Abstract
The neurokinin 1 receptor (NK1R) plays an important role in the pathogenesis of acute pancreatitis (AP). Maropitant is an NK1R antagonist that is widely used as an antiemetic in dogs and cats. In the present study, we investigated the anti-inflammatory action of maropitant in a mouse model of AP. AP was induced in BALB/c mice by intraperitoneal administration of cerulein, and maropitant was administered subcutaneously at a dose of 8 mg/kg. We assessed the mRNA expression levels of NK1R and substance P (SP) in the pancreatic tissue via real-time reverse transcription polymerase chain reaction. In addition, the effect of maropitant on plasma amylase, lipase, and interleukin-6 (IL-6) levels was measured in each mouse. Inflammatory cell infiltration in the pancreas was assessed by myeloperoxidase (MPO) staining. Our results showed that AP induction significantly elevated the mRNA expression of SP in the pancreatic tissue. Treatment with maropitant significantly lowered plasma amylase and IL-6 levels. In addition, treatment with maropitant inhibited the infiltration of MPO-positive cells in the pancreas. The present study suggests that maropitant possesses an anti-inflammatory activity, in addition to its antiemetic action.
Keywords: antiemetic, inflammatory disease, neurokinin 1 receptor, pancreas, substance P
Acute pancreatitis (AP) is a representative inflammatory disease in dogs and cats. The pathogenesis of AP is believed to begin with dysregulated activation of intracellular pancreatic enzymes, which causes injury to pancreatic acinar cells and surrounding tissues, thus triggering an inflammatory response in the abdomen [43]. In severe cases, systemic inflammation induces disseminated intravascular coagulation, followed by multiple organ dysfunction [44]. Therefore, AP can be lethal, and potentially be poor prognosis. In dogs, the mortality rate of AP is reported as 27–58% [28]. The major clinical symptoms of AP are gastrointestinal symptoms, such as vomiting and abdominal pain. The treatment of AP mainly consists of supportive therapy for the alleviation of these clinical symptoms and for the prevention of complications. Based on recent advances in diagnostic tools, the mechanisms underlying the pathogenesis and prognosis of canine and feline AP have been elucidated [6, 44]. However, studies regarding AP therapy are still behind, and there is need to explore candidate medications to improve the outcome of AP.
The neurokinin 1 receptor (NK1R) belongs to the tachykinin family of peptides. NK1R and its main ligand, substance P (SP), regulate various physiological functions, including mood, gastrointestinal transit, vasoconstriction, pain, inflammation, and the vomiting reflex [11]. In particular, SP and NK1R are highly expressed in the vomiting center and the chemoreceptor trigger zone (CTZ), and play an important role in the induction of the vomiting reflex. NK1R antagonists are widely used as antiemetics in both human and veterinary medicine [17]. Maropitant is a highly specific NK1R antagonist that has been used as an antiemetic for dogs and cats [9, 15]. Maropitant performs its antiemetic action both centrally and peripherally by inhibiting the binding of SP to NK1R located in the vomiting center, CTZ, and gastrointestinal tract [37]. Due to its broad-spectrum antiemetic action and its wide safety margin, maropitant is widely prescribed for the treatment of vomiting of varying causes, including AP-induced vomiting [10, 33, 34]
In addition to their antiemetic action, several studies have suggested the novel clinical application of NK1R antagonists as anti-inflammatory agents. SP is a known inflammatory mediator in the acute inflammatory process, and NK1R is expressed in inflammatory cells, such as macrophages and dendritic cells [40]. NK1R antagonists were reported to exhibit anti-inflammatory effects in various inflammatory disease models, including colitis, cystitis, arthritis, asthma, and AP [12, 25, 27, 35]. In particular, SP and NK1R play important roles in the pathogenesis of AP; they are expressed in pancreatic tissue and are involved in the proinflammatory steps of AP by modulating cytokine production, plasma extravasation, edema, and leukocyte infiltration [12, 14]. These findings suggest that maropitant contributes to the management of AP, not only by its antiemetic action, but also by inhibiting the inflammatory process. In the present study, we have investigated the anti-inflammatory action of maropitant in a mouse model of AP.
