Abstract
Scope
Cholesterol efflux capacity of HDL (CEC) is inversely associated with cardiovascular risk. HDL composition, fluidity, oxidation, and size are related with CEC. We aimed to assess which HDL parameters were CEC determinants after virgin olive oil (VOO) ingestion.
Methods and Results
Post-hoc analyses from the VOHF study, a crossover intervention with three types of VOO. We assessed the relationship of 3-week changes in HDL-related variables after intervention periods with independence of the type of VOO. After univariate analyses, mixed linear models were fitted with variables related with CEC and fluidity. Fluidity and Apolipoprotein (Apo)A-I content in HDL was directly associated, and HDL oxidative status inversely, with CEC. A reduction in free cholesterol, an increase in triglycerides in HDL, and a decrease in small HDL particle number or an increase in HDL mean size, were associated to HDL fluidity.
Conclusions
HDL fluidity, ApoA-I concentration, and oxidative status are major determinants for CEC after VOO. The impact on CEC of changes in free cholesterol and triglycerides in HDL, and those of small HDL or HDL mean size, could be mechanistically linked through HDL fluidity. Our work points out novel therapeutic targets to improve HDL functionality in humans through nutritional or pharmacological interventions.
Keywords: BODIPY-cholesterol, HDL cholesterol efflux capacity, fluidity, HDL, Virgin Olive Oil
Introduction
High-density lipoprotein (HDL) cholesterol levels have been shown to be inversely related to cardiovascular disease [1]. However, data from multiple gene association [2] and intervention [3] studies lead to consider the functional quality of HDL as a more important parameter than the circulating quantity of HDL. The main functional property of the HDL particle, the reverse cholesterol transport pathway, is the capacity of HDL to remove the excess of cholesterol in peripheral cells and transport it to the liver cells to be metabolized. In particular, cholesterol efflux, the first step of the reverse cholesterol transport, from macrophages to HDL in atherosclerotic plaques is thought to be critical in the anti-atherogenic effect of HDL [4]. Cholesterol efflux capacity of HDL (CEC) has been shown to be inversely associated with both prevalent coronary disease and incident atherosclerotic cardiovascular disease and it is considered a useful biomarker for cardiovascular risk [5,6].
Several HDL characteristics have been related to its CEC, namely composition, fluidity of the monolayer, oxidative status, and size [7]. Interrelationships among these features have also been described. First, HDL composition deeply affects its function. The distribution of HDL lipids between surface and core is crucial for apolipoprotein (Apo) A-I stability in HDL, a key protein involved in CEC [4], although ApoA-II is also able to efficiently remove cholesterol from macrophages in vivo [8]. From our previous data, changes in HDL composition also modulate HDL fluidity [9, 10], considered an intermediate marker of enhanced HDL functionality [11]. Low levels of phospholipids, or high levels of free cholesterol (FC), on the HDL surface may contribute to a less fluid HDL, and therefore to a less functional one [4]. Second, HDL oxidation also plays a pivotal role in HDL functionality. We have previously reported that oxidized HDL are less fluid, and therefore less able to perform cholesterol efflux from human THP-1 macrophages [11, 12]. This decrease in HDL functionality could be promoted through the impairment of the ApoA-I binding to cholesterol transporters due to ApoA-I/HDL oxidation [13,14]. Finally, the relationship between HDL particle size and CEC remains controversial [15,16]. On one hand, in some in vitro studies small HDL particles were the most efficient mediators of CEC [15]. On the other hand, large HDL particles were the best promoters for CEC in human studies, being also related to coronary endothelial dysfunction [16].
From our data, and those from others, consumption of virgin olive oil (VOO) increases not only the plasma HDL cholesterol concentration levels [17], but also the CEC, and HDL fluidity [10, 18, 19], due, at least in part, to a transcriptomic effect [20]. As we have previously reported in experimental and human studies, phenol-containing VOO, besides to increase HDL fluidity [10, 18], has shown to: modify HDL composition by increasing the HDL content of oleic acid [21] and ApoA-I and ApoA-II levels [22], increase the HDL antioxidant content [23], improve the HDL oxidative status [18, 20, 23], and promote a less atherogenic HDL subclasses profile [18, 24]. However, it still remains unknown how these functional enhancements are inter-related and how they all explain an improvement in HDL functionality. Due to this, in this study we examined the associations between CEC and variables related with HDL composition, fluidity, oxidative/antioxidative status, and particle size after VOO consumption. Our aim was to define which parameters can be the best predictors for CEC after a dietary intervention which enhances it.
Methods
Subjects
Thirty-three hypercholesterolemic (total cholesterol >200 mg/dL) individuals (19 men and 14 women) were recruited from newspaper and University advertisements. Volunteers were preselected when non-smokers, and their clinical record, physical examination, and blood pressure were within a normal range. Next, complete blood count, routine biochemical laboratory analyses, and urinary dipstick tests were performed. Candidates were included when values, other than total and low-density lipoprotein cholesterol (LDL-C), were within the reference range. Exclusion criteria were: LDL-C ≥190 mg/dL, triglycerides ≥350 mg/dL, fasting blood glucose >126 mg/dL, plasma creatinine levels >1.4 mg/dL for women and >1.5 mg/dL for men, body mass index (BMI)>35 kg/m2, smokers (>1 cigarrete/day), athletes with physical activity (>3000 Metabolic Equivalents for Task (METS).min/day), hypertension, multiple allergies, intestinal diseases, chronic diseases (i.e. diabetes, cardiovascular, etc.), or other conditions that would impair the adherence to the study. Participants provided written informed consent, and the local institutional ethics committee approved the protocol (CEIC-IMAS 2009/3347/I).
Design and study procedure
This work was conducted in the frame of the VOHF (Virgin Olive Oil and HDL Functionality) Study. A crossover, double-blind, controlled trial was performed with three types of olive oil: 1) a natural VOO containing its phenolic compounds; 2) this natural VOO enriched with its own phenolic compounds, and 3) the natural VOO enriched with both VOO phenolic compounds plus those from thyme in a 1:1 ratio. The three VOOs did not differ in fat and micronutrient composition, with the exception of the phenolic content (80, 500, and 500 ppm, respectively). Three-week intervention periods (25 mL/day VOO) were preceded by two-week washout periods with refined olive oil. 24-hour urine and blood samples were collected at fasting state at the start of the study and before and after each treatment. A 3-day dietary record was administered at baseline and after each intervention period. Participants were asked to avoid a high intake of rich antioxidant foods (i.e. vegetables, legumes, fruits, etc.). A nutritionist advised to participants for replacing all types of habitually consumed raw fats with the olive oils catered. Plasma EDTA and serum samples were obtained by whole blood centrifugation and preserved at−80°C. The clinical trial was conducted in accordance with the Helsinki Declaration and the Good Clinical Practice for Trials on Medical Products in the European Community (http://ec.europa.eu/health/files/eudralex/vol-10/3cc1aen_en.pdf). The protocol is registered with the International Standard Randomized Controlled Trial register (www.controlled-trials.com: ISRCTN77500181).
