Abstract
Malignant melanoma rarely originates from the female nipple. Tumors that develop on the skin of the breast are often subject to a delayed diagnosis. Cytologic examination provides excellent diagnostic capabilities and is a safe procedure with a lower risk of local implantation, compared to needle or incisional biopsy. We herein report a patient who underwent surgical resection of a primary malignant melanoma of the nipple. An elastic soft nodule was observed on the left nipple, and no abnormal lesions were identified in the breast. Eventually, a malignant melanoma was diagnosed from the clinical and cytological evaluation findings. This bulky tumor was classified as a stage IIIC nodular melanoma, with a thickness of 12 mm. The patient received adjuvant chemotherapy and exhibits no evidence of recurrence 7 years after surgery.
Keywords: Breast, Melanoma, Nipples, Surgical resection
INTRODUCTION
Primary melanomas that arise on the skin of the breast are reported to account for 0.28%–3.8% of all cutaneous melanomas [1,2,3,4]. A primary melanoma originating on the female nipple is an extremely rare variant of malignant melanoma. The incidence of nipple and areola melanomas is approximately 12% of all cutaneous melanomas of the breast [5]. The main symptom is the appearance of a mole on the breast. These tumors appear as areas of brown pigmentation around the nipple. Malignant lesions must be differentiated from Paget's disease, seborrheic keratosis, and benign moles. The American Joint Committee on Cancer (AJCC)/The International Union Against Cancer (UICC) Cancer Staging System Manual published in 2010 included a new staging classification [6]. The clinical course of melanoma is determined by dissemination and depends on the thickness, ulceration, localization, and histology of the primary tumor, as well as the sex of the patient. We herein report a patient who underwent surgical resection of a primary malignant melanoma of the nipple.
CASE REPORT
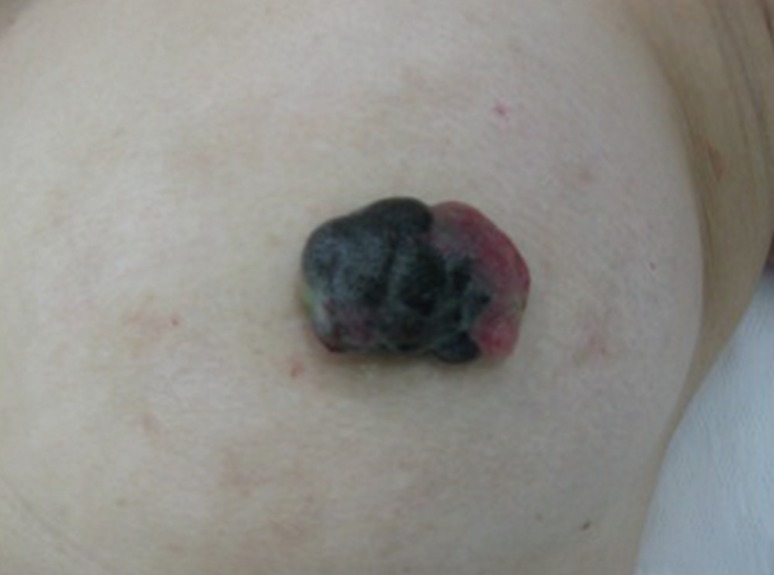
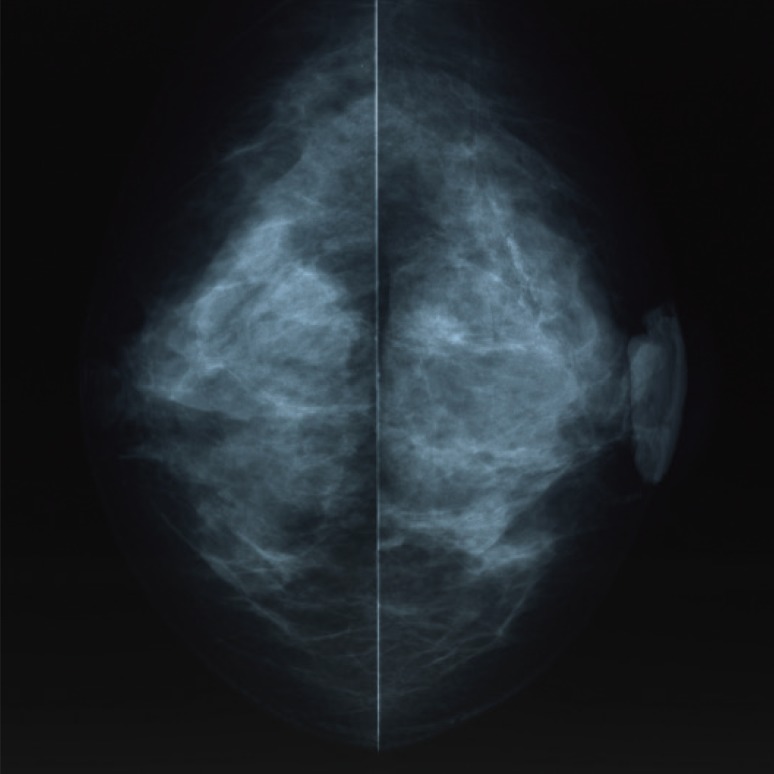
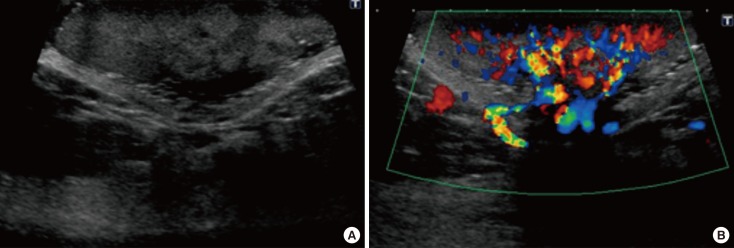
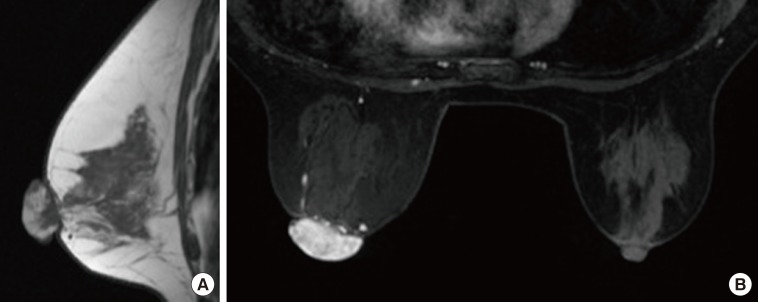