MATERIALS AND METHODS
Animals
The experimental protocols used in this study were approved by the Institutional Animal Care and Use Committee of Azabu University (Approval No.: 1610108-1). Male 8-week-old BALB/c mice (BALB/c SLC: JAPAN SLC INC, Shizuoka, Japan) were kept in polycarbonate cages (CL-0106-1; 310 × 360 × 175 mm; CLEA Japan, Tokyo, Japan) with wood shavings, in a room equipped with a barrier system at the Research Institute of Biosciences, Azabu University. We used BALB/c mice since this strain is reported to show relatively high susceptibility to cerulein [42]. The room was air-conditioned at a temperature of 22 ± 1°C and a humidity of 55 ± 5%, and was lit for 12 hr each day from 06:00 to 18:00. The animals were acclimated to the facility for 1 week before the experiment. Mice were fed a commercial rodent pellet (mouse and rat chow; MC-2, CLEA Japan, Tokyo, Japan) and had access ad libitum to sterilized drinking water provided in a water bottle. All animals were examined and judged healthy before the study, and were fasted overnight before the experiment.
Study procedure
The mice were divided into three groups: Control group, which received neither cerulein nor maropitant; disease group, which received cerulein (AP induction) but not maropitant; and treatment group, which received cerulein (AP induction) and maropitant. In the disease and treatment groups, AP was induced by intraperitoneal administration of 50 µg/kg cerulein (Bachem AG, Switzerland), every hour for 7 hr, as previously described [22]. This model is a well-established rodent AP model that has long been used for assessing the pathophysiology of AP and investigating treatment methods. Maropitant citrate (Pfizer Inc., New York, NY, U.S.A.) was dissolved in saline (2 mg/ml final) and was administered subcutaneously to the treated mice at a dose of 8 mg/kg immediately after the first cerulein injection. Six hours after the last cerulein injection, the mice were euthanized by administration of pentobarbital followed by cervical dislocation. Blood samples were withdrawn from the heart to determine plasma amylase, lipase, and cytokine levels. The entire pancreas was removed from each mouse. The head portion of pancreas was stored with RNAlater® solution (Thermo Fisher Scientific Inc., Waltham, MA, U.S.A) and used for real-time reverse transcription polymerase chain reaction (RT-qPCR) analysis; the body portion of pancreas was immediately frozen and later used for myeloperoxidase (MPO) staining.
NK1R and SP mRNA expression levels
Real time PCR was performed to measure the mRNA expression levels of NK1R and SP in the pancreatic tissue. Total RNA was isolated from the mouse pancreatic tissues using a commercially available kit (RNAspin Mini RNA Isolation Kit; GE Healthcare UK Ltd., Buckinghamshire, U.K.). After the extraction, reverse transcription was performed using a PrimeScript RT Reagent Kit (Takara Bio, Inc., Kusatsu, Japan). Real time PCR was performed with SYBR® Premix Ex Taq™ II (Takara Bio, Inc.) and specific primers. The following primer pairs were used: NK1R (forward primer: 5ʹ-GCTCTGTGCATGGGTCTCTT-3ʹ, reverse primer: 5ʹ-AGGAAGGATGGCTCCAGGAT-3ʹ), SP (forward primer: 5ʹ-ACGCAGTCTCCAAAGAAAGGA-3ʹ, reverse primer: 5ʹ-ATGAAAGCAGAACCAGGGGTA-3ʹ), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (forward primer: 5ʹ-TGTCCCCACCCCCAATGTATC-3ʹ, reverse primer: 5ʹ-CTCCGATGCCTGCTTCACTACCTT-3ʹ) was carried out at 95°C for 10 sec, then 40 cycles of PCR reaction (95°C for 5 sec and 60°C for 30 sec), followed by dissociation (95°C for 15 sec, 60°C for 30 sec and 95°C for 15 sec). The ΔΔCt method was used for the calculation of the gene expression levels. The relative transcript level of the target gene was expressed as the n-fold difference relative to that of the housekeeping gene, GAPDH, as previously described [23, 39].
Plasma levels of pancreatic enzymes and cytokines
Amylase and lipase activities were measured in all plasma samples with commercial kits, according to the manufacturer’s instructions (COBAS6000, Roche Diagnostics, North America, Indianapolis, IN, U.S.A.). In addition, plasma interleukin-6 (IL-6) levels were assessed using a commercially available sandwich enzyme-linked immunosorbent assay (ELISA) kit (Quantikine IL-6 ELISA kit, R&D Systems Inc., Minneapolis, MN, U.S.A.). The absorbance was read at 450 nm with a plate reader (Power Scan HT, DS Pharma Biomedical, Co., Ltd., Osaka, Japan), and IL-6 concentrations were calculated using a four-parameter logistic curve fit.
MPO staining
MPO staining was performed to assess inflammatory cell infiltration. First, frozen tissue samples were sectioned into 6-µm slices using a cryostat (CM3050 S, Leica Biosystems, Germany); then, MPO staining was performed according to the manufacturer’s instructions (New PO-K kit, Muto Pure Chemical, Co., Ltd., Tokyo, Japan). Giemsa staining was performed as a counterstain. As described in a previous report [30], MPO positive cells were counted in 20 consecutive high-power fields (HPFs) on each slide (× 400 magnification). Three slides were randomly selected for each tissue, and the mean number of MPO positive cells was calculated from them.
