Abstract
Cyanobacteria possess a CO2-concentating mechanism that
involves active CO2 uptake and HCO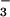 transport. For CO2 uptake, we have identified two systems
in the cyanobacterium Synechocystis sp. strain PCC 6803,
one induced at low CO2 and one constitutive. The low
CO2-induced system showed higher maximal activity and
higher affinity for CO2 than the constitutive system. On
the basis of speculation that separate NAD(P)H dehydrogenase complexes
were essential for each of these systems, we reasoned that inactivation
of one system would allow selection of mutants defective in the other.
Thus, mutants unable to grow at pH 7.0 in air were recovered after
transformation of a ΔndhD3 mutant with a
transposon-bearing library. Four of them had tags within
slr1302 (designated cupB), a homologue of
sll1734 (cupA), which is cotranscribed
with ndhF3 and ndhD3. The
ΔcupB, ΔndhD4, and
ΔndhF4 mutants showed CO2-uptake
characteristics of the low CO2induced system observed
in wild type. In contrast, mutants ΔcupA,
ΔndhD3, and ΔndhF3 showed
characteristics of the constitutive CO2-uptake system.
Double mutants impaired in one component of each of the systems were
unable to take up CO2 and required high CO2 for
growth. Phylogenetic analysis indicated that the
ndhD3/ndhD4-,
ndhF3/ndhF4-, and
cupA/cupB-type genes are present only
in cyanobacteria. Most of the cyanobacterial strains studied possess
the ndhD3/ndhD4-,
ndhF3/ndhF4-, and
cupA/cupB-type genes in pairs. Thus,
the two types of NAD(P)H dehydrogenase complexes essential for low
CO2-induced and constitutive CO2-uptake systems
associated with the NdhD3/NdhF3/CupA-homologues and
NdhD4/NdhF4/CupB-homologues, respectively, appear to be present in
these cyanobacterial strains but not in other organisms.
transport. For CO2 uptake, we have identified two systems
in the cyanobacterium Synechocystis sp. strain PCC 6803,
one induced at low CO2 and one constitutive. The low
CO2-induced system showed higher maximal activity and
higher affinity for CO2 than the constitutive system. On
the basis of speculation that separate NAD(P)H dehydrogenase complexes
were essential for each of these systems, we reasoned that inactivation
of one system would allow selection of mutants defective in the other.
Thus, mutants unable to grow at pH 7.0 in air were recovered after
transformation of a ΔndhD3 mutant with a
transposon-bearing library. Four of them had tags within
slr1302 (designated cupB), a homologue of
sll1734 (cupA), which is cotranscribed
with ndhF3 and ndhD3. The
ΔcupB, ΔndhD4, and
ΔndhF4 mutants showed CO2-uptake
characteristics of the low CO2induced system observed
in wild type. In contrast, mutants ΔcupA,
ΔndhD3, and ΔndhF3 showed
characteristics of the constitutive CO2-uptake system.
Double mutants impaired in one component of each of the systems were
unable to take up CO2 and required high CO2 for
growth. Phylogenetic analysis indicated that the
ndhD3/ndhD4-,
ndhF3/ndhF4-, and
cupA/cupB-type genes are present only
in cyanobacteria. Most of the cyanobacterial strains studied possess
the ndhD3/ndhD4-,
ndhF3/ndhF4-, and
cupA/cupB-type genes in pairs. Thus,
the two types of NAD(P)H dehydrogenase complexes essential for low
CO2-induced and constitutive CO2-uptake systems
associated with the NdhD3/NdhF3/CupA-homologues and
NdhD4/NdhF4/CupB-homologues, respectively, appear to be present in
these cyanobacterial strains but not in other organisms.
Keywords: NAD(P)H dehydrogenase, constitutive CO2 uptake, affinity to CO2, CO2-concentrating mechanism
In cyanobacteria, NAD(P)H dehydrogenase (NDH-1) is essential for both CO2 uptake (1–3) and photosystem-1 (PSI) cyclic electron transport (4). It has been postulated that uptake of CO2 is energized by NDH-1-dependent PSI-cyclic electron transport (1). However, observations that mutants defective in ndhD3 display normal cyclic electron transport but are unable to induce high-affinity CO2 uptake suggest the presence of multiple NDH-1 complexes (5–7). Two types of functionally distinct NDH-1 complexes were recently recognized in Synechocystis sp. strain PCC 6803 with the aid of mutants impaired in one or more subunits of NDH-1 (7). One complex, containing NdhD1 or NdhD2, plays a major role in PSI-cyclic electron flow but is not involved in CO2 uptake (7). When the second type of NDH-1 complex is inactivated (in the double mutant ΔndhD3/ΔndhD4), nearly normal PSI-cyclic electron flow is observed, but the mutant does not take up CO2 and is unable to grow under an air level of CO2 (7). The single mutants ΔndhD3 and ΔndhD4, on the other hand, possess CO2-uptake activity and can grow under low CO2 conditions (7). These results raised the possibility of multiple systems for CO2 uptake. In this report, we bring evidence for the presence of two CO2-uptake systems, one constitutive and one inducible, in Synechocystis sp. PCC 6803, and further identify two genes, sll1734 and slr1302 (designated cupA and cupB for their involvement in CO2 uptake) as essential components of the inducible and constitutive systems, respectively.
