Abstract
An outbreak of botulism occurred over a two-month period beginning July 20, 2016. In all, 697 wild birds were found paralyzed or dead at the Namdong reservoir and 11 Gong-gu. Using a mouse bioassay, type C botulinum toxin was identified in the bird serum, liquid cultures of soil samples, and maggot extracts. To minimize further infection of wild birds, we opened the floodgates of the Namdong reservoir adjacent to the Yellow Sea; this decreased the water temperature and the nutrient load such as nitrogen and phosphorus. The outbreak stopped shortly after taking these actions. It is not known if these efforts decreased the number of dead and diseased wild birds. Our study demonstrates one potential approach to minimize future botulism outbreaks among wild birds and their habitats.
Keywords: avian botulism, type C botulism, wild bird
Avian botulism is a flaccid paralytic disease caused by ingestion of the botulinum neurotoxin (BoNT) produced by Clostridium botulinum (C. botulinum). Avian botulism is the most common cause of death in wild birds worldwide [9] and is a serious emerging disease in poultry [8]. Birds are typically exposed to BoNT through ingestion of invertebrates, after which BoNT accumulates in the blood until it reaches the required threshold to induce signs of paralysis [6]. Seven distinct types of BoNT have been identified, designated by the letters A through G [14]; wild birds are most commonly affected by type C toxin [3]. Outbreaks of avian type C botulism have been reported in the United States [15], Italy [2], Poland [13], and South Korea. The first reported outbreak of type C botulism in Korea occurred in October 2007 in the Tan-cheon, a small branch of the Hangang River [7]. In October 2008, approximately 2,000 birds died from type C botulism in the Namdong reservoir in Incheon, South Korea [12]. In the present study, we describe the diagnostic findings and response to an outbreak of type C botulism that occurred in the Namdong reservoir in July 2016.
Two paralyzed and four dead spot-billed ducks (Anas poecilorhyncha) were discovered in the Namdong reservoir on July 15, 2016. Over the next two months, 697 wild birds were found paralyzed or dead near the Namdong reservoir (519/697) and 11 Gong-gu region, a manufacturing area (178/697) (Fig. 1). Most birds (87.7%; 611/697) were spot-billed ducks (Anas poecilorhyncha). Other species affected included grey herons (Ardea cinerea, 1.3%), vega gulls (Larus argentatus, 8.6%), and sandpiper (Scolopacidae, 2.4%). Eight spot-billed ducks and one gull with signs of depression and flaccid paralysis of the legs, wings, and neck were transported to the National Institute of Environmental Research for necropsy. No specific gross lesions were identified on necropsy. There was no evidence of viral (Avian influenza) or bacterial (Pasteurella multocida and Salmonella spp.) infection. Blood from the heart or wing vein of the nine paralyzed birds was collected into sterile 50 ml conical tubes. A mouse bioassay was used to detect BoNT in the serum and to diagnose avian botulism [4]. Two 6-week-old imprinting control region (ICR) mice were intraperitoneally injected with 0.5 ml of serum. The inoculated mice subsequently developed typical signs of botulism, including fuzzy hair, respiratory failure and a wasp-like narrowed waist, and both mice died from the disease.
Fig. 1.
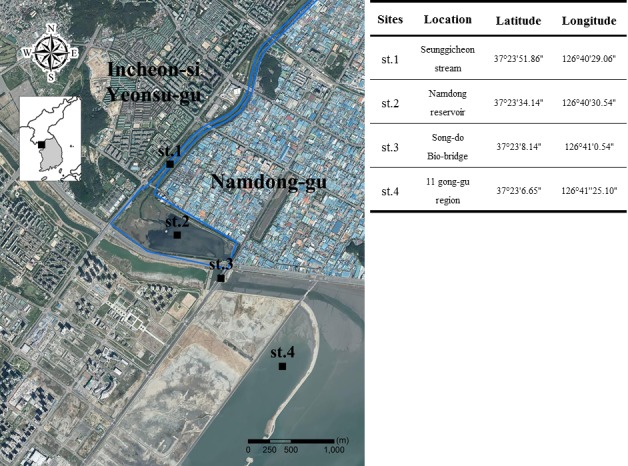
Map of Incheon depicting areas where avian botulism occurred (st.1 and st.4) and soil sample collection sites (st.1, st.2 and st.3).