Data analysis
All analyses were performed using StatMate (ATMS Co., Ltd., Tokyo, Japan). Comparisons of parameters among the three groups were performed using one-way analysis of variance (ANOVA), followed by Tukey’s multiple comparison tests. The results of qPCR were analyzed by Student’s t tests. Data are expressed as the means ± SD. P<0.05 was considered statistically significant.
RESULTS
The mRNA expression levels of SP and NK1R in pancreatic tissues of healthy and affected mice are shown in Fig. 1. SP and NK1R mRNA were detected in the pancreatic tissues of both groups. Notably, the mRNA level of SP was prominently elevated in the tissues with AP, compared to that in normal pancreas. No significant difference in NK1R mRNA level was observed between the tissues.
Fig. 1.
Relative mRNA levels of SP and NK1R in pancreatic tissue. The data are relative mRNA expression levels that were standardized to the corresponding GAPDH value. *P<0.05; N.S., not significant. SP, substance P; NK1R, neurokinin 1 receptor; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; AP, acute pancreatitis.
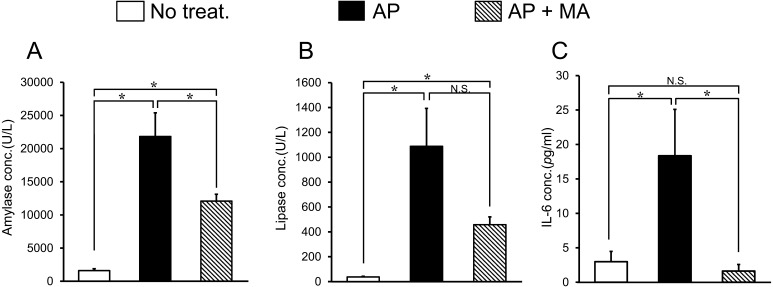
Plasma amylase, lipase and IL-6 levels in each group are shown in Fig. 2. Induction of AP caused a prominent increase in amylase, lipase and IL-6 levels. Treatment with maropitant significantly decreased these levels, especially the IL-6 level, which was lowered in maropitant-treated mice below the value of the healthy mice (Healthy mice: 3.4 ± 1.7 pg/ml, AP mice: 18.4 ± 6.7 pg/ml, AP mice with maropitant treatment: 1.6 ± 0.9 pg/ml).
Fig. 2.
Effect of maropitant on blood amylase, lipase, and IL-6 levels in AP. (A) Plasma amylase level. (B) Plasma lipase level. (C) Plasma IL-6 level. Results are expressed as means ± SD of 8 mice. *P<0.05; N.S., not significant. AP, acute pancreatitis; MA, maropitant; IL-6, interleukin-6.
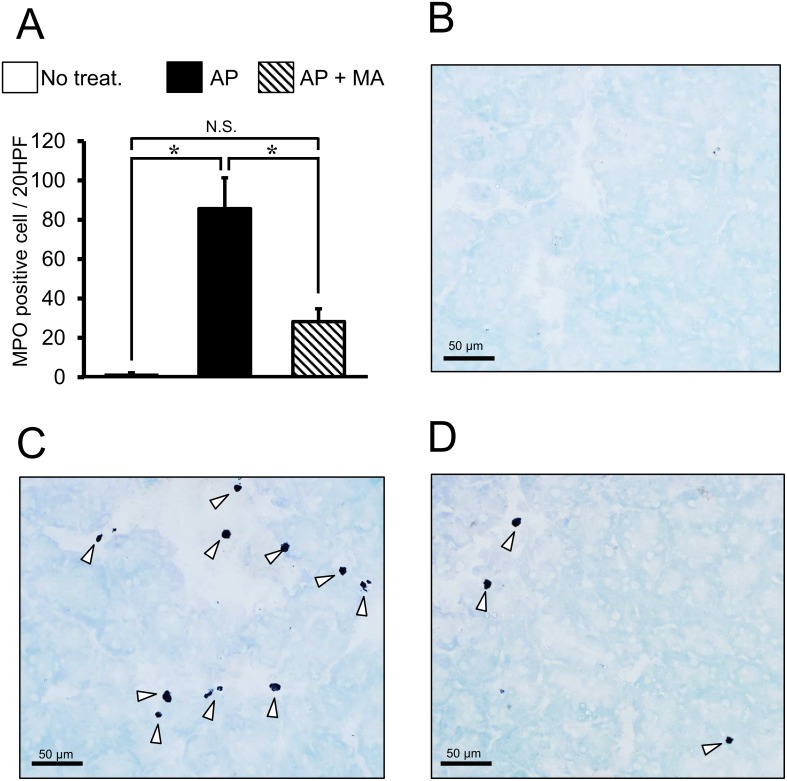
MPO staining was performed to quantify the inflammatory cells in the pancreas (Fig. 3). Treatment with maropitant was found to reduce the cell infiltration (MPO positive cells/20 HPF) in the pancreas (Normal pancreas: 0.4 ± 0.1, AP: 85.7 ± 15.6, AP with maropitant treatment: 28.3 ± 6.6, P<0.05).