To assess the presence of homologous genes encoding constitutive and inducible CO2 uptake systems in other organisms, we used databases available in web sites (ref. 8; http://www.kazusa.or.jp/cyano/; http://www.jgi.doe.gov/tempweb/JGI_ microbial/html/index.html). We also made use of the genome sequence of two cyanobacterial strains, Thermosynechococcus elongatus and Gloeobacter violaceus PCC 7421, recently completed at Kazusa DNA Research Institute. These sequences enabled us to construct phylogenetic trees for genes essential to CO2 uptake in Synechocystis sp. PCC 6803. We show that most of the cyanobacterial strains investigated possess sets of genes encoding components of NDH-1 complexes involved in both the constitutive and the inducible CO2-uptake systems, but that these genes apparently are missing in green algae.
Materials and Methods
Growth Conditions.
Wild-type (WT) and mutant cells of Synechocystis sp. strain PCC 6803 (hereafter Synechocystis 6803) were grown at 30°C in BG11 medium (9), buffered at pH 8.0, and bubbled with either 3% (vol/vol) CO2 in air or air alone, as described (6). Solid medium was BG11 buffered at pH 7.0, supplemented with 1.5% agar and 5 mM sodium thiosulfate. Continuous illumination was provided by fluorescent lamps at 50 μmol photons m−2⋅s−1.
Construction and Isolation of Mutants.
Construction of the ΔndhD3, ΔndhD4, ΔndhF3, ΔndhF4, and ΔcupA mutants has been described in a previous paper (6) and/or deposited in the web site “CyanoMutants” (http://www.kazusa.or.jp/cyano/ mutants/). The constructs used to generate the single mutants were also used to transform appropriate mutants of Synechocystis 6803 to introduce multiple mutations. The sll1732 (designated ndhF3), sll1733 (ndhD3), and sll1734 (cupA) genes are expressed as an operon, and the mutants constructed by inactivating any of these genes grew much more slowly than the WT cells under 50 ppm CO2 (5, 6). This suggested that these genes are essential to the induced high-affinity CO2-uptake system. If so, isolation and analysis of high CO2-requiring mutants after inactivation of ΔndhD3 mutant also should enable us to identify the genes involved in the constitutive low-affinity CO2-uptake system.
By using a Genomic Priming System (New England Biolabs), a transposon containing a gene that confers chloramphenicol resistance (CmR) was randomly inserted into the DNA in each insert of 110 cosmids, which contained DNA fragments of Synechocystis 6803 previously used for genome sequencing (8). The ΔndhD3 strain of Synechocystis 6803 was transformed with this transposon inactivation library, and CmR mutants unable to grow at pH 7.0 in air were isolated. Genomic DNA isolated from each mutant was digested with HhaI and, after self ligation, was used as a template for inverse PCR with primers complementary to the N- and C-terminal regions of the CmR cassette. The exact position of the cassette in the mutant genome was determined by sequencing the PCR product.
CO2 Exchange Measurements.
Cells grown under 3% (vol/vol) CO2 in air (H cells) or acclimated to air for 18 h in the light (L cells) were harvested by centrifugation, resuspended in 25 ml of 20 mM N-Tris(hydroxymethyl)methyl-2-amino-ethanesulfonic acid (TES)-KOH buffer, pH 7.0, containing 15 mM NaCl to a cell density corresponding to 4.3 μg chlorophyll ml−1 and placed in a reaction vessel (10). CO2 exchange of the cell suspension was measured at 30°C by using an open infrared gas-analysis system that records the rate of CO2 exchange as a function of time. N2, O2, and CO2 were mixed (using a standard gas generator model SGGU-712, STECH, Tokyo) to generate N2 gas containing 20% O2 in combination with various concentrations of CO2. The mixed gas was provided to the reaction vessel at a flow rate of 1.0 liter/min. The gas leaving the chamber was dried and its CO2 concentration analyzed by using an infrared CO2 analyzer (model URA-106, Shimadzu). The CO2 concentration in the medium surrounding the cells was calculated from its concentration in the gas produced by the standard gas generator, assuming that CO2 in the gas is in equilibrium with CO2 in the medium.
Determination of Growth Characteristics.
WT and mutant strains grown under 3% CO2 were collected and resuspended in fresh BG11 medium. Cell suspensions (2 μl) were spotted onto BG11 agar plates buffered at pH 7.0, which then were incubated under air for 5 days with continuous illumination by fluorescent lamps at 50 μmol photons m−2⋅s−1. The OD730 nm was measured by using a recording spectrophotometer, model V-550 (Jasco, Tokyo).
