Abstract
Bisphenol A (BPA) is among the better-known endocrine disruptors. BPA is used in various food-contacting materials and is easily eluted into food; as a result, we are exposed to BPA on a daily basis. In adults, BPA is metabolized and eliminated rapidly from the body. However, numerous reports suggest that fetuses and young children are susceptible to BPA. One of the concerning adverse effects of BPA is disruption of behavior, especially anxiety-like behavior. In order to study the mechanism of influences on offspring, it is important to clarify the most vulnerable gestation period. We hypothesized that offspring in late pregnancy would be more susceptible to BPA, because late pregnancy is a critical time for functional brain development. In this study, C57BL/6 mouse fetuses were exposed prenatally by oral dosing of pregnant dams, once daily from gestational day 5.5 to 12.5 (early pregnancy) or 11.5 to 18.5 (late pregnancy), with BPA (0 or 10 mg/kg body weight). Following birth and weaning, the resulting pups were tested using an elevated plus maze at postnatal week 10. The behavior of the offspring was altered by prenatal BPA exposure during late pregnancy but not during early pregnancy. These results indicated that offspring are more vulnerable to exposure to BPA in late pregnancy.
Keywords: anxiety-like behavior, bisphenol A, endocrine disruptors, late pregnancy
Bisphenol A (BPA, 2,2-bis[4-hydroxyphenyl]propane) has weak estrogenic activity and is one of the better-known endocrine disruptors. There are reports suggesting that human health may be affected even with exposure to low levels of BPA [63, 66]. However, the detailed mechanism of BPA influence remains to be elucidated.
BPA is used in various food-contacting materials by incorporation into polycarbonate plastics and epoxy resins. BPA is easily eluted from these materials, resulting in human exposure to BPA on a daily basis. Indeed, BPA has been detected in urine, blood, and fetal samples [7, 8, 17, 55]. Previously, we found that, in adult rats, BPA is rapidly metabolized to BPA-glucuronide (BPA-GA) by UGT2B1, UDP-glucuronosyltransferase (UGT) [68]. In humans, UGT2B7 mediates metabolism of BPA [33]. BPA-GA is a hydrophilic molecule, lacking the estrogenic activity of BPA. Given the high activity of such hepatic UGTs in adults, BPA is rapidly excreted in urine and feces. However, in fetuses, the expression of such a UGT is very weak [27, 36, 40], indicating that fetuses are more susceptible to BPA than adults.
Numerous studies have reported a variety of BPA effects on fetuses and infants, including disruptions of the reproductive system [49, 54, 59], brain [28, 45, 52] and behavior [66]. One of the most infamous behavioral effects of BPA is anxiety-like behavior in rodents. Many studies have conducted behavioral tests to measure anxiety-like behavior of offspring that were exposed to BPA during various gestation periods from mating through weaning [14, 18, 25, 31, 53]. The effects of BPA were observed even when the period of exposure was limited to lactation [15, 44] or pregnancy [10, 67]. Behavioral abnormalities are considered likely to reflect changes in the brain, but the degree and nature of brain development varies during different periods of gestation. The identification of the vulnerable period for BPA exposure would be of great use in elucidating the mechanism of BPA’s influence on the developing brain.
Thus, the purpose of this study was to evaluate the association between the behavioral influence of prenatal BPA exposure and the stage of pregnancy. In this study, pregnant mice were exposed to BPA during different gestation periods, and anxiety-like behavior in their offspring was measured.
MATERIALS AND METHODS
Animals
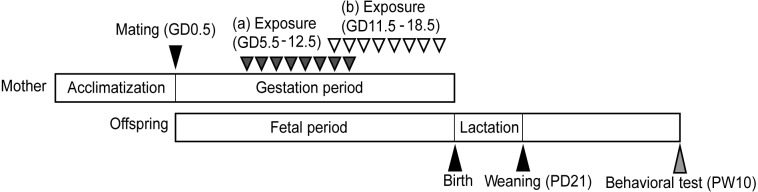
Male and female C57BL/6NCrSlc mice, 7 weeks of age, were purchased from Sankyo Lab Co. (Tokyo, Japan). Our facility’s animal rooms were maintained at controlled temperature (22 ± 2°C) and humidity (50 ± 5%) on a 12-hr light/12-hr dark cycle (lights on at 7:00 AM). Mice were provided with free access to food and water. After acclimatizing for 2 weeks, mating was conducted; the date on which a vaginal plug was discovered was regarded as gestational day (GD) 0.5. In this study, dams (4–6 per group) were randomly assigned to 1 of 4 treatment groups, with 2 groups each dosed with olive oil (vehicle control) or BPA (10 mg/kg bw/d, Kanto Chemical Co., Tokyo, Japan) dissolved in olive oil. For each pair of treatment groups (each pair consisting of one control group and one experimental group), vehicle or BPA were administered once daily by oral gavage (1 ml/kg bw) from GD 5.5 to 12.5 (early pregnancy) (Fig. 1 [a]) or GD 11.5 to 18.5 (late pregnancy) (Fig. 1 [b]). Dams were not dosed at the beginning of each pregnancy (i.e., GD 0.5 until GD 5.5) in order to preclude failure of embryo implantation due to stress [29]. After weaning, at postnatal day (PD) 21, offspring were group housed with same-sex littermates. The elevated plus maze test (EPM) was conducted at postnatal week (PW) 10. The test was recorded and automatically analyzed by a computer equipped with software (TimeEP1 for EPM) purchased from O’Hara & Co., Ltd. (Tokyo, Japan). All experimental procedures were in accordance with the guidelines of the Committee for Animal Welfare at Rakuno Gakuen University (Ethics Committee protocol number VH25A4, approved 26 June 2013).
Fig. 1.
Schematic summary of the experimental procedure. Dams were administered orally with vehicle or BPA during (a) early pregnancy (dark gray arrowheads) or (b) late pregnancy (white arrowheads). Behavioral tests were performed at postnatal week (PW) 10 (light gray arrowheads).