A 59-year-old Japanese female patient presented with painless pigment deposition and diffuse enlargement of the left nipple. The nevus had grown slowly for approximately 3 years, at which time the patient was admitted to our institution. She had no history of breast trauma. The patient had contracted hepatitis type B at 30 years of age. She had no remarkable family history. A physical examination revealed a soft, elastic, pedunculated nodule located on the left nipple (Figure 1). The tumor size was 4.0 cm×3.5 cm×1.5 cm. There were no palpable masses in the breast. The nipple discharge was bloody. There were no palpable axillary or supraclavicular lymph nodes. Dermoscopy revealed an asymmetrical, irregularly structured area accompanied by a 12-mm-thick ulceration, and a blue-whitish veil. The clinical course suggested malignant melanoma, rather than mammary Paget's disease. All laboratory data were unremarkable except for a slightly elevated lactate dehydrogenase level. Regarding tumor markers, the levels of carcinoembryonic antigen and cancer antigen 15-3 were within normal ranges, whereas those of neuron-specific enolase and 5-S-cysteinyldopa were elevated (13.7 ng/mL and 32.1 nmol/L, respectively). Mammography showed a lobulated mass with a well-circumscribed margin in the left nipple (Figure 2). Ultrasonography showed a hypoechoic mass with a lobular shape that measured 4.0 cm×3.5 cm. Internally, the mass was heterogeneously echoic, and color Doppler ultrasound demonstrated a strong increase in blood flow within the lesion (Figure 3). Magnetic resonance imaging showed a heterogeneously distributed lesion with high signal intensity in the left nipple on both T1-weighted and T2-weighted images (Figure 4A). The mass in the nipple was rapidly enhanced following the injection of gadolinium diethylenetriaminepentaacetic acid, and the time-intensity curve showed a rapid plateau pattern (Figure 4B). No other enhanced lesion was detected in the breast area. Positron emission tomography/computed tomography (PET/CT) revealed a lesion with high 18F-fluorodeoxyglucose only in the left nipple. Exfoliative cytology of the left nipple and using Papanicolaou and Giemsa staining revealed anaplastic cells (melanocytes) with intranuclear cytoplasmic invaginations and prominent nucleoli (Figure 5A and 5B). These melanocytes also exhibited strong mmunohistochemical reactions to Melan-A and HMB45, which are highly specific for melanoma cells (Figure 5C and 5D). These findings suggested a very thick nodular malignant melanoma. The patient underwent a mastectomy resection of the left breast. A left-sided sentinel lymph node biopsy revealed an axillary lymph node metastasis, and an axillary lymph node dissection