Results
Genes Encoding Components Involved in Two CO2-Uptake Systems.
Our earlier results showed that the ΔndhD3/ΔndhD4 double mutant could not take up CO2 and required high CO2 for growth (7). The single mutants, on the other hand, could grow under low CO2 (6). These observations suggested that there are functionally distinct NDH-1 complexes and that at least one, containing either NdhD3 or NdhD4, must be functioning for CO2 uptake in Synechocystis 6803 to proceed. To clarify the role of these NDH-1 complexes and identify other components that are essential for CO2 uptake and possibly associated with NDH-1, we transformed mutant ΔndhD3 with a transposon-bearing library, tagging and inactivating many genes. Thirteen new mutants unable to grow under an air level of CO2, at pH 7.0, were isolated. Table 1 summarizes the genes inactivated in these mutants and the positions and directions of the CmR tag. Two of these mutants had interruptions in slr1347, a homologue of icfA in Synechococcus sp. strain PCC 7942, which encodes a carboxysome-localized carbonic anhydrase essential for the growth of cells in an air level of CO2 (11), and four mutants had interruptions in the genes encoding hypothetical proteins. NB-33 had the tag in ndhE, which is present as a single copy in Synechocystis 6803 and encodes a component essential to all types of NDH-1 complexes. Four of the mutants, NB29, 30, 31, and 32, contained the tag at various sites within slr1302 (cupB, Fig. 1A). The putative protein encoded by cupB showed significant amino acid sequence similarity to that encoded by sll1734 (cupA), which is cotranscribed with sll1732 (ndhF3) and sll1733 (ndhD3) (6). No high-CO2-requiring mutants bore the CmR tag within slr1301 or slr1303. These results suggested that the inability of the ΔcupB/ΔndhD3 mutants to grow at pH 7.0 in air (Fig. 1B) was not because of a pleiotropic effect. In the experiments described here (Fig. 1B), we used NB-29 as the ΔcupB/ΔndhD3 mutant. Inactivation of cupB or sll0026 (ndhF4) in the WT (not shown) or in the ΔndhD4 mutant scarcely affected their growth under an air level of CO2 (Fig. 1B). The transcript originating from sll1732–4 is hardly detectable in cells grown under high CO2 conditions but accumulates many-fold during acclimation to low CO2 (12, 13). The results of present (Fig. 1B) and earlier (7) growth experiments lend support to the hypothesis that two CO2-uptake systems occur in Synechocystis 6803. One is induced under low CO2 and involves NdhF3, NdhD3, and CupA, and the other is constitutive and involves NdhF4, NdhD4, and CupB. That the double mutants ΔndhF4/ΔndhD3, ΔcupB/ΔndhD3, and ΔndhD4/ΔndhD3 could not grow at pH 7.0 in air (Fig. 1B; figure 1 in ref. 7) could, among other possibilities, stem from inactivation of both the inducible and the constitutive CO2 uptake systems. These possibilities were further examined by following the CO2 exchange characteristics of the mutants (Figs. 2 and 3).
Table 1.
Mutants unable to grow at pH 7.0 in air isolated after transformation of a ΔndhD3 mutant with a transposon-bearing library
| Mutant names | Tagged genes | Positions interrupted* | Directions† | Gene products‡ |
|---|---|---|---|---|
| NB-29 | slr1302 | 305854 | F | CupB |
| NB-30 | slr1302 | 306595 | R | CupB |
| NB-31 | slr1302 | 306299 | R | CupB |
| NB-32 | slr1302 | 306580 | F | CupB |
| NB-33 | sll0522 | 3267274 | F | NdhE |
| NB-34 | slr1347 | 1742934 | R | CA |
| NB-35 | slr1347 | 1743132 | F | CA |
| NB-38 | sll0247 | 1518083 | R | LHC |
| NB-39 | slr0364 | 2354056 | F | H.P. |
| NB-41 | slr0684 | 429962 | F | H.P. |
| NB-42 | sll1488 | 3379184 | F | H.P. |
| NB-44 | slr0687 | 431822 | F | PleD |
| NB-45 | slr1521 | 1613061 | R | H.P. |
Numbers represent those of nucleotide sequences in the Cyanobase (http://www.kazusa.or.jp/cyano/).
F, forward; R, reverse.
CA, carboxysome-localized CA; LHC, light-harvesting chlorophyll induced under iron stress conditions; H.P., hypothetical protein.
Figure 1.