Fig. 3.
Effect of maropitant on the number of MPO positive cells in AP. A: Results of MPO staining in each treatment group (n=8, each). *P<0.05; N.S., not significant. The arrowheads indicate MPO positive cells in the pancreas. AP, acute pancreatitis; MA, maropitant; MPO, myeloperoxidase. B: histological finding of no treated mice. C: histological finding of AP mice. D: histological finding of AP mice treated with maropitant.
DISCUSSION
In the present study, we demonstrated the anti-inflammatory action of the NK1R antagonist, maropitant, in a mouse model of AP. Among several inflammatory disease models, the association of SP/NK1R signaling with the pathogenesis AP has been well demonstrated. During the early stage of AP, stimulation of sensory nerves induces the release of SP. The released SP binds to NK1R to activate the inflammatory process in AP, causing edema, hyperamylasemia, microcirculatory dysfunction, and ultimately pancreatic tissue injury [12, 19]. A previous study had indicated that the selective NK1R antagonist, CP96345, decreased blood amylase levels and pancreatic MPO activity in the cerulein-induced AP model [21]. Another rat study demonstrated that administration of CP96345 attenuated the inflammation in AP associated with endoscopic retrograde cholangiopancreatography [13]. In the present study, we confirmed the expression of NK1R and SP in the pancreas. Notably, the SP mRNA level was prominently increased in tissues with AP. Based on the present and previous findings, the anti-inflammatory action of maropitant is mediated through the blockade of NK1R in the pancreas.
Several mechanisms of the anti-inflammatory action of maropitant on AP have been proposed. One proposed mechanism is that released SP directly stimulates inflammatory cells to release cytokines, oxygen free radicals, and other inflammatory mediators. This is especially important for TNF-α, IL-1β and IL-6, which play important roles in the initiation of the inflammatory cascade and tissue injury in AP [26]. A previous study indicated that SP and NK1R expressed in monocytes and macrophages [16]. SP promotes the synthesis of TNF-α, IL-1β and IL-6 [4, 24], and enhances the phagocytosis of murine macrophages [2]. SP also induces the degranulation of human neutrophils and activates the oxidative burst, leading to the generation of reactive oxygen species [32].
Furthermore, SP induces IL-6 production via NF kappa B pathway in mast cell [1]. Our results show that maropitant completely inhibited blood IL-6 levels. This result indicated that SP-NK1R system is major regulator of IL-6 production in inflammatory cells in circulating blood. Considering our findings and those of previous studies, maropitant shows anti-inflammatory action by inhibiting the production of proinflammatory cytokines.
Along with the modulation of inflammatory cytokines, SP/NK1R signaling regulates chemotaxis and vascular permeability. SP is a known chemoattractant for monocytes and neutrophils [32]. It has been reported that SP/NK1R signaling results in neutrophil accumulation in the pancreas, and blockade of NK1R suppressed CC and CXC chemokine production in mice [18]. Furthermore, NK1R expressed in blood vessels, and it can regulate extravasation [18]. In a cerulein-induced AP mouse model, superfusion of SP increased microvascular permeability, and NK1R antagonist treatment inhibited the function of SP, thereby improving the microcirculatory dysfunction [19]. Our results show that treatment with maropitant significantly decreased inflammatory cell infiltration. Taken together, the inhibition of SP-induced chemoattraction and extravasation also may be involved in the anti-inflammatory action of maropitant.
Following the previous CP96345 study [21], we demonstrated the anti-inflammatory action of maropitant, a FDA-approved NK1R antagonist, in cerulein-induced AP. CP96345 is first NK1R antagonist developed in 1990s. CP96345 shows high selectivity to NK1R, and has analgesic and anti-inflammatory action [31]. However, CP96345 has not been clinically approved because of its severe hypotensive action caused by interaction with calcium channel [36]. Like CP96345, maropitant shows high selectivity to NK1R [7]. Maropitant shows high brain-blood ratio [8], and has strong central anti-emetic action without prominent adverse reaction [7]. Along with its anti-emetic action, recent findings suggest that maropitant possess peripheral action, including analgesic, antipruritic, and anti-diarrheal action [5, 34, 45]. To author’s knowledge, this is a first report that demonstrated the anti-inflammatory action of maropitant, suggesting its novel clinical application as anti-inflammatory agent. In the present study, cerulein was administered 7 times to mice, and an autopsy of the pancreas was performed 12 hr later. Compared to the previous CP96345 study, AP model used in the present study is mild, because cerulein was administered fewer times. In addition, the present AP model shows early phase of AP. Based on the present finding, further studies are required to confirm the effect of maropitant on pancreatic injury using more severe AP model with later-phase.
