Abstract
Purpose
Postoperative adjuvant chemotherapy is usually prescribed to improve the survival of patients with advanced gastric cancer who undergo curative surgery. This study was designed to determine the impact that the degree of compliance with chemotherapy has on the prognosis of patients with gastric cancer.
Materials and Methods
Among 252 patients with stage III gastric cancer who underwent curative surgery between July 2004 and December 2014, 85 patients were postoperatively treated with S-1, the oral fluoropyrimidine derivative, 23 received no chemotherapy, and 144 received other regimens. Overall survival was compared between the complete compliance group (who received 8 cycles of S-1 chemotherapy, n=44) and the incomplete compliance group (who received less than 8 cycles of S-1 chemotherapy, n=41). Factors that influenced patient compliance with chemotherapy were also analyzed.
Results
The overall 5-year survival rate was significantly different between the complete chemotherapy and incomplete chemotherapy groups (80.0% vs. 42.7%, P<0.001). Based on univariate and multivariate survival analyses of patients who received S-1 chemotherapy, the independent prognostic factors were tumor, node, and metastasis (TNM) stage (IIIa vs. IIIb vs. IIIc) and compliance with chemotherapy. TNM stage and age are significant factors that influence compliance with chemotherapy.
Conclusions
TNM stage and compliance with chemotherapy are independent prognostic factors in patients with stage III gastric cancer who received postoperative chemotherapy. TNM stage and age are significant factors that influence patient compliance with chemotherapy.
Keywords: Compliance, Drug therapy, Stomach neoplasms
INTRODUCTION
Gastric cancer is the third most common cancer and the second leading cause of cancer-related death worldwide [1]. Curative resection is the treatment of choice for patients with gastric cancer; however, according to several recent large-scale studies, only 30%–50% of patients with gastric cancer remain disease-free after undergoing radical resections [2,3]. A large proportion of patients are diagnosed with loco-regionally advanced disease, which results in high loco-regional and distant recurrence rates, with poor survival [4,5]. For patients with potentially curable gastric cancer, randomized trials have indicated that a number of approaches, including postoperative adjuvant chemotherapy, can provide significant survival benefits over surgery alone [6,7]. Following 2 large, randomized clinical trials (CLASSIC and ACTS-GC trials), postoperative chemotherapy with either S-1 or capecitabine + oxaliplatin was determined to be the current standard of care for gastric cancer adjuvant therapy in East Asia [8,9,10]. Patients with stage III gastric cancer generally receive postoperative chemotherapy to improve their survival [11]. Therefore, in East Asia, extended resection followed by adjuvant chemotherapy is the standard of treatment. Based on multiple meta-analyses of adjuvant chemotherapy, postoperative chemotherapy is associated with a survival benefit, especially in East Asian patients [12]. However, there are many patients who stop chemotherapy prematurely for various reasons [13,14]. Nevertheless, the risk factors for poor compliance with adjuvant S-1 chemotherapy are not well known. In this study, we investigated factors affecting compliance with chemotherapy and survival rates according to the compliance of patients with the same disease stage (stage III gastric cancer) who were treated with the same regimen (S-1). This study was designed to determine the impact of the degree of compliance with chemotherapy on the prognosis of patients with gastric cancer.
MATERIALS AND METHODS
Patients
There were 252 patients with stage III (IIIa, IIIb, and IIIc) gastric cancer who underwent curative surgery between July 2004 and December 2014 at Hanyang University Seoul Hospital. All surgeries were performed by a single surgeon, and all patients who were treated with other regimens (144 patients) were excluded. All patients were diagnosed with histologically-proven stage III gastric cancer and received D2 or more extensive lymph-node dissection with R0 surgery; had no hepatic, peritoneal, or distant metastases; were 20 to 80 years of age; had received no previous cancer treatment; and had adequate organ function (leukocyte count of at least 4,000 per cubic millimeter or the lower limit of the normal range, platelet count of at least 100,000 per cubic millimeter, total bilirubin levels of no more than 1.5 mg/dL, aspartate aminotransferase and alanine aminotransferase levels of no more than 2.5 times the upper limit of the normal range, and serum creatinine levels no greater than the upper limit of the normal range).