was performed. Macroscopically, the resected specimen revealed a pigmented tumor measuring approximately 4.5 cm×3.2 cm×2.0 cm (Figure 6). A histological analysis revealed high cellularity, with plump cells containing prominent and pleomorphic nucleoli. The cytoplasm was filled with compact melanosomes. Numerous atypical melanocytes were also observed in the connective tissue (Figure 7A and 7B). Breslow's tumor thickness was 12 mm, which suggested Level IV according to the Clark level of invasion. Strong immunohistochemical reactions for S100 protein and HMB45, a marker of melanocytic neoplasms, confirmed the diagnosis (Figure 7C and 7D). Based on these features, a pathological diagnosis of nodular malignant melanoma was made. Metastasis was identified in the left axillary lymph nodes, and a AJCC/UICC stage of IIIC, T4bN1bM0 was confirmed. The surgical margin was pathologically negative. The patient was given postoperative chemotherapy (DAV-Feron [dacarbazine, nimustine, vincristine, and interferon-β] and subsequent interferon). A lung nodule detected by computed tomography 2 years after surgery was subjected to careful follow-up. The size of the lung nodule did not change for 5 years, and was deemed benign. There has been no evidence of recurrence after 7 years of surgery.
Figure 1. Gross finding. An elastic soft pedunculated nodule located on the left nipple.
Figure 2. Mammography examination of the left breast. The craniocaudal view mammogram showed a lobulated mass in the left nipple.
Figure 3. Ultrasonography (US) examination. (A) US showed a hypoechoic mass with a lobular shape. (B) The color Doppler ultrasound demonstrated highly increased blood flow within the lesion.
Figure 4. Magnetic resonance imaging (MRI). (A) MRI showed the heterogeneously distributed lesion of the high signal intensity in the left nipple on T1-weighted images. (B) The mass of the nipple was enhanced quickly following the injection of gadolinium diethylenetriamine-pentaacetic acid at early phase (2 minutes after A B agent injection).
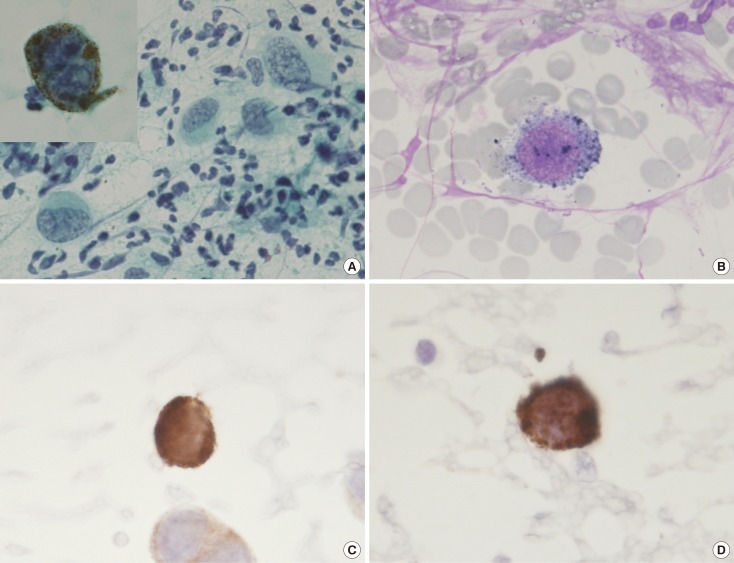
Figure 5. Exfoliative cytology of the left nipple. The anaplastic cells with intranuclear cytoplasmic invaginations, and the prominent nucleoli can be seen using (A) Papanicolaou stain (×400; inset, ×1,000) and (B) Giemsa stain (×1,000). These melanocytes also revealed strong immunohistochemical reactions for (C) Melan-A (×1,000) and (D) HMB45 (×1,000).