(A) A schematic map of the s1r1302(cupB) region and the position and direction of the CmR cassette tags interrupting the genes and (B) growth of WT and mutants on agar plates at pH 7.0 in air. (A) The CmR cassette was inserted at 259, 704, 985, and 1,000 bp downstream of the initiation codon of cupB in NB-29, 31, 30, and 32, respectively. The horizontal arrows indicate the direction of the cassettes. (B) Two microliters of the cell suspensions with the OD730 nm values of 0.1 (Top), 0. 01 (Middle), and 0.001 (Bottom) were spotted on agar plates containing BG11 buffered at pH 7.0 and grown in an air level of CO2.
Figure 2.
CO2 exchange profiles of the WT and various mutants of Synechocystis 6803 on switching on the light. The mutants were grown at 3% CO2 (H) or acclimated to air overnight (L). An open gas-exchange system capable of recording the rate of CO2 exchange as a function of time was used for this analysis (10). The mixed gas containing 100 μl CO2/liter, and 20% O2 was provided to the reaction vessel. Light intensity was 800 μmol photons m−2⋅s−1. Cells were suspended in 25 ml of 20 mM Tes-KOH buffer, pH 7.0, containing 15 mM NaCl, to final concentration corresponding to 4.2 μg chlorophyll/ml.
Figure 3.
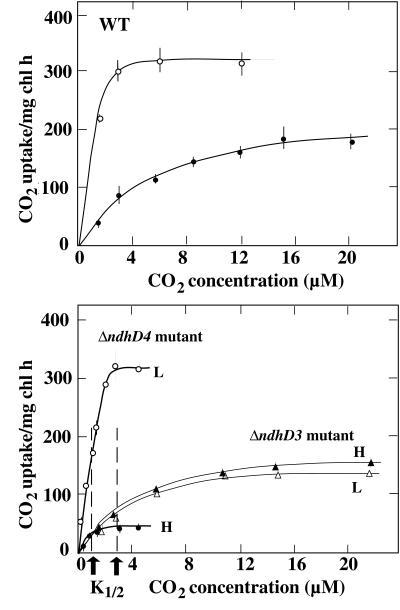
The rate of CO2 uptake by H cells (closed symbols) and L cells (open symbols) of the WT (circles, Upper) and mutant strains of Synechocystis PCC 6803, ΔndhD3 (triangles) and ΔndhD4 (circles, Lower), as a function of CO2 concentrations in the medium. The CO2 concentration in the medium in equilibrium with the gas containing 300 μl CO2/liter was taken as 9.2 μM at 30°C. Other conditions were as in Fig. 2.
Constitutive and Inducible CO2-Uptake Systems Operate in Synechocystis 6803.
Fig. 2 shows the CO2 exchange profiles (measured
in an open gas-exchange system; ref. 10) of cells grown under high
CO2 (H) or acclimated to low
CO2 (Fig. 2, L). On illumination of the cell
suspensions, the rate of CO2 uptake (Fig. 2,
shown by V on curve b) increased and reached a maximum in ≈20 s. The
V exhibited by L cells was much greater than that for H cells in the
case of the WT (Fig. 2, a and b), the ΔndhD4 mutant (Fig.
2, c and d, in which only NdhD3 is present), and the ΔcupB
mutant (Fig. 2, g and h). In contrast, no appreciable difference was
observed between the rates of CO2 uptake in H and
L cells of mutants ΔndhD3 (Fig. 2, e and f) and
ΔcupA (Fig. 2, i and j). Confirming our previous
observation (7), the double mutant
ΔndhD3/ΔndhD4 was unable to take up
CO2 (V virtually zero; Fig. 2, k) after
acclimation to low CO2 conditions This mutant
exhibited high rates of HCO uptake (T.O.,
unpublished work), confirming that the V value represents the rate of
CO2 uptake and is little affected by
HCO
uptake (T.O.,
unpublished work), confirming that the V value represents the rate of
CO2 uptake and is little affected by
HCO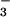 uptake. The double mutants,
ΔcupB/ΔndhD3 (l) and
ΔndhF4/ΔndhD3 (Fig. 2, m) were unable
to take up CO2. The single mutants
ΔcupA and ΔcupB showed
CO2-exchange characteristics similar to those of
ΔnhD3 and ΔndhD4, respectively (Fig. 2,
g–j). The V value for H cells of mutants ΔndhD4 and
ΔcupB was considerably lower than that for WT. Taken
together, the data support the suggestion that two
CO2-uptake systems operate in
Synechocystis 6803. One is constitutive in H cells (impaired
in mutants ΔndhD4 and ΔcupB), and the other
is induced by exposing the cells to low CO2
conditions (impaired in mutants ΔndhD3 and
ΔcupA). The double mutants
ΔndhD3/ΔndhD4,
ΔcupB/ΔndhD3, and
ΔndhF4/ΔndhD3 are apparently deprived
of both the constitutive and the induced CO2
uptake systems and thus are unable to take up
CO2.