Stage classification and evaluation of the resected specimens were performed in accordance with the 7th American Joint Committee on Cancer tumor, node, and metastasis (TNM) staging system guidelines. In the S-1 group, administration of S-1 started 28 to 35 days after surgery and continued for one year. The treatment regimen consisted of 6-week cycles, which, in principle, consisted of 80 mg of oral S-1 per square meter of body-surface area per day for 4 weeks, followed by no chemotherapy for 2 weeks. Specifically, during the treatment weeks, patients with body-surface areas of less than 1.25 m2 received 80 mg daily, those with body-surface areas of 1.25 m2 or more, but less than 1.5 m2, received 100 mg daily, and those with body-surface areas of 1.5 m2 or more received 120 mg daily. This 6-week cycle was repeated during the first year after surgery. If patients had hematologic toxic effects of grades 3 or 4 (highest possible grade) or non-hematologic toxic effects of grades 2, 3, or 4, their daily dose was reduced, from 120 mg to 100 mg, 100 mg to 80 mg, or 80 mg to 50 mg, respectively. Adverse events were assessed according to the Common Toxicity Criteria of the National Cancer Institute (version 2.0). There were 85 patients who were postoperatively treated with S-1, and 23 patients received surgery only. In the postoperative S-1 group, 44 patients completed 8 cycles of S-1 chemotherapy, and 41 received less than 8 cycles of S-1 chemotherapy.
Follow-up
In the S-1 group, the blood test results and clinical findings were assessed at 2-week intervals, during treatment with S-1. Medical history, physical examination, general blood tests, biochemical tests, tumor marker tests, chest radiography, gastroscopic examination, abdominal ultrasonography, and abdominal computed tomography were regularly performed during postsurgical ambulatory follow-up. Patients underwent at least one imaging study (such as abdomen & chest computed tomography and gastrofiberscopy), at 3-month intervals for the first year after surgery, and at 6-month intervals until 5 years after surgery. Individual patients were followed-up for 5 years, from the date of random assignment.
Statistical analysis
Overall survival was defined as the period between surgery and death. All deaths, including those from other diseases, were considered death events. Data for patients who had not had an event were censored at the date of the final observation. The Kaplan-Meier method was used to estimate cumulative survival. The Cox proportional-hazards model was used to calculate the hazard ratios (HR). All P-values calculated in the subgroup analysis were 2-sided and were not adjusted for multiple testing. A P-value less than 0.05 was considered to indicate statistical significance.
RESULTS
Clinical characteristics
In total, 108 patients who underwent surgery between July 2004 and December 2014 were enrolled, including 85 in the S-1 group and 23 in the surgery-only group. The reasons for postoperative adjuvant chemotherapy ineligibility in 23 patients were as follows: laboratory test values at enrollment did not meet the protocol requirements (8 patients), and patient refusal to receive chemotherapy (15 patients). Table 1 shows the baseline clinical characteristics, surgical procedures, and pathological findings. Patients in the chemotherapy group were younger in age and had more advanced disease stages, higher preoperative body mass indices, and lower post-operative weight losses (%) than patients in the surgery-only group, all of which were statistically significant differences.
Table 1. Comparison of clinicopathologic factors in stage III gastric cancer patients between adjuvant chemotherapy group and surgery only group.
| Variables | Chemotherapy group (n=85) | Surgery only group (n=23) | P-value | |
|---|---|---|---|---|
| Sex | NS | |||
| Male | 59 (69.4) | 14 (60.9) | ||
| Female | 26 (30.5) | 9 (39.1) | ||
| Age (yr) | 0.002 | |||
| <60 | 38 (44.7) | 2 (8.7) | ||
| ≥60 | 47 (55.3) | 21 (91.3) | ||
| Performance status | NS | |||
| 0 | 16 (18.8) | 5 (21.7) | ||
| 1 or 2 | 69 (81.2) | 18 (78.3) | ||
| Tumor size (cm) | NS | |||
| ≤5 | 29 (34.1) | 7 (30.4) | ||
| >5 | 56 (65.9) | 16 (69.6) | ||
| Histology | NS | |||
| Differentiated | 25 (29.4) | 5 (21.7) | ||
| Undifferentiated | 60 (70.6) | 18 (78.3) | ||
| TNM stage* | <0.001 | |||
| IIIa | 23 (27.1) | 23 (100) | ||
| IIIb | 29 (34.1) | 0 (0) | ||
| IIIc | 33 (38.8) | 0 (0) | ||
| Body mass index | 0.049 | |||
| <18.5 | 0 (0) | 1 (4.3) | ||
| 18.5–25.0 | 55 (64.7) | 18 (78.3) | ||
| >25.0 | 30 (35.3) | 4 (17.4) | ||
| Hemoglobin (gm/dL) | NS | |||
| <12 | 33 (38.8) | 10 (43.5) | ||
| ≥12 | 52 (61.2) | 13 (56.5) | ||
| Surgery | NS | |||
| Subtotal gastrectomy | 52 (61.2) | 11 (47.8) | ||
| Total gastrectomy | 33 (38.8) | 12 (52.2) | ||
| Postoperative weight loss (%) | <0.001 | |||
| <5 | 0 (0) | 4 (17.4) | ||
| <10 | 5 (5.9) | 2 (8.7) | ||
| ≥10 | 40 (47.1) | 16 (69.6) | ||
Values are presented as number (%).