Elevated plus maze test (EPM)
The EPM is well-established paradigm and one of the most commonly used test for assessing the anxiety in rodents [3]. The EPM has 2 open arms (25 × 5 × 15 cm), 2 closed arms (25 × 5 × 15 cm), and a central region (5 × 5 cm) connecting these 4 arms into a cross shape. The maze was elevated 45 cm above the floor. Individual mice were allowed to search the maze freely during a 5-min interval under 110-lux light intensity. We measured total distance moved (a measure of locomotor activity; locomotor activity to novelty is an animal index of anxiety), along with the time spent in each arm and the frequency of entries in each arm (measures of anxiety). Heightened anxiety was indicated by the following results: a decrease in the total distance moved, a decrease in the amount of time / number of entries in the open arms, or an increase in the amount of time / number of entries in the closed arms. To avoid biasing the evaluation, we excluded data if the offspring slept or remained immobile for the majority of the test duration.
Statistical analysis
Results were compared between groups using a Student’s t test. A P value of less than 0.05 was considered significant. All results are presented as means ± standard errors (SE).
RESULTS
Offspring were divided into 4 groups according to BPA administration and administration period. There was no significant difference between the control and BPA groups for a given dosing period in terms of the number of offspring or the male/female ratio in litters (Table 1). When the offspring reached PW 10, the EPM was used to evaluate the anxiety of offspring exposed to BPA in early or late pregnancy.
Table 1. The number and sex ratio (male/female) of offspring in each group.
| Early pregnancy |
Late pregnancy |
|||
|---|---|---|---|---|
| Number | Sex ratio | Number | Sex ratio | |
| Control | 6.5 ± 0.9 | 1.1 ± 0.3 | 7.0 ± 0.3 | 1.4 ± 0.4 |
| BPA | 7.5 ± 0.5 | 1.3 ± 0.4 | 7.0 ± 1.0 | 1.0 ± 0.2 |
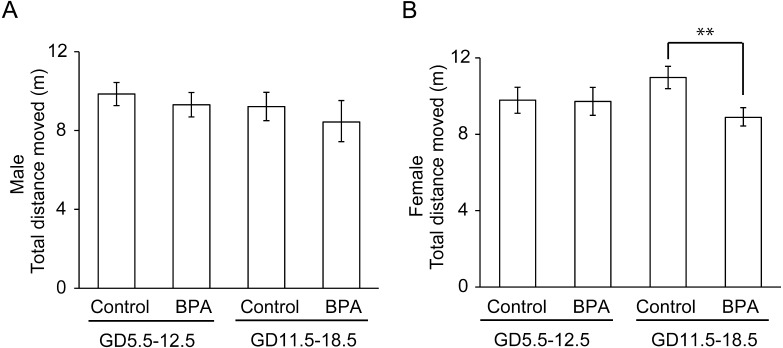
First, the total distance moved in the EPM was calculated (Fig. 2 and Table 2). This parameter is generally used as a measure of locomotor activity, which can be an indicator of anxiety. A significant effect (a decrease in activity compared to the respective control group) was observed in female offspring that had been exposed to BPA during late pregnancy (P<0.01; Fig. 2B).
Fig. 2.
Effects of BPA on locomotor activity of offspring in the elevated plus maze test (EPM). The parameter is the total distance moved (mean ± standard error [SE]). **P<0.01 compared with control.
Table 2. Numerical details for the EPM data.
| Male |
Female |
|||||||
|---|---|---|---|---|---|---|---|---|
| Early pregnancy |
Late pregnancy |
Early pregnancy |
Late pregnancy |
|||||
| Control | BPA | Control | BPA | Control | BPA | Control | BPA | |
| (n=9) | (n=12) | (n=11) | (n=9) | (n=9) | (n=10) | (n=11) | (n=15) | |
| Total distance (m) | 9.9 ± 0.6 | 9.3 ± 0.6 | 9.3 ± 0.7 | 8.5 ± 1.0 | 9.8 ± 0.7 | 9.7 ± 0.7 | 11.0 ± 0.6 | 8.9 ± 0.5 b) |
| Time spent in the OA (%) | 20.4 ± 4.1 | 23.6 ± 4.2 | 28.0 ± 5.0 | 28.8 ± 5.9 | 32.9 ± 4.3 | 36.3 ± 6.3 | 33.4 ± 5.4 | 22.0 ± 4.0 a) |
| Time spent in the CA (%) | 34.9 ± 4.6 | 31.7 ± 3.5 | 30.0 ± 4.3 | 38.9 ± 6.5 | 26.0 ± 4.4 | 25.5 ± 4.3 | 28.9 ± 4.0 | 35.3 ± 3.8 |
| Time spent in the CE (%) | 44.7 ± 3.9 | 44.8 ± 2.9 | 42.0 ± 3.5 | 32.3 ± 3.4 a) | 41.1 ± 3.5 | 38.2 ± 3.1 | 37.7 ± 2.9 | 42.7 ± 2.6 |
| Entries in the OA | 5.9 ± 0.9 | 5.5 ± 0.7 | 5.9 ± 1.4 | 6.2 ± 1.1 | 8.7 ± 1.1 | 7.3 ± 1.1 | 8.1 ± 1.1 | 5.3 ± 0.7 a) |
| Entries in the CA | 9.7 ± 1.2 | 9.4 ± 1.0 | 9.5 ± 1.3 | 7.7 ± 1.5 | 6.2 ± 1.0 | 8 ± 1.2 | 10.0 ± 1.1 | 8.7 ± 1.0 |
OA, open arms; CA, closed arms; CE, center region; a) P<0.05 compared with controls; b) P<0.01 compared with controls.
