Abstract
Canine squamous cell carcinoma (SCC) shows highly invasive and locally destructive growth. In animal models and human cancer cases, periostin plays a critical role in the enhancement of cancer growth; however, the mechanism of involvement in canine cancers remains unknown. The aim of this study was to examine the involvement of periostin in the pathophysiology of SCC in dogs. We examined the localization of periostin and periostin-producing cells in 20 SCC and three squamous papilloma specimens. Furthermore, we focused on transforming growth factor (TGF)-β1, which was assumed to be an inducing factor of periostin, using culture cells. By immunohistochemistry, limited periostin expression in the stroma was observed in all squamous papillomas. In SCC, periostin protein diffusely expressed at the tumor invasion front of cancer growth. In situ hybridization revealed that periostin mRNA was expressed in the stromal fibroblasts in SCC. In vitro analysis determined that canine SCC cells expressed significantly higher levels of TGF-β1 mRNA compared with canine keratinocytes. In addition, recombinant TGF-β1 induced secretion of periostin from cultured dermal fibroblasts. These data suggest that periostin produced by stromal fibroblasts may be involved in the pathophysiology of canine SCC. TGF-β1 derived from SCC cells may stimulate fibroblasts to produce periostin.
Keywords: dog, immunohistochemistry, in situ hybridization, periostin, squamous cell carcinoma
The spontaneous occurrence of cancers in dogs has received attention and suggested the use of canine model for human cancer biology and translational cancer therapeutics [27]. Squamous cell carcinoma (SCC) is the second most common cutaneous malignant neoplasm occurring in dogs [6, 11]. In general, canine SCC is highly invasive, locally destructive, and shares similarities with human invasive SCC [9, 11, 26]. Canine SCC is classified into three groups: 1) well-differentiated; 2) moderately differentiated; and 3) poorly differentiated; however, statistics on histological grading or subtypes are rarely given [11].
The tumors consist of not only neoplastic cells but also the tumor microenvironment, such as fibroblasts, inflammatory cells, vasculature, extracellular matrix (ECM), and extracellular molecules [5, 17]. Matricellular proteins are secreted ECM proteins, which modulate cell function by interacting with cell-surface receptors, bioactive molecules, and matrix components, such as collagen [2]. Matricellular proteins are generally present at low levels in most adult tissues but are highly expressed at sites of neoplasm or inflammation in adult humans and experimental animals [2, 17].
Periostin is a recently characterized secretory protein belonging to the group of matricellular proteins [4]. Current studies on animal models and human cases demonstrated that periostin is involved in the pathophysiology of various diseases, including atopic dermatitis (AD), asthma, and other inflammatory diseases, as well as cancer [3, 8, 12, 13, 18, 21, 24, 25]. The expression of periostin is increased in various human neoplastic tissues, including cancers of the stomach, colon, pancreas, skin, and other organs [1, 5, 8, 12,13,14]. Several studies have shown that periostin plays a critical role in tumor angiogenesis, growth of neoplastic components, cancer cell motility, and adhesion [12, 13].
We have previously shown that periostin expression was more intense in the skin tissues of AD dogs and correlated with the severity score of chronic histopathological changes (epidermal thickening and fibrosis) and CD3+ cell number in the dermis, similar to that reported in human AD patients [20]. We also reported that IL-13 possibly derived from T cells stimulates periostin production in both keratinocytes and fibroblasts, and then periostin may play a critical role in the pathophysiology of canine AD, particularly in the enhancement and chronicity of skin lesions [19]. However, in canine cancer, periostin expression has not been demonstrated, and its role in cancer is not known. In the present study, we focused on canine SCC, which is a highly invasive cancer [11]. We examined the expression patterns of the periostin protein in SCC of dogs as well as squamous papilloma for comparison. Furthermore, to investigate the source of periostin in SCC, we examined the localization of periostin mRNA by in situ hybridization (ISH) in SCC. In addition, in vitro analysis, we investigated the relationship between periostin and transforming growth factor (TGF)-β1, which is known as a major trigger of periostin production [10, 25]. Finally, we discussed the possible involvement of periostin in the pathophysiology of SCC in dogs.
MATERIALS AND METHODS
Pathologic examination
Squamous papilloma (n=3) and SCC (n=20) specimens were obtained from surgical or necropsy samples. The clinical data of 20 dogs of various breeds included in the present study is listed in Table 1. Their ages ranged from seven to 16 years, and the cohort included 10 males and 10 females. These specimens were fixed in 10% neutral buffered formalin, embedded in paraffin, cut, and stained with hematoxylin and eosin (HE). The pathological diagnosis was based on the World Health Organization classification systems [9]. The cases of SCC were subdivided into well-differentiated subtypes (n=19) and an acantholytic subtype (n=1), based on the histological criteria proposed by Gross [11].