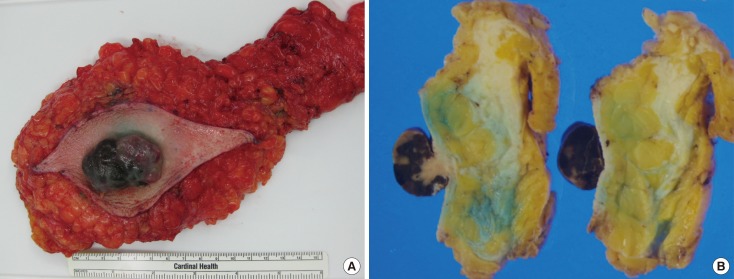
Figure 6. Gross appearance of the mastectomy specimen. (A) The resected specimen revealed a black color tumor. (B) The cut slice through the nipple of the mastectomy specimen.
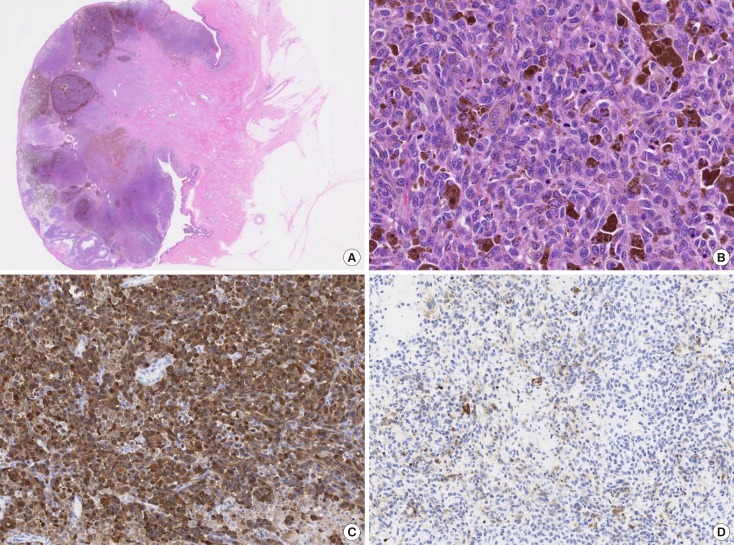
Figure 7. Histopathologic findings. (A) Microscopic findings revealed numerous atypical melanocytes in the connective tissue (H&E stain, low magnification view). (B) The cytoplasm was filled with compact melanosomes (H&E stain, ×200). The immunohistochemistry image for (C) S100 protein (×100) and (D) HMB45 (×100) were strongly positive.
