Abstract
Purpose
Peroxisome proliferator-activated receptor γ (PPARγ) is involved in the pathology of numerous diseases including atherosclerosis, diabetes, obesity, and cancer. Matrix metalloproteinases (MMPs) play a significant role in tissue remodeling related to various processes such as morphogenesis, angiogenesis, tissue repair, invasion, and metastasis. We investigated the effects of PPARγ on MMP expression and invasion in breast cancer cells.
Methods
MCF-7 cells were cultured and then cell viability was monitored in an MTT assay. Western blotting, gelatin zymography, real-time polymerase chain reaction, and luciferase assays were performed to investigate the effect of the synthetic PPARγ ligand troglitazone on MMP expression. Transcription factor DNA binding was analyzed by electrophoretic mobility shift assay. A Matrigel invasion assay was used to assess the effects of troglitazone on MCF-7 cells.
Results
Troglitazone did not affect MCF-7 cell viability. 12-O-tetradecanoylphorbol-13-acetate (TPA) induced MMP-9 expression and invasion in MCF-7 cell. However, these effects were decreased by troglitazone. TPA increased nuclear factor κB and activator protein-1 DNA binding, while troglitazone inhibited these effects. The selective PPARγ antagonist GW9662 reversed MMP-9 inhibition by troglitazone in TPA-treated MCF-7 cells.
Conclusion
Troglitazone inhibited nuclear factor κB and activator protein-1-mediated MMP-9 expression and invasion of MCF-7 cells through a PPARγ-dependent mechanism.
Keywords: Matrix metalloproteinases, MCF-7 cells, NF-kappa B, PPAR gamma, Transcription factor AP-1
INTRODUCTION
Breast cancer is the most common malignant tumor in women worldwide, and its incidence has increased tremendously in the past several decades [1]. Despite earlier diagnosis and advances in treatment, breast cancer is a heterogeneous disease with a nearly 30% mortality rate because of disease progression. Cancer invasion and metastasis are pathol-ogical hallmarks of breast cancer and indicate poor prognosis [2].
Matrix metalloproteinases (MMPs) are key enzymes for invasion and metastasis, which are the major causes of mortality in breast cancer patients [3]. Among them, MMP-9 expression is linked with increased metastasis in various tumors, including the brain, prostate, and breast [4]. 12-O-tetradecanoylphorbol-13-acetate (TPA) induces MMP expression by activating transcription factors such as nuclear factor κB (NF-κB) and activator protein-1 (AP-1) [5]. Induction of MMP-9 is critical for TPA-induced breast tumor cell migration and invasion [6]. Thus, controlling MMP-9 in tumors is considered a promising strategy for inhibiting metastasis and improving the survival of breast cancer patients.
Peroxisome proliferator-activated receptors (PPARs) are nuclear receptors that function as transcription factors to regulate the expression of genes involved in lipid oxidation, transport, and storage [7]. Among the three PPAR isoforms (α, β, and γ), PPARγ forms heterodimer with the retinoid X receptor and optimal activation of the heterodimer requires both ligands [8]. The natural ligands of PPARγ are hydroxyoctadecanoic acids and the cyclopentane prostaglandin 15-deoxy-Δ-12, 14 prostaglandin J2; synthetic ligands include troglitazone, rosiglitazone, and ciglitazone [9].
Because abnormal energy metabolism and biological disorder are characteristics of tumors, metabolic control may be effective for cancer management [10]. Normal and cancer cells show large differences in energy metabolism [11]. In addition, increased aerobic glycolysis and elevated oxidative stress are important biochemical features of cancer cells according to the Warburg hypothesis [12]. Although sex and age are key risk factors for breast cancer, a high-fat diet and obesity are also associated with its development [13]. Obesity and associated adipocyte pathology were recently suggested to occur because of downregulation of PPARγ activity [9]. These data as well as the association of obesity with increased breast cancer risk suggest a role for PPARγ activators in the prevention or treatment of breast cancer. We tested a PPARγ activator as a new therapeutic agent for breast cancer. In this study, the effects of troglitazone on MMP-9 expression and invasion in MCF-7 cells were evaluated.