Table 1. Signalment of dogs with squamous cell carcinoma, histopathological subtypes and the results of immunohistochemical examination of periostin.
| Case No. | Signalment of dogs |
Histopathological Subtypes | Immunohistochemical score and localization of periostinb) |
Remark | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Site | Breed | Sex/Agea) | Non-marginal region of cancer |
Marginal region of cancer |
|||||||
| BMc) | Mesh-liked) | Diffusee) | BMc) | Mesh-liked) | Diffusee) | ||||||
| 1 | Nose | Mixed | F/7y | Well-differented | 0 | 0 | 1+ | 1+ | 1+ | 2+ | |
| 2 | Armpit | Shih Tzu | F/10y | Well-differented | 0 | 0 | 3+ | 0 | 0 | 3+ | |
| 3 | Eyelid | Labrador Retriever | F/13y | Well-differented | 1+ | 1+ | 2+ | 0 | 0 | 1+ | |
| 4 | Hind leg | Welsh Corgi | M/10y | Well-differented | 0 | 0 | 1+ | 0 | 0 | 3+ | |
| 5 | Eyelid | Shetland Sheepdog | F/9y | Well-differented | 3+ | 3+ | 0 | NEf) | NEf) | NEf) | Tru-cutg) |
| 6 | Face | Bulldog | M/11y | Well-differented | 1+ | 0 | 1+ | 1+ | 0 | 1+ | |
| 7 | Chest | Mixed | F/16y | Well-differented | 0 | 0 | 1+ | 0 | 0 | 3+ | |
| 8 | Nail bed | Bulldog | M/9y | Well-differented | 0 | 0 | 1+ | 0 | 0 | 1+ | |
| 9 | Abdomen | Great Pyrenees | C/8y | Acantholytic | 0 | 0 | 1+ | 0 | 0 | 2+ | |
| 10 | Mandible | Jack Russell Terrier | S/11y | Well-differented | 0 | 0 | 0 | 0 | 0 | 0 | |
| 11 | Nail bed | Labrador Retriever | S/9y | Well-differented | 0 | 0 | 1+ | 0 | 0 | 2+ | |
| 12 | Penis | Mixed | M/15y | Well-differented | 0 | 0 | 0 | 0 | 0 | 2+ | |
| 13 | Neck | Shih Tzu | S/9y | Well-differented | 0 | 0 | 0 | 0 | 0 | 0 | |
| 14 | Armpit | Shiba | C/13y | Well-differented | 0 | 0 | 0 | 0 | 0 | 1+ | |
| 15 | Nail bed | Mixed | M/unknown | Well-differented | 0 | 0 | 1+ | NEf) | NEf) | NEf) | Small sample |
| 16 | Back | Mixed | S/13y | Well-differented | 0 | 0 | 1+ | 0 | 0 | 3+ | |
| 17 | Neck | Labrador Retriever | M/13y | Well-differented | 0 | 0 | 1+ | 0 | 0 | 1+ | |
| 18 | Nail bed | Labrador Retriever | M/9y | Well-differented | 0 | 0 | 0 | 0 | 0 | 0 | |
| 19 | Face | Bernese Mountain Dog | F/11y | Well-differented | 0 | 0 | 0 | 0 | 0 | 1+ | |
| 20 | Neck | Labrador Retriever | M/11y | Well-differented | 0 | 0 | 0 | 0 | 0 | 0 | |
a) M, male; F, female; C, castrated male; S, spayed female; y, years old, b) 0<10%, 1+10–50%, 2+50–80%, 3+>80%, c) BM, basement membrane pattern, d) Mesh-like pattern, e) Diffuse pattern, f) NE, Not estimated, g) Biopsy was done by Tru-Cut Needle. It’s difficult to analyse periostin immunolabeling in marginal region of cancer.
Immunohistochemistry
Immunohistochemistry (IHC) using an immunoenzyme polymer method with a rabbit anti-periostin antibody (1:500, Abcam, Cambridge, U.K.) was performed as previously described [20].
For quantitative morphometric analysis, the localization and distribution of periostin were assessed within two regions of the 18 SCCs: 1) the marginal region, which indicated an invasive front area and 2) the non-marginal region (case Nos. 1–4, 6–14 and 16–20). For two small samples, it was difficult to analyze periostin immunolabeling in marginal region of cancers (case Nos. 5 and 15).
The three deposition patterns of periostin were defined as follows: 1) the basement membrane (BM) pattern; 2) the mesh-like pattern, and 3) the diffuse pattern. The three deposition patterns of periostin were graded according to rate of periostin deposition from (−) to (3+), with (−) indicating no or low expression (<10%), (1+) indicating weak deposition (10–50%), (2+) indicating moderate deposition (50–80%), and (3+) indicating high periostin deposition (>80%) (periostin protein expression scores).
In situ hybridization
ISH was performed using QuantiGene® ViewRNA ISH Tissue Assay (Affymetrix, Santa Clara, CA, U.S.A.) according to the manufacturer’s protocol. Periostin probes were used in type 1/Fast Red. A red color assay against periostin mRNA was performed as previously described [20].
