Abstract
The present study was designed to clarify phosphodiesterase 9 (PDE9) expression in bovine tracheal smooth muscle tissue, and to elucidate that PDE9 may contribute to the regulation of airway relaxation. PDE9 mRNA expression was detected in bovine tracheal smooth muscle. Sodium nitroprusside (an NO donor) and BAY 73-6691 (a selective PDE9 inhibitor) reduced high K+- and carbachol-induced contraction. BAY 73-6691 relaxed tracheal tissue on the same level with vardenafil (a selective PDE5 inhibitor). These results support our hypothesis that PDE9 plays functional role in the tracheal smooth muscle relaxation. PDE9 inhibitors are expected to be a novel target of the add-on treatment of airway hyperresponsiveness.
Keywords: BAY 73-6691, PDE9, relaxation, tracheal smooth muscle
Of importance to asthma therapeutics is the belief that β2-adrenoceptor agonists produce bronchodilation by elevating cyclic adenosine monophosphate (cAMP) content and activating protein kinase A (PKA) [17]. In addition, xanthine derivatives (including theophylline), identified as non-selective phosphodiesterase (PDE) inhibitors, are clinically used as bronchodilators for an add-on controller and/or a reliever of asthma and chronic obstructive pulmonary diseases (COPD) with β agonists or corticosteroids [1].
Currently PDEs are classified into 11 families, by their functional properties and responses to specific effectors [2, 4, 7, 11]. PDEs degrade cAMP and cyclic guanosine monophosphate (cGMP), which are important second messengers and have been associated with smooth muscle relaxation. Each PDE has substrate specificity, some PDEs specifically hydrolyse cAMP (PDE4, PDE7 and PDE8), whereas others specifically hydrolyse cGMP (PDE5, PDE6 and PDE9), and some hydrolyse both (PDE1, PDE2, PDE3, PDE10 and PDE11) [2]. Distributions of PDE isozymes vary in tissue; Bovine airway smooth muscle contains isoenzymes of the PDE1, 2, 3, 4 and 5 families [14, 17]. PDE3 and PDE4 were the primary therapeutic targets, their inhibitors were discovered to bronchodilatory activities. PDE4 selective inhibitors were developed to treat COPD and asthma, but were not approved owing to lack of efficacy and the presence of intolerable side effects [13].
Recent reports suggest that cGMP mediated relaxation mechanism may act as a bronchodilator. For example, inducible NO synthase mRNA is upregulated in PDE5 inhibitors, including sildenafil may act as a bronchodilator in guinea-pig asthma models, show that cGMP also have bronchodilate potential as cAMP [18]. However, little is known whether other cGMP related PDEs are effective in bronchodilation.
PDE9 is one of a novel identified isozyme [8], which is expressed in brain [19], spleen [6], skeletal muscles [3], urinary tract [12] and corpus cavernosum [5]. Recently, PDE9 inhibitors have received much attention as potential therapeutics for the treatment of Alzheimer’s disease [16, 21]. Furthermore, PDE9 inhibitors have been shown to be driven by expectations of treatment effects for dysuria via enhancement of intracellular cGMP levels in urinary tissue [12]. However, functions of PDE9 remain poorly understood. BAY 73-6691 (1-(2-chlorophenyl)-6-[(2R)-3,3,3-trifluoro-2-methylpropyl]-1,5-dihydro-4H-pyrazolo[3,4-d]pyrimidine-4-one), recently described as a PDE9 inhibitor, was found to have high affinity for human and murine PDE9 (IC50 of 55 and 100 nM, respectively) and high selectivity for PDE9 versus other PDEs in vitro [20]. Therefore, this study aimed to characterize the PDE9 mRNA expression in bovine tracheal smooth muscle, and investigate the effects of BAY 73-6691 in high K+- and carbachol- induced contraction to clarify the role of PDE9-related pathway in regulation of tracheal smooth muscle relaxation.