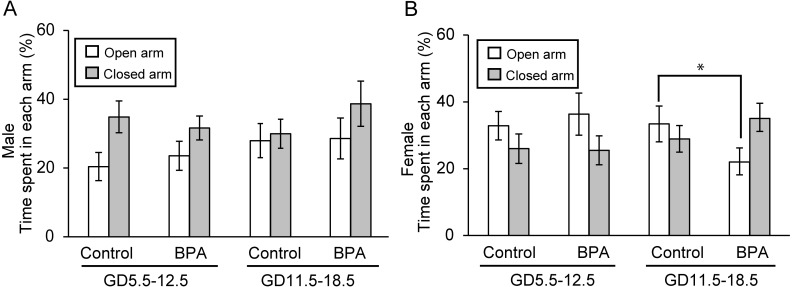
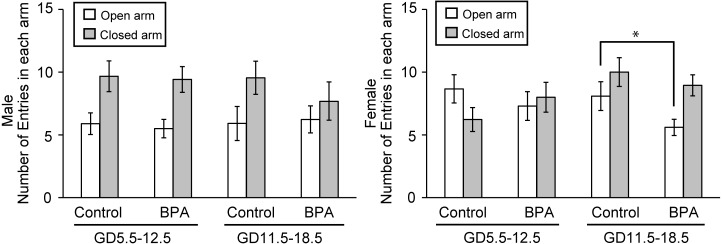
Second, the time spent and the frequency of entries in each arm were assessed (Figs. 3 and 4, respectively). Table 2 provides the numerical data. For most measures in the control groups, the results revealed sexual dimorphism; control males tended to spend more time and make more entries in the closed arms than in the open arms and control females showed roughly opposite behavior. This observation was in agreement with literature results showing that adult exploration of the EPM is sexually dimorphic, with males typically spending less time and making less entries in the open arms [23]. For animals exposed to BPA during early pregnancy, no significant effect was found and typical sexually dimorphic behavior was maintained among the offspring (Figs. 3 and 4). However, female offspring exposed to BPA during late pregnancy exhibited a significant decrease in the time spent in the open arms (P<0.05; Fig. 3B) and the frequency of entries in the open arms (P<0.05; Fig. 4B) compared to the respective control group. In male, offspring exposed to BPA during late pregnancy showed significant decrease of the time spent in the center region (P<0.05; Table 2). In addition, the time spent in the closed arm increased by almost as much as the difference.
Fig. 3.
Effects of BPA on anxiety-like behavior of offspring in the elevated plus maze test (EPM). The parameter is the percentage of the time spent in each arm for males (A) and females (B) (mean ± SE). *P<0.05 compared with control in the results for the open arm.
Fig. 4.
Effects of BPA on anxiety-like behavior of offspring in the elevated plus maze test (EPM). The parameter is the frequency of total entries in each arm for males (A) and females (B) (mean ± SE). *P<0.05 compared with control in the results for the open arm.
These findings indicate that BPA exposure during late pregnancy is associated with heightened anxious behavior in offspring; notably, this effect appears to abolish the typical sexual dimorphism of the assessed behavioral patterns.
DISCUSSION
Previous reports suggested that maternal exposure to BPA causes behavioral disorder in offspring. However, none of those reports compared behavioral effects of prenatal BPA exposure during different periods of pregnancy. The development of the brain is greatly different, and the outcome of the prenatal chemical insult can vary widely, depending on the stage of pregnancy [35, 65]. Identification of the pregnancy period during which animals are most sensitive to BPA exposure would be of great use for tracing the mechanism of BPA’s prenatal influence on brain function. In the present study, the administration period was from GD 5.5 to 12.5 (early pregnancy) or from GD 11.5 to 18.5 (late pregnancy). The resulting offspring were subjected to the EPM in adulthood to assess anxious behavior. In Male offspring exposed to BPA during late pregnancy, the time spent in the closed arms had a tendency to increase by as much as the decreasing the time spent in the center region. It might have been difficult to observe significant influence of BPA on male offspring because control males originally prefer staying in the closed arms to staying in open arms. Female offspring exposed to BPA during late pregnancy displayed significant decreases in the time spent and the frequency of entries in the open arms of the maze. Although control females tended to stay in the open arms, female offspring exposed to BPA during late pregnancy tended to stay in the closed arms like males. The results suggested that offspring exposed to BPA during late pregnancy showed higher anxiety than controls, corresponding to an apparent loss of sexual dimorphism of anxiety-like behavior.
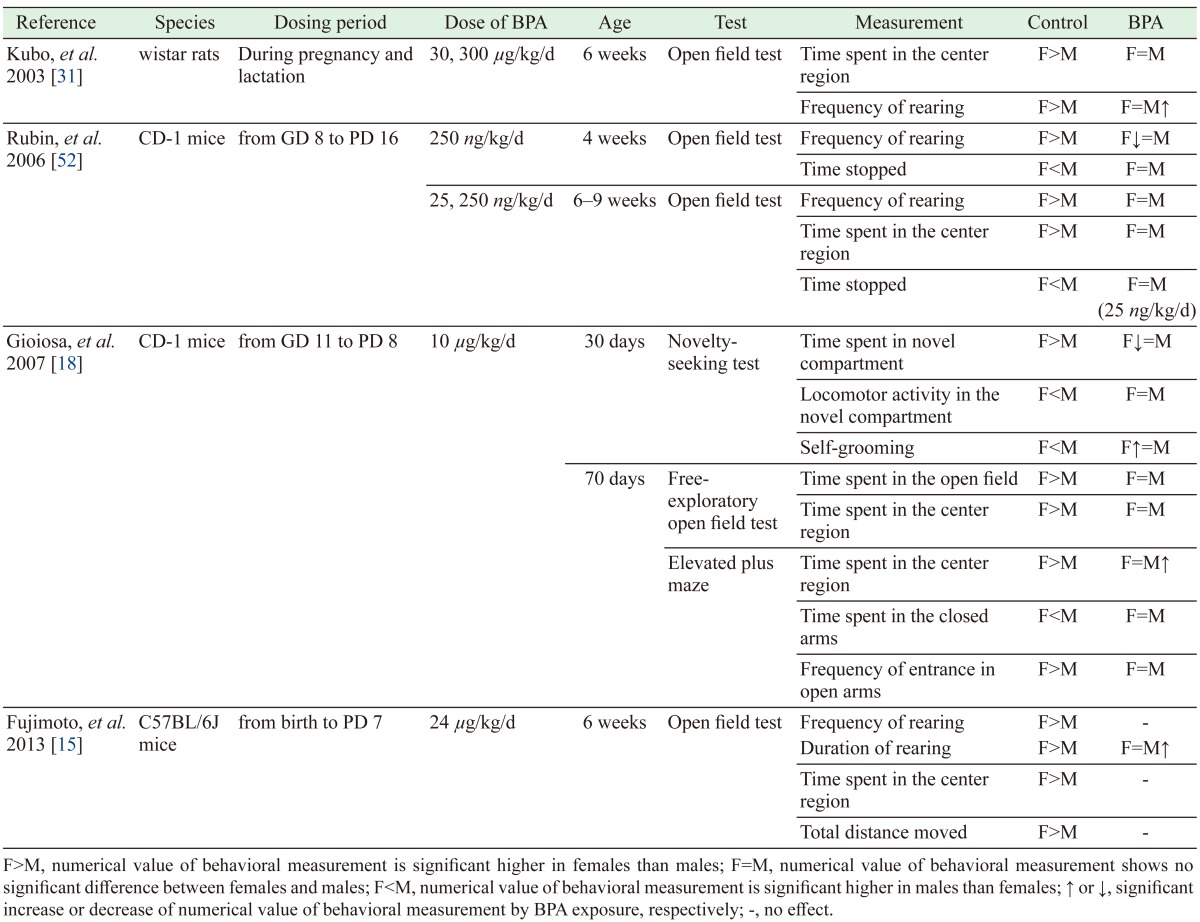
Some previous reports indicated that gestational BPA exposure induces loss of sexual dimorphism of anxiety-like behavior, though the BPA dosing period differed among the specific studies (Table 3) [15, 18, 31, 52]. Our results are consistent with these reports of loss of sexual dimorphism of anxiety-like behavior, and extend those results by indicating that late pregnancy is the period of greater vulnerability to BPA exposure.