In the present study, maropitant was subcutaneously administered to mice immedietly after the first cerulein administration. We administered maropitant at single dose since this drug is reported to show long duration of action [3]. Previous reports have investigated the pharmacological action of maropitant in mice. One study demonstrated that 1 mg/kg of maropitant alleviated pruritus caused by ulcerative dermatitis [45]. Other investigators found that 10 mg/kg of maropitant inhibited gastrointestinal transit in a mouse model of postoperative ileus, but they did not observe anti-inflammatory activity [29]. This difference in pharmacological action may be due to the degree of involvement of the NK1R in inflammatory process. In the present study, the dose of maropitant was adjusted to 8 mg/kg, which is the maximum oral dose in dogs [7]. Based on the present study, additional studies are needed to investigate the anti-inflammatory action of maropitant in dogs as well.
Along with SP/NK1R pathway, 5-HT/5-HT3R signaling plays an important role in the pathogenesis of AP. We have previously shown that 5-HT3R antagonist ondansetron has anti-inflammatory action in AP [39]. Recently, it is demonstrated that activation of 5-HT3R promotes SP-NK1R system in acute inflammatory process in DSS-induced colitis model. In the report, DSS treatment increased 5-HT3R and SP double positive colonic nerve fiber, and up-regulate the colonic SP level. In addition, these responses were attenuated by NK1R antagonist aprepitant [41].Considering previous and present findings, there is a possibility that anti-inflammatory action of 5-HT3R antagonist ondansetron on AP can be associated with SP/NK1R pathway.
Emesis is a major clinical symptom of canine AP, and in many cases, antiemetics are used in the management of this symptom. Maropitant is a representative antiemetic agent used for AP-induced vomiting. In addition to maropitant, dopamine D2 receptor antagonists, such as metoclopramide, and 5-HT3 receptor antagonists, such as ondansetron and dolasetron, can be used [20, 43]. In a previous study, the dopamine D2 receptor antagonist, domperidone, worsened the pancreatic injury in a rat AP model [38]. In addition, we recently showed that the 5-HT3 receptor antagonist, ondansetron, had anti-inflammatory activity in a mouse AP model [39]. These findings suggest that the selection of antiemetics affects the outcome of AP. Based on our results, further studies are warranted to investigate the association between antiemetic agents and the prognosis of canine AP.
CONFLICT OF INTERESTS
All authors have no conflict of interests to declare.
Acknowledgments
This study was supported by grants from the project grant (Young Scientist Research Training Award funded by Azabu University Research Service, and by a Grant-in-Aid for Scientific Research provided by the Japan Society for the Promotion of Science (MH, 24248050)
REFERENCES
- 1.Azzolina A., Bongiovanni A., Lampiasi N.2003. Substance P induces TNF-alpha and IL-6 production through NF kappa B in peritoneal mast cells. Biochim. Biophys. Acta 1643: 75–83. doi: 10.1016/j.bbamcr.2003.09.003 [DOI] [PubMed] [Google Scholar]
- 2.Bar-Shavit Z., Goldman R., Stabinsky Y., Gottlieb P., Fridkin M., Teichberg V. I., Blumberg S.1980. Enhancement of phagocytosis - a newly found activity of substance P residing in its N-terminal tetrapeptide sequence. Biochem. Biophys. Res. Commun. 94: 1445–1451. doi: 10.1016/0006-291X(80)90581-1 [DOI] [PubMed] [Google Scholar]
- 3.Benchaoui H. A., Cox S. R., Schneider R. P., Boucher J. F., Clemence R. G.2007. The pharmacokinetics of maropitant, a novel neurokinin type-1 receptor antagonist, in dogs. J. Vet. Pharmacol. Ther. 30: 336–344. doi: 10.1111/j.1365-2885.2007.00877.x [DOI] [PubMed] [Google Scholar]