The present study is a post-hoc analysis with samples of the participants in the VOHF study assessing, with independence of the type of olive oil consumed, the relationship of the 3-week changes after all intervention periods among CEC, the fluidity of the HDL monolayer, and variables related to HDL composition, oxidative/antioxidative status, and size.
Data collection
Anthropometric variables were recorded. Blood pressure was measured with a mercury sphygmomanometer after at least a 10-min rest in the seated position. Physical activity, recorded at baseline and at the end of the study was assessed by the Minnesota Leisure Time Physical Activity Questionnaire which has been validated for its use in Spanish men and women [25]. Plasma glucose, total cholesterol (TC), and triglycerides (TG) were measured by standard enzymatic automated methods, plasma HDL-cholesterol by an accelerator selective detergent one (ABX-Horiba Diagnostics, Montpellier, France), and ApoA-I and ApoA-II by immunoturbidimetry, in a PENTRA-400 automated analyzer (ABX-Horiba Diagnostics, Montpellier, France). Low density lipoproteins (LDL) cholesterol was calculated by the Friedewald equation.
HDL isolation
HDL was isolated from plasma by ultracentrifugation with a density gradient preparation method [26], using at once two solutions of different densities, 1.006 g/mL and 1.21 g/mL. To ensure the purity of the HDL fractions, ApoB100 and albumin levels were determined in the samples by automatic immunoturbidimetric methods (ABX-Horiba Diagnostics).
HDL composition
Fatty acids in HDL
HDL lipids were trans-esterified and after methanolysis, 1 mL of saturated NaCl solution was added to stop the reaction and 0.75 mL of hexane to extract the fatty acid methyl esters. Samples were centrifuged at 2212 g for 10 min and the supernatant injected into the chromatograph. The analysis was performed by gas chromatography (Agilent 7890A Series) using a capillary SP-2330 column (30 m × 0.25 mm × 0.2 µm) (Supelco, Bellefonte, USA), coupled to a flame ionization detector.
Lipids and proteins in HDL
Total (TC) and free (FC) cholesterol, TG, and phospholipids in HDL were quantified by automatic enzymatic-colorimetric methods, and ApoA-I and ApoA-II by automatic immunoturbidimetric methods. Determinations were performed in a Cobas-Mira Plus automated analyzer (Roche, Basel, Switzerland) with reagents from Spinreact (Barcelona, Spain). Esterified cholesterol (EC) was quantified as TC minus FC. The TG content of the HDL core was assessed as the ratio between TG and EC in HDL [18].
HDL oxidative/antioxidative status
HDL resistance to oxidation
It was determined by the conjugated dienes formation after copper oxidation of isolated HDL [27]. Briefly, dialyzed HDL (10 mg/dL of HDL cholesterol) was incubated with cupric sulphate (5 µM) in phosphate buffered saline at 37°C for 4h. Absorbance at 234 nm was continuously monitored at 3 min intervals in an INFINITE M200 reader (Tecan Group Ltd). The length of the lag phase was defined as the time (minutes) to the intercept of the tangent of the absorbance curve in the propagation phase with baseline. Propagation rate was expressed as the slope of the tangent (change in absorbance/min). Maximum dienes formation was calculated by the maximum increase of the absorbance at 234 nm using the molar absorbance ε234nm for conjugated dienes (29.5000 · L · mol−1 · cm−1). Determinations were performed in duplicate. We used an HDL pool from healthy volunteers as between assay control. Data from HDL resistance to oxidation were only available from a subsample of 25 volunteers.
Antioxidant compounds in HDL
Carotenoids, retinol, ubiquinol, tocopherols, and phenolic compounds from olive oil and thyme were analyzed by high-pressure liquid chromatography (HPLC) [28, 29]. Compounds were identified by their retention time compared with pure standards or, when unavailable (lutein and β-cryptoxanthin), with compounds obtained and purified in the laboratory. Determinations were run in duplicate.
Paraoxonase 1(PON1) activity assessment
PON1-associated lactonase activity was measured in human sera using 5-thiobutyl butyrolactone (a synthetic lactone) as substrate as previously described [30, 31]. This activity is expressed as U/L (1 U = 1µmol of substrate hydrolyzed per minute).
Nuclear magnetic resonance spectroscopy (NMR) HDL lipoprotein particle count and subclasses
Serum samples were shipped to the National Heart Lung and Blood Institute, National Institutes of Health (NIH; Bethesda, MD, USA). Lipoprotein subclasses measurement was performed by NMR in a Vantera clinical spectrometer, produced by LipoScience (Raleigh, NC, USA). The NMR LipoProfile test by LipoScience involves measurement of the 400 MHz proton NMR spectrum of samples and uses the characteristic signal amplitude of the lipid methyl group broadcasted by every lipoprotein subfraction as the basis for quantification. LipoProfile-3 algorithm was performed to quantify the mean particle size and concentrations of HDL lipoproteins. Subparticle concentrations were determined for three HDL subclasses (large: 8.8–13 nm; medium: 8.2– 8.8 nm; and small: 7.3–8.2 nm) [32].
HDL monolayer fluidity
HDL particle fluidity was determined by the steady-state anisotropy of 1,6-diphenyl-1,3,5-hexatriene (DPH) [18]. Briefly, HDL samples were incubated with DPH 1µM (30 min) and stimulated with a vertically polarized light at 360 nm. Fluorescent emission intensities were detected at 460 nm in a Perkin-Elmer LS5OB spectrofluorometer (Perkin Elmer, Waltham, MA, USA), through a polarizer orientated in parallel and perpendicular to the direction of polarization of the emitted beam. The steady-state fluorescence anisotropy (r) was calculated with the Ip values, and with the grating correction factor of the monochromator (G), using the following formula: r = (Ivv-GIvh)/(Ivv+2GIvh). The steady-state anisotropy refers to the rigidity of the sample, therefore the inverse value of this parameter (1/r) is the fluidity index. Determinations were performed in duplicate.
Cholesterol Efflux determination
Murine J-774A.1 macrophages were labeled for 1 h with TopFluor-Cholesterol, a fluorescent cholesterol probe in which the cholesterol molecule is linked to boron dipyrromethene difluoride (BODIPY) moiety (Avanti Polar Lipids, USA). The labeling of cells was performed in Dulbecco's Modified Eagle's medium (DMEM) containing 0.125 mM of total cholesterol, where TopFluor-Cholesterol accounted for 20 % of total cholesterol. Labeled cells were incubated for 18 h with TO-901317 (3 µM; Sigma-Aldrich, USA) to upregulate ATP-binding cassette (ABC) transporters, in low-glucose DMEM supplemented with bovine serum albumin (BSA; 0.2 %). This assay determines CEC mediated predominantly via ABCA1 but also via ABCG1. Cells were then incubated for 24 h with volunteers’ HDL (100 µg/mL). The Acyl-CoA cholesterol acyltransferase (ACAT) inhibitor Sandoz 58-035 (5 µM; Sigma-Aldrich, USA) was present during the whole experimental procedure. All incubations were performed at 37°C in a humidified incubator. Cells were solubilized with cholic acid (1 %) and mixed on a plate shaker for 3 h at room temperature. Fluorescence intensity of media and cells was monitored in Microplate Reader Synergy HT (BioTekInstruments; USA) at λEx/Em=485/528 nm. CEC was calculated as: [media fluorescence/(media fluorescence + cells fluorescence)] *100. Background efflux was subtracted from values obtained in the presence of HDL. Data were adjusted by the HDL particle number. We used an HDL pool from healthy volunteers as a between assay control. All conditions were run in triplicate. Intra- and inter-assay coefficients of variability (CV%) of the method were 4.87% and 6.61% respectively.
