Abstract
To examine outbreaks of mange in raccoon dogs (Nyctereutes procyonoides) with respect to population density, we analyzed camera trap videos, and isolated mites from raccoon dog carcasses. In a camera trapping survey, we categorized the skin condition of raccoon dogs, and used a number of independent videos to calculate the relative abundance index (RAI). The RAI of raccoon dogs with alopecia increased following an increase in the RAI of those without alopecia. Among 27 raccoon dog carcasses, 12 showed mange-compatible skin lesions. Sarcoptes scabiei was isolated from 11 of these raccoon dogs, indicating that sarcoptic mange was endemic in our study area. Therefore, a high relative population density may be a factor underlying epizootics of sarcoptic mange in raccoon dogs.
Keywords: camera trapping, population density, raccoon dog, Sarcoptes scabiei, sarcoptic mange
Sarcoptic mange is a parasitic skin disease caused by Sarcoptes scabiei, and has been reported in 104 species of mammals [1, 17]. In Japan, there have been many reports of sarcoptic mange in raccoon dogs (Nyctereutes procyonoides) [10, 24, 28, 31]. Kido et al. [7] and Shibata and Kawamichi [25] considered the effect of sarcoptic mange epizootics on the population dynamics of raccoon dogs. In general, mange is more prevalent when host animal population densities are high [17, 18]. Population density is therefore a suspected factor associated with epizootics of mange in raccoon dogs [7, 24, 25]. However, a limitation of these previous studies [7, 24, 25] is that they considered the number of captured or rescued raccoon dogs as an index of population density. The number of captured animals does not precisely reflect the population density because it depends on the amount of effort expended [16]. Therefore, it is necessary to use an index of population density different from those used in previous studies to analyze both current outbreaks of mange and raccoon dog population density.
Camera traps are widely used in wildlife studies [14, 15, 20, 33] because they are less invasive, less time-consuming, and more economical than the long-term direct observation of wildlife [2]. In addition, the number of records per trap can be used as a relative abundance index (RAI), and this index is directly related to independently derived estimates of population density [14]. Epidermal hyperkeratosis and alopecia [1, 17] are clinical signs of mange that can potentially be observed visually in camera trap images to estimate trends [15]. However, we cannot use camera trap data alone to make a definitive diagnosis of mange, which requires the isolation of mites, and its incidence may be overestimated due to the inclusion of animals with alopecia caused by other factors [8, 19, 26]. Hence, it is necessary to validate the diagnostic accuracy of camera trap data for mange; this could be accomplished by isolating mites from collected animals. Such validation would demonstrate the usefulness of camera trap data for inferring trends in mange, making it possible to evaluate both outbreaks of mange, and changes in the population density of raccoon dogs, using camera trapping data.
The objective of this study was to investigate the relationship between mange epizootics in raccoon dogs and their population density. We analyzed camera trap videos of raccoon dogs to evaluate population density and identify clinical signs of mange.
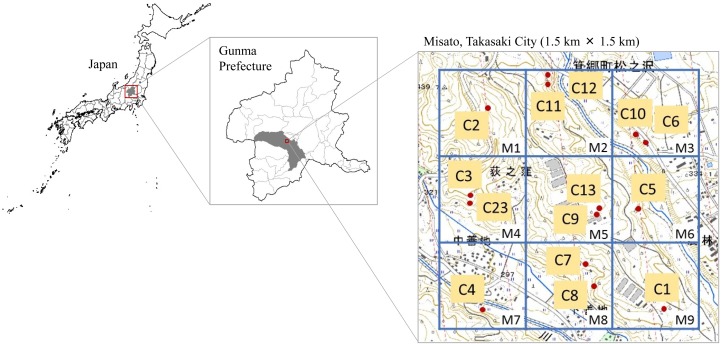
The study area for the camera trapping survey was a 1.5 × 1.5 km area located in Misato, Takasaki City, Gunma Prefecture, Japan. The area was divided into a 3 × 3 grid composed of 500 × 500 m squares (Fig. 1). One or more passive infrared camera traps (Model 119435; Trophy Camera Brown, Bushnell, Overland Park, KS, U.S.A.) were set up in each zone. The cameras were installed 1 m above the ground along animal trails, and were set to record 10 sec of video at an interval of 10 sec. The camera trapping survey was carried out from June 25, 2011 to August 29, 2016. The skin condition of raccoon dogs recorded on video was evaluated to identify those with and without alopecia. When the skin condition was difficult to identify in a video, it was defined as “indeterminable.” Further, the number of individual raccoon dogs obtained by the camera trapping survey was counted. When multiple raccoon dogs were recorded on a video simultaneously, the largest number of raccoon dogs recorded at once was determined. To avoid overestimation, successive observations were defined as independent when they were separated by more than 3 min [13]. The camera trap data were divided into four seasons: spring (March–May), summer (June–August), autumn (September–November), and winter (December–February). To estimate raccoon dog population densities, the number of independent video observations for each individual per 100 Camera-Nights (one camera active for one night=one Camera-Night) was estimated as the RAI using the following formula [14]: RAI=(number of individual raccoon dog observations)/(number of days a camera was active)×100 Camera‐Nights.