NS = not significant; TNM = tumor, node, and metastasis; AJCC = American Joint Committee on Cancer.
*Classification according to the 7th edition of the AJCC staging system.
Chemotherapy compliance-related characteristics
Table 2 shows the distribution of clinicopathological factors between the incomplete treatment and the complete treatment groups. The incomplete group showed older median age (64 years vs. 60 years) and higher TNM stage compared to the complete group. These differences were statistically significant.
Table 2. Distribution of clinicopathological factors according to the compliance of S-1 chemotherapy.
| Variables | Incomplete group (cycle<8) (n=41) | Complete group (cycle=8) (n=44) | P-value | |
|---|---|---|---|---|
| Sex | NS | |||
| Male | 29 (70.7) | 30 (68.2) | ||
| Female | 12 (29.3) | 14 (31.8) | ||
| Age (yr) | 0.037 | |||
| Median | 64 | 60 | ||
| Performance status | NS | |||
| 0 | 7 (17.1) | 9 (20.6) | ||
| 1–2 | 34 (82.9) | 35 (79.4) | ||
| Surgery | NS | |||
| Subtotal gastrectomy | 25 (61.0) | 27 (61.4) | ||
| Total gastrectomy | 16 (39.0) | 17 (38.6) | ||
| TNM stage* | 0.035 | |||
| IIIa | 6 (14.6) | 17 (38.6) | ||
| IIIb | 15 (36.6) | 14 (31.8) | ||
| IIIc | 20 (48.8) | 13 (29.6) | ||
| Histology | NS | |||
| Differentiated | 12 (29.3) | 13 (29.5) | ||
| Undifferentiated | 29 (70.7) | 31 (70.5) | ||
| BMI (kg/m2) | NS | |||
| Median | 22.70 | 23.60 | ||
| Hemoglobin (g/dL) | NS | |||
| Median | 12.00 | 12.95 | ||
| Weight loss (%) | NS | |||
| Median | 12.00 | 9.15 | ||
Values are presented as number (%).
NS = not significant; BMI = body mass index; TNM = tumor, node, and metastasis; AJCC = American Joint Committee on Cancer.
*Classification according to the 7th edition of the AJCC staging system.
Table 3 shows the HRs for mortality according to number of completed cycles of adjuvant S-1 chemotherapy. The HR for death in patients in the S-1 group who completed 8 cycles, compared to those in the surgery-only group, was 0.107 (95% confidence interval [CI], 0.023–0.500; P=0.004). The HRs for death in patients in the S-1 group who completed 1 to 7 cycles versus the surgery-only group were not significantly different. Table 4 lists the causes of non-compliance with chemotherapy. Treatment was withdrawn due to detection of metastases or relapse in 16 patients, and doctors terminated chemotherapy due to adverse events, including leukopenia, anemia, and thrombocytopenia, in 15 patients. Patient refusal (because of economic difficulty or patient fatigue) to continue chemotherapy was observed in 10 patients.