METHODS
Cell culture and reagents
Human breast cancer MCF-7 cell lines were obtained from the American Type Culture Collection (Manassas, USA). Cells were grown in high-glucose Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% antibiotics (10,000 U/mL penicillin and 10,000 µg/mL streptomycin) at 37℃ in a 5% CO2 incubator. Troglitazone was purchased from ALEXIS Biochemicals (Lausen, Switzerland) and TPA, 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-tetrazolium bromide assay (MTT), and β-actin antibody were obtained from Sigma-Aldrich (St. Louis, USA). Phosphorylated (p)-c-Jun, nuclear factor of κ light polypeptide gene enhancer in B cells inhibitor (p-IκB) α, IκB kinase (p-IKK) α/β, c-Jun N-terminal kinases (JNK), p-JNK, extracellular signal-regulated kinases (ERK), p-ERK, and p-protein kinase B (AKT) antibodies were purchased from Cell Signaling Technology (Danvers, USA). MMP-9, p50, p65, proliferating cell nuclear antigen, IκBα, AKT, and horseradish peroxidase (HRP)-conjugated IgG antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, USA). The mitogen-activated protein kinase (MAPK) inhibitors PD98059 (ERK inhibitor) and SP600125 (JNK inhibitor) and phosphoinositol-3-kinase (PI3K) inhibitors LY294002 and wortmannin were purchased from Merck Millipore (Billerica, USA). NF-κB and AP-1 consensus oligonucleotides were obtained from Promega (Madison, USA). [α-32P] deoxycytidine triphosphate (dCTP) was purchased from Amersham (Buckinghamshire, UK) and DMEM, FBS, and phosphate-buffered saline (PBS) were obtained from Gibco-BRL (Grand Island, USA).
MTT assay
Cell viability was monitored in an MTT assay. MCF-7 cells were seeded in a 96-well plate and incubated at 37℃ for 24 hours to allow attachment. The attached cells were either untreated or treated with different concentrations of troglitazone at 37℃. After inoculation for 24 hours, the cells were washed with PBS before the addition of MTT (0.5 mg/mL) and incubated for 30 minutes at 37℃. Dimethyl sulfoxide was added to dissolve the formazan crystals and absorbance at 570 nm was measured with a microplate reader (Bio-Rad, Hercules, USA).
Nuclear extraction
MCF-7 cells were treated with troglitazone in the presence or absence of TPA for 4 hours. Subsequently, the cells were washed and scraped into PBS (pH 7.5), and pelleted for 3 minutes. Cytoplasmic and nuclear extracts were obtained using NE-PER Nuclear and Cytoplasmic Extraction Reagents (Pierce Biotechnology, Rockford, USA).
Western blotting
After treatment with troglitazone, cells were incubated with TPA for 24 hours at 37℃. These cells were lysed with M-PER Mammalian Protein Extraction Reagent (Pierce Biotechnology). The cell lysates were loaded and resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and proteins were transferred to Hybond™-polyvinylidene fluoride membranes (GE Healthcare Life Sciences, Little Chalfont, UK). Membrane blocking was performed using 2% bovine serum albumin or 5% skim milk and followed by the incubation with primary antibody overnight at 4℃. HRP-conjugated IgG (1:2,000 dilution) was used as the secondary antibody for 1 hour at 4℃. A Fujifilm image analyzer (Tokyo, Japan) was used to determine protein levels. Immunoreactive signals were visualized using the western chemiluminescent HRP substrate (Sigma-Aldrich).
Zymography assay
Conditioned media were collected after 24 hours of treatment, mixed with non-reducing sample buffer, and separated by PAGE containing 0.1% gelatin. Gels were washed for 30 minutes at 18℃ to 23℃ (room temperature) with 2.5% Triton X-100 and then incubated for 16 hours in developing buffer at 37℃. Gels were stained with 0.25% Coomassie brilliant blue (40% methanol and 7% acetic acid) for 30 minutes and photographed using a Fujifilm image analyzer. For densitometric analysis, we used Multi Gauge Image Analysis software (Fujifilm).
Real-time polymerase chain reaction
Total RNA was isolated from cells with TRIzol reagent (Takara, Shiga, Japan) according to the manufacturer's protocol and extracted using FastPure™ RNA Kits (Takara). cDNA was synthesized using a PrimeScript™ RT Reagent Kits (Takara) at 37℃ for 15 minutes and 85℃ for 5 seconds. MMP-9 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA were determined by real-time polymerase chain reaction (PCR) using the ABI PRISM 7900 sequence detection system and SYBR® Green PDR Master Mix (Applied Biosystems, Foster City, USA). Primer sequences for MMP-9 (NM 004994) and GAPDH (NM002046) were as follows: MMP-9: forward, CCTGGAGACCTGAGAACCAATCT; reverse, CCACCCGAGTGTAACCATAGC; GAPDH: forward, ATGGAAATCCCATCACCATCTT; reverse, CGCCCCACTTGATTTTGG. The results were normalized to the expression of GAPDH for variation control in mRNA concentration. The relative expression of each gene was calculated and normalized using the 2−ΔΔCt method. These experiments were repeated at least three times independently.