Fig. 1.
Locations of camera traps in Misato, Takasaki City, Gunma Prefecture, Japan. The study area was divided into a 3 × 3 grid composed of 500 × 500 m squares. Red dots represent the locations of cameras. M is the grid identification (ID) and C is the camera ID.
The proportion of raccoon dogs with alopecia was calculated as follows: Proportion of raccoon dogs with alopecia=(RAI of raccoon dogs with alopecia)/(RAI of all raccoon dogs)×100.
To determine seasonal and long-term changes in camera trap data for each category, we compared RAI values for each category among seasons using the Kruskal-Wallis test. Statistical analyzes were performed using R 3.1.2 software (https://www.r-project.org/).
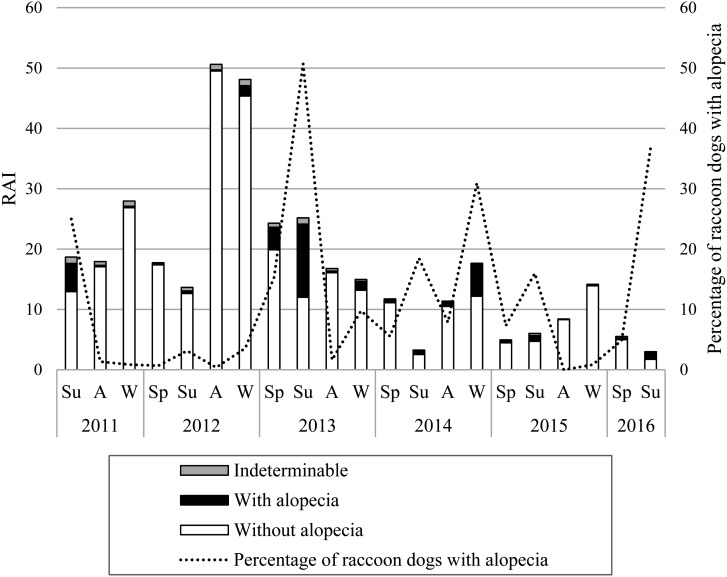
A total of 2,945 observations of raccoon dogs were obtained, of which 2,596 showed raccoon dogs without alopecia (Fig. 3a), 278 videos showed raccoon dogs with alopecia (Fig. 3b), and 71 videos showed raccoon dogs of indeterminable status. RAIs for raccoon dogs with and without alopecia changed significantly both yearly and seasonally (with alopecia: χ2=37.9, df=20, P<0.05; without alopecia: χ2=45.4, df=20, P<0.05; Fig. 4). On the other hand, RAIs for animals in the indeterminable group did not differ significantly over time (χ2=21.2, df=20, P>0.05, Fig. 4). The RAIs of raccoon dogs with alopecia increased in the summers of 2011 and 2013, and the winter of 2014. In particular, the proportion of raccoon dogs with alopecia in the summer of 2013 was 50.6%. The RAI of raccoon dogs without alopecia showed an increasing trend in the autumn and winter of 2012 (Fig. 4); it is possible that the raccoon dog population density increased.