DISCUSSION
Malignant melanoma is a neoplasm of neural crest-derived melanocytes that arises in the epidermis and other organs such as the oral mucosa, gastrointestinal mucosa, uvea, and meninges. Malignant melanomas may develop in exposed or unexposed areas of the skin. The incidence of malignant melanoma is increasing relative to that of other cancers, both in Japan and elsewhere [7]. However, a primary melanoma originating on the female nipple remains an extremely rare variant of malignant melanoma. Haagensen [1] reported a primary melanoma of the nipple in 1971. The reported incidence of nipple and areola melanomas among all cutaneous melanomas of the breast is 12% [5]. A mole-like malignant melanoma of the breast must be differentiated from Paget's disease, seborrheic keratosis, and benign mole. Such tumors are surrounded by pigmented skin and covered by clothing. Although dermoscopy largely contributes to the differential diagnosis of tumors by facilitating the evaluation of melanocytic lesions, highly sensitive diagnostic techniques are required. Therefore, these tumors tend to receive a delayed diagnosis. For a definite diagnosis, procedures such as fine-needle aspiration biopsy, exfoliative cytology, core needle biopsy, or open (incisional, excisional) biopsy are selected on the basis of accuracy. However, as needle biopsy may carry a risk of malignant cell dissemination and hematogenous metastasis, we only performed exfoliative cytology of the ulcerated tumor in the present case. Malignant melanomas are classified according to the criteria of Clark [8], which distinguishes the following types: superficial spreading melanoma (SSM), nodular melanoma (NM), lentigo maligna melanoma, and acro-lentiginous melanoma. Dermoscopy reveals morphologic structures that are invisible to the naked eye and thus adds valuable clinical information used to evaluate moles. Nodular melanoma, the most common subtype, may have nonspecific dermoscopic features. In our case, the tumor appeared to be a papillary nodule accompanied by a blackish-brown unstructured area. The observation of a bluish-white veil and irregularly centered black dots made it difficult to distinguish this lesion from pigmented mammary Paget's disease. However, histology confirmed melanosis of the nipple and areola in this case, leading to a classification of NM. NM is known to account for 21% of all melanomas in Japan, and is considered clinically distinct and high risk.
The standard treatment for melanoma comprises surgical excision with a safety margin around the primary tumor. An appropriate surgical margin is more important in terms of prognosis rather than aesthetics. In an analysis of randomized controlled trials, Lens et al. [9] demonstrated the lack of statistically significant differences in 5-year overall and disease-free survival when comparing wide excision groups (margins of 3–5 cm) to narrow excision groups (margins of 1–2 cm). Surgical excision with a margin no larger than 2 cm yielded a prognosis to that achieved with wider excision [9,10]. As no trials support a 2-cm margin rather than a 1-cm margin, the optimal surgical margin around a primary melanoma with a tumor thickness >2 mm should be 2 cm [10]. Hence, the optimal width of an excision margin remains unclear [11]. Mastectomy offers no advantage over wide local excision (i.e., breast-conserving surgery) of the primary lesion. Sentinel lymph node biopsy is the standard procedure by which regional lymph node involvement of breast cancer and malignant melanoma is determined, and helps to reduce adverse effects [12]. In our case, the tumor was located at the nipple and had a thickness exceeding 2 mm; accordingly, a total mastectomy was performed.
Systemic adjuvant therapy to target melanoma micrometastases is indicated for postoperative patients at a high risk of advanced disease development. DAV combination chemotherapy was widely used in Japan as an adjuvant treatment for malignant melanoma. The results of a Japanese clinical trial conducted from 1988 to 1995 suggest that combination therapy with DAV and Feron was superior to DAV monotherapy [13]. In our case, we selected DAV-Feron therapy after carefully considering the stage, age, and tumor thickness. Although recent studies have identified several oncogenes associated with melanoma, no adjuvant therapy currently improves the prognosis dramatically, given the potential side effects.
The clinical features of nipple and areola melanomas remain largely unclear because few studies have evaluated the histological features of these tumors. Papachristou et al. [5] described favorable behaviors in cutaneous breast melanomas. Clark [14] reported several independent prognostic factors of clinical stage I cutaneous melanoma, such as the mitotic rate per square millimeter, tumor-infiltrating lymphocytes, tumor thickness, anatomic site of primary melanoma, sex of the patient, and histologic regression. The tumor thickness has been identified as the most important prognostic factor for patients with primary cutaneous melanomas. In our case, the tumor thickness was 12 mm, and a previous study reported a thickness cutoff point of >4 mm as indicating a highly significant increase in the relative risk of death, compared with a baseline thickness of ≤1 mm [15]. In Japan, NM is associated with the worst prognosis, whereas SSM has the most favorable prognosis [7].
To the best of our knowledge, this is the first English report of a diagnosed nipple/areola melanoma with a remarkably large tumor thickness. Although the clinical features of nipple and areola melanomas remain unclear, the tumor location, rather than the thickness and subtype, may have influenced the prognosis of our case. The patient has had a favorable course with no recurrences for 7 years after undergoing surgery and chemotherapy.