Luciferase assay
MCF-7 cells were inoculated into 24-well plates. Cells were transfected with NF-κB or AP-1 reporter plasmids using Lipofectamine 2000 (Invitrogen, Carlsbad, USA) at 70%–80% confluence. Transfected cells were pretreated with troglitazone at the indicated concentrations for 1 hour and then with 100 nM TPA. Dual Luciferase Reporter Assay Kits (Promega) and Lumat LB 9507 luminometer (EG&G Berthold, Gaithersburg, USA) were used to detect luciferase activity.
Invasion assay
For the invasion assay, we used 8-µm pore sized 24-well chambers coated with Matrigel (BD Biosciences, Franklin Lakes, USA). Cells were added to the upper chamber and lower compartment containing conditioned medium. After incubation for 24 hours, cells in the upper chamber were removed using cotton swabs. Migrated cells were fixed and stained with Toluidine blue. Invading cells were counted in five random areas of the membrane with a light microscope.
Electrophoretic mobility shift assay
Nuclear extracts were prepared as described previously. Oligonucleotides containing the NF-κB (5′CCGGTTAACAGAGGGGGCTTTCCGAG-3′) or AP-1 (5′CGCTTGATG AGTCAGCCGGAA-3′) binding sites were used as probes (Promega). Complementary strands were labeled with [α-32P]dCTP. Labeled oligonucleotides, 10 µg nuclear extracts and binding buffer were incubated for 30 minutes at room temperature. Reaction products were analyzed using 4% PAGE in 0.5×Tris-borate buffer. A 50-fold excess of NF-κB or AP-1 oligonucleotide was used a control to confirm the specific bands.
Statistical analysis
Data from three or more independent experiments were presented as the mean±standard error of the mean. Statistical analyses were performed by analysis of variance and Duncan's tests using the Microsoft 2010 Excel program (Redmond, USA). In the experiment, p<0.05 was considered as statistically significant.
RESULTS
Effect of troglitazone on MMP-9 expression in MCF-7 cells
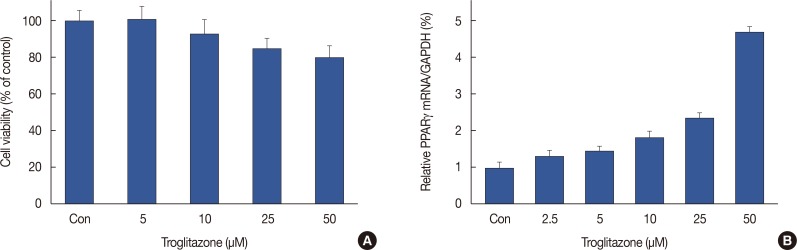
MCF-7 cells were treated with troglitazone (0–50 µM) for 24 hours and toxicity was analyzed using an established MTT assay. Troglitazone treatment did not significantly change MCF-7 cell viability (Figure 1A). Therefore, we used in a nontoxic concentration (25 and 50 µM) of troglitazone. We also found that troglitazone induced PPARγ expression in MCF-7 cells (Figure 1B).
Figure 1. Effect of troglitazone on MCF-7 cell viability and peroxisome proliferator-activated receptor γ (PPARγ). (A) MCF-7 cells were cultured in 96-well plates until 70% confluence, and various concentrations of troglitazone were added to cells for 24 hours. An established MTT assay was used to detect the viability of the cells. The optical density value of control was regarded as 100%. (B) MCF-7 cells were treated with various concentrations of troglitazone for 12 hours, and mRNA levels of PPARγ were analyzed by real-time polymerase chain reaction, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal control. Data are the mean±standard error of the mean of three independent experiments.
Con=control.