uptake. The double mutants,
ΔcupB/ΔndhD3 (l) and
ΔndhF4/ΔndhD3 (Fig. 2, m) were unable
to take up CO2. The single mutants
ΔcupA and ΔcupB showed
CO2-exchange characteristics similar to those of
ΔnhD3 and ΔndhD4, respectively (Fig. 2,
g–j). The V value for H cells of mutants ΔndhD4 and
ΔcupB was considerably lower than that for WT. Taken
together, the data support the suggestion that two
CO2-uptake systems operate in
Synechocystis 6803. One is constitutive in H cells (impaired
in mutants ΔndhD4 and ΔcupB), and the other
is induced by exposing the cells to low CO2
conditions (impaired in mutants ΔndhD3 and
ΔcupA). The double mutants
ΔndhD3/ΔndhD4,
ΔcupB/ΔndhD3, and
ΔndhF4/ΔndhD3 are apparently deprived
of both the constitutive and the induced CO2
uptake systems and thus are unable to take up
CO2.
Rates of CO2 uptake (V) are plotted as a function of CO2 concentrations in Fig. 3. During acclimation of high-CO2-grown WT cells to low CO2 conditions, the maximal rate of CO2 uptake increased substantially (from 190 to 320 μmol/mg chlorophyll h), and the K1/2 (CO2) decreased from 3.3 to 1.2 μM. H cells of ΔndhD4 (Fig. 3 Lower) and ΔcupB (not shown) mutants exhibited a much lower capacity for CO2 uptake than H cells of the WT (Fig. 3 Upper). On the other hand, when grown under low CO2, the ΔndhD4 and ΔcupB mutants exhibited CO2 uptake characteristics similar to WT. Unlike the case of mutants ΔndhD4 and ΔcupB, there was no significant difference between the maximal rates and the K1/2 (CO2) values observed in H and L cells of mutants ΔndhD3 (Fig. 3 Lower) and ΔcupA (not shown). The K1/2 (CO2) and maximal rates of CO2 uptake in both H and L cells of mutants ΔndhD3 and ΔcupA were similar to those of WT H cells, but the maximal rates were significantly higher than those of H cells of ΔndhD4 and ΔcupB. The K1/2 (CO2) values in ΔndhD4 and ΔndhD3 mutants were 0.9 and 2.8 μM, respectively, regardless of the CO2 concentration during growth.
Taken together, these data clearly support the hypothesis that there are two types of CO2 uptake systems in Synechocystis 6803: a constitutive, NdhD4-dependent system and a low-CO2-induced, NdhD3-dependent system. The inducible NdhD3-dependent system, exhibited a maximal rate of CO2 uptake 2-fold higher, and a K1/2 (CO2) values 3-fold lower, than the corresponding values for the constitutive, NdhD4-dependent system.
Phylogenetic Analysis.
The present and earlier (7) studies implicate four of the ndh genes, ndhD3, ndhD4, ndhF3, and ndhF4, as well as ΔcupA and ΔcupB, in CO2 uptake by Synechocystis 6803. It was interesting to examine whether other photosynthetic microorganisms rely on homologous genes for CO2 uptake. A phylogenetic tree for NdhD/NdhF (Fig. 4A) indicates that NdhD and NdhF proteins are both members of a larger family and may be related by an ancient gene duplication event. Three lineages were propagated from the ndhD line. One of them, which branched to the ndhD3- and ndhD4-types, is present only in cyanobacteria. An evolutionary relationship between cyanobacterial ndhD1/ndhD2-type and ndhD genes in chloroplast genomes is noted. On the basis of the relationship shown in Fig. 4A, the proteins designated as NdhD5 and NdhD6 according to previous papers (7, 14) should probably be designated NdhF. Prochlorococcus marinus possesses only the ndhD1-type gene, whereas Gloeobacter violaceus, a cyanobacterial strain that supposedly diverged at an early stage of evolution (15), possesses both the ndhD3- and ndhD4-type genes.
Figure 4.
Phylogenetic trees of NdhD/NdhF (A) and CupA/CupB (B). Multiple sequence alignments were performed by using clustal (26). Ana, Anabaena sp. PCC 7120; Glo, Gloeobacter violaceus PCC 7421; MSy, Marine Synechococcus sp. WH8502; Nos, Nostoc punctiforme; Pro, Prochlorococcus marinus MED4; Syn, Synechocystis sp. PCC 6803; Tsy, Thermosynechococcus elongatus; Cmy, Chlamydomonas reinhardtii; Ara, Arabidopsis thaliana; Mar, Marchantia polymorpha; Tob, Nicotiana tabacum; Zea, Zea mays. D and F after the organism names in A indicate ndhD and ndhF. These genes were denoted as ndhD1 (slr0331 in Synechocystis 6803), ndhD2 (slr1291), ndhD3 (sll1733), ndhD4 (sll0027), ndhD5 (slr2007), ndhD6 (slr2009), ndhF1 (sll0844), ndhF3 (sll1732), and ndhF4 (sll0026) (7, 14). A and B after the organism names in B indicate CupA (sll1734 in Synechocystis 6803) and CupB (slr1302), respectively. C and M in parentheses indicate the genes in chloroplast and mitochondrial genomes, respectively.