Establishment of a canine SCC cell line (Sqc-1)
Canine SCC cell line (Sqc-1) was established from a tumor taken from the abdominal skin of a female mixed-breed dog. The tumor was cauliflower-like and ulcerated, measured 25 × 25 × 8 mm, and had a necrotic center. One half of the specimen was fixed in 10% neutral-buffered formalin, embedded in paraffin, cut, and stained with hematoxylin and eosin. The mass was comprised many neoplastic lobules and showed an invasive nature. Each lobule was composed of epithelial neoplastic cells, which showed squamous cell differentiation. The mitotic activity of neoplastic cells was moderate. The second half of the specimen was immediately placed in phosphate buffered saline (PBS). The specimen was rinsed five times with PBS and digested with a 0.2% trypsin solution in PBS at 31–32°C for 1 hr. The dispersed cells were then filtered through gauze. The collected cells were washed thrice with Eagle’s minimal essential medium (EMEM, Gibco, Carlsbad, CA, U.S.A.), and a 5 × 106 cell suspension was then inoculated in 5 ml of EMEM supplemented with 15% fetal bovine serum, 0.24 mg/ml tryptose phosphate broth, 2 mM glutamine, 6.6 mg/l spectinomycin, and 0.8 mM NaHCO3 in 60 mm plastic dishes. These cells were cultured until confluence at 37°C in a humidified incubator with 5% CO2. Serial cultivation was performed approximately once a week. The cells were examined daily and grew as adherent monolayers with characteristic epithelioid morphological features. We determined that the cells showed these characteristics even after long-term culture. Sqc-1 was transplanted into nude mice in the 300th generation, and the formation of SCC was histologically confirmed. After the cells were subcultured at the 620th generation, they were used for the experiments.
Comparison of TGF-β1 mRNA expression levels of Sqc-1 and CPEK
Commercially available canine keratinocyte cell line (CPEK, CELLnTEC Advanced Cell Systems, Bern, Switzerland) was also used as control in the present study. Sqc-1 or CPEK were grown in 24-well plates (AGC Techno Glass Co., Ltd., Shizuoka, Japan); after washing with PBS to remove all sera, then the cells were serum-starved for 24 hr. Total RNA was extracted from cultured cells in triplicate using the RNeasy Plus Micro Kit (Qiagen). Extracted total RNA was used in RT-PCR employing the SuperScript® VILO™ cDNA Synthesis Kit (Invitrogen Life Technologies, Carlsbad, CA, U.S.A.).
Real-time reverse transcription (RT)-PCR was performed on samples extracted from Sqc-1 and CPEK. Total RNA was extracted from Sqc-1 and CPEK using the RNeasy Plus Micro Kit. Extracted total RNA was used in RT-PCR employing the SuperScript® VILO™ cDNA Synthesis Kit. Quantification of TGF-β1 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA expression was performed using StepOne™ Real-Time PCR Systems (Applied Biosystems, Foster City, CA, U.S.A.) and TaqMan Universal PCR Master Mix (Applied Biosystems), with sample cDNA in a final volume of 20 µl per reaction. Canine TGF-β1 mRNA primers and probes were designed by Applied Biosystems and supplied as Taqman Gene Expression Assays Mix containing a 20× mix of unlabeled PCR forward and reverse primers, as well as a Taqman MGB probe (Assay ID: Cf02623325_m1). The PCR reaction of periostin mRNA was performed in duplicate for each sample and mean values of the gene expression were calculated as a ratio to those of GAPDH according to the ΔΔCT method.
Cell culture of canine dermal fibroblast
A primary culture of canine dermal fibroblasts was performed as previously described [19]. Briefly, full-depth skin samples obtained from the chest region of normal dogs were immediately placed in PBS with 1% Antibiotic Antimycotic (Invitrogen Life Technologies). Skin samples were rinsed seven times with PBS and were then fragmented in 5-mm2 portions. These skin fragments were laid onto the surface of 60 × 15 mm Petri dishes with Dulbecco’s modified Eagle medium: Nutrient Mixture F-12 (DMEM: F12, 1:1; Invitrogen Life Technologies) containing 10% fetal bovine serum (FBS; Hana-nesco Bio. Co., Tokyo, Japan) and 1% Antibiotic Antimycotic. Cultures were incubated at 37°C in a humidified incubator with 5% CO2. The culture medium was changed every three days. Satisfactory proliferation of fibroblasts was observed in approximately twenty days. Fibroblasts between 3rd and 5th passages were used for the experiments.
Stimulation of fibroblasts
Fibroblasts were grown in 24-well plates; after washing with PBS to remove all sera, cells were serum-starved for 24 hr.