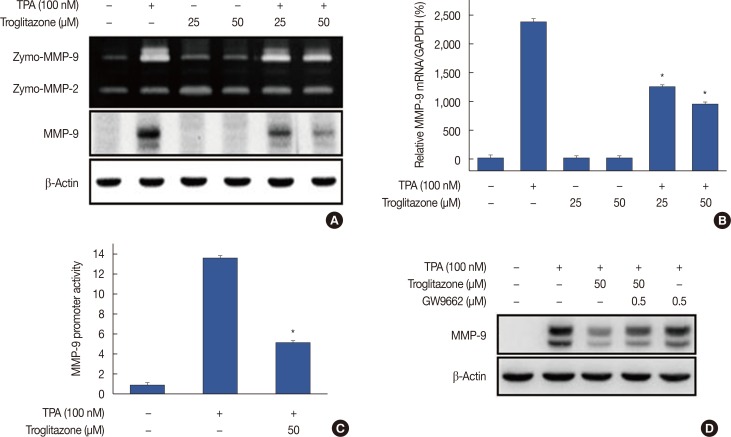
Zymography showed that TPA increased MMP-9 expression in MCF-7 cells and that troglitazone dose-dependently suppressed TPA-induced MMP-9 expression. However, MMP-2 expression was not affected by troglitazone. Western blotting and real-time PCR revealed that troglitazone suppressed TPA-induced MMP-9 expression at both protein and mRNA levels (Figure 2A and 2B). The luciferase assay showed that treatment of MCF-7 cells with troglitazone suppressed TPA-induced MMP-9 promoter activity (Figure 2C). To address whether troglitazone had inhibitory effects via a PPARγ-dependent or PPARγ-independent pathway, we confirmed that suppression of MMP-9 expression after pretreatment with troglitazone was recovered by treatment of MCF-7 cells with the PPARγ antagonist GW9662 (Figure 2D). In addition GW9662 treatment did not affect TPA-induced MMP-9 expression.
Figure 2. Troglitazone inhibits 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced matrix metalloproteinase-9 (MMP-9) expression in MCF-7 cells. MCF-7 cells were pretreated with troglitazone and then TPA was added for 24 hours. (A) MMP-9 secretion by gelatin zymography (top). MMP-9 protein expression was determined by western blot. β-Actin was the internal control (bottom). (B) MMP-9 mRNA was analyzed by real-time polymerase chain reaction with glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as the internal control. (C) Wild type MMP-9-luc reporters and a Renilla luciferase thymidine kinase reporter vector were co-transfected into MCF-7 cells. Cells were treated with troglitazone and TPA and MMP-9 promoter activity was measured with dual-luciferase reporter assays. (D) Peroxisome proliferator-activated receptor γ antagonist GW9662 was added to cells for 30 minutes before troglitazone treatment. Lysates were analyzed by western blot with anti-MMP-9. β-Actin was the loading control. Values are mean±standard error of the mean of three independent experiments.
*p<0.01 vs. TPA.
Effects of troglitazone on ERK/JNK and PI3K/AKT activation in MCF-7 cells
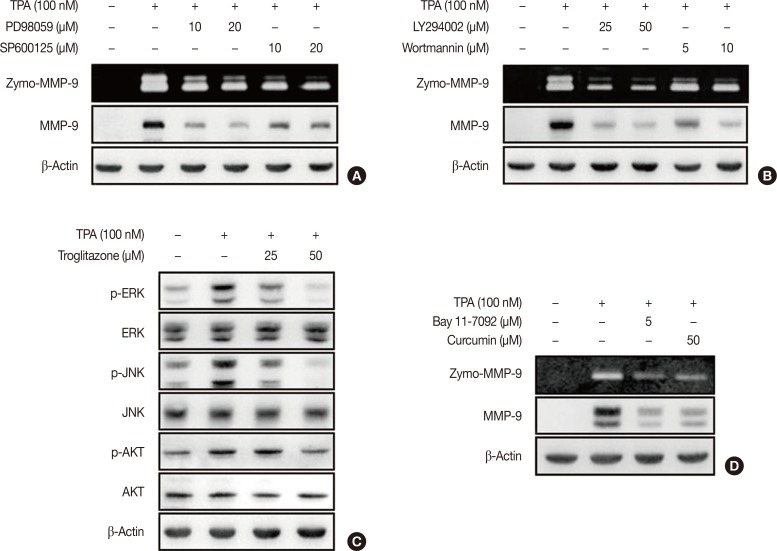
To investigate the effect of troglitazone on ERK/JNK and PI3K/AKT activity, we performed zymography and western blotting. MCF-7 cells were pretreated with inhibitors of ERK (PD98059), JNK (SP600125), or PI3K (LY294002 and wortmannin). The inhibition of ERK, JNK, or PI3K blocked TPA-induced MMP-9 protein expression in MCF-7 cells (Figure 3A and 3B). In addition, TPA significantly enhanced the phosphorylation of ERK, JNK and AKT in MCF-7 cells; this effect was blocked by troglitazone (Figure 3C). MCF-7 cells were also pretreated with an inhibitor of NF-κB (Bay 11-7092) and AP-1 (curcumin). The inhibition of NF-κB and AP-1 suppressed TPA-induced MMP-9 expression in MCF-7 cells (Figure 3D).