Table 3. Mortality hazard ratios according to number of completed cycles of adjuvant S-1 chemotherapy, multivariate-adjusted.
| Chemotherapy group | No. of patients (%) | Overall survival | ||
|---|---|---|---|---|
| HR | 95% CI | P-value | ||
| 0 cycle | 23 | 1.000 | 0.004 | |
| 1 cycle | 4 (5) | 4.576 | 0.670–31.241 | 0.121 |
| 2 cycles | 10 (12) | 0.560 | 0.120–2.605 | 0.460 |
| 3 cycles | 4 (5) | 0.384 | 0.053–2.799 | 0.345 |
| 4 cycles | 9 (11) | 0.601 | 0.124–3.340 | 0.601 |
| 5 cycles | 5 (6) | 0.153 | 0.013–1.986 | 0.153 |
| 6 cycles | 8 (9) | 0.176 | 0.025–1.233 | 0.080 |
| 7 cycles | 1 (1) | 0.000 | 0.000 | 0.980 |
| 8 cycles (complete) | 44 (51) | 0.107 | 0.023–0.500 | 0.004 |
HR = hazard ratio; CI = confidence interval.
Table 4. Causes of non-compliance of chemotherapy and number of cycles.
| Causes | No. of cycles | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 cycle | 2 cycles | 3 cycles | 4 cycles | 5 cycles | 6 cycles | 7 cycles | Total | |
| Disease progression | 1 (25) | 4 (40) | 2 (50) | 7 (78) | 0 (0) | 2 (25) | 0 (0) | 16 (39) |
| Toxic complication | 2 (50) | 3 (30) | 1 (25) | 1 (11) | 3 (60) | 5 (63) | 0 (0) | 15 (37) |
| Patient's refusal | 1 (25) | 3 (30) | 1 (25) | 1 (11) | 2 (40) | 1 (12) | 1 (100) | 10 (24) |
| Total | 4 | 10 | 4 | 9 | 5 | 8 | 1 | 41 |
Values are presented as number (%).
Univariate survival analysis of patients who underwent postoperative adjuvant chemotherapy
Table 5 lists factors that correlate with survival outcomes. Among the postoperative adjuvant chemotherapy group, the number of cycles of chemotherapy (less than 8 cycles vs. 8 cycles; P=0.001) and TNM stage (IIIa vs. IIIb vs. IIIc; P=0.005) were statistically significant differences.
Table 5. Univariate survival analysis in postoperative adjuvant chemotherapy patients.
| Variables | No. of patients (%) (n=85) | Mean±standard deviation | P-value | |
|---|---|---|---|---|
| Sex | NS | |||
| Male | 59 (69.4) | 73.5±6.7 | ||
| Female | 26 (30.6) | 83.7±11.8 | ||
| Age (yr) | NS | |||
| <60 | 38 (44.7) | 95.3±9.3 | ||
| ≥60 | 47 (55.3) | 62.4±7.6 | ||
| Performance status | NS | |||
| 0 | 16 (18.8) | 70.0±10.9 | ||
| 1 or 2 | 69 (81.2) | 85.5±7.7 | ||
| Surgery | NS | |||
| Subtotal gastrectomy | 52 (61.2) | 79.5±6.8 | ||
| Total gastrectomy | 33 (38.8) | 73.2±10.7 | ||
| Size (cm) | NS | |||
| ≤5 | 29 (34.1) | 83.8±8.5 | ||
| >5 | 56 (65.9) | 79.5±8.4 | ||
| Histology | NS | |||
| Differentiated | 25 (29.4) | 55.8±8.5 | ||
| Undifferentiated | 60 (70.6) | 90.6±8.0 | ||
| BMI (kg/m2) | NS | |||
| 18.5–25.0 | 55 (64.7) | 75.3±8.4 | ||
| >25.0 | 30 (35.3) | 88.2±8.2 | ||
| Hemoglobin (gm/dL) | NS | |||
| <12 | 33 (38.8) | 71.3±9.1 | ||
| ≥12 | 52 (61.2) | 85.9±8.4 | ||
| Postoperative weight loss (%) | NS | |||
| <10 | 45 (52.9) | 93.3±9.8 | ||
| ≥10 | 40 (47.1) | 71.0±8.5 | ||
| Cycles of chemotherapy | 0.001 | |||
| 1–7 | 41 (48.2) | 64.6±9.7 | ||
| 8 | 44 (51.8) | 86.5±6.6 | ||
| TNM stage* | 0.005 | |||
| IIIa | 23 (27.1) | 84.5% | ||
| IIIb | 29 (34.1) | 78.0% | ||
| IIIc | 33 (38.8) | 36.6% | ||
NS = not significant; BMI = body mass index; TNM = tumor, node, and metastasis; AJCC = American Joint Committee on Cancer.