For real-time quantitative reverse transcription polymerase chain reaction (RT-PCR) analysis, fibroblasts were cultured containing 0.2% FBS with or without 10 ng/ml recombinant human TGF-β1 (Peprotech, Rochy Hill, NJ, U.S.A.) for six or 24 hr. After stimulation, total RNA was extracted from cell cultures using the RNeasy Plus Micro Kit Extracted total RNA was used in RT-PCR employing the SuperScript® VILO™ cDNA Synthesis Kit.
Quantification of periostin and GAPDH mRNA expression was performed using the StepOne™ Real-Time PCR Systems and the TaqMan Universal PCR Master Mix, as previously described [20]. The PCR reaction of periostin mRNA was performed in duplicate for each sample, and mean values of the gene expression were calculated as a ratio to those of GAPDH according to the ΔΔCT method.
Statistical analyses
Statistical analyses were performed using GraphPad Prism (ver. 5.0, GraphPad Software, La Jolla, CA, U.S.A.). The unpaired t-test was used to determine whether there was a statistically significant difference in the periostin protein expression scores between the marginal and non-marginal regions. Comparisons between Sqc-1 and CPEK in analysis of periostin mRNA expression were performed by the unpaired t-test. The data were analyzed using the unpaired t-test comparing periostin mRNA expression levels between stimulated and non-stimulated cells at the same culture period. All values of P<0.05 were considered significant.
RESULTS
Expression and localization of periostin mRNA and protein in squamous papilloma and SCC of dogs
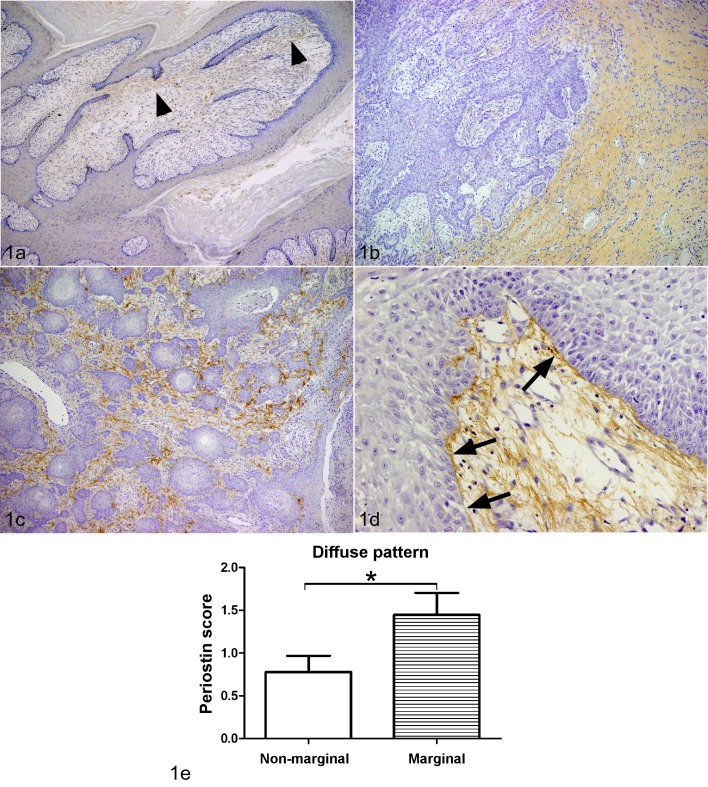
Limited periostin expression in the stroma was observed in all squamous papillomas (Fig. 1a). Periostin protein expression in SCC was intense in the cancer stroma. The expression of periostin was categorized into three localization patterns of periostin protein: 1) the diffuse type (Fig. 1b); 2) the reticular type (Fig. 1c); and 3) the basement membrane (BM) type (Fig. 1d). In general, periostin protein expression showed a diffuse pattern at the tumor invasion front of cancerous growths, whereas BM or reticular patterns were detected in the center of cancerous growths (Fig. 1e; Table 1). In 14 of 18 SCCs, periostin protein diffusely expressed at the tumor invasion front of cancer growth. Periostin expression levels were no obvious association with sex, age and bleeds. No or faint staining of periostin was observed in neoplastic cells.
Fig. 1.
Immunohistochemistry (IHC) of periostin. The brown color indicates positive staining for the periostin protein (a–d). a. Squamous papilloma, dog, skin. Limited periostin protein expression was observed in stroma (arrow heads) (42 ×). b. Squamous cell carcinoma (SCC), skin, dog, case No. 16. The deposition of periostin was diffusely observed in the cancer stroma, particularly at the marginal region (42 ×). c. SCC, skin, dog, case No. 8. Mesh-like pattern periostin protein expression was observed in the neoplastic stroma at the non-infiltrative area (100 ×). d. SCC, skin, dog, case No. 5. The periostin was expressed in the cancer stroma, particularly along the basement membrane (arrows) (200 ×). e. The deposition patterns of periostin were graded according to rate of periostin deposition from (−) to (3+), with (−) indicating no or low expression (<10%), (1+) indicating weak deposition (10–50%), (2+) indicating moderate deposition (50–80%), and (3+) indicating high periostin deposition (>80%) (periostin protein expression scores). There was a significant difference in the periostin protein expression scores in diffuse pattern between marginal region and non-marginal regions (*P<0.05; unpaired t-test).