Statistical analyses
Normality of continuous variables was evaluated by probability plots. Non-normally distributed variables were log transformed. Studentt’s T test for related measurements was used for change comparisons. Univariate associations were examined by Pearson’s correlation coefficients. Stepwise mixed linear models were fitted with variables significantly associated with 3-week changes in CEC or fluidity in the univariate analyses. When collinearity between variables exists, separate models were fitted. Models were adjusted by age and sex, and individual level of test subjects as random effect. Significance was defined at the 5% level using a two-tailed test. Analyses were carried out using SPSS software version 21.0 (SPSS, Inc., Chicago, IL).
Results
Baseline characteristics of the subjects are shown in Table 1. Subjects under antilipidemic medication did not change either their dose or type of medication through the study. No changes in physical activity were observed from the beginning to the end of the study. No changes in daily energy intake or those of micro or macronutrients were observed among intervention periods. Table 2 shows pre- and post-intervention values in HDL related variables. A significant increase at post-intervention versus pre-intervention values was observed in CEC (p=0.042), HDL oleic acid concentration (p=0.009), HDL ApoA-I concentration (p=0.014), and antioxidants in HDL: α-tocopherol (p=0.017), β-cryptoxanthin (p<0.001), coenzyme-Q (p=0.005), lutein (p<0.001), retinol (p=0.011), and phenolic compounds (p<0.001).
Table 1.
Baseline characteristics of the participants
| Variable | Mean ±SD |
|---|---|
| Age, years | 55.6 ± 10.0 |
| Gender, male/female | 19/14 |
| Body mass index, kg/m2 | 26.6 ± 4.5 |
| Physical activity, METs.min/w* | 2423 (897–4544) |
| Systolic blood pressure, mmHg | 128.2 ± 14.6 |
| Diastolic blood pressure, mmHg | 73.15 ± 10.0 |
| Glucose, pl, mg/dL | 90.8 ± 11.6 |
| Cholesterol ,pl, mg/dL | |
| Total | 226.4 ± 35.2 |
| LDL | 148.3 ± 28.8 |
| HDL | 53.01 ± 11.13 |
| Triglycerides, pl, mg/dL* | 114.2 (85.5 ± 145.0) |
| Use of antilipemic medication, yes/no | 14/19 |
Values expressed as median (25th-75th percentile).
pl, plasma.
Table 2.
Pre- and post-intervention values in HDL related variables
| Variable | Pre-intervention | Post-intervention |
|---|---|---|
|
| ||
| HDL mediated cholesterol efflux, % | 3.93 ± 1.16 | 4.13 ± 1.40* |
| Fluidity of the HDL monolayer, 1/AU | 4.96 ± 0.48 | 5.00 ± 0.34 |
| HDL composition | ||
| Fatty acids in HDL, % | ||
| Arachidonic | 10.32 ± 5.13 | 10.61 ± 7.06 |
| Eicosatreanoic | 0.77 ± 1.56 | 0.61 ± 1.15 |
| Linoleic | 29.40 ± 9.55 | 27.78 ± 15.36 |
| Oleic | 19.61±7.26 | 20.78 ± 7.15† |
| Palmitic | 22.33 ±14.13 | 23.61 ± 7.27 |
| Stearic | 10.93 ±10.04 | 11.55 ± 6.85 |
| Proteins in HDL, g/L | ||
| Apolipoprotein A-I | 0.63 ± 0.16 | 0.66 ± 0.17† |
| Apolipoprotein A-II | 0.17 ± 0.05 | 0.17 ± 0.04 |
| Lipids in HDL, mg/dL | ||
| Total Cholesterol | 31.84 ± 11.60 | 31.82 ± 12.05 |
| Free Cholesterol | 13.42 ± 6.25 | 12.73 ± 5.86 |
| Esterified cholesterol | 18.41 ± 8.05 | 18.45 ± 8.07 |
| Triglycerides | 7.22 ± 2.09 | 7.28 ± 2.24 |
| Phospholipids | 61.13 ± 18.44 | 60.66 ± 17.71 |
| Oxidative status of HDLa | ||
| Lag time, minb | 34.09 (26.60–52.78) | 35.38 (26.94–46.07) |
| Maximum absorbance, Abs | 0.48 ± 0.10 | 0.47 ± 0.14 |
| Oxidation rate, Abs/s | 7.96 ± 2.52 | 8.14 ± 2.52 |
| Antioxidative status of HDL, µmols/L | ||
| Total phenolic contentb c | 14.41 (0–144.5) | 63.78 (0–585.3)‡ |
| α-tocopherol | 12.64 ± 0.70 | 12.77 ± 0.79* |
| γ-tocopherol | 0.13 ± 0.35 | 0.15 ± 0.44 |
| β-caroteneb | 1.27 (0–3.18) | 1.79 (0–3.45) |
| β -cryptoxanthin | 7.95 ± 2.96 | 8.73 ± 3.44‡ |
| Coenzime-Q | 299.2 ± 192.2 | 324.2 ± 191.1† |
| Luteine | 2.26 ± 1.93 | 2.58 ± 2.02 |
| Retinol | 3.39 ± 1.67 | 3.71 ± 2.01 |
| PON1 activity, U/Ld | 5.18 ± 1.58 | 5.31± 1.74 |
| HDL particle size, µmol/L | ||
| HDL mean size (total)e | 9.24 ± 0.46 | 9.27 ± 0.45 |
| Small HDL particles | 17.71 ± 6.03 | 16.93 ± 5.32 |
| Medium HDL particles | 10.80 ± 6.09 | 9.35 ± 4.96 |
| Large HDL particles | 7.07 ± 3.68 | 7.09 ± 3.53 |
Values expressed as mean and standard deviation. AU, arbitrary units
Conjugated dienes formation curve.
Values expressed as median (25th-75th percentile)
Expressed in nmol/L.
PON1, Paraoxonase-1.
Expressed in nanometers.
P<0.05;
P<0.01;
P<0.001
Cholesterol efflux associations
Table 3 shows the Pearson’s correlation coefficients for the association between changes in CEC and in HDL fluidity, and changes in variables related to HDL composition, oxidative/antioxidative status, and size. CEC was directly related with the fluidity of the HDL (p=0.004). CEC was inversely related with concentration of linoleic acid in HDL (p=0.034) and with that of small HDL particles (p=0.001), but directly related to the concentration in HDL of stearic acid (p=0.003), ApoA-I (p=0.004), ApoA-II (p=0.001), TC (p=0.008), EC (p=0.40), TG (p=0.021), phospholipids (p<0.001), lag time of conjugated dienes formation (p=0.006), concentration in HDL of β-cryptoxanthin (p=0.008), and concentration of medium (p=0.012) and large (p=0.041) HDL particles.