*Classification according to the 7th edition of the AJCC staging system.
Multivariate analyses of survival HRs in patients who underwent postoperative chemotherapy
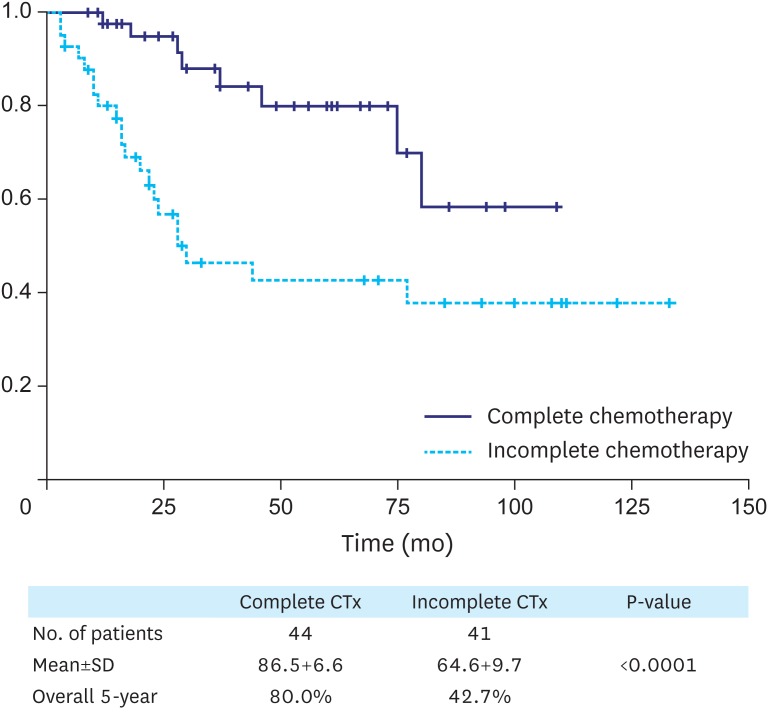
Table 6 shows the survival HRs of patients with stage III gastric cancer. The number of chemotherapy cycles and TNM stage showed statistically significant differences. The complete compliance group had an HR that was 3 times greater than that of the incomplete compliance group (HR, 3.457; P=0.005). Fig. 1 shows the Kaplan-Meier survival analysis for patients with stage III gastric cancer who were treated with 1–7 cycles or 8 cycles of S-1 adjuvant chemotherapy. According to this analysis, patients of the complete chemotherapy group showed a survival advantage compared to patients who received incomplete chemotherapy.
Table 6. Survival hazard ratios in postoperative chemotherapy patients.
| Variables | P-value | HR | 95% CI | |
|---|---|---|---|---|
| TNM stage* | ||||
| IIIa | - | - | - | |
| IIIb | 0.225 | 0.456 | 0.128–1.623 | |
| IIIc | 0.002 | 0.247 | 0.100–0.611 | |
| No. of cycles (chemotherapy) | ||||
| 1–7 | 0.005 | 3.475 | 1.462–8.257 | |
| 8 | - | - | - | |
HR = hazard ratio; CI = confidence interval; TNM = tumor, node, and metastasis; AJCC = American Joint Committee on Cancer.
*Classification according to the 7th edition of the AJCC staging system.
Fig. 1.
Kaplan-Meier survival analysis of patients with stage III gastric cancer who were treated with 1–7 cycles (incomplete chemotherapy, blue) or 8 cycles (complete chemotherapy, green) of S-1 adjuvant chemotherapy.
Ctx = cytotoxic chemotherapy; SD = standard deviation.
DISCUSSION
The use of S-1 chemotherapy for patients with stage III gastric cancer has been thought to effectively improve their survival. However, a study on chemotherapy compliance has not been previously conducted on patients with stage III gastric cancer. To our knowledge, our study is the first investigation into the relationship between the degree of chemotherapy compliance and the prognosis of patients with gastric cancer.