Tracheas from adult bovines of either sex were obtained from a local abattoir. The smooth muscle was excised from the cartilage, and the epithelium and connective tissues were removed. The muscle strips were about 2 mm in width and 7 mm in length and were incubated with physiological salt solution (PSS) containing (in mM) 136.8 NaCl, 5.4 KCl, 2.5 CaCl2, 1.0 MgCl2, 11.9 NaHCO3 and 5.6 glucose. The PSS was aerated with 95% O2 and 5% CO2 at 37°C to adjust the pH to 7.2. Muscle tension was recorded isometrically as described [10]. Briefly, each strip was bound to a glass holder and the other end was connected to a strain-gauge transducer (Nihon Kohden, Tokyo, Japan) in an organ bath containing PSS with a resting tension of 3 g. The muscle strips were equilibrated for 30 min to obtain a stable contractility induced by hyperosmotic addition of 65 mM KCl (high K+). The developed tension was expressed as a percentage by assuming the values at rest in normal PSS to be 0% and those at 15 min after application of high K+ or carbachol to be 100%. All chemicals for the muscle tension measurements were purchased from Sigma Aldrich Japan (Tokyo, Japan).
The total RNA was extracted from bovine tracheal smooth muscle tissue. The first strand of cDNA was synthesized using random 9 mer RT-primer and ReverTra Ace (Takara Bio, Kusatsu, Japan) at 30°C for 10 min, 42°C for 60 min, 99°C for 5 min and 4°C for 5 min. PCR amplification was performed using synthetic gene-specific primers for glyceraldehyde 3-phosphate dehydrogenase (GAPDH), PDE5 and PDE9 (as shown in Table 1) and ExTaq DNA polymerase (Takara Bio). After 38 cycles of amplification at 98°C for 10 sec, at 57°C for 30 sec and at 72°C for 60 sec by a thermal cycler (Takara Bio), the PCR products were electrophoresed onto a 2% agarose gel containing ethidium bromide at 0.2 µg/ml. The detectable fluorescence bands were visualized using a photo-imager (ATTO, Tokyo, Japan).
Table 1. PCR primer sequences and optimal product sizes.
| Target (accession No.) | Sequences (5′ to 3′) | Size (bp) | |
|---|---|---|---|
| GAPDH (NM_001034034.2) | Forward | GACCCCTTCATTGACCTTCA | 80 |
| Reverse | TTGACTGTGCCGTTGAACTT | ||
| PDE5 (NM_174417.2) | Forward | CAGCTGCAGAGGAAGAAACC | 130 |
| Reverse | CACAGTGCTGTTTCCAGGTC | ||
| PDE9 (BC102958.1) | Forward | GCGTCGAATTGGAAGGACTA | 151 |
| Reverse | CGAGGCGTCAACTTCTTGTT |
All data are expressed as the mean ± S.E.M. The two-tailed Student’s t test were used for statistical analyses. P<0.05 was considered significant. Half maximal (50%) inhibitory concentration (IC50) was calculated by 4 Parameters Logistic Regression using SigmaPlot (Systat Software, San Jose, CA, U.S.A.).