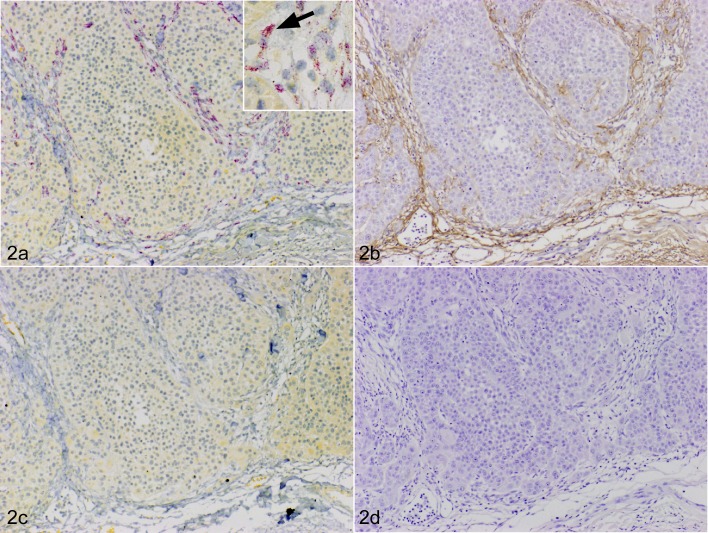
Positive signals for periostin mRNA were detected in fibroblasts in the stroma (Fig. 2a) together with protein expression (Fig. 2b). No signals were detected in tissues hybridized for the sense probe or the ISH solution without the probe (Fig. 2c). No staining was observed with non-immune rabbit IgG instead of anti-periostin antibody in cancer stroma (Fig. 2d).
Fig. 2.
Squamous cell carcinoma (SCC), skin, dog, case No. 7. a. The red color indicates a positive signal for periostin mRNA (fast red). The periostin mRNA was expressed in fibroblasts surrounding cancer (100 ×). Inset: high-power magnification view of periostin mRNA positive cells (arrow). In situ hybridization (ISH) for periostin. b. The brown color indicates positive staining for the periostin protein. Periostin protein expression was prominent in the peripheral stroma (100 ×). Immunohistochemistry (IHC) for periostin. c. No periostin mRNA signal was observed with the sense probe (100 ×). ISH (sense probe). d. No staining was observed with non-immune rabbit IgG in neoplastic stroma (100 ×). IHC using non-immune rabbit IgG.
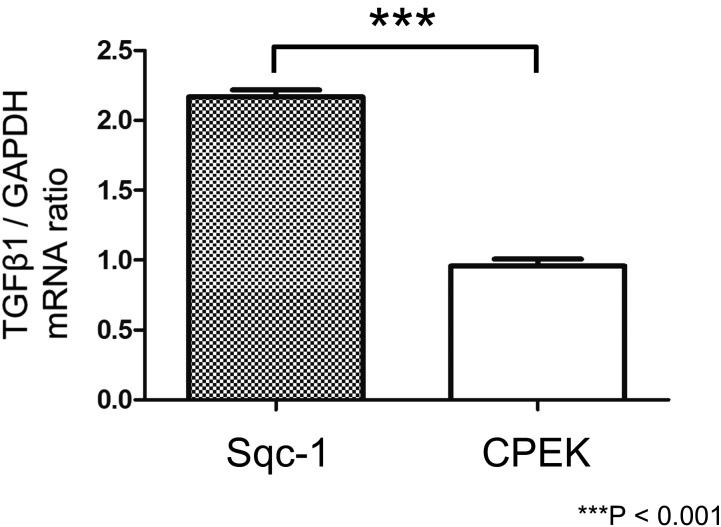
Canine SCC cells expressed significantly higher levels of TGF-β1 mRNA compared with canine keratinocytes
The ratio of TGF-β1/GAPDH mRNA expression was significantly higher in Sqc-1 than CPEK (Fig. 3).
Fig. 3.
Quantitative analysis of mRNA levels of transforming growth factor (TGF)-β1 in canine squamous cell carcinoma cell line (Sqc-1) and canine keratinocyte cell line (CPEK). The ratio of TGF-β1/GAPDH mRNA expression was significantly higher in Sqc-1 than CPEK (***P<0.001; unpaired t-test). Real-time quantitative reverse transcription polymerase chain reaction (RT-PCR).