The phylogenetic tree shows evolutionary lineages for ndhF1, ndhF3, and ndhF4 propagated from the ndhF line (Fig. 4A). Whereas the ndhF1 is related to the chloroplast ndhF, the ndhF3/ndhF4-type genes are present only in cyanobacteria. The cupA/cupB-type genes are also confined to the cyanobacteria (Fig. 4B). All of the cyanobacterial strains studied except the marine Synechococcus possess both ndhF3 and ndhF4 genes as well as cupA- and cupB-type genes. The ndhD3/ndhF3/cupA-type genes were not found in the marine Synechococcus. However, because the genome sequencing of this strain has not yet been completed, it is too early to conclude the absence of these genes. It appears that the presence of two types of NDH-1 complexes is common in cyanobacteria.
Discussion
Data presented here show that two NDH-1-dependent CO2-uptake systems operate in Synechocystis 6803. One of them, involving NdhD3, NdhF3, and CupA, is induced during acclimation to low CO2 conditions. The other system, involving NdhD4, NdhF4 and CupB, exhibits a 3-fold lower affinity for CO2, and its maximal activity (approximately one-half that of the induced system) is not significantly affected by acclimation of the cells to low CO2 (Fig. 3). The conclusion that two separate NDH-1-dependent systems are involved is based on the results shown in Figs. 1–3, in which the growth and CO2 uptake characteristics of single and double mutants with lesions in the above components are presented. Mutants impaired in a component of the induced system, either NdhD3, NdhF3, or CupA, exhibited CO2 uptake characteristics similar to those observed in H cells of the WT regardless of the CO2 concentration during growth. L cells of mutants in which a component of the constitutive system was inactivated were able to take up CO2 like L cells of the WT, but uptake was severely depressed under high CO2. Double mutants, in which the components of the induced and the constitutive systems were inactivated, were unable to take up CO2 and required high CO2 for growth.
The inability of a mutant to increase CO2 uptake after exposure to low CO2 might stem either from a defect in the sensing/signal transduction process or from lack of an essential component of the induced CO2 uptake system. As an example, the direct involvement of NdhD3 in CO2 uptake indicates that ΔndhD3 mutants of Synechococcus PCC 7002 (5) and Synechocystis 6803 (6) are deprived of a component of the specific CO2 uptake system that operates under low CO2 conditions rather than being unable to acclimate to low CO2.
Inhibition of CO2 uptake by an aquaporin
blocker suggested that CO2 enters the cells
passively (16) rather than by active transport (17). Intracellular
conversion of the entering CO2 to
HCO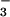 , the inorganic carbon species that accumulates
in the cells, is mediated by a carbonic anhydrase-like activity
(18–21). The direct energy source for the energy-requiring energy
HCO
, the inorganic carbon species that accumulates
in the cells, is mediated by a carbonic anhydrase-like activity
(18–21). The direct energy source for the energy-requiring energy
HCO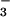 formation and release to the cytoplasm is
photosynthetically generated ΔμH+ rather than
ATP hydrolysis (16). The observation that NDH-1 components essential
for CO2 uptake (ref. 6 and Figs. 1–3) are
located on the thylakoid (22) provides strong evidence that
conversion of CO2 to bicarbonate at the
thylakoid provides the driving force for inward diffusion of
CO2 across the cytoplasmic membrane.
formation and release to the cytoplasm is
photosynthetically generated ΔμH+ rather than
ATP hydrolysis (16). The observation that NDH-1 components essential
for CO2 uptake (ref. 6 and Figs. 1–3) are
located on the thylakoid (22) provides strong evidence that
conversion of CO2 to bicarbonate at the
thylakoid provides the driving force for inward diffusion of
CO2 across the cytoplasmic membrane.
Participation of NdhD3/NdhD4-type NDH-1 complexes in the respiratory and cyclic PSI electron transfer (from NADPH to plastoquinone pool) appears to be small, because these processes were scarcely impaired in the ΔndhD3/ΔndhD4 mutants (7), even though CO2 uptake was depressed. In contrast, NdhD1/NdhD2 types of NDH-1 are essential for cyclic PSI electron transport, but their absence hardly affected CO2 uptake (7). It was earlier suggested that cyclic PSI electron transport is essential for CO2 uptake. To reconcile the data from mutants with this suggestion, we proposed (16) that multiple PSI types or alternative routes of cyclic electron transport (23) operate in Synechocystis 6803. The present conclusion that NdhD3 and NdhD4 actually belong to two functionally distinct NDH-I complexes, induced and constitutive, respectively, supports the suggestion of multiple routes for electron transport.