Many randomized trials [6,7,15,16] have shown survival rate benefits with adjuvant chemotherapy for patients with advanced gastric cancer after D2 gastrectomy, compared to patients who underwent surgery only. Based on the results of these trials, postoperative adjuvant chemotherapy is generally administered to patients with stage III gastric cancer [17]. However, planned chemotherapy could be terminated early, due to cancer recurrence, severe side effects, patient refusal, and other causes [18,19]. Our data show that patients with stage III gastric cancer who completed S-1 chemotherapy showed better survival than those who failed to complete the regimen. To investigate the effects of continuing adjuvant S-1 for one year on the prognosis of patients with gastric cancer, Eun et al. [20] analyzed the survival of 49 patients with curatively resected stage II and III gastric cancers. Patients who continued S-1 for one year had significantly increased rates of disease-free survival (P<0.001) and overall survival (P=0.001) compared to patients who discontinued S-1 during the first year. The multivariate analysis indicated poor outcomes for patients with stage III disease and those who discontinued S-1 treatment. The investigators concluded that discontinuing S-1 treatment may be an unfavorable factor in the prevention of recurrence and stressed that S-1 adjuvant treatment should be continued for one year, if possible, with the proper management of toxicities. Aoyama et al. [21] reported that creatinine clearance (CCr) was the only significant independent factor for predicting the 6-month continuation of adjuvant chemotherapy with S-1. The 6-month continuation rate was 72.9% in patients with a CCr of at least 60 mL/min and 40.0% in patients with a CCr <60 mL/min (P=0.015). Adverse events occurred more frequently and earlier in patients with a CCr <60 mL/min than in those with a CCr ≥60 mL/min. In another study, Aoyama et al. [22] analyzed the importance of postoperative weight loss as an independent risk factor for the continuation of adjuvant chemotherapy with S-1. The 5-year overall survival rate for patients with a weight loss (BWL) of <15% was 59.9%, while that of patients with a BWL of ≥15% was 36.4% (P=0.004). Univariate and multivariate analyses for overall survival demonstrated that pathological T factor and BWL were significant risk factors. The authors concluded that severe postoperative BWL, which is closely associated with poor S-1 compliance, is an important risk factor for survival. In a multicenter retrospective study, Yamashita et al. [23] analyzed risk factors for poor compliance with adjuvant S-1 chemotherapy for gastric cancer. Of 359 patients, 252 (70.2%) continued adjuvant S-1 chemotherapy until 12 months after surgery. Older age (>65 years) and postoperative infectious complications (Clavien-Dindo grade III or higher) correlated significantly with low compliance with S-1 for 12 months (P=0.008 and P=0.042, respectively). These 2 factors also showed significant associations with low cumulative continuation rates (log-rank P<0.001 and P=0.018). They concluded that older age, particularly age over 65 years, and postoperative infectious complications were independent risk factors for poor compliance with adjuvant S-1 chemotherapy for gastric cancer.
Di Costanzo et al. [24] reported that the key factors that are predictive of adjuvant chemotherapy non-completion are patient age and performance status. In our study, the completion rate for patients who received S-1 chemotherapy was only 51%. The reasons for withdrawal of S-1 chemotherapy included patient refusal to continue treatment, due to unbearable side effects or other factors (61%, 25 patients), and doctor decisions to terminate treatment because of disease progression (39%, 16 patients). In the comparative analysis according to compliance with S-1 chemotherapy, the group that completed chemotherapy was composed of younger patients (P=0.037) and those with lower disease stage (P=0.035) than those in the group that did not complete chemotherapy. The median BWL was lower in the complete group than in the incomplete group (9.15% vs. 12.0%), but this difference was not statistically significant. In the univariate and multivariate analyses, TNM substage and compliance with chemotherapy were independent prognostic factors in patients with stage III gastric cancer who were treated with postoperative S-1 chemotherapy. Significant influencing factors for the compliance with chemotherapy were TNM substage and age. Our study confirms the findings of previous reports, that patients with stage III gastric cancer who fail to complete adjuvant S-1 chemotherapy show worse survival than those who complete this treatment.
To confirm whether completion of 5FU-based adjuvant chemotherapy is a major prognostic factor for the survival of elderly patients with stage III colon cancer, Morris et al. [25] previously studied a population-based series from Australia. In their study, patients who failed to complete chemotherapy showed worse cancer-specific survival than those who completed treatment (HR, 2.24; 95% CI, 1.66–3.03; P<0.001) and those who were treated with surgery alone (HR, 1.37; 95% CI, 1.09–1.72; P=0.008). Their analyses found that treatment in district or rural hospitals, low socioeconomic index, and treatment by a low-volume surgeon were independent predictors for failure to complete chemotherapy. The authors suggested that further prospective studies are needed to better identify the physiological and socioeconomic factors that are responsible for failure to complete chemotherapy, so that appropriate health service delivery improvements can be made.