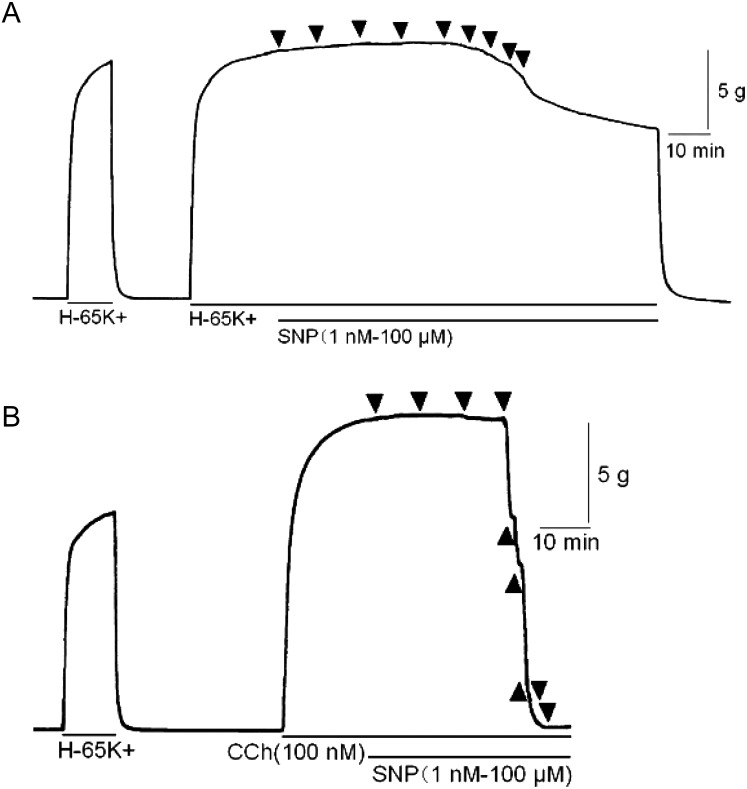
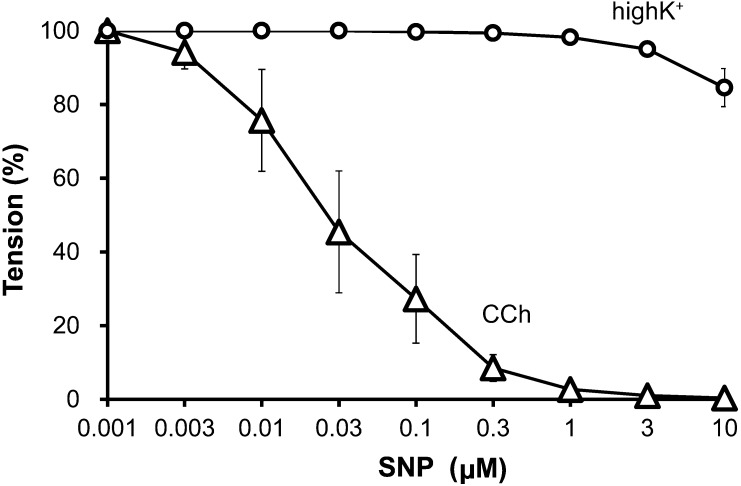
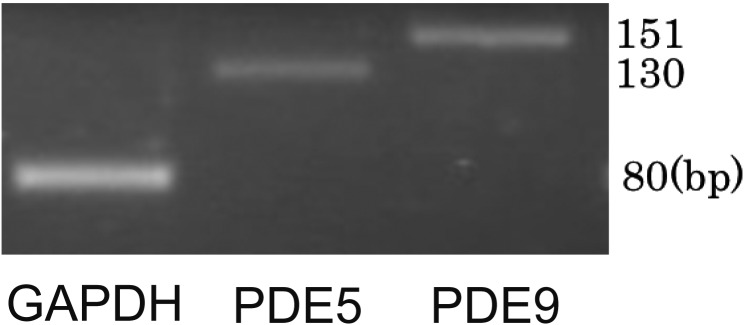
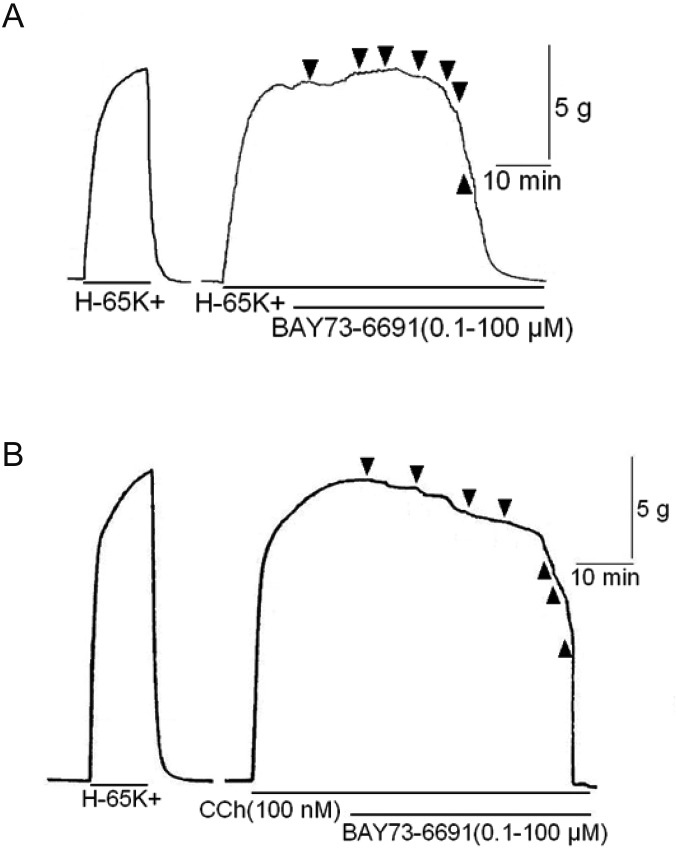
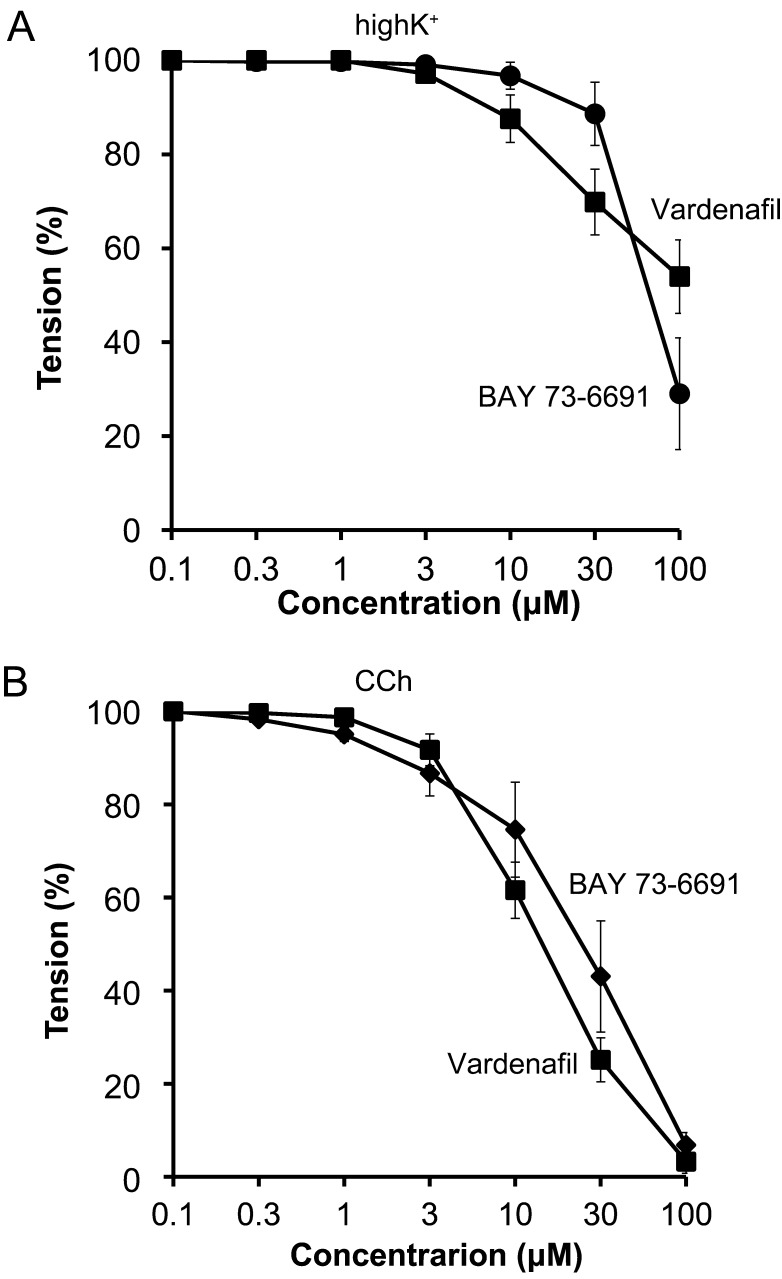
Priory, we confirmed cGMP dependent relaxation pathway exists in bovine tracheal smooth muscle, we applied sodium nitroprusside (SNP, an NO donor) cumulatively to the pre-contracted bovine tracheal smooth muscle with high K+ and carbachol (CCh, a muscarinic receptor agonist). SNP slightly decreased high K+-induced contraction in high concentration (Fig. 1A). On the other hand, SNP attenuated CCh (100 nM)-induced contraction (Fig. 1B) in concentration dependent manner (Fig. 2). RT-PCR revealed that bovine tracheal smooth muscle expressed mRNAs for PDE9 as well as PDE5 (Fig. 3). To determine PDE9 is involved in bovine tracheal smooth muscle relaxation, a PDE9 selective inhibitor was applied. BAY 73-6691 attenuated high K+-induced contraction (Fig. 4A) and CCh-induced contraction (Fig. 4B) in concentration dependent manner (Fig. 5). Refer to vardenafil (a PDE5 selective inhibitor), BAY 73-6691 equally effective to inhibit high K+- and CCh-induced contraction (Table 2).