Table 3.
Correlation coefficients (R) between 3-week changes in HDL cholesterol efflux and fluidity of the HDL monolayer and HDL characteristics after olive oil ingestion
| Cholesterol efflux | Fluidity | |||
|---|---|---|---|---|
|
|
||||
| Variable (3-week changes) | R | P value | R | P value |
| Cholesterol efflux, % | 1 | 1 | 0.308 | 0.004 |
| Fluidity, 1/anisotropy (AU) | 0.308 | 0.004 | 1 | 1 |
| HDL Composition | ||||
| Fatty acids in HDL, % | ||||
| Arachidonic | 0.043 | 0.700 | 0.097 | 0.387 |
| Eicosatreanoic | 0.084 | 0.456 | 0.033 | 0.768 |
| Linoleic | −0.235 | 0.034 | 0.057 | 0.613 |
| Oleic | 0.025 | 0.823 | 0.004 | 0.972 |
| Palmitic | 0.128 | 0.256 | −0.155 | 0.156 |
| Stearic | 0.328 | 0.003 | 0.140 | 0.213 |
| Proteins in HDL, g/L | ||||
| Apolipoprotein A-I | 0.309 | 0.004 | 0.123 | 0.264 |
| Apolipoprotein A-II | 0.364 | 0.001 | 0.070 | 0.537 |
| Lipids in HDL, mg/dL | ||||
| Total Cholesterol | 0.287 | 0.008 | −0.010 | 0.927 |
| Free Cholesterol | 0.073 | 0.509 | −0.322 | 0.003 |
| Esterified Cholesterol | 0.225 | 0.040 | 0.229 | 0.036 |
| Triglycerides | 0.251 | 0.021 | 0.383 | <0.001 |
| Phospholipids | 0.382 | <0.001 | 0.186 | 0.091 |
| Triglycerides in HDL core* | −0.041 | 0.713 | 0.220 | 0.044 |
| Oxidative/Antioxidative status of HDL | ||||
| Oxidative status† | ||||
| Lag time, min | 0.331 | 0.006 | −0.049 | 0.606 |
| Maxim absorbance, Abs | 0.053 | 0.708 | 0.125 | 0.375 |
| Oxidation rate, Abs/s | −0.168 | 0.189 | 0.175 | 0.215 |
| Antioxidative status of HDL, µmol/L | ||||
| α-tocopherol | −0.021 | 0.859 | −0.001 | 0.903 |
| γ-tocopherol | −0.022 | 0.850 | 0.069 | 0.549 |
| β-carotene | 0.044 | 0.701 | 0.016 | 0.892 |
| β-cryptoxanthin | 0.306 | 0.008 | 0.034 | 0.772 |
| Coenzime-Q | 0.128 | 0.291 | 0.075 | 0.539 |
| Luteine | 0.211 | 0.066 | 0.065 | 0.575 |
| Retinol | 0.158 | 0.170 | −0.179 | 0.120 |
| Phenolic compounds‡ | 0.138 | 0.182 | 0.127 | 0.271 |
| PON1 activity, U/L | 0.139 | 0.210 | 0.108 | 0.332 |
| HDL particle size, µmol/L | ||||
| HDL mean size (total)¥ | 0.076 | 0.494 | 0.379 | <0.001 |
| Small HDL particles | −0.349 | 0.001 | −0.635 | <0.001 |
| Medium HDL particles | 0.273 | 0.012 | 0.241 | 0.027 |
| Large HDL particles | 0.224 | 0.041 | 0.155 | 0.158 |
AU, arbitrary units. PON1, Paraoxonase-1.
Calculated as triglycerides/esterified cholesterol ratio.
Conjugated dienes formation curve.
nmol/L.
nanometers
Results of the stepwise mixed linear models are shown in Table 4. Given that determinations of the lag time of conjugated dienes were not available in the whole sample we performed Model 1 with all variables which had significance in the univariate analyses, but without entering the lag time in the model. Model 2 shows the results with all variables that had significance in the univariate analyses. Results of these models point out an increase in HDL fluidity, ApoA-I, and resistance against oxidation (lag time of conjugated dienes) as main determinants for CEC. Model 3 shows the results without entering HDL fluidity in the full model. In this case, small HDL particle number appear inversely and significantly related, indicating that it could be a variable which reflects the effect of fluidity on CEC. Thus, we performed the same analyses with fluidity as the dependent variable.
Table 4.
Determinants of 3-week changes in cholesterol efflux in hypercholesterolemic subjects after virgin olive oil ingestion.
| Predictor variable (3-week changes) |
B coefficient | SE | T | P value |
|---|---|---|---|---|
| Model 1* | ||||
| Fluidity of the HDL monolayer | 0.731 | 0.279 | 2.62 | 0.010 |
| Apolipoprotein A1 | 2.54 | 0.73 | 3.45 | 0.001 |
| Model 2† | ||||
| Fluidity of the HDL monolayer | 0.777 | 0.289 | 2.69 | 0.009 |
| Apolipoprotein A1 | 2.47 | 0.892 | 2.77 | 0.007 |
| Lag time of conjugated dienes | 0.008 | 0.004 | 2.36 | 0.022 |
| Model 3‡ | ||||
| Apolipoprotein A1 | 1.92 | 0.94 | 2.04 | 0.046 |
| Lag time of conjugated dienes | 0.008 | 0.004 | 2.26 | 0.027 |
| Small HDL particle number | −0.042 | 0.016 | −2.68 | 0.009 |
Stepwise mixed linear models adjusted for age and sex, and individual level of test subjects as a random effect. SE, standard error.
Model 1, without the lag time of conjugated dienes formation;
Model 2, including all variables;
Model 3, without fluidity of the HDL monolayer
HDL fluidity associations
Table 3 shows the Pearson’s correlation coefficients for the univariate association between changes in HDL fluidity and in CEC, and changes of those variables related to HDL composition, oxidative/antioxidative status, and size. HDL fluidity was inversely related to the concentration of FC (p= 0.003) in HDL, and that of small HDL particles (p<0.001). HDL fluidity was directly related to the concentration of EC in HDL (p=0.036), total TG content (p<0.001), TG in HDL core (p=0.044), HDL mean size (p< 0.001), and with the concentration of medium HDL particles (p=0.027).
Results of the stepwise mixed linear model including all variables which reached significance in the univariate analyses showed that the content of FC and TG in HDL and the concentration of small and medium HDL particles appeared as main determinants of the fluidity of HDL monolayer. However, the strong inverse relationship between small and medium HDL particles number (r= −0.752, p<0.001) (see Supplemental Figure 1) promotes a collinearity in the model distorting the results. Due to this, two models were fitted including or not small or medium HDL particles in them. Results of the models are shown in Table 5. An increase in TG and a decrease in FC levels in HDL, together with a decrease in small HDL particle number or an increase in the HDL mean size appear as main determinants of the fluidity of the HDL particles.