The mechanism for CO2 uptake and the role
of the NDH-1 complexes in this process are not fully understood. A
recent model (18) proposed that CO2 uptake by
cyanobacteria and its intracellular conversion to
HCO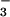 is energized by photosynthetic electron
transport via the formation of alkaline domains on the stromal face of
the thylakoid membrane. The formation of these putative domains would
enable the vectoral conversion of CO2 to
HCO
is energized by photosynthetic electron
transport via the formation of alkaline domains on the stromal face of
the thylakoid membrane. The formation of these putative domains would
enable the vectoral conversion of CO2 to
HCO , which accumulates in the cytoplasm (17, 18).
The carbonic anhydrase-like moiety essential for accelerating the
conversion of CO2 to HCO
, which accumulates in the cytoplasm (17, 18).
The carbonic anhydrase-like moiety essential for accelerating the
conversion of CO2 to HCO has
not been identified, and the roles of the components of the induced and
constitutive CO2 uptake systems in the route of
electron transport have not yet been clarified. The presence of two
types of CO2 uptake systems suggests the
involvement of two proteins (or protein complexes) that bind
CO2 followed by hydration and release of
HCO
has
not been identified, and the roles of the components of the induced and
constitutive CO2 uptake systems in the route of
electron transport have not yet been clarified. The presence of two
types of CO2 uptake systems suggests the
involvement of two proteins (or protein complexes) that bind
CO2 followed by hydration and release of
HCO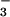 to the cytoplasm. Hydrophobicity analyses (not
shown) indicated that NdhD3, NdhD4, NdhF3, and NdhF4 are most likely
embedded in the membrane, whereas CupA and CupB are not. Because CupA
and CupB are functionally associated with NDH-1 complexes involved in
CO2 uptake, they may have a role in the
conversion of CO2 to HCO
to the cytoplasm. Hydrophobicity analyses (not
shown) indicated that NdhD3, NdhD4, NdhF3, and NdhF4 are most likely
embedded in the membrane, whereas CupA and CupB are not. Because CupA
and CupB are functionally associated with NDH-1 complexes involved in
CO2 uptake, they may have a role in the
conversion of CO2 to HCO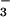 .
However, neither CupA nor CupB shows significant homology to any of the
presently known carbonic anhydrases.
.
However, neither CupA nor CupB shows significant homology to any of the
presently known carbonic anhydrases.
Recent studies (24) indicated significant oxidation of NADPH in
cyanobacteria even when CO2 fixation is
completely inhibited, under which conditions massive Ci cycling in the
form of CO2 uptake and HCO release still persists (16). These data raised the possibility that the
reduction of NADP+ to NADPH may serve as the
direct source of OH− for the conversion of
CO2 to HCO
release still persists (16). These data raised the possibility that the
reduction of NADP+ to NADPH may serve as the
direct source of OH− for the conversion of
CO2 to HCO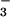 . This possibility
is being examined.
. This possibility
is being examined.
All of the cyanobacterial strains studied except the marine Synechochoccus possess genes encoding components associated with the two types of NDH-1 complexes involved in the induced and constitutive CO2 uptake systems (Fig. 4), suggesting that their presence is common in cyanobacteria. Gloeobacter possesses two copies of ndhD4- and cupB-type genes, suggesting that this strain has two constitutive CO2 uptake systems. Although Prochlorococcus did not have any genes related to the CO2 uptake systems, we are unable to conclude the absence of such genes in Prochlorococcus at present because the genome sequencing of this strain has not been completed. Green algae also possess mechanisms to concentrate CO2 (25). However, the absence of genes homologous to ndhD3(F3), ndhD4(F4), and cupA(B) in algae indicates that different mechanism(s) may be involved in CO2 uptake by algae.
Acknowledgments
We thank Ms. Natsu Hagino, Ms. Akiko Tachibana, and Mr. Yasunori Matsui for technical assistance. This study was supported by a Grant-in-Aid for Scientific Research (B) (2)(12440228) and a grant from the Human Frontier Science Program (RG0051/1997 M) to T.O., a Grant-in-Aid from for Scientific Research (No. 12660300) to H.F., a grant for “Research for the Future” Program (JSPS-RFTF97R16001) to T.O. and H.F., and grants from the Israeli Ministry of Science and Technology (MOST), the U.S.A.–Israel Binational Science Foundation, and Program MARS2, a cooperation between MOST and the German Ministerium für Bildung, Wissenschaft, Forschung, und Technologie (BMBF) to A.K.