Figure 3. Troglitazone inhibits 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced activation of extracellular signal-regulated kinases (ERK), c-Jun N-terminal kinases (JNK), and protein kinase B (AKT) in MCF-7 cells. (A) MCF-7 cells were pretreated with inhibitors of ERK (PD98059), JNK (SP600125) and then TPA was added for 24 hours. Matrix metalloproteinase-9 (MMP-9) secretion was analyzed by gelatin zymography and protein was detected by western blot, respectively. (B) MCF-7 cells were pretreated with inhibitor of phosphoinositide 3-kinase (PI3K) (LY294002 and wortmannin) and then TPA was added for 24 hours. MMP-9 secretion was analyzed by gelatin zymography and protein was detected by western blot, respectively. (C) MCF-7 cells were pretreated with troglitazone and TPA was added for 24 hours. Phosphorylation of ERK, JNK, and AKT was analyzed by western blot. Total ERK, JNK, and AKT were the internal controls. Values are mean±standard error of the mean (SEM) of three independent experiments. (D) MCF-7 cells were pretreated with inhibitors of nuclear factor κB (Bay 11-7092) and activator protein-1 (curcumin) and then TPA was added for 24 hours. MMP-9 secretion was analyzed by gelatin zymography and protein was detected by western blot, respectively. Values are mean±SEM of three independent experiments.
Effect of troglitazone on TPA-induced NF-κB and AP-1 activation in MCF-7 cells
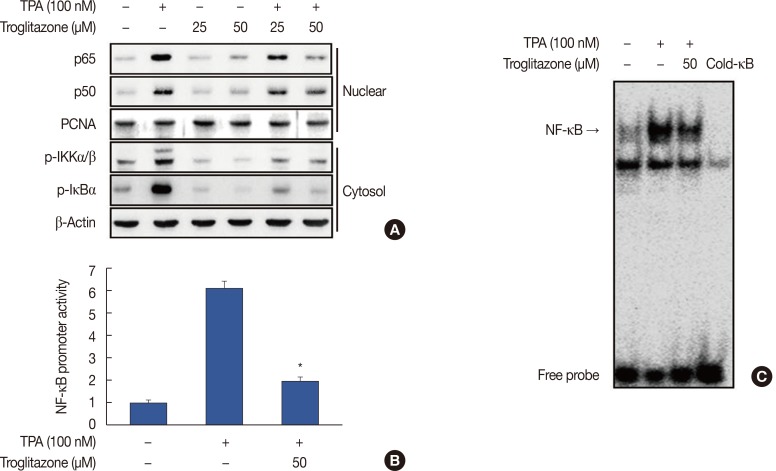
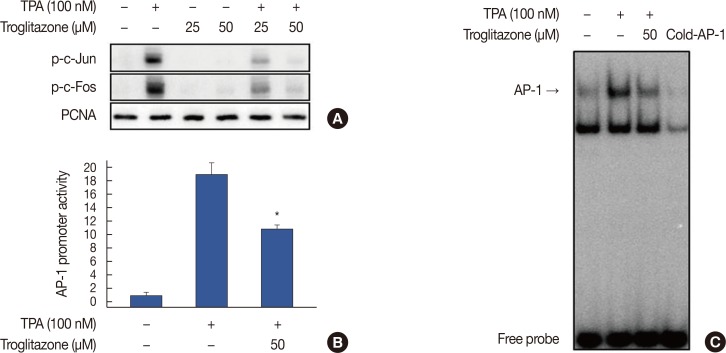
To understand the mechanism by which troglitazone inhibited MMP-9 transcription, we tested whether troglitazone inhibited NF-κB and AP-1 activation in MCF-7 cells after TPA stimulation. Troglitazone inhibited nuclear translocation of NF-κB p65/p50 and phosphorylation of IKKα/β and IκBα as determined by western blotting of MCF-7 cells (Figure 4A). The luciferase assay revealed that troglitazone inhibited the promoter activity of TPA-induced NF-κB (Figure 4B). In addition, electrophoretic mobility shift assay showed that troglitazone significantly inhibited TPA-induced NF-κB DNA binding (Figure 4C). Troglitazone inhibited phosphorylation of c-Jun and c-Fos and the promoter activities of TPA-induced AP-1 (Figure 5A and 5B). Troglitazone also inhibited TPA-induced AP-1 DNA binding (Figure 5C).