C. botulinum is an anaerobic organism found in the soil [5], and wild birds ingest the toxin by consuming invertebrates such as maggots, which play a critical role in the carcass-maggot cycle of avian botulism [10]. Therefore, we collected soil samples at a depth of 15–30 cm from the Namdong reservoir (n=5), Seunggicheon stream (n=5), and Song-do Bio-bridge (n=5) (Fig. 1). In addition, maggots (n=3) from carcasses found at the Namdong reservoir were collected for BoNT identification.
Cecal content samples from the birds, soil samples, and maggots were incubated for three days in cooked meat medium under anaerobic conditions. The supernatants were mixed with trypsin and used in the mouse bioassay. Supernatants of one of the three cecal content samples (33.3%), seven of the fifteen soil samples (46.7%), and all three maggot samples (100%) were positive for BoNT. The toxin type was determined using a neutralizing test with specific antitoxins. For this test, 25 µl of antitoxin (A, B, C and D, 100 units/ml) was mixed with 1 ml of serum and 1 ml of culture supernatant. After incubation for 30 min at 37°C, 500 µl of the mixture was used for the mouse neutralization test. Type C botulinum toxin was identified in the serum (9/9), cecal contents (1/3), and maggot extracts (3/3). BoNT types A through D were identified in the soil samples (Table 1).
Table 1. Results of the mouse bioassay used to detect BoNTs.
| Sample | Antitoxin type used | No. positive samples/ No. samples examined |
|---|---|---|
| Serum | C | 9 / 9 |
| Cecum content culture supernatanta) | C | 1 / 3 |
| Maggot culture supernatantb) | C | 3 / 3 |
| Soil samplec) | ABCD | 7 / 15 |
a) Supernatants of homogenized cecal contents were heated at 80°C for 20 min, and cultured in deaerated cooked meat medium (CMM) at 30°C for 5 days under anaerobic conditions. The supernatant was centrifuged at 5,000 × g for 30 min at 4°C, sterilized using 0.45 µm filters, and used in the mouse bioassay. b) Supernatants of homogenized maggots were heated at 80°C for 20 min and cultured in de-aerated CMM at 30°C for 5 days under anaerobic conditions. c) Soil sediments were mixed 1:10 with distilled water and added 1:1 (v:v) to a twofold concentrate of CMM (25 g/100 ml), followed by heat treatment. After five days, cultured supernatants were used in the mouse bioassay.
It is known that temperature can play a critical role in the proliferation of C. botulinum, because the optimal growth of C. botulinum in the laboratory occurs between 25 and 40°C [10]. Furthermore, C. botulinum growth is promoted by the high biomass levels and anaerobic conditions found in wetlands [1]. Abnormally high temperatures and low precipitation rates occurred in South Korea in 2016 (http://www.kma.go.kr/weather/climate/past_table.jsp). During the outbreak, the water temperature was above average from June to August 2016 (Table 2). The average monthly temperature was 27.3 to 30.7°C, which was 2.5 to 3.7°C higher than normal. High ambient temperatures can elevate both the soil and water temperature. In addition, the precipitation rate in the Namdong reservoir was significantly below average in 2016 compared with the previous 11 years. In July and August 2016, the monthly precipitation was 300.5 and 26.5 mm, which was 120.5 and 183.1 mm less than normal, respectively (Table 2). Moreover, a sewage treatment plant surrounds the Namdong reservoir, which can increase the presence of decaying organic matter, providing an energy source for C. botulinum. Many elemental substances such as dissolved oxygen (DO), total nitrogen (TN), total phosphorus (TP) indicate water quality of Namdong reservoir. The average of DO was 9.45 ± 0.86 mg/l, TN was 7.45 ± 2.25 mg/l and TP was 0.57 ± 0.19 mg/l from 2013 to 2016. It is likely that these epidemiological factors (high water temperature, decreased precipitation, and abundant organic matter) contributed to the botulism outbreak in the Namdong Reservoir.