Table 5.
Determinants of 3-week changes in the HDL fluidity in hypercholesterolemic subjects after virgin olive oil ingestion.
| Predictor variable (3-week changes) |
β coefficient | SE | T | P value |
|---|---|---|---|---|
| Model 1* | ||||
| Free cholesterol in HDL | −0.032 | 0.007 | −4.73 | <0.001 |
| Triglycerides in HDL | 0.035 | 0.013 | 2.68 | 0.010 |
| Small HDL particles | −0.025 | 0.004 | −6.28 | <0.001 |
| Model 2† | ||||
| Free cholesterol in HDL | −0.035 | 0.077 | −4.47 | <0.001 |
| Triglycerides in HDL | 0.061 | 0.014 | 4.25 | <0.001 |
| HDL mean size | 0.290 | 0.104 | 2.79 | 0.007 |
Stepwise mixed linear model adjusted for age and sex, and individual level of test subjects as a random effect. SE, standard error.
Model 1, without medium HDL particles.
Model 2, without small HDL particles.
Discussion
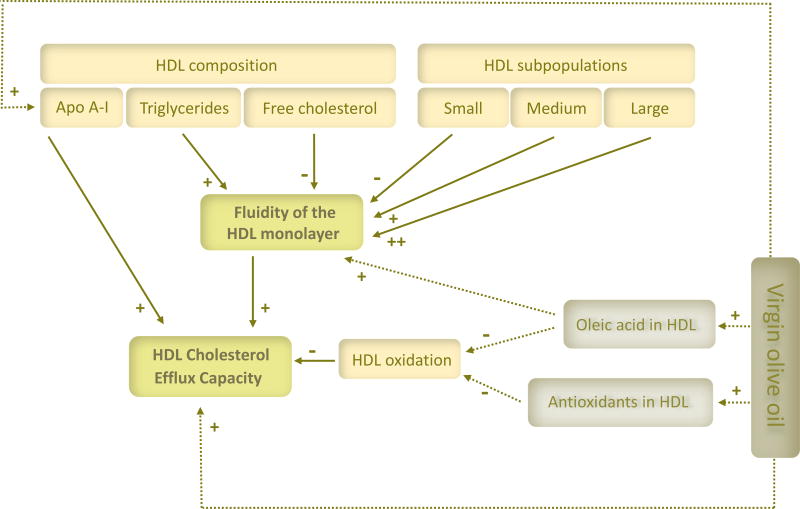
In this study, we examined the association between 3-week changes in CEC and HDL fluidity after VOO ingestion, as well as the association of the changes in both variables with those of several characteristics of the HDL. Our results point out an increase in HDL fluidity and in the concentration of ApoA-I in HDL, and a decrease in HDL oxidative status as major determinants of CEC. A reduction in FC together with an increase in TG in HDL, and a decrease in the concentration of small HDL particle number or an increase in HDL mean size appeared to be the main determinants for HDL fluidity. A schema of the interrelationships is depicted in Figure 1.
Figure 1.
A proposal model for the interrelationship among the main determinants (dense arrows) of HDL cholesterol efflux capacity in hypercholesterolemic subjects after virgin olive oil ingestion. Intermittent arrows point out the expected contribution of the main virgin olive oil components.
The molecular determinants of CEC are still largely unknown, and multiple pathways, which can be modulated by HDL composition and modification, are involved.[33] Mechanisms accounting for CEC include passive diffusion process as well as active pathways mediated by ABCA1, ABCG1, and the scavenger receptor class B type I (SR-BI). The post-transcriptional regulation of cholesterol efflux via ABCA1 includes mechanisms that involve the stabilization of ABCA1 protein by ApoA-I.[4,13] In this sense, oxidation of HDL and ApoA-I results in a selective inhibition of ABCA1-dependent cholesterol efflux from macrophages.[13] The ABCA-1/ApoA-I complex absorbs antioxidants (i.e lutein, zeaxanthin, etc.) which are LDL-protecting molecules, thus contributing to the formation of nascent HDL.[34] ABCA1/ApoA-I activity is fundamental for the formation of the nascent HDL (pre-β1 fractions), which are also efficient acceptors of cell cholesterol via ABCG1.[4] In agreement with our past findings, HDL oxidation decreases HDL fluidity and concomitantly CEC.[11,12] Lipoprotein oxidation promotes the linkage between fatty acids reducing the mobility of the chains which increases the rigidity of the lipid monolayer.[35] In turn, the fluidity of the HDL monolayer influences ApoA-I conformation and binding to HDL.[36] Thus, a close interrelationship exists between HDL oxidative status, ApoA-I, and HDL fluidity, the three main factors which appear related to CEC in our study.
Changes within the HDL lipid core can also promote HDL dysfunction. The main constituents of the HDL lipidome are the phospholipids, followed by EC, and by TG and FC. They are spatially organized according to their hydrophilic properties: phospholipids and FC in the external hydrophilic monolayer which encloses a hydrophobic core rich in EC and TG.[37] An upregulation of the activity of the cholesterol ester transfer protein (CETP), like occurs in insulin resistance states, alters the TG/EC ratio in HDL, the index used to assess the quantity of TG in the core of the HDL.[18] Higher TG contents in the lipid core impair transfer of EC through SR-BI, hindering CEC.[38] Despite all this referred before, data from human in vivo studies show that to maintain a critical pool size of triglyceride-rich lipoproteins, that affects cholesterol from HDL, promotes the efficiency of CEC.[39] A positive correlation between the capacity of plasma to effect cholesterol efflux and plasma levels of TG has been demonstrated in several studies.[39] Thus, the controversy concerning the role of TG on CEC lead us to hypothesize that maintaining an equilibrium in the TG content of HDL versus other lipids could be the clue for the HDL functionality. From our data, the influence of TG and FC on CEC could be mediated through their effect on the HDL fluidity. We have previously described a positive association between the content of TG, oleic acid, and phospholipids in HDL and the fluidity of the lipoprotein, FC content of the HDL being negatively associated.[9] Fluidity depends on the length and saturation of the fatty acids present in the phospholipids of the HDL monolayer. In this sense, a decrease in acyl chain length and an increase in chain unsaturation, such as in the case of oleic acid, increase the fluidity of the lipoprotein.[40,41] In contrast, FC complexes with phospholipids form the lipid rafts which are known to harden the membrane and to decrease its fluidity.[41] An increase in TG, a well know effector for increasing lipid membranes fluidity,[42] and a decrease in FC in HDL were major determinants for the HDL fluidity in our population.