Fig. 3.
a. A raccoon dog without alopecia recorded on video by camera trapping. Skin lesions were not detected. b. A raccoon dog with alopecia recorded on video by camera trapping. Skin lesions, including alopecia, were observed.
Fig. 4.
Changes in the relative abundance index (RAI) of raccoon dogs. Bars represent the RAI of raccoon dogs, presented according to skin condition. Line indicates the proportion of raccoon dogs with alopecia. The horizontal axis represents the year and season of the camera trapping survey. Sp, spring; Su, summer; A, autumn; W, winter. RAIs for raccoon dogs with and without alopecia changed significantly over time (Kruskal-Wallis test, with alopecia: χ2=37.9, df=20, P<0.05; without alopecia: χ2=45.4, df=20, P<0.05). RAIs for those in the indeterminable group did not change significantly (Kruskal-Wallis test, χ2=21.2, df=20, P>0.05).
Some previous studies have reported raccoon dogs with alopecia being infested with S. scabiei [7, 24, 25]. However, alopecia is also caused by other factors besides sarcoptic mange [8, 19, 26]. Therefore, to confirm that sarcoptic mange in raccoon dogs was endemic in our study area, we isolated mites from raccoon dog carcasses. Between March 28, 2014 and March 31, 2015, 27 raccoon dog carcasses were collected in the Misato, Miyazawa, and Jumonji areas of Takasaki City, Gunma Prefecture. These animals were captured as part of pest control measures for the prevention of agricultural damage, and were killed by licensed hunters on behalf of Takasaki City. Collected raccoon dogs were classified, based on skin condition, into those with or without mange-compatible lesions, such as alopecia, on their skin. Skin lesion samples were collected and frozen until used to identify mite species. Using a scalpel for scraping, samples were placed in a 15% potassium hydroxide (KOH) and 40% dimethyl sulfoxide (DMSO) solution [30], and mounted on glass slides for microscopic observation. Isolated mites were identified as S. scabiei according to Fain’s criteria [3].
Mange-compatible lesions in the skin were found in 12 of the 27 raccoon dog carcasses. Mites were isolated from 11 of 12 raccoon dogs that showed lesions. All mites were identified as S. scabiei (Fig. 2). Thus, 91.7% of raccoon dogs with alopecia were infested with S. scabiei, indicating that sarcoptic mange in raccoon dogs was endemic in our study area.
Fig. 2.
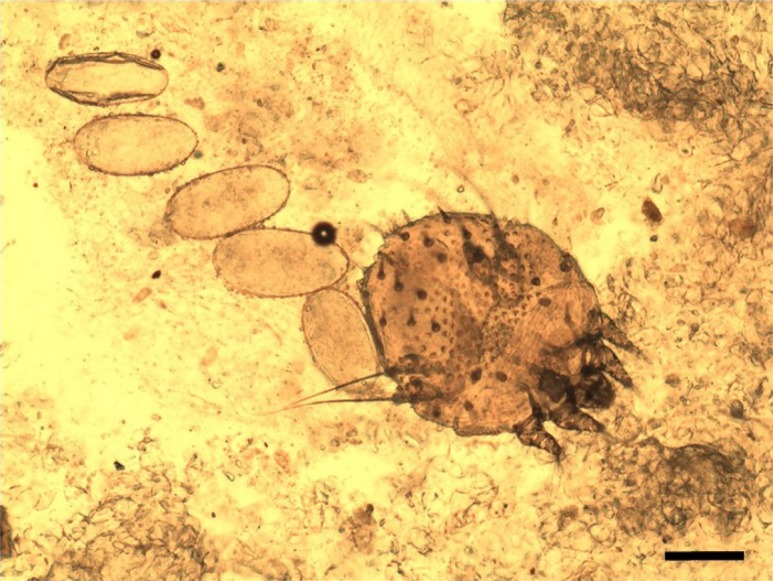
Microscope images of an adult mite and eggs of Sarcoptes scabiei isolated from raccoon dogs. Bar=100 µm.