Fig. 1.
Effect of sodium nitroprusside (SNP) on contractile force in bovine tracheal smooth muscle stimulated with high K+ (65 mM, A) and carbachol (100 nM, B). After the effect of KCl or carbachol was stabled, sodium nitroprusside were added cumulatively (1 nM to 100 µM).
Fig. 2.
Concentration-response curves of bovine tracheal strips pre-contracted with 65 mM KCl (high K+, ○) and 100 nM carbachol (CCh, ∆) to sodium nitroprusside. Mean ± SEM (n=4). Pre-contraction induced by high K+ at 10 min was considered to be 100% or resting tension was considered to be 0%.
Fig. 3.
mRNA expression of PDE9 and PDE5 in bovine tracheal smooth muscle tissue. Representative of n=4 experiments. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Fig. 4.
Effect of BAY 73-6691 on contractile force in bovine tracheal smooth muscle stimulated with high K+ (65 mM, A) and carbachol (100 nM, B). After the effect of KCl or carbachol was stabled, BAY73-6691 was added cumulatively (0.1 to 100 µM).
Fig. 5.
Concentration-response curves of bovine tracheal strips pre-contracted with 65 mM KCl (high K+, A) and 100 nM carbachol (CCh, B) to BAY 73-6691 (●) and vardenafil (■). Mean ± SEM (n=4). Pre-contraction induced by high K+ at 10 min was considered to be 100% or resting tension was considered to be 0%.
Table 2. IC50 values for high K+ or carbachol induced contraction with PDE inhibitors in bovine tracheal smooth muscle.
| Inhibitors | IC50 (μM) |
|
|---|---|---|
| High K+ | Carbachol | |
| Vardenafil | 100 < | 17.9 ± 2.8 |
| BAY 73-6691 | 72.1 ± 14.5 | 24.7 ± 3.2 |
These results indicate that PDE9 is expressed in bovine tracheal smooth muscle, further PDE9 plays major role as a potent regulator of tracheal smooth muscle relaxation.
SNP-mediated relaxation in bovine tracheal smooth muscle is consistent with previous reports that 3-morpholinosydnonimine (NO donor) provides relaxation of canine [9] and porcine [15] tracheal smooth muscles. Moreover, substrate selectivity of PDE9 for cGMP is higher than that of PDE5 and PDE6 [16, 21]. Taken together, PDE9 inhibitor probably raises intracellular cGMP level following protein kinase G activation in bovine tracheal smooth muscle.
