMed] [Google Scholar]
- 13.Hua X., Goedert J. J., Pu A., Yu G., Shi J.2015. Allergy associations with the adult fecal microbiota: Analysis of the American Gut Project. EBioMedicine 3: 172–179. doi: 10.1016/j.ebiom.2015.11.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ichinohe T., Pang I. K., Kumamoto Y., Peaper D. R., Ho J. H., Murray T. S., Iwasaki A.2011. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc. Natl. Acad. Sci. U.S.A. 108: 5354–5359. doi: 10.1073/pnas.1019378108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Islam K. B. M. S., Fukiya S., Hagio M., Fujii N., Ishizuka S., Ooka T., Ogura Y., Hayashi T., Yokota A.2011. Bile acid is a host factor that regulates the composition of the cecal microbiota in rats. Gastroenterology 141: 1773–1781. doi: 10.1053/j.gastro.2011.07.046 [DOI] [PubMed] [Google Scholar]
- 16.Leclercq S., Mian F. M., Stanisz A. M., Bindels L. B., Cambier E., Ben-Amram H., Koren O., Forsythe P., Bienenstock J.2017. Low-dose penicillin in early life induces long-term changes in murine gut microbiota, brain cytokines and behavior. Nat. Commun. 8: 15062. doi: 10.1038/ncomms15062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahowald M. A., Rey F. E., Seedorf H., Turnbaugh P. J., Fulton R. S., Wollam A., Shah N., Wang C., Magrini V., Wilson R. K., Cantarel B. L., Coutinho P. M., Henrissat B., Crock L. W., Russell A., Verberkmoes N. C., Hettich R. L., Gordon J. I.2009. Characterizing a model human gut microbiota composed of members of its two dominant bacterial phyla. Proc. Natl. Acad. Sci. U.S.A. 106: 5859–5864. doi: 10.1073/pnas.0901529106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L.1986. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 136: 2348–2357. [PubMed] [Google Scholar]
- 19.Nishijima S., Suda W., Oshima K., Kim S. W., Hirose Y., Morita H., Hattori M.2016. The gut microbiome of healthy Japanese and its microbial and functional uniqueness. DNA Res. 23: 125–133. doi: 10.1093/dnares/dsw002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olsen G. J., Woese C. R., Overbeek R.1994. The winds of (evolutionary) change: breathing new life into microbiology. J. Bacteriol. 176: 1–6. doi: 10.1128/jb.176.1.1-6.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oyama N., Sudo N., Sogawa H., Kubo C.2001. Antibiotic use during infancy promotes a shift in the T(H)1/T(H)2 balance toward T(H)2-dominant immunity in mice. J. Allergy Clin. Immunol. 107: 153–159. doi: 10.1067/mai.2001.111142 [DOI] [PubMed] [Google Scholar]
- 22.Penders J., Stobberingh E. E., van den Brandt P. A., Thijs C.2007. The role of the intestinal microbiota in the development of atopic disorders. Allergy 62: 1223–1236. doi: 10.1111/j.1398-9995.2007.01462.x [DOI] [PubMed] [Google Scholar]
- 23.Stefka A. T., Feehley T., Tripathi P., Qiu J., McCoy K., Mazmanian S. K., Tjota M. Y., Seo G. Y., Cao S., Theriault B. R., Antonopoulos D. A., Zhou L., Chang E. B., Fu Y. X., Nagler C. R.2014. Commensal bacteria protect against food allergen sensitization. Proc. Natl. Acad. Sci. U.S.A. 111: 13145–13150. doi: 10.1073/pnas.1412008111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thompson J. A., Oliveira R. A., Djukovic A., Ubeda C., Xavier K. B.2015. Manipulation of the quorum sensing signal AI-2 affects the antibiotic-treated gut microbiota. Cell Reports 10: 1861–1871. doi: 10.1016/j.celrep.2015.02.049 [DOI] [PubMed] [Google Scholar]
- 25.Thorburn A. N., McKenzie C. I., Shen S., Stanley D., Macia L., Mason L. J., Roberts L. K., Wong C. H., Shim R., Robert R., Chevalier N., Tan J. K., Mariño E., Moore R. J., Wong L., McConville M. J., Tull D. L., Wood L. G., Murphy V. E., Mattes J., Gibson P. G., Mackay C. R.2015. Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nat. Commun. 6: 7320. doi: 10.1038/ncomms8320 [DOI] [PubMed] [Google Scholar]
- 26.Watanabe J., Fujiwara R., Sasajima N., Ito S., Sonoyama K.2010. Administration of antibiotics during infancy promoted the development of atopic dermatitis-like skin lesions in NC/Nga mice. Biosci. Biotechnol. Biochem. 74: 358–363. doi: 10.1271/bbb.90709 [DOI] [PubMed] [Google Scholar]
- 27.Watanabe S., Narisawa Y., Arase S., Okamatsu H., Ikenaga T., Tajiri Y., Kumemura M.2003. Differences in fecal microflora between patients with atopic dermatitis and healthy control subjects. J. Allergy Clin. Immunol. 111: 587–591. doi: 10.1067/mai.2003.105 [DOI] [PubMed] [Google Scholar]
- 28.Yatunennko T., Ray F. E., Manary M. J., Trehan I., Dominguez-Bello M. G., Contreras M., Magris M., Hidalgo G., Baldassano R. N., Anokhin A. P., Heath A. C., Warner B., Reeder J., Kuczynski J., Caporaso J. G., Lozupone C. A., Lauber C., Clemente J. C., Knights D., Knight R., Gordon J. I.2012. Human gut microbiome viewed across age and geography. Nature 486: 222–227. [DOI] [PMC free article] [PubMed] [Google Scholar]