These results suggest that it is reasonable to assume that raccoon dogs with alopecia recorded in camera trap videos are infested with S. scabiei. The mating season of raccoon dogs starts in February, offspring are born in April–May, and the dispersion of juveniles starts in early autumn [5, 29]. Therefore, the RAI of raccoon dogs without alopecia tended to increase toward autumn each year (Fig. 4). The home range of raccoon dogs between spring and summer tends to decrease because both females and males participate in offspring care [5, 29]. This seasonal behavioral pattern may explain the decrease in RAI from spring to early summer. Thus, seasonal changes in the RAI of raccoon dogs may largely be related to breeding habits. However, the RAI of raccoon dogs without alopecia exhibited the greatest increase in the autumn and winter of 2012 (Fig. 4), and it is reasonable to assume that the raccoon dog population density increased during this period. Therefore, sarcoptic mange epizootics were detected in the summer of 2013, following increases in population density in the autumn and winter of 2012; in other words, a high relative population density may be a factor in epizootics of sarcoptic mange in raccoon dogs. It is also possible that the RAI of raccoon dogs without alopecia increased prior to the summer of 2011.
Various factors, such as roadkill rates, hunting pressure, and availability of food resources, have an effect on the population density of raccoon dogs [4, 21,22,23]. It is possible that factors other than sarcoptic mange were related; however, in our study area, the number of raccoon dog carcasses attributed to pest control did not exhibit significant changes [27]. Therefore, the epizootic of sarcoptic mange may have been linked to changes in population density in this area. On the other hand, after the summer of 2013, a few raccoon dogs with alopecia were consistently observed, and the RAI of all raccoon dogs reached a minimum in the summer of 2016. This suggested that sarcoptic mange persisted in the raccoon dog population and may have influenced changes in population density over an extended period of time.
The results of this study indicate that epizootics of sarcoptic mange in raccoon dogs may be related to a high relative population density. Considering that the home range of raccoon dogs is approximately 0.1–6 km2 [6, 22, 32], a larger study area with longer surveillance periods might be required for more detailed analyzes. It remains unclear how S. scabiei is transmitted and epizootics of mange are triggered when the population density is high.
Epizootic of sarcoptic mange in raccoon dogs may increase the risk of S. scabiei transmission to other wild mammals and/or domestic animals. Previous studies have reported that S. scabiei derived from raccoon dogs, domestic dogs, and other wild animals are very closely related at the genetic level [9, 11, 12]. Epizootics of sarcoptic mange in raccoon dogs have important implications for veterinary medicine and wildlife conservation. Therefore, it is necessary to comprehensively understand the transmission patterns of mites and the risk of mange transmission to other animal taxa.
Acknowledgments
We are extremely grateful to the Wildlife Damage Control Support Center of Gunma Prefecture, the Gunma Chuo Hunting Association, the Agriculture and Forestry Section of Takasaki City, the Industry Section of Misato Branch, the Industrial Tourism Section of Haruna Branch, the Gunma Prefectural Institute of Agriculture and Forestry, and the members of the Division of Wildlife Science, Nippon Veterinary and Life Science University.