Our study has some limitations that should be considered when interpreting the results. One of the main limitations of our study is its retrospective design, and there were a variety of factors in each group. Since the number of patients who did not complete chemotherapy was limited, the effects of incomplete chemotherapy were limited. Considering the various factors that we investigated, further research is required to identify the factors that could increase postoperative adjuvant chemotherapy completion rates and ultimately improve the prognosis of patients with gastric cancer.
In conclusion, higher TNM stage is associated with poorer prognoses, and higher compliance with chemotherapy is associated with improved prognoses, in patients with stage III gastric cancer who received postoperative chemotherapy. Higher compliance with chemotherapy is in turn associated with lower TNM stage and younger age. As compliance is associated with improved prognosis, providers should consider educating patients who are at higher risk of failing to complete chemotherapy on the importance of completing the procedure.
ACKNOWLEDGMENTS
We would like to thank Editage (www.editage.co.kr) for English language editing.
Footnotes
- Conceptualization: K.S.J.
- Data curation: J.Y.J.
- Formal analysis: K.S.J.
- Investigation: K.M.G.
- Writing - original draft: J.S.H.
- Writing - review & editing: J.S.H., J.Y.J., K.M.G.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Rugge M, Fassan M, Graham D. Epidemiology of gastric cancer. In: Strong VE, editor. Gastric Cancer: Principles and Practice. Cham: Springer; 2015. pp. 23–34. [Google Scholar]
- 2.Orditura M, Galizia G, Sforza V, Gambardella V, Fabozzi A, Laterza MM, et al. Treatment of gastric cancer. World J Gastroenterol. 2014;20:1635–1649. doi: 10.3748/wjg.v20.i7.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wagner AD, Unverzagt S, Grothe W, Kleber G, Grothey A, Haerting J, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. 2010:CD004064. doi: 10.1002/14651858.CD004064.pub3. [DOI] [PubMed] [Google Scholar]
- 4.Kilic L, Ordu C, Yildiz I, Sen F, Keskin S, Ciftci R, et al. Current adjuvant treatment modalities for gastric cancer: from history to the future. World J Gastrointest Oncol. 2016;8:439–449. doi: 10.4251/wjgo.v8.i5.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fuentes E, Ahmad R, Hong TS, Clark JW, Kwak EL, Rattner DW, et al. Adjuvant therapy completion rates in patients with gastric cancer undergoing perioperative chemotherapy versus a surgery-first approach. J Gastrointest Surg. 2016;20:172–179. doi: 10.1007/s11605-015-2954-5. [DOI] [PubMed] [Google Scholar]
- 6.Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KW, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy CLASSIC: a phase 3 open-label, randomised controlled trial. Lancet. 2012;379:315–321. doi: 10.1016/S0140-6736(11)61873-4. [DOI] [PubMed] [Google Scholar]
- 7.Diaz-Nieto R, Orti-Rodríguez R, Winslet M. Post-surgical chemotherapy versus surgery alone for resectable gastric cancer. Cochrane Database Syst Rev. 2013:CD008415. doi: 10.1002/14651858.CD008415.pub2. [DOI] [PubMed] [Google Scholar]
- 8.Lee JH, Kim JG, Jung HK, Kim JH, Jeong WK, Jeon TJ, et al. Clinical practice guidelines for gastric cancer in Korea: an evidence-based approach. J Gastric Cancer. 2014;14:87–104. doi: 10.5230/jgc.2014.14.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noh SH, Park SR, Yang HK, Chung HC, Chung IJ, Kim SW, et al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy CLASSIC: 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:1389–1396. doi: 10.1016/S1470-2045(14)70473-5. [DOI] [PubMed] [Google Scholar]