A huge body of data, somewhat contradictory, exists concerning HDL size and CEC. On the basis of the phospholipid content, small HDL more potently promote CEC,[15,43,44] whereas on a particle number basis, large HDL are more effective.[16,43] In this sense, recent studies in peri-menopausal women and elderly adults show CEC to be directly related to large and medium HDL particle concentration and inversely to that of small HDL particle number in multivariate adjusted models.[45,46] In agreement with these data, in our lineal mixed model small HDL particles were inversely related with CEC, when fluidity was not present in the model. When entering the fluidity of the HDL in the model, small HDL particle number was no longer associated with CEC, but it was, however, inversely related with HDL fluidity in the mixed model when HDL fluidity was the dependent variable. In a similar way that occurred for the lipid HDL composition, in our models the size of the HDL lipoprotein seem to exert their effect on CEC through changes in the HDL monolayer fluidity, with an improvement at larger HDL particle size. The fluidity of the HDL, as well as its CEC, is considered to be inversely related to the sphingomyelin content of the HDL. The sphingomyelin content of the HDL subpopulations remains, at present, controversial.[47] The complexity of sphingomyelin species, their distribution in the different HDL particles, and their role in HDL fluidity and CEC is considered a promising target for future studies.[47]
Our study has limitations. First, cholesterol efflux was measured ex vivo, but the transport of the cholesterol to the liver, the second part of the reverse cholesterol transport was not assessed. Because our CEC assay involved the use of cell lines[33] our results may not reflect the real in vivo situation. However, CEC as measured by a similar method with J744 cells, but using ApoB depleted plasma as acceptor, has been shown to be inversely related with cardiovascular risk.[6] A determining limitation in the study is the method used to isolate HDL for measuring CEC. This method limits HDL density to 1.063–1.210 g/mL and does not accurately represent the contribution of pre-β HDL, which is found in the density range 1.210–1.250 g/mL.[48] We have measured ApoA-I concentration as a surrogated marker for ApoA-I functionality in our study. Also, we have related 3-week changes in HDL variables with independence of the olive oil intervention period, the possible confounding effect avoided by the introduction of the individual level of test subjects as random effect in the model. Although this approach has advantages in mitigating the effect from confounding factors, it could buffer the pre-post differences. Also, the fact that our population was hypercholesterolemic subjects, can limit the extrapolation of the results to a more general population. A major strength of this study was its crossover, double-blind, placebo controlled design, which helps to limit the effect from confounding factors such as differences in physical activity or diets in tests subjects. As essential strength of our work, it is one of the first comprehensive association studies between HDL functional characteristics (also covering an intensive lipidomic and metabolomic profile) and the most relevant HDL functionality trait, CEC.
Among factors which can modify CEC and HDL characteristics, besides VOO alone, we have reported that diets rich in VOO and antioxidants such as the Mediterranean diet have shown to increase the CEC together with improvements in HDL oxidative status, composition, ApoA-I, and HDL particle size.[49–51] Also, novel pharmaceutical approaches are being developed for improving HDL characteristics and functionality. Therapies based on the infusion of ApoA-I formulations (rHDL) in humans, increased ApoA-I levels and cholesterol esterification, and caused a transient accumulation of very small HDL species, followed by an enlargement of particles present within medium and large HDL. These changes were concomitant with an increase in the CEC.[52]
In summary, by using a model as VOO consumption in which improvements in HDL characteristics and functionality have been reported,[10,17–24] we looked for the major determinants of the CEC. From our results, an increase in the fluidity of the HDL and in the concentration of ApoA-I in HDL, and a decrease in HDL oxidative status appear as major determinants of CEC. The impact of a reduction in FC, together with an increase in TG in HDL on CEC could be mediated by their effect increasing the fluidity of the HDL monolayer. The same occurs for the decrease in small HDL particle number or the increase in the HDL mean size. Our work point out several promising new therapeutic targets, besides HDL cholesterol levels, for improving HDL functionality in humans through dietary, nutraceutical, or pharmaceutical interventions.
Supplementary Material
Acknowledgments
This study was supported by the Spanish Ministry of Economy (MINECO) financing the projects AGL2009-13517-457 C03-01, C03-02 and C03-3, AGL2012-40144-C01-03, C02-03 and C03-03, and the FPI-fellowship (BES-2010-040766). L.R. is Sara Borell contract (CD14/00275) co-financed by and IISPV. U.C. is a PERIS contract (Plaestratègic de recerca i innovació en salut). A.P. is a Torres Quevedo contract (Subprograma Estatal de Incorporación, Plan Estatal de Investigación Científica y Técnica y de Innovación) co-financed by Eurecat-CTNS. O.C. was supported by a research contract of the ISCIII JR14/00008. CIBEROBN is an initiative of the Health Institute Carlos III. Work done by ATR was supported by the intramural National Heart, Lung and Blood Institute of the National Institutes of Health, in Bethesda, MD. We thank NUPROAS HB for their substantial contribution interpreting the data and revising the manuscript critically, Borges Mediterranean Group for providing the VOO, and Doctor Claude Motta for his priceless help in teaching the fluidity measurement methods.
Footnotes
Conflicts of interest
None declared
References
- 1.Castelli WP, Doyle JT, Gordon T, Hames CG, et al. HDL cholesterol and other lipids in coronary heart disease. The cooperative lipoprotein phenotyping study. Circulation. 1977;55:767–772. doi: 10.1161/01.cir.55.5.767. [DOI] [PubMed] [Google Scholar]
- 2.Voight BF, Peloso GM, Orho-Melander M, Frikke-Schmidt R, et al. Plasma HDL cholesterol and risk of myocardial infarction: A mendelian randomisation study. Lancet. 2012;380:572–580. doi: 10.1016/S0140-6736(12)60312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barter P, Caulfield M, Eriksson M, Grundy S, et al. Effects of Torcetrapib in Patients at High Risk for Coronary Events. N. Engl. J. Med. 2007;357:2109–2122. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 4.Favari E, Chroni A, Tietge UJF, Zanotti I, et al. Cholesterol Efflux and Reverse Cholesterol Transport. Handb. Exp. Pharmacol. 2015;224:181–206. doi: 10.1007/978-3-319-09665-0_4. [DOI] [PubMed] [Google Scholar]