- 4.Bill A., Stjernschantz J., Mandahl A., Brodin E., Nilsson G.1979. Substance P: release on trigeminal nerve stimulation, effects in the eye. Acta Physiol. Scand. 106: 371–373. doi: 10.1111/j.1748-1716.1979.tb06412.x [DOI] [PubMed] [Google Scholar]
- 5.Chambers E. C., Rehm C. D., Correra J., Garcia L. E., Marquez M. E., Wylie-Rosett J., Parsons A.2017. Factors in Placement and Enrollment of Primary Care Patients in YMCA’s Diabetes Prevention Program, Bronx, New York, 2010-2015. Prev. Chronic Dis. 14: E28. doi: 10.5888/pcd14.160486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chartier M. A., Hill S. L., Sunico S., Suchodolski J. S., Robertson J. E., Steiner J. M.2014. Pancreas-specific lipase concentrations and amylase and lipase activities in the peritoneal fluid of dogs with suspected pancreatitis. Vet. J. 201: 385–389. doi: 10.1016/j.tvjl.2014.07.014 [DOI] [PubMed] [Google Scholar]
- 7.Conder G. A., Sedlacek H. S., Boucher J. F., Clemence R. G.2008. Efficacy and safety of maropitant, a selective neurokinin 1 receptor antagonist, in two randomized clinical trials for prevention of vomiting due to motion sickness in dogs. J. Vet. Pharmacol. Ther. 31: 528–532. doi: 10.1111/j.1365-2885.2008.00990.x [DOI] [PubMed] [Google Scholar]
- 8.de la Puente-Redondo V., Tingley F. D., 3rd., Schneider R. P., Hickman M. A.2007. The neurokinin-1 antagonist activity of maropitant, an antiemetic drug for dogs, in a gerbil model. J. Vet. Pharmacol. Ther. 30: 281–287. doi: 10.1111/j.1365-2885.2007.00847.x [DOI] [PubMed] [Google Scholar]
- 9.de la Puente-Redondo V. A., Tilt N., Rowan T. G., Clemence R. G.2007. Efficacy of maropitant for treatment and prevention of emesis caused by intravenous infusion of cisplatin in dogs. Am. J. Vet. Res. 68: 48–56. doi: 10.2460/ajvr.68.1.48 [DOI] [PubMed] [Google Scholar]
- 10.de la Puente-Redondo V. A., Siedek E. M., Benchaoui H. A., Tilt N., Rowan T. G., Clemence R. G.2007. The anti-emetic efficacy of maropitant (Cerenia) in the treatment of ongoing emesis caused by a wide range of underlying clinical aetiologies in canine patients in Europe. J. Small Anim. Pract. 48: 93–98. doi: 10.1111/j.1748-5827.2006.00321.x [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Recio S., Gascón P.2015. Biological and Pharmacological Aspects of the NK1-Receptor. BioMed Res. Int. 2015: 495704. doi: 10.1155/2015/495704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grady E. F., Yoshimi S. K., Maa J., Valeroso D., Vartanian R. K., Rahim S., Kim E. H., Gerard C., Gerard N., Bunnett N. W., Kirkwood K. S.2000. Substance P mediates inflammatory oedema in acute pancreatitis via activation of the neurokinin-1 receptor in rats and mice. Br. J. Pharmacol. 130: 505–512. doi: 10.1038/sj.bjp.0703343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He Z. J., Winston J. H., Yusuf T. E., Micci M. A., Elfert A., Xiao S. Y., Pasricha P. J.2003. Intraductal administration of an NK1 receptor antagonist attenuates the inflammatory response to retrograde infusion of radiological contrast in rats: implications for the pathogenesis and prevention of ERCP-induced pancreatitis. Pancreas 27: e13–e17. doi: 10.1097/00006676-200307000-00018 [DOI] [PubMed] [Google Scholar]
- 14.Hegde A., Bhatia M.2005. Neurogenic inflammation in acute pancreatitis. JOP 6: 417–421. [PubMed] [Google Scholar]
- 15.Hickman M. A., Cox S. R., Mahabir S., Miskell C., Lin J., Bunger A., McCall R. B.2008. Safety, pharmacokinetics and use of the novel NK-1 receptor antagonist maropitant (Cerenia) for the prevention of emesis and motion sickness in cats. J. Vet. Pharmacol. Ther. 31: 220–229. doi: 10.1111/j.1365-2885.2008.00952.x [DOI] [PubMed] [Google Scholar]