Table 3. Literature reports of effects of BPA on sexually dimorphic anxiety-like behavior.
In early pregnancy, embryogenesis is initiated and organogenesis occurs. Early pregnancy includes periods of high sensitivity to embryonic lethality and teratogenic action in the brain, eye, heart, and axial skeleton. As for palate and urogenital organs, their highest sensitivity occurs in late pregnancy. Major processes during late pregnancy include brain development and functional maturation [35, 65]. In previous studies, BPA was not found to have effects on the survival number of offspring per litter except at fairly high doses of BPA (e.g., 500 mg/kg) [61, 62]. On the other hand, BPA’s influence on behavior and development of the urogenital system has been observed even at low doses [49, 66]. In the present study, litter sizes and sex ratios were not significantly altered by prenatal BPA exposure, consistent with previous reports [61, 62]. Notably, our results demonstrated that the observed behavioral changes were associated with BPA exposure during late pregnancy, but not with exposure during early pregnancy. Additionally, our results suggested that the window for behavioral effects of prenatal BPA exposure likely occurs from GD13.5, given that dosing on GD11.5 and GD12.5 occurred in both the early and late pregnancy cohorts in our study.
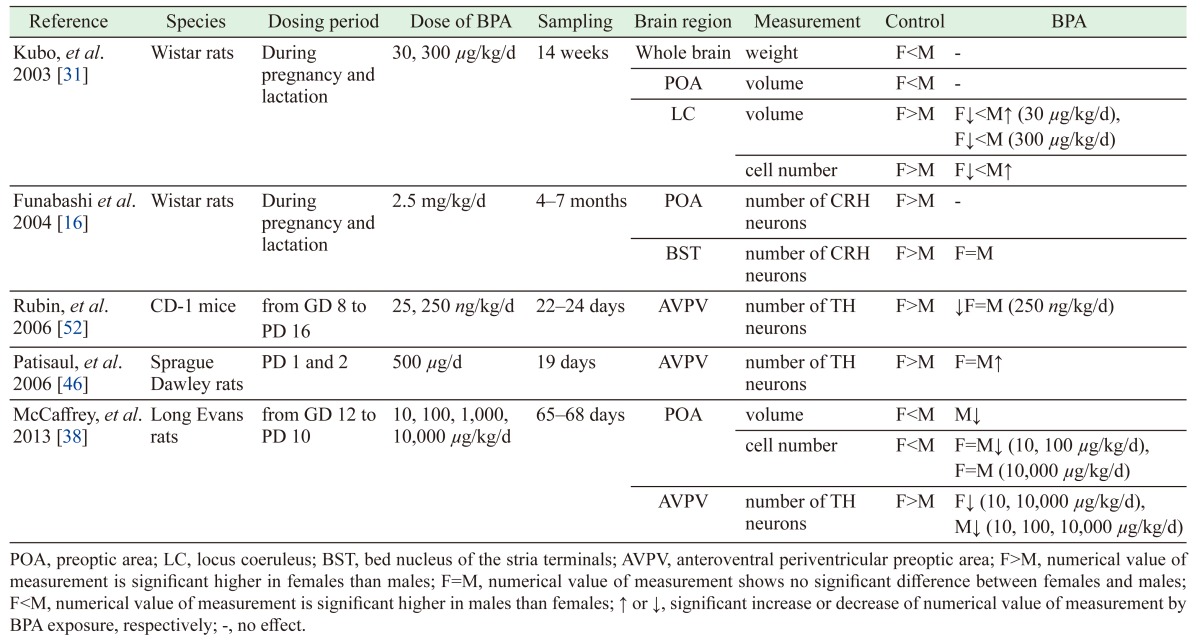
Our study demonstrated loss of sexual dimorphism in anxiety-related behavior, as assessed by the EPM. With regard to BPA’s influences on the prenatal brain, the compound has been reported to cause loss of sex differences in some areas of the brain, including the size of the locus coeruleus (LC) [31], the population of corticotropin-releasing hormone neurons in the bed nucleus of the stria terminals (BST) [16], and the number of neurons in the anteroventral periventricular preoptic area (AVPV) [52]. The influence of BPA on the sexual dimorphism of these brain regions are summarized in Table 4 [16, 31, 38, 46, 52]. The critical period for development of sexually dimorphic regions is known to correspond to the late pregnancy and early postnatal periods [34]. For example, neurogenesis of the AVPV occurs between GD 13 and GD 18 [41]. During this critical period, gonadal steroids such as estrogen play a role in regulating the sexual dimorphism of the brain [26, 34, 39, 56]. Some studies have reported that gonadal steroids influence the volume and number of neurons in some brain areas [2, 26, 50], including the AVPV [43], LC [19] and BST [11, 20]. In one study, gonadal steroid exposure during the perinatal period resulted in abolishment of sexually dimorphic behavior, as assessed by the open-field test (an initial screening test for anxiety-related behavior in rodents) [6]. Prenatal BPA exposure has been demonstrated to exhibit the same influence in the open-field test [30, 31, 52]. The estrogen activity of BPA may influence estrogen-sensitive cells and thereby disrupt sexual differentiation of the brain. Estrogen receptors (ERs) such as ERα and ERβ have region-specific distribution and sex-specific expression patterns [39, 43, 56]. Decreased ERβ levels are associated with enhanced anxiety [21, 24, 58, 60], and prenatal BPA exposure has been reported to result in decreases of ERβ in the amygdala, a brain area known to be associated with anxiety [47]. In addition, estrogens have effects on neuronal migration and differentiation in the developing brain [5, 64], and prenatal BPA exposure has been shown to influence neurogenesis in the developing neocortex [28]. Although the mechanism whereby BPA affects anxiety-related behavior remains unknown, ERs are thought to play a role. Indeed, some studies have reported that BPA exposure yields changes in the expression of ERs in various regions of the brain [9, 32, 51]. BPA is thought to influence multiple segments of the brain during periods critical to the complex interconnection of various brain areas, resulting in behavioral changes, including processes that affect anxious behavior.