- 5.Mody P, Joshi PH, Khera A, Ayers CR, et al. Beyond Coronary Calcification, Family History, and C-Reactive Protein: Cholesterol Efflux Capacity and Cardiovascular Risk Prediction. J. Am. Coll. Cardiol. 2016;67:2480–2487. doi: 10.1016/j.jacc.2016.03.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rohatgi A, Khera A, Berry JD, Givens EG, et al. HDL Cholesterol Efflux Capacity and Incident Cardiovascular Events. N. Engl. J. Med. 2014;371:2383–2393. doi: 10.1056/NEJMoa1409065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hernáez A, Farràs M, Fitó M. Olive oil phenolic compounds and high-density lipoprotein function. Curr. Opin. Lipidol. 2016;27:47–53. doi: 10.1097/MOL.0000000000000261. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Niimi M, Nishijima K, Waqar AB, et al. Human apolipoprotein A-II protects against diet-induced atherosclerosis in transgenic rabbits. Arterioscler. Thromb. Vasc. Biol. 2013;33:224–231. doi: 10.1161/ATVBAHA.112.300445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Solà R, Baudet MF, Motta C, Maillé M, et al. Effects of dietary fats on the fluidity of human high-density lipoprotein: Influence of the overall composition and phospholipid fatty acids. Biochim. Biophys. Acta (BBA)/Lipids Lipid Metab. 1990;1043:43–51. doi: 10.1016/0005-2760(90)90108-a. [DOI] [PubMed] [Google Scholar]
- 10.Solà R, Motta C, Maille M, Bargalló M, et al. Dietary Monounsaturated Fatty Acids enhance cholesterol efflux from human fibroblasts. Atheroscler. Thromobosisbosis. 1993;13:958–966. doi: 10.1161/01.atv.13.7.958. [DOI] [PubMed] [Google Scholar]
- 11.Bonnefont-Rousselot D, Motta C, Khalil A, Sola R, et al. Physicochemical changes in human high-density lipoproteins (HDL) oxidized by gamma radiolysis-generated oxyradicals. Effect on their cholesterol effluxing capacity. Biochim. Biophys. Acta. 1995;1255:23–30. doi: 10.1016/0005-2760(94)00211-g. [DOI] [PubMed] [Google Scholar]
- 12.Girona J, LaVille AE, Solà R, Motta C, et al. HDL derived from the different phases of conjugated diene formation reduces membrane fluidity and contributes to a decrease in free cholesterol efflux from human THP-1 macrophages. Biochim. Biophys. Acta - Mol. Cell Biol. Lipids. 2003;1633:143–148. doi: 10.1016/s1388-1981(03)00108-2. [DOI] [PubMed] [Google Scholar]
- 13.Besler C, Lüscher TF, Landmesser U. Molecular mechanisms of vascular effects of High-density lipoprotein: Alterations in cardiovascular disease. EMBO Mol. Med. 2012;4:251–268. doi: 10.1002/emmm.201200224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng L, Nukuna B, Brennan ML, Sun M, et al. Apolipoprotein A-I is a selective target for myeloperoxidase-catalyzed oxidation and function impairment in subjects with cardiovascular disease. J. Clin. Invest. 2004;114:529–541. doi: 10.1172/JCI21109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kontush A, Chapman MJ. Functionally defective high-density lipoprotein: a new therapeutic target at the crossroads of dyslipidemia, inflammation, and atherosclerosis. Pharmacol. Rev. 2006;58:342–74. doi: 10.1124/pr.58.3.1. [DOI] [PubMed] [Google Scholar]
- 16.Monette JS, Hutchins PM, Ronsein GE, Wimberger J, et al. Patients with coronary endothelial dysfunction have impaired cholesterol efflux capacity and reduced HDL particle concentration. Circ. Res. 2016;119:83–90. doi: 10.1161/CIRCRESAHA.116.308357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Covas M, Nyyssönen K, Poulsen HE, Kaikkonen J, et al. The Effect of Polyphenols in Olive Oil on Heart Disease Risk Factors. Ann. Intern. Med. 2006;145:333–341. doi: 10.7326/0003-4819-145-5-200609050-00006. [DOI] [PubMed] [Google Scholar]
- 18.Hernáez Á, Fernández-Castillejo S, Farràs M, Catalán Ú, et al. Olive oil polyphenols enhance high-density lipoprotein function in humans: A randomized controlled trial. Arterioscler. Thromb. Vasc. Biol. 2014;34:2115–2119. doi: 10.1161/ATVBAHA.114.303374. [DOI] [PubMed] [Google Scholar]
- 19.Helal O, Berrougui H, Loued S, Khalil A. Extra-virgin olive oil consumption improves the capacity of HDL to mediate cholesterol efflux and increases ABCA1 and ABCG1 expression in human macrophages. Br.J.Nutr. 2013;109:1844–1855. doi: 10.1017/S0007114512003856. [DOI] [PubMed] [Google Scholar]
- 20.Farràs M, Valls RM, Fernández-Castillejo S, Giralt M, et al. Olive oil polyphenols enhance the expression of cholesterol efflux related genes in vivo in humans. A randomized controlled trial. J. Nutr. Biochem. 2013;24:1334–9. doi: 10.1016/j.jnutbio.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 21.Solà R, La Ville AE, Richard JL, Motta C, et al. Oleic acid rich diet protects against the oxidative modification of high density lipoprotein. Free Radic. Biol. Med. 1997;22:1037–45. doi: 10.1016/s0891-5849(96)00490-x. [DOI] [PubMed] [Google Scholar]
- 22.Pedret A, Catalán Ú, Fernández-Castillejo S, Farràs M, et al. Impact of Virgin Olive Oil and Phenol-Enriched Virgin Olive Oils on the HDL Proteome in Hypercholesterolemic Subjects: A Double Blind, Randomized, Controlled, Cross-Over Clinical Trial (VOHF Study) PLoS One. 2015;10:e0129160. doi: 10.1371/journal.pone.0129160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farràs M, Castañer O, Martín-Peláez S, Hernáez Á, et al. Complementary phenol-enriched olive oil improves HDL characteristics in hypercholesterolemic subjects. A randomised, double-blind, crossover, controlled trial. The VOHF study. Mol. Nutr. Food Res. 2015:1758–1770. doi: 10.1002/mnfr.201500030. [DOI] [PubMed] [Google Scholar]
- 24.Fernández-Castillejo S, Valls R-M, Castañer O, Rubió L, et al. Polyphenol rich olive oils improve lipoprotein particle atherogenic ratios and subclasses profile: a randomized, crossover, controlled trial. Mol. Nutr. Food Res. 2016;60:1544–54. doi: 10.1002/mnfr.201501068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elosua R, Marrugat J, Molina L, Pons S, et al. Validation of the Minnesota Leisure Time Physical Activity Questionnaire in Spanish men. The MARATHOM Investigators. Am. J. Epidemiol. Epidemiol. 1994;139:1197–2019. doi: 10.1093/oxfordjournals.aje.a116966. [DOI] [PubMed] [Google Scholar]
- 26.Chapman MJ, Goldstein S, Lagrange D, Laplaud PM. A density gradient ultracentrifugal procedure for the isolation of the major lipoprotein classes from human serum. J. Lipid Res. 1981;22:339–358. [PubMed] [Google Scholar]
- 27.Esterbauer H, Gebicki J, Puhl H, Jürgens G. The role of lipid peroxidation and antioxidants in oxidative modification of LDL. Free Radic. Biol. Med. 1992;13:341–90. doi: 10.1016/0891-5849(92)90181-f. [DOI] [PubMed] [Google Scholar]