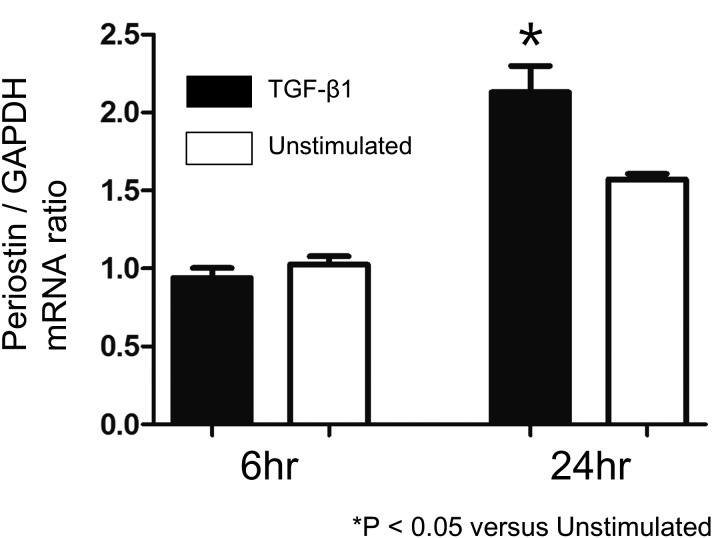
TGF-β1 stimulated dermal fibroblasts to produce periostin mRNA
To investigate the effects of TGF-β1 on periostin expression in cultured dermal fibroblasts, we performed real-time RT-PCR. Following 24 hr of TGF-β1 treatment, mRNA expression of periostin was significantly increased as compared with unstimulated fibroblasts (Fig. 4).
Fig. 4.
Quantitative analysis of mRNA levels of periostin in the cultured dermal fibroblasts at six and 24 hr after stimulation with or without 10 ng/ml recombinant TGF-β1 (*P<0.05; vs Unstimulated cells; unpaired t-test). Real-time quantitative reverse transcription polymerase chain reaction (RT-PCR).
DISCUSSION
In the present study, we found that periostin was deposited intensely in the cancer stroma, particularly at the tumor invasion front. In human and animal models of cancer, periostin is a critical element of the tumor microenvironment as it contributes to the initiation of tumorigenesis and metastasis [1, 5, 7, 8, 12,13,14]. Periostin is a ligand for αvβ3 and αvβ5 integrins and promotes neoplastic cell motility and migration [8]. Kikuchi et al. [12] reported that pericryptal or mesh-like patterns of periostin were observed in normal and inflammatory mucosa of the human colon, and a diffuse pattern was evident in carcinoma that was correlated with invasiveness. These reports suggested that periostin may be involved in the infiltration of human cancer. Therefore, from the results of the present study, we suspect that periostin may additionally play an important role in the invasion of SCC in dogs.
By ISH analysis, we detected periostin mRNA in the stromal fibroblasts together with periostin protein deposition in canine SCC. These correlations strongly suggest that fibroblasts are the main source of periostin in canine SCC. Recent investigations have additionally demonstrated that periostin is overexpressed by stromal fibroblasts in human cancers [12, 13]. We revealed that Sqc-1 cells expressed significantly higher levels of TGF-β1 mRNA compared with CPEK. In addition, we confirmed the expression of the transforming growth factor beta-receptor 1 (TGF-βR1) gene in cultured canine dermal fibroblasts (date not shown), and recombinant TGF-β1 induced secretion of periostin in these fibroblasts. Previous reports revealed that TGF-β1 was found to be overexpressed in various neoplastic cells such as in breast cancer [22], prostate carcinoma [23] and SCC [15, 16]. Lewis et al. [15] reported that neoplastic cell-derived TGF-β1 promotes HGF/SF-dependent invasion of SCC cells in human. Our results suggested that TGF-β1 is associated with invasion of canine SCC cells as inducing factor of periostin.
In conclusion, we suggest the existence of a paracrine periostin loop between stromal fibroblasts and SCC cells. In other words, TGF-β1 derived from SCC cells stimulates fibroblasts to produce periostin. Periostin may promote invasion of cancer cells. In vitro analyses using cultured Sqc-1 may provide valuable information regarding the functional roles of periostin. In vivo analysis targeting TGF-β1 using SCC specimens of various histopathological grades may also provide useful information. The results of the present study provide a molecular basis for periostin as a potential therapeutic target for SCC in dogs.
Acknowledgments
We gratefully thanks to Sanritsu Zelkova Veterinary Laboratory who provided specimens. We thank Dr. Kyohei Yasuno, Dr. Naoyuki Aihara, Dr. Mariko Shirota, Dr. Mariko Okamoto, Dr. Noriaki Okamoto, Dr. Yoko Kakinuma, Ms. Yuka Isayama and Ms. Kanako Sato at Azabu University for their excellent assistance. We also thank Dr. Sadatoshi Maeda, Department of Veterinary Internal Medicine, Gifu University, for his valuable advice regarding quantitative PCR methods.