Figure 4. Troglitazone inhibits 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced nuclear factor κB (NF-κB) activation in MCF-7 cells. (A) Cells were treated with troglitazone with TPA and nuclear extracts were prepared after 3 hours. Translocation of p65 and p50 into the nucleus and phosphorylation of IκB kinase (IKK) α/β and nuclear factor of κ light polypeptide gene enhancer in B cells inhibitor (IκB) α in cytosol was determined by western blots. Proliferating cell nuclear antigen (PCNA) was the loading control for nuclear proteins. (B) NF-κB-luc reporters and a Renilla luciferase thymidine kinase reporter vector were co-transfected into MCF-7 cells. Cells were treated with troglitazone with TPA and NF-κB promoter activity was measured with dual-luciferase reporter assays. (C) Cells were treated with troglitazone with TPA and nuclear extracts made after 3 hours. NF-κB DNA binding was analyzed by electrophoretic mobility shift assay. Values are mean±standard error of the mean of three independent experiments.
*p<0.01 vs. TPA.
Figure 5. Troglitazone inhibits 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced activator protein-1 (AP-1) activation in MCF-7 cells. (A) Phosphorylation of c-Jun, a major subunit of AP-1, was determined by western blots with proliferating cell nuclear antigen (PCNA) as the loading control for nuclear proteins. (B) AP-1-luc reporters and a Renilla luciferase thymidine kinase reporter vector were co-transfected into MCF-7 cells. Cells were treated with troglitazone with TPA and AP-1 promoter activity was measured with dual-luciferase reporter assays. (C) Cells were treated with troglitazone with TPA and nuclear extracts were prepared after 3 hours. AP-1 DNA binding was analyzed by electrophoretic mobility shift assay. Values are mean±standard error of the mean of three independent experiments.
*p<0.01 vs. TPA.
Effect of troglitazone on in vitro invasion of TPA-induced MCF-7 cells
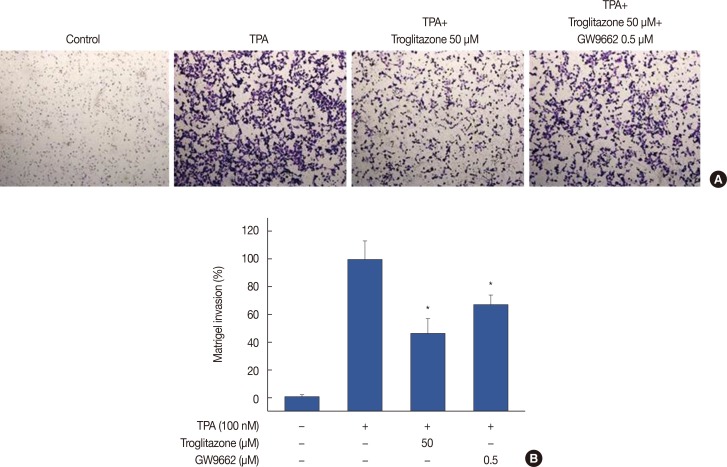
A Matrigel invasion assay was used to elucidate the inhibition by troglitazone of invasion on MCF-7 cells. A troglitazone concentration of 50 µM effectively inhibited the invasion of MCF-7 cells by approximately 55% compared to untreated control cells. However, inhibition of only 30% was observed with troglitazone and GW9662, indicating that troglitazone suppresses the invasive potential of MCF-7 cells via PPARγ activation (Figure 6).
Figure 6. Troglitazone inhibits 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced Matrigel invasion. (A) Matrigel migration assays were carried out on cells treated with TPA, troglitazone and GW9662. Cell were seeded onto the upper chamber with TPA, troglitazone and GW9662 in the well. After 24 hours, cells on the bottom of the filter were fixed. The migrated cells were stained with crystal violet and photographed microscope (×40 magnification). (B) Results were quantified by counting the migrated cells in five randomly selected regions. The data presented as the mean number of migrated cells. Values are mean±standard error of the mean of three independent experiments.
*p<0.01 vs. TPA.
DISCUSSION
We found that troglitazone, a synthetic PPARγ ligand, blocks TPA-induced MMP-9 expression and invasion of MCF-7 cells through a PPARγ-dependent mechanism. Troglitazone blocked cancer cell invasion by suppressing NF-κB/AP-1-mediated MMP-9 expression. Thus, troglitazone may be developed as a novel therapeutics to prevent breast cancer invasion.
PPARγ ligands are potential targets for the prevention and treatment of various human cancers [14]. PPARγ ligands inhibit proliferation and induce apoptosis of breast cancer cells [15,16], indicating their potential for breast cancer treatment and prevention. PPARγ protein is expressed in breast cancer cell lines including MCF-7 and MDA-MB-231 cells [17]. Troglitazone induces PPARγ expression in many tumors including breast, brain, and liver [18]. We found that troglitazone induces PPARγ-dependent G1 arrest and apoptosis of breast cancer cells [19], indicating that PPARγ-regulates genes that are pivotal in breast carcinogenesis.