Table 2. Average monthly precipitation in Incheon city and water temperature in Seunggi-cheon (2005–2016).
| Parameter | Year | Jun. | Jul. | Aug. |
|---|---|---|---|---|
| Precipitation (mm) | 2005–2015 | 117.7 | 421.0 | 209.6 |
| 2016 | 19.5 | 300.5 | 26.5 | |
| Water temperature (°C) | 2005–2015 | 24.8 | 25.3 | 27.0 |
| 2016 | 27.3 | 28.8 | 30.7 |
To decrease the water temperature and dilute the sewage concentration, the Namdong reservoir was filled with sea water by opening the floodgates at rising tide for 5 hr. After flooding, the floodgates were closed and kept flooding for 5 days (from August 17 to 22, 2016). The sea water added by flooding was released by re-opening the floodgates at low tide. After flooding, the water temperature in the Namdong reservoir decreased from 30.7 ± 0.8°C to 27.6 ± 0.4°C. The elemental substances including DO, TN and TP decreased to 5.66 ± 0.50, 0.65 ± 0.11 and 0.13 ± 0.01 mg/l, respectively (Table 3). In addition, the carcasses were promptly removed to disrupt the carcass-maggot cycle of botulism outbreaks [14]. Because BoNT was detected in the maggot extracts obtained from the carcasses of infected birds (Table 1), the prompt removal of carcasses may have helped minimize the spread of botulism by decreasing the number of maggots that spread the disease. Opening the floodgates and removing the carcasses coincided with a decrease in the number of dead birds (Fig. 2) [11]. The unprecedented high temperature, low precipitation, and water pollution, along with the ecosystem stress of industrialization may have been the most important factors for this avian outbreak of botulism. While it is unclear exactly how management of the water and carcasses inhibited the spread of botulism, the present study does demonstrate one potential way to minimize future botulism outbreaks among wild birds and their habitats in this ecosystem.
Table 3. Average water quality index in the Namdong reservoir.
| Parameter | Period | DO (mg/l) | TN (mg/l) | TP (mg/l) |
|---|---|---|---|---|
| Water quality | 2013–2016 | 9.45 ± 0.86 | 7.45 ± 2.25 | 0.57 ± 0.19 |
| After flooding | 5.66 ± 0.50 | 0.65 ± 0.11 | 0.13 ± 0.01 |
DO, Dissolved Oxygen; TN, Total Nitrogen; TP, Total Phosphorus.
Fig. 2.
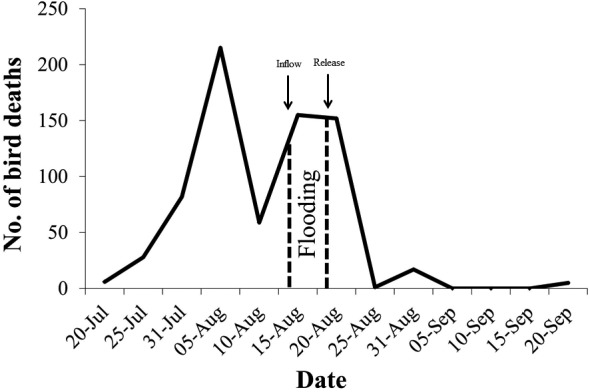
The number of wild bird deaths over time, from July 20 to September 20, 2016. Inflow denotes start of flooding; Release denotes end of flooding in the Namdong reservoir.
Acknowledgments
This study was funded by the National Institute of Environmental Research (NIER), Grant No. 2016-01-01-033, which had no role in the study design, data collection, data interpretation, or the decision to submit this work for publication.