PDE3 and PDE4 are considered as major pharmaceutical targets to control the hyperresponsiveness in asthmatic airways, thus theophylline is clinically used primarily as a bronchodilator in the treatment of pulmonary diseases such as asthma, chronic bronchitis, emphysema and COPD. On the other hand, PDE9 is reported to be insensitive to major non-selective PDE inhibitors including theophylline and 3-isobutyl-1-methylxantine (IBMX) [8, 11]. Therefore, our results suggest that PDE9 inhibitors should be considered as a novel candidate for an add-on controller of asthmatic airways.
In conclusion, PDE9 contributes to regulation of bovine tracheal smooth muscle relaxation, therefore PDE9 inhibitors may be effective to improve airway hyperresponsiveness as an add-on treatments with other bronchodilators.
Acknowledgments
This work was supported in part by Grant-in-Aid for Young Scientists (B) (No. 24248050 to T. Tajima) from the Japan Society for the Promotion of Science.
REFERENCES
- 1.Barnes P. J.2013. Corticosteroid resistance in patients with asthma and chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 131: 636–645. doi: 10.1016/j.jaci.2012.12.1564 [DOI] [PubMed] [Google Scholar]
- 2.Beavo J. A.1995. Cyclic nucleotide phosphodiesterases: functional implications of multiple isoforms. Physiol. Rev. 75: 725–748. doi: 10.1152/physrev.1995.75.4.725 [DOI] [PubMed] [Google Scholar]
- 3.Bloom T. J.2002. Cyclic nucleotide phosphodiesterase isozymes expressed in mouse skeletal muscle. Can. J. Physiol. Pharmacol. 80: 1132–1135. doi: 10.1139/y02-149 [DOI] [PubMed] [Google Scholar]
- 4.Conti M., Nemoz G., Sette C., Vicini E.1995. Recent progress in understanding the hormonal regulation of phosphodiesterases. Endocr. Rev. 16: 370–389. doi: 10.1210/edrv-16-3-370 [DOI] [PubMed] [Google Scholar]
- 5.da Silva F. H., Pereira M. N., Franco-Penteado C. F., De Nucci G., Antunes E., Claudino M. A.2013. Phosphodiesterase-9 (PDE9) inhibition with BAY 73-6691 increases corpus cavernosum relaxations mediated by nitric oxide-cyclic GMP pathway in mice. Int. J. Impot. Res. 25: 69–73. doi: 10.1038/ijir.2012.35 [DOI] [PubMed] [Google Scholar]
- 6.Diederen R. M., La Heij E. C., Markerink-van Ittersum M., Kijlstra A., Hendrikse F., de Vente J.2007. Selective blockade of phosphodiesterase types 2, 5 and 9 results in cyclic 3‘5’ guanosine monophosphate accumulation in retinal pigment epithelium cells. Br. J. Ophthalmol. 91: 379–384. doi: 10.1136/bjo.2006.100628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fawcett L., Baxendale R., Stacey P., McGrouther C., Harrow I., Soderling S., Hetman J., Beavo J. A., Phillips S. C.2000. Molecular cloning and characterization of a distinct human phosphodiesterase gene family: PDE11A. Proc. Natl. Acad. Sci. U.S.A. 97: 3702–3707. doi: 10.1073/pnas.97.7.3702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fisher D. A., Smith J. F., Pillar J. S., St Denis S. H., Cheng J. B.1998. Isolation and characterization of PDE9A, a novel human cGMP-specific phosphodiesterase. J. Biol. Chem. 273: 15559–15564. doi: 10.1074/jbc.273.25.15559 [DOI] [PubMed] [Google Scholar]
- 9.Jones K. A., Lorenz R. R., Warner D. O., Katusić Z. S., Sieck G. C.1994. Changes in cytosolic cGMP and calcium in airway smooth muscle relaxed by 3-morpholinosydnonimine. Am. J. Physiol. 266: L9–L16. [DOI] [PubMed] [Google Scholar]