- 28.Gleize B, Steib M, André M, Reboul E. Simple and fast HPLC method for simultaneous determination of retinol, tocopherols, coenzyme Q10 and carotenoids in complex samples. Food Chem. 2012;134:2560–2564. doi: 10.1016/j.foodchem.2012.04.043. [DOI] [PubMed] [Google Scholar]
- 29.Rubió L, Serra A, Macià A, Borràs X, et al. Validation of determination of plasma metabolites derived from thyme bioactive compounds by improved liquid chromatography coupled to tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2012;905:75–84. doi: 10.1016/j.jchromb.2012.07.040. [DOI] [PubMed] [Google Scholar]
- 30.Marsillach J, Aragonès G, Beltrán R, Caballeria J, et al. The measurement of the lactonase activity of paraoxonase-1 in the clinical evaluation of patients with chronic liver impairment. Clin. Biochem. 2009;42:91–8. doi: 10.1016/j.clinbiochem.2008.09.120. [DOI] [PubMed] [Google Scholar]
- 31.Fernández-Castillejo S, García-Heredia A-I, Solà R, Camps J, et al. Phenol-enriched olive oils modify paraoxonase-related variables: A randomized, crossover, controlled trial. Mol. Nutr. Food Res. 2017 doi: 10.1002/mnfr.201600932. [DOI] [PubMed] [Google Scholar]
- 32.Jeyarajah EJ, Cromwell WC, Otvos JD. Lipoprotein Particle Analysis by Nuclear Magnetic Resonance Spectroscopy. Clin. Lab. Med. 2006;26:847–870. doi: 10.1016/j.cll.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 33.Ronsein GE, Vaisar T. Inflammation, remodeling, and other factors affecting HDL cholesterol efflux. Curr. Opin. Lipidol. 2017;28:52–59. doi: 10.1097/MOL.0000000000000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niesor EJ, Chaput E, Mary JL, Staempfli A, et al. Effect of compounds affecting ABCA1 expression and CETP activity on the HDL pathway involved in intestinal absorption of lutein and zeaxanthin. Lipids. 2014;49:1233–1243. doi: 10.1007/s11745-014-3958-8. [DOI] [PubMed] [Google Scholar]
- 35.Motta BYC, Gueux E, Mazur A, Rayssiguier Y. Lipid fluidity of triacylglycerol-rich lipoproteins isolated from copper-deficient rats. Br. J. Nutr. 1996;75:767–773. doi: 10.1079/bjn19960180. [DOI] [PubMed] [Google Scholar]
- 36.Pakkanen KI, Duelund L, Qvortrup K, Pedersen JS, et al. Mechanics and dynamics of triglyceride-phospholipid model membranes: Implications for cellular properties and function. Biochim. Biophys. Acta - Biomembr. 2011;1808:1947–1956. doi: 10.1016/j.bbamem.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 37.Salazar J, Olivar LC, Ramos E, Chávez-Castillo M, et al. Dysfunctional High-Density Lipoprotein: An Innovative Target for Proteomics and Lipidomics. Cholesterol. 2015;2015 doi: 10.1155/2015/296417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Greene DJ, Skeggs JW, Morton RE. Elevated Triglyceride Content Diminishes the Capacity of High Density Lipoprotein to Deliver Cholesteryl Esters via the Scavenger Receptor Class B Type I (SR-BI) J. Biol. Chem. 2001;276:4804–4811. doi: 10.1074/jbc.M008725200. [DOI] [PubMed] [Google Scholar]
- 39.Chan DC, Hoang A, Barrett PHR, Wong ATY, et al. Apolipoprotein B-100 and ApoA-II kinetics as determinants of cellular cholesterol efflux. J. Clin. Endocrinol. Metab. 2012;97:1658–1666. doi: 10.1210/jc.2012-1522. [DOI] [PubMed] [Google Scholar]
- 40.Davidson W, Gillotte K, Lund-Katz S, Johnson W, et al. The effect of high density lipoprotein phospholipid acyl chain composition on the efflux of cellular free cholesterol. J. Biol. Chem. 1995;270:5882–5890. doi: 10.1074/jbc.270.11.5882. [DOI] [PubMed] [Google Scholar]
- 41.Simopoulos A, Cleland L. Omega-6/Omega-3 Essential Fatty Acid Ratio. The Scientific Evidence; Karger: 2003. [Google Scholar]
- 42.Hong SS, Kim SH, Lim SJ. Effects of triglycerides on the hydrophobic drug loading capacity of saturated phosphatidylcholine-based liposomes. Int. J. Pharm. 2015;483:142–150. doi: 10.1016/j.ijpharm.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 43.Camont L, Chapman MJ, Kontush A. Biological activities of HDL subpopulations and their relevance to cardiovascular disease. Trends Mol. Med. 2011;17:594–603. doi: 10.1016/j.molmed.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 44.Sankaranarayanan S, Oram JF, Asztalos BF, Vaughan AM, et al. Effects of acceptor composition and mechanism of ABCG1-mediated cellular free cholesterol efflux. J. Lipid Res. 2009;50:275–84. doi: 10.1194/jlr.M800362-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.El Khoudary SR, Hutchins PM, Matthews KA, Brooks MM, et al. Cholesterol efflux capacity and subclasses of HDL particles in healthy women transitioning through menopause. J. Clin. Endocrinol. Metab. 2016;101:3419–3428. doi: 10.1210/jc.2016-2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mutharasan RK, Thaxton CS, Berry J, Daviglus ML, et al. HDL Efflux Capacity, HDL Particle Size and High-Risk Carotid Atherosclerosis in a Cohort of Asymptomatic Older Adults: The Chicago Healthy Aging Study. J. Lipid Res. 2017 doi: 10.1194/jlr.P069039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martínez-Beamonte Roberto, Lou-Bonafonte JM, Martínez-Gracia MV, et al. Sphingomyelin in High-Density Lipoproteins: Structural Role and Biological Function. Int. J. Mol. Sci. 2013;14:7716–7741. doi: 10.3390/ijms14047716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosenson RS, Brewer HB, Ansell BJ, Barter P, et al. Dysfunctional HDL and atherosclerotic cardiovascular disease. Nat. Rev. Cardiol. 2016;13:48–60. doi: 10.1038/nrcardio.2015.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Solá R, Fitó M, Estruch R, Salas-Salvadó J, et al. Effect of a traditional Mediterranean diet on apolipoproteins B, A-I, and their ratio: A randomized, controlled trial. Atherosclerosis. 2011;218:174–180. doi: 10.1016/j.atherosclerosis.2011.04.026. [DOI] [PubMed] [Google Scholar]
- 50.Hernáez Á, Castañer O, Elosua R, Pintó X, et al. Mediterranean Diet Improves High-Density Lipoprotein Function in High-Cardiovascular-Risk Individuals: A Randomized Controlled Trial. Circulation. 2017;135:633–643. doi: 10.1161/CIRCULATIONAHA.116.023712. [DOI] [PubMed] [Google Scholar]
- 51.Damasceno NRT, Sala-Vila A, Cofán M, Pérez-Heras AM, et al. Mediterranean diet supplemented with nuts reduces waist circumference and shifts lipoprotein subfractions to a less atherogenic pattern in subjects at high cardiovascular risk. Atherosclerosis. 2013;230:347–353. doi: 10.1016/j.atherosclerosis.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 52.Diditchenko S, Gille A, Pragst I, Stadler D, et al. Novel formulation of a reconstituted high-density lipoprotein (CSL112) dramatically enhances ABCA1-dependent cholesterol efflux. Arterioscler. Thromb. Vasc. Biol. 2013;33:2202–2211. doi: 10.1161/ATVBAHA.113.301981. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.