REFERENCES
- 1.Bornstein S., Mörner T., Samuel W. M.2001. Sarcoptes scabiei and sarcoptic mange. pp. 107–119. In: Parasitic Diseases of Wild Mammals, 2nd ed. (Samuel, W. M., Pybus, M. J. and Kocan, A. A. eds.), Iowa State University Press, Iowa. [Google Scholar]
- 2.Cutler T. L., Swann D. E.1999. Using remote photography in wildlife ecology: a review. Wildl. Soc. Bull. 27: 571–581. [Google Scholar]
- 3.Fain A.1968. Etude de la Variabilite de Sarcoptes scabiei avec une Revision des Sarcoptidae. Acta Zool. Pathol. Antverp. 47: 1–196. [Google Scholar]
- 4.Helle E., Kauhala K.1995. Reproduction in the raccoon dog in Finland. J. Mammal. 76: 1036–1046. doi: 10.2307/1382597 [DOI] [Google Scholar]
- 5.Ikeda H.1983. Development of young and parental care of the raccoon dog, Nyctereutes procyonoides viverrinus TEMMINCK, in captivity. J. Mammal. Soc. Jpn. 9: 229–236. [Google Scholar]
- 6.Ikeda H., Eguchi K., Ono Y.1979. Home range utilization of a raccoon dog, Nyctereutes procyonoides viverrinus, TEMMINCK, in a small islet in western Kyushu. Jap. J. Ecol. 29: 35–48. [Google Scholar]
- 7.Kido N., Itabashi M., Takahashi M., Futami M.2013. Epidemiology of sarcoptic mange in free-ranging raccoon dogs (Nyctereutes procyonoides) in Yokohama, Japan. Vet. Parasitol. 191: 102–107. doi: 10.1016/j.vetpar.2012.07.026 [DOI] [PubMed] [Google Scholar]
- 8.Machida N., Kiryu K., Oh-ishi K., Kanda E., Izumisawa N., Nakamura T.1993. Pathology and epidemiology of canine distemper in raccoon dogs (Nyctereutes procyonoides). J. Comp. Pathol. 108: 383–392. doi: 10.1016/S0021-9975(08)80210-1 [DOI] [PubMed] [Google Scholar]
- 9.Makouloutou P., Suzuki K., Yokoyama M., Takeuchi M., Yanagida T., Sato H.2015. Involvement of two genetic lineages of Sarcoptes scabiei mites in a local mange epizootic of wild mammals in Japan. J. Wildl. Dis. 51: 69–78. doi: 10.7589/2014-04-094 [DOI] [PubMed] [Google Scholar]
- 10.Matsumoto I., Takashima K., Yamane T., Yamane Y., Okano T., Asano M.2011. Sarcoptic mange in wild raccoon dogs in western and central Tottori prefecture. Dobutsu Rinsho Igaku 20: 13–17 (In Japanese). [Google Scholar]
- 11.Matsuyama R.2016. Molecular epidemiology on populations of Sarcoptes scabiei in domestic dogs and wild animals in Japan. Doctoral thesis, Doctoral course of the united graduate school of veterinary sciences, Gifu University (In Japanese).
- 12.Matsuyama R., Yabusaki T., Kuninaga N., Morimoto T., Okano T., Suzuki M., Asano M.2015. Coexistence of two different genotypes of Sarcoptes scabiei derived from companion dogs and wild raccoon dogs in Gifu, Japan: The genetic evidence for transmission between domestic and wild canids. Vet. Parasitol. 212: 356–360. doi: 10.1016/j.vetpar.2015.06.023 [DOI] [PubMed] [Google Scholar]
- 13.Moll R. J., Millspaugh J. J., Beringer J., Sartwell J., He Z., Eggert J. A., Zhao X.2009. A terrestrial animal-borne video system for large mammals. Comput. Electron. Agric. 66: 133–139. doi: 10.1016/j.compag.2009.01.001 [DOI] [Google Scholar]
- 14.O’Brien T. G., Kinnaird M. F., Wibisono H. T.2003. Crouching tigers hidden prey: Sumatran tiger and prey populations in a tropical forest landscape. Anim. Conserv. 6: 131–139. doi: 10.1017/S1367943003003172 [DOI] [Google Scholar]
- 15.Oleaga A., Casais R., Balseiro A., Espí A., Llaneza L., Hartasánchez A., Gortázar C.2011. New techniques for an old disease: sarcoptic mange in the Iberian wolf. Vet. Parasitol. 181: 255–266. doi: 10.1016/j.vetpar.2011.04.036 [DOI] [PubMed] [Google Scholar]
- 16.Ohtsuka-Ito E., Kanzaki N.1998. Population trends of the Japanese wild boar during the Showa era. Wildl. Conserv. Jap. 3: 95–105 (In Japanese). [Google Scholar]