NF-κB and AP-1 are well-known, major transcriptional factors that regulate MMP-9 expression in cancer cells [20]. MAPK and PI3K/AKT are signaling transducers upstream of NF-κB and AP-1; their activation stimulates the growth and invasion of cancer cells [21,22,23]. Our data suggest that troglitazone inhibits the NF-κB, AP-1, MAPK, and PI3K/AKT signaling pathways in MCF-7 cells. This finding is important for understanding breast cancer progression, as these signaling pathways increase tumor cell invasiveness because of increased MMP-9 expression [24]. We found that troglitazone inhibited MMP-9 expression in MCF-7 cells and suppressed the invasion of breast cancer cells. Troglitazone inhibited tumor invasion by suppressing NF-κB-, AP-1-, MAPK-, and PI3K/AKT-dependent MMP-9 expression and invasion of MCF-7 cells. In addition, suppression of MMP-9 expression by troglitazone in MCF-7 cells was recovered by GW9662, an inhibitor of PPARγ, indicating that the troglitazone inhibitory effects involve a PPARγ-dependent pathway. Thus, we suggest that troglitazone involves PPARγ-dependent inhibitory mechanisms affecting MMP-9 expression in various cancer cell lines.
In conclusion, we investigated whether MMP-9 expression and invasion of breast cancer cells is modulated by PPARγ ligands. MCF-7 cells treated with troglitazone displayed inhibited MMP-9 expression, which plays a critical role in cancer invasion. Moreover, troglitazone caused a PPARγ-dependent decrease of MMP-9 expression in MCF-7 cells. Our results suggest that troglitazone affects estrogen receptor-positive breast cancer metastasis and shows potential for the prevention and treatment of estrogen receptor-positive breast cancer. However, the therapeutic effects of troglitazone on estrogen receptor-negative breast cancer requires further analysis.
Footnotes
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2013R1A1A2011718) and Chonbuk National University Hospital Research Fund 2010. This work was supported by a NRF funded by the Korean government (No. 2008-0062279).
CONFLICT OF INTEREST: The authors declare that they have no competing interests.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Takaoka M, Naomoto Y, Ohkawa T, Uetsuka H, Shirakawa Y, Uno F, et al. Heparanase expression correlates with invasion and poor prognosis in gastric cancers. Lab Invest. 2003;83:613–622. doi: 10.1097/01.lab.0000067482.84946.bd. [DOI] [PubMed] [Google Scholar]
- 3.Wang L, Ling Y, Chen Y, Li CL, Feng F, You QD, et al. Flavonoid baicalein suppresses adhesion, migration and invasion of MDA-MB-231 human breast cancer cells. Cancer Lett. 2010;297:42–48. doi: 10.1016/j.canlet.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 4.Yao J, Xiong S, Klos K, Nguyen N, Grijalva R, Li P, et al. Multiple signaling pathways involved in activation of matrix metalloproteinase-9 (MMP-9) by heregulin-beta1 in human breast cancer cells. Oncogene. 2001;20:8066–8074. doi: 10.1038/sj.onc.1204944. [DOI] [PubMed] [Google Scholar]
- 5.Liacini A, Sylvester J, Li WQ, Zafarullah M. Inhibition of interleukin-1-stimulated MAP kinases, activating protein-1 (AP-1) and nuclear factor kappa B (NF-kappa B) transcription factors down-regulates matrix metalloproteinase gene expression in articular chondrocytes. Matrix Biol. 2002;21:251–262. doi: 10.1016/s0945-053x(02)00007-0. [DOI] [PubMed] [Google Scholar]
- 6.Lee WT, Lee TH, Cheng CH, Chen KC, Chen YC, Lin CW. Antroquinonol from Antrodia Camphorata suppresses breast tumor migration/invasion through inhibiting ERK-AP-1- and AKT-NF-kappaB-dependent MMP-9 and epithelial-mesenchymal transition expressions. Food Chem Toxicol. 2015;78:33–41. doi: 10.1016/j.fct.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 7.Schoonjans K, Staels B, Auwerx J. Role of the peroxisome proliferator-activated receptor (PPAR) in mediating the effects of fibrates and fatty acids on gene expression. J Lipid Res. 1996;37:907–925. [PubMed] [Google Scholar]