Table 4. Literature reports of effects of BPA on sexually dimorphic brain regions.
Notably, however, the binding affinity of BPA to ERα and ERβ is much weaker (by a factor of approximately 4 to 5 logs) than that of estrogen [1, 4]. It is difficult to imagine that all of BPA’s influences on brain development come solely from effects on ERα and ERβ. Recently, it was reported that BPA exhibits strong binding to estrogen-related receptor γ (ERRγ) [37, 42]. ERRγ is a member of the ERR subfamily, proteins known as orphan nuclear receptors because their physiological activating ligands are unknown. ERRγ is expressed in the central nervous system during mouse fetal development [22, 57], and ERRγ has been reported to regulate neuronal metabolism involved in memory formation by the brain [48]. These reports suggest that ERRγ may be involved in the differentiation of the brain. Details of how ERRγ regulates brain development remain unknown, but BPA’s interaction with ERRγ may influence (and disrupt) brain development.
BPA’s placental migration is also believed to play an interesting role in BPA’s influences on the fetus. Previously, Domoradzki et al. [12] administered 14C-BPA (10 mg/kg bw) orally to Sprague-Dawley rats at different stages of pregnancy, and found that the highest fetal free-BPA concentrations occurred with administration during late pregnancy [12]. In our own work, we have demonstrated that BPA-GA can pass through the placenta, with subsequent release of free-BPA in the fetus as a result of deconjugation of GA from BPA-GA [40]. We speculate that placental transfer of BPA is an important factor in adverse effects on fetuses in late pregnancy.
In summary, we demonstrated that the BPA sensitivity of the fetus is elevated in late pregnancy, as assessed by behavioral effects observed in pups exposed at different prenatal periods. In human studies, BPA and BPA-GA have been detected in fetuses and amniotic fluid [13, 69]. In humans, the fetus may be especially vulnerable to BPA exposure because the length of human gestation provides an extended period for BPA exposure in late pregnancy. In order to clarify the mechanism of fetal influence of BPA, it is necessary to investigate the BPA influence of more limited period of the late pregnancy and to focus on any factor other than simple estrogenic activity of BPA.
REFERENCES
- 1.Andersen H. R., Andersson A. M., Arnold S. F., Autrup H., Barfoed M., Beresford N. A., Bjerregaard P., Christiansen L. B., Gissel B., Hummel R., Jørgensen E. B., Korsgaard B., Le Guevel R., Leffers H., McLachlan J., Møller A., Nielsen J. B., Olea N., Oles-Karasko A., Pakdel F., Pedersen K. L., Perez P., Skakkeboek N. E., Sonnenschein C., Soto A. M., Sumpter J. P., Thorpe S. M., Grandjean P.1999. Comparison of short-term estrogenicity tests for identification of hormone-disrupting chemicals. Environ. Health Perspect. 107 Suppl 1: 89–108. doi: 10.1289/ehp.99107s189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnold A. P., Gorski R. A.1984. Gonadal steroid induction of structural sex differences in the central nervous system. Annu. Rev. Neurosci. 7: 413–442. doi: 10.1146/annurev.ne.07.030184.002213 [DOI] [PubMed] [Google Scholar]
- 3.Bailey K. R., Crawley J. N.2009. Anxiety-related behaviors in mice. pp. 77–101. In: Methods of Behavior Analysis in Neuroscience. 2nd ed. (Buccafusco, J. J. ed.), CRC Press/Taylor & Francis LCC, Boca Raton. [Google Scholar]
- 4.Barkhem T., Carlsson B., Nilsson Y., Enmark E., Gustafsson J., Nilsson S.1998. Differential response of estrogen receptor alpha and estrogen receptor beta to partial estrogen agonists/antagonists. Mol. Pharmacol. 54: 105–112. doi: 10.1124/mol.54.1.105 [DOI] [PubMed] [Google Scholar]
- 5.Beyer C.1999. Estrogen and the developing mammalian brain. Anat. Embryol. (Berl.) 199: 379–390. doi: 10.1007/s004290050236 [DOI] [PubMed] [Google Scholar]
- 6.Blizard D. A., Lippman H. R., Chen J. J.1975. Sex differences in open-field behavior in the rat: the inductive and activational role of gonadal hormones. Physiol. Behav. 14: 601–608. doi: 10.1016/0031-9384(75)90188-2 [DOI] [PubMed] [Google Scholar]
- 7.Bushnik T., Haines D., Levallois P., Levesque J., Van Oostdam J., Viau C.2010. Lead and bisphenol A concentrations in the Canadian population. Health Rep. 21: 7–18. [PubMed] [Google Scholar]
- 8.Calafat A. M., Ye X., Wong L. Y., Reidy J. A., Needham L. L.2008. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003−2004. Environ. Health Perspect. 116: 39–44. doi: 10.1289/ehp.10753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao J., Rebuli M. E., Rogers J., Todd K. L., Leyrer S. M., Ferguson S. A., Patisaul H. B.2013. Prenatal bisphenol A exposure alters sex-specific estrogen receptor expression in the neonatal rat hypothalamus and amygdala. Toxicol. Sci. 133: 157–173. doi: 10.1093/toxsci/kft035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cox K. H., Gatewood J. D., Howeth C., Rissman E. F.2010. Gestational exposure to bisphenol A and cross-fostering affect behaviors in juvenile mice. Horm. Behav. 58: 754–761. doi: 10.1016/j.yhbeh.2010.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.del Abril A., Segovia S., Guillamón A.1987. The bed nucleus of the stria terminalis in the rat: regional sex differences controlled by gonadal steroids early after birth. Brain Res. 429: 295–300. doi: 10.1016/0165-3806(87)90110-6 [DOI] [PubMed] [Google Scholar]