- 17.Pence D. B., Ueckermann E.2002. Sarcoptic manage in wildlife. Rev. - Off. Int. Epizoot. 21: 385–398 (International Office of Epizootics). doi: 10.20506/rst.21.2.1335 [DOI] [PubMed] [Google Scholar]
- 18.Pence D. B., Windberg L. A.1994. Impact of a sarcoptic mange epizootic on a coyote population. J. Wildl. Manage. 58: 624–633. doi: 10.2307/3809675 [DOI] [Google Scholar]
- 19.Rijnberk A., Kinderen, P. J. der., Thijssen J. H. H., 1969. Canine Cushing’s syndrome. Zentralbl. Veterinarmed. A 16: 13–28. doi: 10.1111/j.1439-0442.1969.tb01035.x [DOI] [PubMed] [Google Scholar]
- 20.Rovero F., Zimmermann F., Berzi D., Meek P.2013. “Which camera trap type and how many do I need?” A review of camera features and study designs for a range of wildlife research applications. Hystrix 24: 148–156. [Google Scholar]
- 21.Saeki M., Macdonald D. W.2004. The effects of traffic on the raccoon dog (Nyctereutes procyonoides viverinus) and other mammals in Japan. Biol. Conserv. 118: 559–571. doi: 10.1016/j.biocon.2003.10.004 [DOI] [Google Scholar]
- 22.Saeki M., Johnson P. J., Macdonald D. W.2007. Movements and habitat selection of raccoon dogs (Nyctereutes procyonoides) in a mosaic landscape. J. Mammal. 88: 1098–1111. doi: 10.1644/06-MAMM-A-208R1.1 [DOI] [Google Scholar]
- 23.Seki Y., Koganezawa M.2013. Does sika deer overabundance exert cascading effects on the raccoon dog population? J. For. Res. 18: 121–127. doi: 10.1007/s10310-011-0332-z [DOI] [Google Scholar]
- 24.Shibata A.2004. Studies on mange of wildlife in Japan. Doctoral Thesis, Doctoral Course in Veterinary Medicine, Nippon Veterinary and Life Science University, Tokyo (In Japanese).
- 25.Shibata F., Kawamichi T.1999. Decline of raccoon dog populations resulting from sarcoptic mange epizootics. Mammalia 63: 281–290. doi: 10.1515/mamm.1999.63.3.281 [DOI] [Google Scholar]
- 26.Sue P.2010. Skin Diseases of the Dog and Cat. Interzoo, Tokyo (In Japanese). [Google Scholar]
- 27.System of statistical information in Gunma Prefecture website. 2017. The number of captured wild animals by hunting and pest control in each municipality. Available at: http://toukei.pref.gunma.jp/chouju/sichoson.html [Accessed 25 May 2017].
- 28.Takahashi M., Nogami S., Misumi H., Maruyama S., Shiibashi T., Yamamoto Y., Sakai T.2001. Mange caused by Sarcoptes scabiei (Acari: Sarcoptidae) in wild raccoon dogs, Nyctereutes procyonoides, in Kanagawa Prefecture, Japan. J. Vet. Med. Sci. 63: 457–460. doi: 10.1292/jvms.63.457 [DOI] [PubMed] [Google Scholar]
- 29.Tanaka H.2009. Rearing behavior of raccoon dogs (Nyctereutes procyonoides viverrinus) in Yamaguchi City, Yamaguchi, Japan. Bull. Yamaguchi Mus. 35: 25–32 (In Japanese). [Google Scholar]
- 30.Tani K.2006. Clinical sign, diagnosis and immunology for canine demodicosis. ViVeD 2: 299–303 (In Japanese). [Google Scholar]
- 31.Yamamoto S., Takahashi M., Nogami S.1998. Scabies in wild raccoon dogs, Nyctereutes procyonoides at the Tomioka-Kanra district in Gunma Prefecture, Japan. Med. Entomol. Zool. 49: 217–222 (In Japanese). doi: 10.7601/mez.49.217 [DOI] [Google Scholar]
- 32.Yamamoto Y., Terao K., Horiguchi T., Morita M., Yachimori S.1994. Home range and dispersal of the raccoon dog (Nyctereutes procyonoides viverrinus) in Mt. Nyugasa, Nagano Prefecture, Japan. Nat. Environ. Sci. Res. 7: 53–61 (In Japanese). [Google Scholar]
- 33.Yasuda M.2004. Monitoring diversity and abundance of mammals with camera traps: a case study on Mount Tsukuba, central Japan. Mammal Study 29: 37–46. doi: 10.3106/mammalstudy.29.37 [DOI] [Google Scholar]