REFERENCES
- 1.Bao S., Ouyang G., Bai X., Huang Z., Ma C., Liu M., Shao R., Anderson R. M., Rich J. N., Wang X. F.2004. Periostin potently promotes metastatic growth of colon cancer by augmenting cell survival via the Akt/PKB pathway. Cancer Cell 5: 329–339. doi: 10.1016/S1535-6108(04)00081-9 [DOI] [PubMed] [Google Scholar]
- 2.Bornstein P.2009. Matricellular proteins: an overview. J. Cell Commun. Signal. 3: 163–165. doi: 10.1007/s12079-009-0069-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corren J., Lemanske R. F., Jr., Hanania N. A., Korenblat P. E., Parsey M. V., Arron J. R., Harris J. M., Scheerens H., Wu L. C., Su Z., Mosesova S., Eisner M. D., Bohen S. P., Matthews J. G.2011. Lebrikizumab treatment in adults with asthma. N. Engl. J. Med. 365: 1088–1098. doi: 10.1056/NEJMoa1106469 [DOI] [PubMed] [Google Scholar]
- 4.Egbert M., Ruetze M., Sattler M., Wenck H., Gallinat S., Lucius R., Weise J. M.2014. The matricellular protein periostin contributes to proper collagen function and is downregulated during skin aging. J. Dermatol. Sci. 73: 40–48. doi: 10.1016/j.jdermsci.2013.08.010 [DOI] [PubMed] [Google Scholar]
- 5.Erkan M., Kleeff J., Gorbachevski A., Reiser C., Mitkus T., Esposito I., Giese T., Büchler M. W., Giese N. A., Friess H.2007. Periostin creates a tumor-supportive microenvironment in the pancreas by sustaining fibrogenic stellate cell activity. Gastroenterology 132: 1447–1464. doi: 10.1053/j.gastro.2007.01.031 [DOI] [PubMed] [Google Scholar]
- 6.Espinosa de los Monteros A., Aguirre-Sanceledonio M., Ramírez G. A., Castro P., Rodríguez F.2003. Signet-ring squamous cell carcinoma in a dog. Vet. Rec. 153: 90–92. doi: 10.1136/vr.153.3.90 [DOI] [PubMed] [Google Scholar]
- 7.Fukushima N., Kikuchi Y., Nishiyama T., Kudo A., Fukayama M.2008. Periostin deposition in the stroma of invasive and intraductal neoplasms of the pancreas. Mod. Pathol. 21: 1044–1053. doi: 10.1038/modpathol.2008.77 [DOI] [PubMed] [Google Scholar]
- 8.Gillan L., Matei D., Fishman D. A., Gerbin C. S., Karlan B. Y., Chang D. D.2002. Periostin secreted by epithelial ovarian carcinoma is a ligand for alpha(V)beta(3) and alpha(V)beta(5) integrins and promotes cell motility. Cancer Res. 62: 5358–5364. [PubMed] [Google Scholar]
- 9.Goldschmidt M. H., Dunstan R. W., Stannard A. A., von Tscharner C., Walder E. J., Yager J. A.1998. Histological classification of epithelial and melanocytic tumors of the skin of domestic animals, 2nd ser., vol. 3. Armed Forces Institute of Pathology. Washington, D.C. [Google Scholar]
- 10.Gordon E. D., Sidhu S. S., Wang Z. E., Woodruff P. G., Yuan S., Solon M. C., Conway S. J., Huang X., Locksley R. M., Fahy J. V.2012. A protective role for periostin and TGF-β in IgE-mediated allergy and airway hyperresponsiveness. Clin. Exp. Allergy 42: 144–155. doi: 10.1111/j.1365-2222.2011.03840.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gross T. L., Ihrke P. J., Walder E. J., Affolter V. K.2005. Epidermal Tumors. pp. 562–603. In: Skin diseases of the dog and cat, 2nd ed. Blackwell Publishing, Oxford. [Google Scholar]
- 12.Kikuchi Y., Kashima T. G., Nishiyama T., Shimazu K., Morishita Y., Shimazaki M., Kii I., Horie H., Nagai H., Kudo A., Fukayama M.2008. Periostin is expressed in pericryptal fibroblasts and cancer-associated fibroblasts in the colon. J. Histochem. Cytochem. 56: 753–764. doi: 10.1369/jhc.2008.951061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kikuchi Y., Kunita A., Iwata C., Komura D., Nishiyama T., Shimazu K., Takeshita K., Shibahara J., Kii I., Morishita Y., Yashiro M., Hirakawa K., Miyazono K., Kudo A., Fukayama M., Kashima T. G.2014. The niche component periostin is produced by cancer-associated fibroblasts, supporting growth of gastric cancer through ERK activation. Am. J. Pathol. 184: 859–870. doi: 10.1016/j.ajpath.2013.11.012 [DOI] [PubMed] [Google Scholar]
- 14.Kudo Y., Ogawa I., Kitajima S., Kitagawa M., Kawai H., Gaffney P. M., Miyauchi M., Takata T.2006. Periostin promotes invasion and anchorage-independent growth in the metastatic process of head and neck cancer. Cancer Res. 66: 6928–6935. doi: 10.1158/0008-5472.CAN-05-4540 [DOI] [PubMed] [Google Scholar]