- 8.Chandra V, Huang P, Hamuro Y, Raghuram S, Wang Y, Burris TP, et al. Structure of the intact PPAR-gamma-RXR- nuclear receptor complex on DNA. Nature. 2008;456:350–356. doi: 10.1038/nature07413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berger J, Moller DE. The mechanisms of action of PPARs. Annu Rev Med. 2002;53:409–435. doi: 10.1146/annurev.med.53.082901.104018. [DOI] [PubMed] [Google Scholar]
- 10.Seyfried TN, Mukherjee P. Targeting energy metabolism in brain cancer: review and hypothesis. Nutr Metab (Lond) 2005;2:30. doi: 10.1186/1743-7075-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsu PP, Sabatini DM. Cancer cell metabolism: Warburg and beyond. Cell. 2008;134:703–707. doi: 10.1016/j.cell.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 12.Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 13.Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab. 2001;280:E745–E751. doi: 10.1152/ajpendo.2001.280.5.E745. [DOI] [PubMed] [Google Scholar]
- 14.Allred CD, Kilgore MW. Selective activation of PPARgamma in breast, colon, and lung cancer cell lines. Mol Cell Endocrinol. 2005;235:21–29. doi: 10.1016/j.mce.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Fenner MH, Elstner E. Peroxisome proliferator-activated receptor-gamma ligands for the treatment of breast cancer. Expert Opin Investig Drugs. 2005;14:557–568. doi: 10.1517/13543784.14.6.557. [DOI] [PubMed] [Google Scholar]
- 16.Yin Y, Russell RG, Dettin LE, Bai R, Wei ZL, Kozikowski AP, et al. Peroxisome proliferator-activated receptor delta and gamma agonists differentially alter tumor differentiation and progression during mammary carcinogenesis. Cancer Res. 2005;65:3950–3957. doi: 10.1158/0008-5472.CAN-04-3990. [DOI] [PubMed] [Google Scholar]
- 17.Elstner E, Müller C, Koshizuka K, Williamson EA, Park D, Asou H, et al. Ligands for peroxisome proliferator-activated receptorgamma and retinoic acid receptor inhibit growth and induce apoptosis of human breast cancer cells in vitro and in BNX mice. Proc Natl Acad Sci U S A. 1998;95:8806–8811. doi: 10.1073/pnas.95.15.8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu J, Qiao L, Zimmermann L, Ebert MP, Zhang H, Lin W, et al. Troglitazone inhibits tumor growth in hepatocellular carcinoma in vitro and in vivo. Hepatology. 2006;43:134–143. doi: 10.1002/hep.20994. [DOI] [PubMed] [Google Scholar]
- 19.Yu HN, Lee YR, Noh EM, Lee KS, Kim JS, Song EK, et al. Induction of G1 phase arrest and apoptosis in MDA-MB-231 breast cancer cells by troglitazone, a synthetic peroxisome proliferator-activated receptor gamma (PPARgamma) ligand. Cell Biol Int. 2008;32:906–912. doi: 10.1016/j.cellbi.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 20.Bond M, Fabunmi RP, Baker AH, Newby AC. Synergistic upregulation of metalloproteinase-9 by growth factors and inflammatory cytokines: an absolute requirement for transcription factor NF-kappa B. FEBS Lett. 1998;435:29–34. doi: 10.1016/s0014-5793(98)01034-5. [DOI] [PubMed] [Google Scholar]
- 21.Chiang PC, Lin SC, Pan SL, Kuo CH, Tsai IL, Kuo MT, et al. Antroquinonol displays anticancer potential against human hepatocellular carcinoma cells: a crucial role of AMPK and mTOR pathways. Biochem Pharmacol. 2010;79:162–171. doi: 10.1016/j.bcp.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 22.Hwang YP, Yun HJ, Choi JH, Han EH, Kim HG, Song GY, et al. Suppression of EGF-induced tumor cell migration and matrix metalloproteinase-9 expression by capsaicin via the inhibition of EGFR-mediated FAK/Akt, PKC/Raf/ERK, p38 MAPK, and AP-1 signaling. Mol Nutr Food Res. 2011;55:594–605. doi: 10.1002/mnfr.201000292. [DOI] [PubMed] [Google Scholar]
- 23.Yang YT, Weng CJ, Ho CT, Yen GC. Resveratrol analog-3,5,4’-trimethoxy-trans-stilbene inhibits invasion of human lung adenocarcinoma cells by suppressing the MAPK pathway and decreasing matrix metalloproteinase-2 expression. Mol Nutr Food Res. 2009;53:407–416. doi: 10.1002/mnfr.200800123. [DOI] [PubMed] [Google Scholar]
- 24.Westermarck J, Kähäri VM. Regulation of matrix metalloproteinase expression in tumor invasion. FASEB J. 1999;13:781–792. [PubMed] [Google Scholar]