Abbreviations
- H cells
cells grown under 3% (vol/vol) CO2 in air
- L cells
cells acclimated to air for 18 h in the light
- NDH-1
NAD(P)H dehydrogenase
- WT
wild type
- PSI
photosystem-1
- CmR
chloramphenicol resistance
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Ogawa T. Proc Natl Acad Sci USA. 1991;88:4275–4279. doi: 10.1073/pnas.88.10.4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogawa T. Plant Physiol. 1991;96:280–284. doi: 10.1104/pp.96.1.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marco M, Ohad N, Schwarz R, Lieman-Hurwitz J, Gabay C, Kaplan A. Plant Physiol. 1993;101:1047–1053. doi: 10.1104/pp.101.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mi H, Endo T, Schreiber U, Ogawa T, Asada K. Plant Cell Physiol. 1992;33:1233–1238. [Google Scholar]
- 5.Klughammer B, Sültemeyer D, Badger M R, Price G D. Mol Microbiol. 1999;32:1305–1315. doi: 10.1046/j.1365-2958.1999.01457.x. [DOI] [PubMed] [Google Scholar]
- 6.Ohkawa H, Price G D, Badger M R, Ogawa T. J Bacteriol. 2000;182:2591–2596. doi: 10.1128/jb.182.9.2591-2596.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohkawa H, Pakrasi H B, Ogawa T. J Biol Chem. 2000;275:31630–31634. doi: 10.1074/jbc.M003706200. [DOI] [PubMed] [Google Scholar]
- 8.Kaneko T, Sato S, Kotani H, Tanaka A, Asamizu E, Nakamura Y, Miyajima N, Hirosawa M, Sugiura M, Sasamoto S, et al. DNA Res. 1996;3:109–136. doi: 10.1093/dnares/3.3.109. [DOI] [PubMed] [Google Scholar]
- 9.Stanier R Y, Kunisawa R, Mandel M, Cohen-Bazire G. Bacterial Rev. 1971;35:171–205. doi: 10.1128/br.35.2.171-205.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ogawa T, Miyano A, Inoue Y. Biochim Biophys Acta. 1985;808:77–84. [Google Scholar]
- 11.Fukuzawa H, Suzuki E, Komukai Y, Miyachi S. Proc Natl Acad Sci USA. 1992;89:4437–4441. doi: 10.1073/pnas.89.10.4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohkawa H, Sonoda M, Katoh H, Ogawa T. Can J Bot. 1998;76:1035–1042. [Google Scholar]
- 13.Figge R M, Cassier-Chauvat C, Chauvat F, Cerff R. Mol Microbiol. 2001;39:455–469. doi: 10.1046/j.1365-2958.2001.02239.x. [DOI] [PubMed] [Google Scholar]
- 14.Price G D, Sültemeyer D, Klughammer B, Badger M R. Can J Bot. 1998;76:973–1002. [Google Scholar]
- 15.Honda D, Yokota A, Sugiyama J. J Mol Evol. 1999;48:723–739. doi: 10.1007/pl00006517. [DOI] [PubMed] [Google Scholar]
- 16.Tchernov D, Helman Y, Keren N, Luz B, Ohad I, Reinhold L, Ogawa T, Kaplan A. J Biol Chem. 2001;276:23450–23455. doi: 10.1074/jbc.M101973200. [DOI] [PubMed] [Google Scholar]
- 17.Miller A G, Espie G E, Canvin D T. Can J Bot. 1991;69:925–935. [Google Scholar]
- 18.Kaplan A, Reinhold L. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:539–570. doi: 10.1146/annurev.arplant.50.1.539. [DOI] [PubMed] [Google Scholar]
- 19.Volokita M, Zenvirth D, Kaplan A, Reinhold L. Plant Physiol. 1984;76:599–602. doi: 10.1104/pp.76.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abe T, Tsuzuki M, Kaplan A, Miyachi S. Plant Cell Physiol. 1987;28:671–677. [Google Scholar]
- 21.Price G D, Badger M R. Plant Physiol. 1989;91:505–513. doi: 10.1104/pp.91.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohkawa H, Sonoda M, Shibata M, Ogawa T. J Bacteriol. 2001;183:4938–4939. doi: 10.1128/JB.183.16.4938-4939.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeanjean R, Bedu S, Havaux M, Matthijs H C P, Joset F. FEMS Microbiol Lett. 1998;167:131–137. doi: 10.1111/j.1574-6968.2002.tb11294.x. [DOI] [PubMed] [Google Scholar]
- 24.Mi H, Klughammer C, Schreiber U. Plant Cell Physiol. 2000;41:1129–1135. doi: 10.1093/pcp/pcd038. [DOI] [PubMed] [Google Scholar]
- 25.Badger M R, Spalding M H. In: Advances in Photosynthesis. Leegood R C, Sharkey T D, von Caemmerer S, editors. Vol. 9. Dordrecht, The Netherlands: Kluwer; 2000. pp. 399–434. [Google Scholar]
- 26.Higgins D G, Thompson J D, Dibson T J. Methods Enzymol. 1996;266:383–402. doi: 10.1016/s0076-6879(96)66024-8. [DOI] [PubMed] [Google Scholar]