- 15.Lewis M. P., Lygoe K. A., Nystrom M. L., Anderson W. P., Speight P. M., Marshall J. F., Thomas G. J.2004. Tumour-derived TGF-β1 modulates myofibroblast differentiation and promotes HGF/SF-dependent invasion of squamous carcinoma cells. Br. J. Cancer 90: 822–832. doi: 10.1038/sj.bjc.6601611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liss C., Fekete M. J., Hasina R., Lam C. D., Lingen M. W.2001. Paracrine angiogenic loop between head-and-neck squamous-cell carcinomas and macrophages. Int. J. Cancer 93: 781–785. doi: 10.1002/ijc.1407 [DOI] [PubMed] [Google Scholar]
- 17.Liu A. Y., Zheng H., Ouyang G.2014. Periostin, a multifunctional matricellular protein in inflammatory and tumor microenvironments. Matrix Biol. 37: 150–156. doi: 10.1016/j.matbio.2014.04.007 [DOI] [PubMed] [Google Scholar]
- 18.Masuoka M., Shiraishi H., Ohta S., Suzuki S., Arima K., Aoki S., Toda S., Inagaki N., Kurihara Y., Hayashida S., Takeuchi S., Koike K., Ono J., Noshiro H., Furue M., Conway S. J., Narisawa Y., Izuhara K.2012. Periostin promotes chronic allergic inflammation in response to Th2 cytokines. J. Clin. Invest. 122: 2590–2600. doi: 10.1172/JCI58978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mineshige T., Kamiie J., Sugahara G., Shirota K.2017. A study on periostin involvement in the pathophysiology of canine atopic skin. J. Vet. Med. Sci. 80: 103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mineshige T., Kamiie J., Sugahara G., Yasuno K., Aihara N., Kawarai S., Yamagishi K., Shirota M., Shirota K.2015. Expression of Periostin in Normal, Atopic, and Nonatopic Chronically Inflamed Canine Skin. Vet. Pathol. 52: 1118–1126. doi: 10.1177/0300985815574007 [DOI] [PubMed] [Google Scholar]
- 21.Nishiyama T., Kii I., Kashima T. G., Kikuchi Y., Ohazama A., Shimazaki M., Fukayama M., Kudo A.2011. Delayed re-epithelialization in periostin-deficient mice during cutaneous wound healing. PLoS ONE 6: e18410. doi: 10.1371/journal.pone.0018410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rønnov-Jessen L., Petersen O. W., Bissell M. J.1996. Cellular changes involved in conversion of normal to malignant breast: importance of the stromal reaction. Physiol. Rev. 76: 69–125. doi: 10.1152/physrev.1996.76.1.69 [DOI] [PubMed] [Google Scholar]
- 23.Rowley D. R.1998–1999. What might a stromal response mean to prostate cancer progression? Cancer Metastasis Rev. 17: 411–419. doi: 10.1023/A:1006129420005 [DOI] [PubMed] [Google Scholar]
- 24.Shiraishi H., Masuoka M., Ohta S., Suzuki S., Arima K., Taniguchi K., Aoki S., Toda S., Yoshimoto T., Inagaki N., Conway S. J., Narisawa Y., Izuhara K.2012. Periostin contributes to the pathogenesis of atopic dermatitis by inducing TSLP production from keratinocytes. Allergol. Int. 61: 563–572. doi: 10.2332/allergolint.10-OA-0297 [DOI] [PubMed] [Google Scholar]
- 25.Takayama G., Arima K., Kanaji T., Toda S., Tanaka H., Shoji S., McKenzie A. N., Nagai H., Hotokebuchi T., Izuhara K.2006. Periostin: a novel component of subepithelial fibrosis of bronchial asthma downstream of IL-4 and IL-13 signals. J. Allergy Clin. Immunol. 118: 98–104. doi: 10.1016/j.jaci.2006.02.046 [DOI] [PubMed] [Google Scholar]
- 26.Toll A., Masferrer E., Hernández-Ruiz M. E., Ferrandiz-Pulido C., Yébenes M., Jaka A., Tuneu A., Jucglà A., Gimeno J., Baró T., Casado B., Gandarillas A., Costa I., Mojal S., Peña R., de Herreros A. G., García-Patos V., Pujol R. M., Hernández-Muñoz I.2013. Epithelial to mesenchymal transition markers are associated with an increased metastatic risk in primary cutaneous squamous cell carcinomas but are attenuated in lymph node metastases. J. Dermatol. Sci. 72: 93–102. doi: 10.1016/j.jdermsci.2013.07.001 [DOI] [PubMed] [Google Scholar]
- 27.Vail D. M., MacEwen E. G.2000. Spontaneously occurring tumors of companion animals as models for human cancer. Cancer Invest. 18: 781–792. doi: 10.3109/07357900009012210 [DOI] [PubMed] [Google Scholar]