- 10.Miceli R, Tomasello G, Bregni G, Di Bartolomeo M, Pietrantonio F. Adjuvant chemotherapy for gastric cancer: current evidence and future challenges. World J Gastroenterol. 2014;20:4516–4525. doi: 10.3748/wjg.v20.i16.4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4) Gastric Cancer. 2017;20:1–19. doi: 10.1007/s10120-016-0622-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foo M, Leong T. Adjuvant therapy for gastric cancer: current and future directions. World J Gastroenterol. 2014;20:13718–13727. doi: 10.3748/wjg.v20.i38.13718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takahari D, Hamaguchi T, Yoshimura K, Katai H, Ito S, Fuse N, et al. Survival analysis of adjuvant chemotherapy with S-1 plus cisplatin for stage III gastric cancer. Gastric Cancer. 2014;17:383–386. doi: 10.1007/s10120-013-0264-8. [DOI] [PubMed] [Google Scholar]
- 14.Fujitani K, Yang HK, Mizusawa J, Kim YW, Terashima M, Han SU, et al. Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor REGATTA: a phase 3, randomised controlled trial. Lancet Oncol. 2016;17:309–318. doi: 10.1016/S1470-2045(15)00553-7. [DOI] [PubMed] [Google Scholar]
- 15.Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29:4387–4393. doi: 10.1200/JCO.2011.36.5908. [DOI] [PubMed] [Google Scholar]
- 16.Zhao SL, Fang JY. The role of postoperative adjuvant chemotherapy following curative resection for gastric cancer: a meta-analysis. Cancer Invest. 2008;26:317–325. doi: 10.1080/07357900701834686. [DOI] [PubMed] [Google Scholar]
- 17.Allum WH, Blazeby JM, Griffin SM, Cunningham D, Jankowski JA, Wong R. Guidelines for the management of oesophageal and gastric cancer. Gut. 2011;60:1449–1472. doi: 10.1136/gut.2010.228254. [DOI] [PubMed] [Google Scholar]
- 18.Saif MW, Makrilia N, Zalonis A, Merikas M, Syrigos K. Gastric cancer in the elderly: an overview. Eur J Surg Oncol. 2010;36:709–717. doi: 10.1016/j.ejso.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 19.Imamura H, Kishimoto T, Takiuchi H, Kimura Y, Morimoto T, Imano M, et al. Phase II study of S-1 monotherapy in patients over 75 years of age with advanced gastric cancer (OGSG0404) J Chemother. 2014;26:57–61. doi: 10.1179/1973947813Y.0000000116. [DOI] [PubMed] [Google Scholar]
- 20.Eun H, Hur H, Byun CS, Son SY, Han SU, Cho YK. Effects of continuing adjuvant S-1 for 1 year on the prognosis of gastric cancer patients: results from a prospective single center study. J Gastric Cancer. 2015;15:113–120. doi: 10.5230/jgc.2015.15.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aoyama T, Yoshikawa T, Hayashi T, Kuwabara H, Mikayama Y, Ogata T, et al. Risk factors for 6-month continuation of S-1 adjuvant chemotherapy for gastric cancer. Gastric Cancer. 2013;16:133–139. doi: 10.1007/s10120-012-0158-1. [DOI] [PubMed] [Google Scholar]
- 22.Aoyama T, Sato T, Maezawa Y, Kano K, Hayashi T, Yamada T, et al. Postoperative weight loss leads to poor survival through poor S-1 efficacy in patients with stage II/III gastric cancer. Int J Clin Oncol. 2017;22:476–483. doi: 10.1007/s10147-017-1089-y. [DOI] [PubMed] [Google Scholar]
- 23.Yamashita K, Kurokawa Y, Yamamoto K, Hirota M, Kawabata R, Mikami J, et al. Risk factors for poor compliance with adjuvant S-1 chemotherapy for gastric cancer: a multicenter retrospective study. Ann Surg Oncol. 2017;24:2639–2645. doi: 10.1245/s10434-017-5923-2. [DOI] [PubMed] [Google Scholar]
- 24.Di Costanzo F, Gasperoni S, Manzione L, Bisagni G, Labianca R, Bravi S, et al. Adjuvant chemotherapy in completely resected gastric cancer: a randomized phase III trial conducted by GOIRC. J Natl Cancer Inst. 2008;100:388–398. doi: 10.1093/jnci/djn054. [DOI] [PubMed] [Google Scholar]
- 25.Morris M, Platell C, Fritschi L, Iacopetta B. Failure to complete adjuvant chemotherapy is associated with adverse survival in stage III colon cancer patients. Br J Cancer. 2007;96:701–707. doi: 10.1038/sj.bjc.6603627. [DOI] [PMC free article] [PubMed] [Google Scholar]