Footnotes
CONFLICT OF INTEREST: The authors declare that they have no competing interests.
References
- 1.Haagensen CD. Diseases of the Breast. 3rd ed. Philadelphia: WB Saunders; 1986. pp. 352–356. [Google Scholar]
- 2.Ariel IM, Caron AS. Diagnosis and treatment of malignant melanoma arising from the skin of the female breast. Am J Surg. 1972;124:384–390. doi: 10.1016/0002-9610(72)90047-5. [DOI] [PubMed] [Google Scholar]
- 3.Roses DF, Harris MN, Stern JS, Gumport SL. Cutaneous melanoma of the breast. Ann Surg. 1979;189:112–115. doi: 10.1097/00000658-197901000-00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenberg BM, Hamilton R, Rothkopf DM, Balk M, Clark WH, Jr, LaRossa D. Management of cutaneous melanomas of the female breast. Plast Reconstr Surg. 1987;80:409–415. doi: 10.1097/00006534-198709000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Papachristou DN, Kinne D, Ashikari R, Fortner JG. Melanoma of the nipple and areola. Br J Surg. 1979;66:287–288. doi: 10.1002/bjs.1800660424. [DOI] [PubMed] [Google Scholar]
- 6.Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishihara K, Saida T, Otsuka F, Yamazaki N Prognosis and Statistical Investigation Committee of the Japanese Skin Cancer Society. Statistical profiles of malignant melanoma and other skin cancers in Japan: 2007 update. Int J Clin Oncol. 2008;13:33–41. doi: 10.1007/s10147-007-0751-1. [DOI] [PubMed] [Google Scholar]
- 8.Mihm MC, Jr, Clark WH, Jr, From L. The clinical diagnosis, classification and histogenetic concepts of the early stages of cutaneous malignant melanomas. N Engl J Med. 1971;284:1078–1082. doi: 10.1056/NEJM197105132841907. [DOI] [PubMed] [Google Scholar]
- 9.Lens MB, Dawes M, Goodacre T, Bishop JA. Excision margins in the treatment of primary cutaneous melanoma: a systematic review of randomized controlled trials comparing narrow vs wide excision. Arch Surg. 2002;137:1101–1105. doi: 10.1001/archsurg.137.10.1101. [DOI] [PubMed] [Google Scholar]
- 10.Haigh PI, DiFronzo LA, McCready DR. Optimal excision margins for primary cutaneous melanoma: a systematic review and meta-analysis. Can J Surg. 2003;46:419–426. [PMC free article] [PubMed] [Google Scholar]
- 11.Søndergaard K, Schou G. Therapeutic and clinico-pathological factors in the survival of 1,469 patients with primary cutaneous malignant melanoma in clinical stage I: a multivariate regression analysis. Virchows Arch A Pathol Anat Histopathol. 1985;408:249–258. doi: 10.1007/BF00707987. [DOI] [PubMed] [Google Scholar]
- 12.Morton DL, Thompson JF, Cochran AJ, Mozzillo N, Elashoff R, Essner R, et al. Sentinel-node biopsy or nodal observation in melanoma. N Engl J Med. 2006;355:1307–1317. doi: 10.1056/NEJMoa060992. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto A, Ishihara K. Clinical study of DAV+IFN-beta therapy (combination adjuvant therapy with intravenous DTIC, ACNU and VCR, and local injection of IFN-beta) for malignant melanoma. Int J Immunother. 1996;12:73–78. [Google Scholar]
- 14.Clark WH, Jr, Elder DE, Guerry D, 4th, Braitman LE, Trock BJ, Schultz D, et al. Model predicting survival in stage I melanoma based on tumor progression. J Natl Cancer Inst. 1989;81:1893–1904. doi: 10.1093/jnci/81.24.1893. [DOI] [PubMed] [Google Scholar]
- 15.Büttner P, Garbe C, Bertz J, Burg G, d'Hoedt B, Drepper H, et al. Primary cutaneous melanoma: optimized cutoff points of tumor thickness and importance of Clark's level for prognostic classification. Cancer. 1995;75:2499–2506. doi: 10.1002/1097-0142(19950515)75:10<2499::aid-cncr2820751016>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]