- 12.Domoradzki J. Y., Pottenger L. H., Thornton C. M., Hansen S. C., Card T. L., Markham D. A., Dryzga M. D., Shiotsuka R. N., Waechter J. M., Jr.2003. Metabolism and pharmacokinetics of bisphenol A (BPA) and the embryo-fetal distribution of BPA and BPA-monoglucuronide in CD Sprague-Dawley rats at three gestational stages. Toxicol. Sci. 76: 21–34. doi: 10.1093/toxsci/kfg206 [DOI] [PubMed] [Google Scholar]
- 13.Edlow A. G., Chen M., Smith N. A., Lu C., McElrath T. F.2012. Fetal bisphenol A exposure: concentration of conjugated and unconjugated bisphenol A in amniotic fluid in the second and third trimesters. Reprod. Toxicol. 34: 1–7. doi: 10.1016/j.reprotox.2012.03.009 [DOI] [PubMed] [Google Scholar]
- 14.Farabollini F., Porrini S., Dessì-Fulgherit F.1999. Perinatal exposure to the estrogenic pollutant bisphenol A affects behavior in male and female rats. Pharmacol. Biochem. Behav. 64: 687–694. doi: 10.1016/S0091-3057(99)00136-7 [DOI] [PubMed] [Google Scholar]
- 15.Fujimoto T., Kubo K., Nishikawa Y., Aou S.2013. Postnatal exposure to low-dose bisphenol A influences various emotional conditions. J. Toxicol. Sci. 38: 539–546. doi: 10.2131/jts.38.539 [DOI] [PubMed] [Google Scholar]
- 16.Funabashi T., Kawaguchi M., Furuta M., Fukushima A., Kimura F.2004. Exposure to bisphenol A during gestation and lactation causes loss of sex difference in corticotropin-releasing hormone-immunoreactive neurons in the bed nucleus of the stria terminalis of rats. Psychoneuroendocrinology 29: 475–485. doi: 10.1016/S0306-4530(03)00055-6 [DOI] [PubMed] [Google Scholar]
- 17.Ginsberg G., Rice D. C.2009. Does rapid metabolism ensure negligible risk from bisphenol A? Environ. Health Perspect. 117: 1639–1643. doi: 10.1289/ehp.0901010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gioiosa L., Fissore E., Ghirardelli G., Parmigiani S., Palanza P.2007. Developmental exposure to low-dose estrogenic endocrine disruptors alters sex differences in exploration and emotional responses in mice. Horm. Behav. 52: 307–316. doi: 10.1016/j.yhbeh.2007.05.006 [DOI] [PubMed] [Google Scholar]
- 19.Guillamón A., de Blas M. R., Segovia S.1988. Effects of sex steroids on the development of the locus coeruleus in the rat. Brain Res. 468: 306–310. doi: 10.1016/0165-3806(88)90143-5 [DOI] [PubMed] [Google Scholar]
- 20.Guillamón A., Segovia S., del Abril A.1988. Early effects of gonadal steroids on the neuron number in the medial posterior region and the lateral division of the bed nucleus of the stria terminalis in the rat. Brain Res. Dev. Brain Res. 44: 281–290. doi: 10.1016/0165-3806(88)90226-X [DOI] [PubMed] [Google Scholar]
- 21.Handa R. J., Ogawa S., Wang J. M., Herbison A. E.2012. Roles for oestrogen receptor β in adult brain function. J. Neuroendocrinol. 24: 160–173. doi: 10.1111/j.1365-2826.2011.02206.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hermans-Borgmeyer I., Süsens U., Borgmeyer U.2000. Developmental expression of the estrogen receptor-related receptor gamma in the nervous system during mouse embryogenesis. Mech. Dev. 97: 197–199. doi: 10.1016/S0925-4773(00)00422-6 [DOI] [PubMed] [Google Scholar]
- 23.Imhof J. T., Coelho Z. M. I., Schmitt M. L., Morato G. S., Carobrez A. P.1993. Influence of gender and age on performance of rats in the elevated plus maze apparatus. Behav. Brain Res. 56: 177–180. doi: 10.1016/0166-4328(93)90036-P [DOI] [PubMed] [Google Scholar]
- 24.Imwalle D. B., Gustafsson J. A., Rissman E. F.2005. Lack of functional estrogen receptor beta influences anxiety behavior and serotonin content in female mice. Physiol. Behav. 84: 157–163. doi: 10.1016/j.physbeh.2004.11.002 [DOI] [PubMed] [Google Scholar]
- 25.Jašarević E., Sieli P. T., Twellman E. E., Welsh T. H., Jr., Schachtman T. R., Roberts R. M., Geary D. C., Rosenfeld C. S.2011. Disruption of adult expression of sexually selected traits by developmental exposure to bisphenol A. Proc. Natl. Acad. Sci. U.S.A. 108: 11715–11720. doi: 10.1073/pnas.1107958108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelly S. J., Ostrowski N. L., Wilson M. A.1999. Gender differences in brain and behavior: hormonal and neural bases. Pharmacol. Biochem. Behav. 64: 655–664. doi: 10.1016/S0091-3057(99)00167-7 [DOI] [PubMed] [Google Scholar]
- 27.King C. D., Rios G. R., Assouline J. A., Tephly T. R.1999. Expression of UDP-glucuronosyltransferases (UGTs) 2B7 and 1A6 in the human brain and identification of 5-hydroxytryptamine as a substrate. Arch. Biochem. Biophys. 365: 156–162. doi: 10.1006/abbi.1999.1155 [DOI] [PubMed] [Google Scholar]
- 28.Komada M., Asai Y., Morii M., Matsuki M., Sato M., Nagao T.2012. Maternal bisphenol A oral dosing relates to the acceleration of neurogenesis in the developing neocortex of mouse fetuses. Toxicology 295: 31–38. doi: 10.1016/j.tox.2012.02.013 [DOI] [PubMed] [Google Scholar]
- 29.Kondoh E., Okamoto T., Higuchi T., Tatsumi K., Baba T., Murphy S. K., Takakura K., Konishi I., Fujii S.2009. Stress affects uterine receptivity through an ovarian-independent pathway. Hum. Reprod. 24: 945–953. doi: 10.1093/humrep/den461 [DOI] [PubMed] [Google Scholar]
- 30.Kubo K., Arai O., Ogata R., Omura M., Hori T., Aou S.2001. Exposure to bisphenol A during the fetal and suckling periods disrupts sexual differentiation of the locus coeruleus and of behavior in the rat. Neurosci. Lett. 304: 73–76. doi: 10.1016/S0304-3940(01)01760-8 [DOI] [PubMed] [Google Scholar]
- 31.Kubo K., Arai O., Omura M., Watanabe R., Ogata R., Aou S.2003. Low dose effects of bisphenol A on sexual differentiation of the brain and behavior in rats. Neurosci. Res. 45: 345–356. doi: 10.1016/S0168-0102(02)00251-1 [DOI] [PubMed] [Google Scholar]