- 16.Ho W. Z., Lai J. P., Zhu X. H., Uvaydova M., Douglas S. D.1997. Human monocytes and macrophages express substance P and neurokinin-1 receptor. J. Immunol. 159: 5654–5660. [PubMed] [Google Scholar]
- 17.Huang S. C., Korlipara V. L.2010. Neurokinin-1 receptor antagonists: a comprehensive patent survey. Expert Opin. Ther. Pat. 20: 1019–1045. doi: 10.1517/13543776.2010.495121 [DOI] [PubMed] [Google Scholar]
- 18.Hutter M. M., Wick E. C., Day A. L., Maa J., Zerega E. C., Richmond A. C., Jordan T. H., Grady E. F., Mulvihill S. J., Bunnett N. W., Kirkwood K. S.2005. Transient receptor potential vanilloid (TRPV-1) promotes neurogenic inflammation in the pancreas via activation of the neurokinin-1 receptor (NK-1R). Pancreas 30: 260–265. doi: 10.1097/01.mpa.0000153616.63384.24 [DOI] [PubMed] [Google Scholar]
- 19.Ito Y., Lugea A., Pandol S. J., McCuskey R. S.2007. Substance P mediates cerulein-induced pancreatic microcirculatory dysfunction in mice. Pancreas 34: 138–143. doi: 10.1097/01.mpa.0000246663.30751.24 [DOI] [PubMed] [Google Scholar]
- 20.Jensen K. B., Chan D. L.2014. Nutritional management of acute pancreatitis in dogs and cats. J. Vet. Emerg. Crit. Care (San Antonio) 24: 240–250. doi: 10.1111/vec.12180 [DOI] [PubMed] [Google Scholar]
- 21.Lau H. Y., Wong F. L., Bhatia M.2005. A key role of neurokinin 1 receptors in acute pancreatitis and associated lung injury. Biochem. Biophys. Res. Commun. 327: 509–515. doi: 10.1016/j.bbrc.2004.12.030 [DOI] [PubMed] [Google Scholar]
- 22.Lerch M. M., Gorelick F. S.2013. Models of acute and chronic pancreatitis. Gastroenterology 144: 1180–1193. doi: 10.1053/j.gastro.2012.12.043 [DOI] [PubMed] [Google Scholar]
- 23.Livak K. J., Schmittgen T. D.2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 24.Lotz M., Vaughan J. H., Carson D. A.1988. Effect of neuropeptides on production of inflammatory cytokines by human monocytes. Science 241: 1218–1221. doi: 10.1126/science.2457950 [DOI] [PubMed] [Google Scholar]
- 25.Luber-Narod J., Austin-Ritchie T., Hollins C., 3rd., Menon M., Malhotra R. K., Baker S., Carraway R. E.1997. Role of substance P in several models of bladder inflammation. Urol. Res. 25: 395–399. doi: 10.1007/BF01268854 [DOI] [PubMed] [Google Scholar]
- 26.Makhija R., Kingsnorth A. N.2002. Cytokine storm in acute pancreatitis. J. Hepatobiliary Pancreat. Surg. 9: 401–410. doi: 10.1007/s005340200049 [DOI] [PubMed] [Google Scholar]
- 27.Makino A., Sakai A., Ito H., Suzuki H.2012. Involvement of tachykinins and NK1 receptor in the joint inflammation with collagen type II-specific monoclonal antibody-induced arthritis in mice. J. Nippon Med. Sch. 79: 129–138. doi: 10.1272/jnms.79.129 [DOI] [PubMed] [Google Scholar]
- 28.Mansfield C., Beths T.2015. Management of acute pancreatitis in dogs: a critical appraisal with focus on feeding and analgesia. J. Small Anim. Pract. 56: 27–39. doi: 10.1111/jsap.12296 [DOI] [PubMed] [Google Scholar]
- 29.Mikawa S., Yamamoto S., Islam M. S., Kaji N., Murata T., Mizuno R., Ozaki H., Hori M.2015. Anti-emetic drug maropitant induces intestinal motility disorder but not anti-inflammatory action in mice. J. Vet. Med. Sci. 77: 1195–1199. doi: 10.1292/jvms.15-0182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moreno C., Nicaise C., Gustot T., Quertinmont E., Nagy N., Parmentier M., Louis H., Devière J.2006. Chemokine receptor CCR5 deficiency exacerbates cerulein-induced acute pancreatitis in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 291: G1089–G1099. doi: 10.1152/ajpgi.00571.2005 [DOI] [PubMed] [Google Scholar]
- 31.Nagahisa A., Kanai Y., Suga O., Taniguchi K., Tsuchiya M., Lowe J. A., 3rd., Hess H. J.1992. Antiinflammatory and analgesic activity of a non-peptide substance P receptor antagonist. Eur. J. Pharmacol. 217: 191–195. doi: 10.1016/0014-2999(92)90847-W [DOI] [PubMed] [Google Scholar]