- 10.Kaneda T., Fujieda T., Eto Y., Nagai Y., Sasaki N., Tajima T., Urakawa N., Shimizu K.2012. Key role of glycogen storage in high K+-induced contraction of the smooth muscles of the bovine trachea. J. Vet. Med. Sci. 74: 1277–1282. doi: 10.1292/jvms.12-0020 [DOI] [PubMed] [Google Scholar]
- 11.Maurice D. H., Ke H., Ahmad F., Wang Y., Chung J., Manganiello V. C.2014. Advances in targeting cyclic nucleotide phosphodiesterases. Nat. Rev. Drug Discov. 13: 290–314. doi: 10.1038/nrd4228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagasaki S., Nakano Y., Masuda M., Ono K., Miki Y., Shibahara Y., Sasano H.2012. Phosphodiesterase type 9 (PDE9) in the human lower urinary tract: an immunohistochemical study. BJU Int. 109: 934–940. doi: 10.1111/j.1464-410X.2011.10429.x [DOI] [PubMed] [Google Scholar]
- 13.Schudt C., Hatzelmann A., Beume R., Tenor H.2011. Phosphodiesterase inhibitors: history of pharmacology. Handb. Exp. Pharmacol. 2011: 1–46. [DOI] [PubMed] [Google Scholar]
- 14.Smith S. J., Brookes-Fazakerley S., Donnelly L. E., Barnes P. J., Barnette M. S., Giembycz M. A.2003. Ubiquitous expression of phosphodiesterase 7A in human proinflammatory and immune cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 284: L279–L289. doi: 10.1152/ajplung.00170.2002 [DOI] [PubMed] [Google Scholar]
- 15.Stuart-Smith K., Bynoe T. C., Lindeman K. S., Hirshman C. A.1994. Differential effects of nitrovasodilators and nitric oxide on porcine tracheal and bronchial muscle in vitro. J. Appl. Physiol. 77: 1142–1147. doi: 10.1152/jappl.1994.77.3.1142 [DOI] [PubMed] [Google Scholar]
- 16.Su T., Zhang T., Xie S., Yan J., Wu Y., Li X., Huang L., Luo H. B.2016. Discovery of novel PDE9 inhibitors capable of inhibiting Aβ aggregation as potential candidates for the treatment of Alzheimer’s disease. Sci. Rep. 6: 21826. doi: 10.1038/srep21826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Torphy T. J.1998. Phosphodiesterase isozymes: molecular targets for novel antiasthma agents. Am. J. Respir. Crit. Care Med. 157: 351–370. doi: 10.1164/ajrccm.157.2.9708012 [DOI] [PubMed] [Google Scholar]
- 18.Toward T. J., Smith N., Broadley K. J.2004. Effect of phosphodiesterase-5 inhibitor, sildenafil (Viagra), in animal models of airways disease. Am. J. Respir. Crit. Care Med. 169: 227–234. doi: 10.1164/rccm.200211-1372OC [DOI] [PubMed] [Google Scholar]
- 19.van Staveren W. C., Glick J., Markerink-van Ittersum M., Shimizu M., Beavo J. A., Steinbusch H. W., de Vente J.2002. Cloning and localization of the cGMP-specific phosphodiesterase type 9 in the rat brain. J. Neurocytol. 31: 729–741. doi: 10.1023/A:1025704031210 [DOI] [PubMed] [Google Scholar]
- 20.Wang H., Luo X., Ye M., Hou J., Robinson H., Ke H.2010. Insight into binding of phosphodiesterase-9A selective inhibitors by crystal structures and mutagenesis. J. Med. Chem. 53: 1726–1731. doi: 10.1021/jm901519f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu Y. F., Huang Y. D., Zhang C., Wu X. N., Zhou Q., Wu D., Wu Y., Luo H. B.2017. Discovery of Novel Pyrazolopyrimidinone Derivatives as Phosphodiesterase 9A Inhibitors Capable of Inhibiting Butyrylcholinesterase for Treatment of Alzheimer’s Disease. ACS Chem. Neurosci. 8: 2522–2534. doi: 10.1021/acschemneuro.7b00268 [DOI] [PubMed] [Google Scholar]