- 32.Kundakovic M., Gudsnuk K., Franks B., Madrid J., Miller R. L., Perera F. P., Champagne F. A.2013. Sex-specific epigenetic disruption and behavioral changes following low-dose in utero bisphenol A exposure. Proc. Natl. Acad. Sci. U.S.A. 110: 9956–9961. doi: 10.1073/pnas.1214056110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mackenzie P. I., Owens I. S., Burchell B., Bock K. W., Bairoch A., Bélanger A., Fournel-Gigleux S., Green M., Hum D. W., Iyanagi T., Lancet D., Louisot P., Magdalou J., Chowdhury J. R., Ritter J. K., Schachter H., Tephly T. R., Tipton K. F., Nebert D. W.1997. The UDP glycosyltransferase gene superfamily: recommended nomenclature update based on evolutionary divergence. Pharmacogenetics 7: 255–269. doi: 10.1097/00008571-199708000-00001 [DOI] [PubMed] [Google Scholar]
- 34.MacLusky N. J., Naftolin F.1981. Sexual differentiation of the central nervous system. Science 211: 1294–1302. doi: 10.1126/science.6163211 [DOI] [PubMed] [Google Scholar]
- 35.Manson J. M., Wise L. D.1993. Teratogens. pp. 226–254. In: Casarett and Doull’s toxicology: The Basic Science of Poisons, 4th ed. (Amdur, M. O., Doull, J. and Klaassen, C. D. eds.), McGraw-Hill, New York. [Google Scholar]
- 36.Matsumoto J., Yokota H., Yuasa A.2002. Developmental increases in rat hepatic microsomal UDP-glucuronosyltransferase activities toward xenoestrogens and decreases during pregnancy. Environ. Health Perspect. 110: 193–196. doi: 10.1289/ehp.02110193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsushima A., Kakuta Y., Teramoto T., Koshiba T., Liu X., Okada H., Tokunaga T., Kawabata S., Kimura M., Shimohigashi Y.2007. Structural evidence for endocrine disruptor bisphenol A binding to human nuclear receptor ERR gamma. J. Biochem. 142: 517–524. doi: 10.1093/jb/mvm158 [DOI] [PubMed] [Google Scholar]
- 38.McCaffrey K. A., Jones B., Mabrey N., Weiss B., Swan S. H., Patisaul H. B.2013. Sex specific impact of perinatal bisphenol A (BPA) exposure over a range of orally administered doses on rat hypothalamic sexual differentiation. Neurotoxicology 36: 55–62. doi: 10.1016/j.neuro.2013.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCarthy M. M.2008. Estradiol and the developing brain. Physiol. Rev. 88: 91–124. doi: 10.1152/physrev.00010.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nishikawa M., Iwano H., Yanagisawa R., Koike N., Inoue H., Yokota H.2010. Placental transfer of conjugated bisphenol A and subsequent reactivation in the rat fetus. Environ. Health Perspect. 118: 1196–1203. doi: 10.1289/ehp.0901575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nishizuka M., Sumida H., Kano Y., Arai Y.1993. Formation of neurons in the sexually dimorphic anteroventral periventricular nucleus of the preoptic area of the rat: effects of prenatal treatment with testosterone propionate. J. Neuroendocrinol. 5: 569–573. doi: 10.1111/j.1365-2826.1993.tb00523.x [DOI] [PubMed] [Google Scholar]
- 42.Okada H., Tokunaga T., Liu X., Takayanagi S., Matsushima A., Shimohigashi Y.2008. Direct evidence revealing structural elements essential for the high binding ability of bisphenol A to human estrogen-related receptor-gamma. Environ. Health Perspect. 116: 32–38. doi: 10.1289/ehp.10587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patchev A. V., Götz F., Rohde W.2004. Differential role of estrogen receptor isoforms in sex-specific brain organization. FASEB J. 18: 1568–1570. doi: 10.1096/fj.04-1959fje [DOI] [PubMed] [Google Scholar]
- 44.Patisaul H. B., Bateman H. L.2008. Neonatal exposure to endocrine active compounds or an ERbeta agonist increases adult anxiety and aggression in gonadally intact male rats. Horm. Behav. 53: 580–588. doi: 10.1016/j.yhbeh.2008.01.008 [DOI] [PubMed] [Google Scholar]
- 45.Patisaul H. B., Polston E. K.2008. Influence of endocrine active compounds on the developing rodent brain. Brain Res. Brain Res. Rev. 57: 352–362. doi: 10.1016/j.brainresrev.2007.06.008 [DOI] [PubMed] [Google Scholar]
- 46.Patisaul H. B., Fortino A. E., Polston E. K.2006. Neonatal genistein or bisphenol-A exposure alters sexual differentiation of the AVPV. Neurotoxicol. Teratol. 28: 111–118. doi: 10.1016/j.ntt.2005.11.004 [DOI] [PubMed] [Google Scholar]
- 47.Patisaul H. B., Sullivan A. W., Radford M. E., Walker D. M., Adewale H. B., Winnik B., Coughlin J. L., Buckley B., Gore A. C.2012. Anxiogenic effects of developmental bisphenol A exposure are associated with gene expression changes in the juvenile rat amygdala and mitigated by soy. PLoS One 7: e43890. doi: 10.1371/journal.pone.0043890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pei L., Mu Y., Leblanc M., Alaynick W., Barish G. D., Pankratz M., Tseng T. W., Kaufman S., Liddle C., Yu R. T., Downes M., Pfaff S. L., Auwerx J., Gage F. H., Evans R. M.2015. Dependence of hippocampal function on ERRγ-regulated mitochondrial metabolism. Cell Metab. 21: 628–636. doi: 10.1016/j.cmet.2015.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peretz J., Vrooman L., Ricke W. A., Hunt P. A., Ehrlich S., Hauser R., Padmanabhan V., Taylor H. S., Swan S. H., VandeVoort C. A., Flaws J. A.2014. Bisphenol a and reproductive health: update of experimental and human evidence, 2007−2013. Environ. Health Perspect. 122: 775–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pilgrim C., Hutchison J. B.1994. Developmental regulation of sex differences in the brain: can the role of gonadal steroids be redefined? Neuroscience 60: 843–855. doi: 10.1016/0306-4522(94)90267-4 [DOI] [PubMed] [Google Scholar]