REFERENCES
- 1.Anza I., Vidal D., Laguna C., Díaz-Sánchez S., Sánchez S., Chicote A., Florín M., Mateo R.2014. Eutrophication and bacterial pathogens as risk factors for avian botulism outbreaks in wetlands receiving effluents from urban wastewater treatment plants. Appl. Environ. Microbiol. 80: 4251–4259. doi: 10.1128/AEM.00949-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Defilippo F., Luppi A., Maioli G., Marzi D., Fontana M. C., Paoli F., Bonilauri P., Dottori M., Merialdi G.2013. Outbreak of type C botulism in birds and mammals in the Emilia Romagna region, northern Italy. J. Wildl. Dis. 49: 1042–1046. doi: 10.7589/2013-03-072 [DOI] [PubMed] [Google Scholar]
- 3.Forrester D. J., Wenner K. C., White F. H., Greiner E. C., Marion W. R., Thul J. E., Berkhoff G. A.1980. An epizootic of avian botulism in a phosphate mine settling pond in northern Florida. J. Wildl. Dis. 16: 323–327. doi: 10.7589/0090-3558-16.3.323 [DOI] [PubMed] [Google Scholar]
- 4.Hatheway C. L.1988. Botulism. pp. 111–133. In: Laboratory Diagnosis of Infectious Diseases: Principles and Practice (Balows, W. J., Hausler, W. J. J., Ohashi, M. and Turano, A. eds.), Springer-Verlag., New York. [Google Scholar]
- 5.Huss H. H.1980. Distribution of Clostridium botulinum. Appl. Environ. Microbiol. 39: 764–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hyun S. H., Sakaguchi G.1988. Cell-bound toxin of Clostridium botulinum type C and its pathogenic significance. Nippon Juigaku Zasshi 50: 495–501. doi: 10.1292/jvms1939.50.495 [DOI] [PubMed] [Google Scholar]
- 7.Kang M. S., Kim B. Y., Yung M. J., Kim S. J., Joh Y. J., Lee Y. H., Bong N. R., Shin C.2008. An outbreak of botulism in wild ducks in the Tancheon. In Proceedings of the 2008 KSVS Conference and General Meeting, Korean Journal of Veterinary Science. Korean Society of Veterinary Science, Gwangju, Korea, September 25–26, p. 281.
- 8.Lindberg A., Skarin H., Knutsson R., Blomqvist G., Båverud V.2010. Real-time PCR for Clostridium botulinum type C neurotoxin (BoNTC) gene, also covering a chimeric C/D sequence--application on outbreaks of botulism in poultry. Vet. Microbiol. 146: 118–123. doi: 10.1016/j.vetmic.2010.04.030 [DOI] [PubMed] [Google Scholar]
- 9.Mitchell W. R., Rosendal S.1987. Type C botulism: The agent, host susceptibility, and predisposing factors. pp. 55–71. In: Avian Botulism: an International Perspective (Eklund, W. and Dowell, V. R. Jr. eds.), Charles C. Thomas, Springfield. [Google Scholar]
- 10.Rocke T. E., Bollinger T. K.2007. Avian botulism. In: Infectious diseases of Wild Birds (Nancy, J., Thomas, D., Hunter, B. and Atkinson, C. T. eds.), Blackwell Publishng, Ames. [Google Scholar]
- 11.Shayegani M., Stone W. B., Hannett G. E.1984. An outbreak of botulism in waterfowl and fly larvae in New York State. J. Wildl. Dis. 20: 86–89. doi: 10.7589/0090-3558-20.2.86 [DOI] [PubMed] [Google Scholar]
- 12.Shin N. R., Byun S. H., Chun J. H., Shin J. H., Kim Y. J., Kim J. H., Rhie G. E., Chung H. M., Mo I. P., Yoo C. K.2010. An outbreak of type C botulism in waterbirds: Incheon, Korea. J. Wildl. Dis. 46: 912–917. doi: 10.7589/0090-3558-46.3.912 [DOI] [PubMed] [Google Scholar]
- 13.Wlodarczyk R., Minias P., Kukier E., Grenda T., Smietanka K., Janiszewski T.2014. The first case of a major avian type C botulism outbreak in Poland. Avian Dis. 58: 488–490. doi: 10.1637/10669-091913-Case.1 [DOI] [PubMed] [Google Scholar]
- 14.Wobeser G.1997. Avian botulism—another perspective. J. Wildl. Dis. 33: 181–186. doi: 10.7589/0090-3558-33.2.181 [DOI] [PubMed] [Google Scholar]
- 15.Work T. M., Klavitter J. L., Reynolds M. H., Blehert D.2010. Avian botulism: a case study in translocated endangered Laysan Ducks (Anas laysanensis) on Midway Atoll. J. Wildl. Dis. 46: 499–506. doi: 10.7589/0090-3558-46.2.499 [DOI] [PubMed] [Google Scholar]