- 32.O’Connor T. M., O’Connell J., O’Brien D. I., Goode T., Bredin C. P., Shanahan F.2004. The role of substance P in inflammatory disease. J. Cell. Physiol. 201: 167–180. doi: 10.1002/jcp.20061 [DOI] [PubMed] [Google Scholar]
- 33.Ramsey D. S., Kincaid K., Watkins J. A., Boucher J. F., Conder G. A., Eagleson J. S., Clemence R. G.2008. Safety and efficacy of injectable and oral maropitant, a selective neurokinin 1 receptor antagonist, in a randomized clinical trial for treatment of vomiting in dogs. J. Vet. Pharmacol. Ther. 31: 538–543. doi: 10.1111/j.1365-2885.2008.00992.x [DOI] [PubMed] [Google Scholar]
- 34.Rau S. E., Barber L. G., Burgess K. E.2010. Efficacy of maropitant in the prevention of delayed vomiting associated with administration of doxorubicin to dogs. J. Vet. Intern. Med. 24: 1452–1457. doi: 10.1111/j.1939-1676.2010.0611.x [DOI] [PubMed] [Google Scholar]
- 35.Rijnierse A., van Zijl K. M., Koster A. S., Nijkamp F. P., Kraneveld A. D.2006. Beneficial effect of tachykinin NK1 receptor antagonism in the development of hapten-induced colitis in mice. Eur. J. Pharmacol. 548: 150–157. doi: 10.1016/j.ejphar.2006.07.010 [DOI] [PubMed] [Google Scholar]
- 36.Schmidt A. W., McLean S., Heym J.1992. The substance P receptor antagonist CP-96,345 interacts with Ca2+ channels. Eur. J. Pharmacol. 219: 491–492. doi: 10.1016/0014-2999(92)90498-S [DOI] [PubMed] [Google Scholar]
- 37.Sedlacek H. S., Ramsey D. S., Boucher J. F., Eagleson J. S., Conder G. A., Clemence R. G.2008. Comparative efficacy of maropitant and selected drugs in preventing emesis induced by centrally or peripherally acting emetogens in dogs. J. Vet. Pharmacol. Ther. 31: 533–537. doi: 10.1111/j.1365-2885.2008.00991.x [DOI] [PubMed] [Google Scholar]
- 38.Sikirić P., Rotkvić I., Mise S., Krizanac S., Gjuris V., Jagić V., Suchanek E., Petek M., Udovicić I., Geber J., et al. 1988. The influence of dopamine receptor agonists and an antagonist on acute pancreatitis in rats. Eur. J. Pharmacol. 147: 321–326. doi: 10.1016/0014-2999(88)90164-1 [DOI] [PubMed] [Google Scholar]
- 39.Tsukamoto A., Sugimoto T., Onuki Y., Shinoda H., Mihara T., Hori M., Inomata T.2017. The 5-HT3 Receptor Antagonist Ondansetron Attenuates Pancreatic Injury in Cerulein-Induced Acute Pancreatitis Model. Inflammation 40: 1409–1415. doi: 10.1007/s10753-017-0584-7 [DOI] [PubMed] [Google Scholar]
- 40.Tuluc F., Lai J. P., Kilpatrick L. E., Evans D. L., Douglas S. D.2009. Neurokinin 1 receptor isoforms and the control of innate immunity. Trends Immunol. 30: 271–276. doi: 10.1016/j.it.2009.03.006 [DOI] [PubMed] [Google Scholar]
- 41.Utsumi D., Matsumoto K., Amagase K., Horie S., Kato S.2016. 5-HT3 receptors promote colonic inflammation via activation of substance P/neurokinin-1 receptors in dextran sulphate sodium-induced murine colitis. Br. J. Pharmacol. 173: 1835–1849. doi: 10.1111/bph.13482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang J., Ohmuraya M., Suyama K., Hirota M., Ozaki N., Baba H., Nakagata N., Araki K., Yamamura K.2010. Relationship of strain-dependent susceptibility to experimentally induced acute pancreatitis with regulation of Prss1 and Spink3 expression. Lab. Invest. 90: 654–664. doi: 10.1038/labinvest.2010.44 [DOI] [PubMed] [Google Scholar]
- 43.Washabau R. J.2013. Canine and Feline Gastroenterology, 1st ed., Saunders, Philadelphia. [Google Scholar]
- 44.Watson P.2015. Pancreatitis in dogs and cats: definitions and pathophysiology. J. Small Anim. Pract. 56: 3–12. doi: 10.1111/jsap.12293 [DOI] [PubMed] [Google Scholar]
- 45.Williams-Fritze M. J., Carlson Scholz J. A., Zeiss C., Deng Y., Wilson S. R., Franklin R., Smith P. C.2011. Maropitant citrate for treatment of ulcerative dermatitis in mice with a C57BL/6 background. J. Am. Assoc. Lab. Anim. Sci. 50: 221–226. [PMC free article] [PubMed] [Google Scholar]