- 51.Rebuli M. E., Cao J., Sluzas E., Delclos K. B., Camacho L., Lewis S. M., Vanlandingham M. M., Patisaul H. B.2014. Investigation of the effects of subchronic low dose oral exposure to bisphenol A (BPA) and ethinyl estradiol (EE) on estrogen receptor expression in the juvenile and adult female rat hypothalamus. Toxicol. Sci. 140: 190–203. doi: 10.1093/toxsci/kfu074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rubin B. S., Lenkowski J. R., Schaeberle C. M., Vandenberg L. N., Ronsheim P. M., Soto A. M.2006. Evidence of altered brain sexual differentiation in mice exposed perinatally to low, environmentally relevant levels of bisphenol A. Endocrinology 147: 3681–3691. doi: 10.1210/en.2006-0189 [DOI] [PubMed] [Google Scholar]
- 53.Ryan B. C., Vandenbergh J. G.2006. Developmental exposure to environmental estrogens alters anxiety and spatial memory in female mice. Horm. Behav. 50: 85–93. doi: 10.1016/j.yhbeh.2006.01.007 [DOI] [PubMed] [Google Scholar]
- 54.Salian S., Doshi T., Vanage G.2009. Impairment in protein expression profile of testicular steroid receptor coregulators in male rat offspring perinatally exposed to Bisphenol A. Life Sci. 85: 11–18. doi: 10.1016/j.lfs.2009.04.005 [DOI] [PubMed] [Google Scholar]
- 55.Schönfelder G., Wittfoht W., Hopp H., Talsness C. E., Paul M., Chahoud I.2002. Parent bisphenol A accumulation in the human maternal-fetal-placental unit. Environ. Health Perspect. 110: A703–A707. doi: 10.1289/ehp.021100703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Simerly R. B.2002. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu. Rev. Neurosci. 25: 507–536. doi: 10.1146/annurev.neuro.25.112701.142745 [DOI] [PubMed] [Google Scholar]
- 57.Süsens U., Hermans-Borgmeyer I., Borgmeyer U.2000. Alternative splicing and expression of the mouse estrogen receptor-related receptor gamma. Biochem. Biophys. Res. Commun. 267: 532–535. doi: 10.1006/bbrc.1999.1976 [DOI] [PubMed] [Google Scholar]
- 58.Tetel M. J., Pfaff D. W.2010. Contributions of estrogen receptor-α and estrogen receptor-ß to the regulation of behavior. Biochim. Biophys. Acta 1800: 1084–1089. doi: 10.1016/j.bbagen.2010.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Timms B. G., Howdeshell K. L., Barton L., Bradley S., Richter C. A., vom Saal F. S.2005. Estrogenic chemicals in plastic and oral contraceptives disrupt development of the fetal mouse prostate and urethra. Proc. Natl. Acad. Sci. U.S.A. 102: 7014–7019. doi: 10.1073/pnas.0502544102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tomihara K., Soga T., Nomura M., Korach K. S., Gustafsson J. A., Pfaff D. W., Ogawa S.2009. Effect of ER-beta gene disruption on estrogenic regulation of anxiety in female mice. Physiol. Behav. 96: 300–306. doi: 10.1016/j.physbeh.2008.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tyl R. W., Myers C. B., Marr M. C., Sloan C. S., Castillo N. P., Veselica M. M., Seely J. C., Dimond S. S., Van Miller J. P., Shiotsuka R. N., Beyer D., Hentges S. G., Waechter J. M., Jr.2008. Two-generation reproductive toxicity study of dietary bisphenol A in CD-1 (Swiss) mice. Toxicol. Sci. 104: 362–384. doi: 10.1093/toxsci/kfn084 [DOI] [PubMed] [Google Scholar]
- 62.Tyl R. W., Myers C. B., Marr M. C., Thomas B. F., Keimowitz A. R., Brine D. R., Veselica M. M., Fail P. A., Chang T. Y., Seely J. C., Joiner R. L., Butala J. H., Dimond S. S., Cagen S. Z., Shiotsuka R. N., Stropp G. D., Waechter J. M.2002. Three-generation reproductive toxicity study of dietary bisphenol A in CD Sprague-Dawley rats. Toxicol. Sci. 68: 121–146. doi: 10.1093/toxsci/68.1.121 [DOI] [PubMed] [Google Scholar]
- 63.vom Saal F. S., Hughes C.2005. An extensive new literature concerning low-dose effects of bisphenol A shows the need for a new risk assessment. Environ. Health Perspect. 113: 926–933. doi: 10.1289/ehp.7713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang L., Andersson S., Warner M., Gustafsson J. A.2003. Estrogen receptor (ER)beta knockout mice reveal a role for ERbeta in migration of cortical neurons in the developing brain. Proc. Natl. Acad. Sci. U.S.A. 100: 703–708. doi: 10.1073/pnas.242735799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wilson J. G.1973. Environment and Birth Defects. Academic Press, New York. [Google Scholar]
- 66.Wolstenholme J. T., Rissman E. F., Connelly J. J.2011. The role of Bisphenol A in shaping the brain, epigenome and behavior. Horm. Behav. 59: 296–305. doi: 10.1016/j.yhbeh.2010.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wolstenholme J. T., Taylor J. A., Shetty S. R. J., Edwards M., Connelly J. J., Rissman E. F.2011. Gestational exposure to low dose bisphenol A alters social behavior in juvenile mice. PLoS ONE 6: e25448. doi: 10.1371/journal.pone.0025448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yokota H., Iwano H., Endo M., Kobayashi T., Inoue H., Ikushiro S., Yuasa A.1999. Glucuronidation of the environmental oestrogen bisphenol A by an isoform of UDP-glucuronosyltransferase, UGT2B1, in the rat liver. Biochem. J. 340: 405–409. doi: 10.1042/bj3400405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang J., Cooke G. M., Curran I. H. A., Goodyer C. G., Cao X. L.2011. GC-MS analysis of bisphenol A in human placental and fetal liver samples. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 879: 209–214. doi: 10.1016/j.jchromb.2010.11.031 [DOI] [PubMed] [Google Scholar]