Abstract
Purpose
It has been reported that the survival of patients with locally advanced gastric cancer (LAGC) is better in East Asia countries than in developed western countries; however, the prognosis of LAGC remains poor. This study aimed to evaluate the effects of perioperative chemotherapy on the long-term survival of East Asia patients with LAGC.
Materials and Methods
From October 2006 through August 2008, 43 patients with LAGC received perioperative S-1 combined with weekly docetaxel in a phase II study (neoadjuvant group). These patients were matched using propensity scores to patients who underwent surgery without neoadjuvant chemotherapy during the same period (surgery group). The surgical outcomes and long-term survivals were compared between the 2 groups.
Results
After matching, 43 and 86 patients were included in the neoadjuvant and surgery groups, respectively, and there was no significant difference in their baseline characteristics. Although the operating time was longer in the neoadjuvant group, there was no significant difference in postoperative complications between the 2 groups. The neoadjuvant group had a significantly higher 5-year overall survival (OS) rate (73.3% vs. 51.1%, P=0.005) and a trend towards higher 5-year progression-free survival (PFS) (62.8% vs. 49.9%, P=0.145). In the multivariate analysis, perioperative chemotherapy was an independent factor for OS, with a hazard ratio of 0.4 (P=0.005) and a marginal effect on the PFS (P=0.054).
Conclusions
Perioperative chemotherapy was associated with better long-term survival without increasing postoperative complications in the setting of D2 surgery for patients with LAGC, suggesting that perioperative chemotherapy can be a therapeutic option in East Asia countries.
Keywords: Stomach neoplasm, Neoadjuvant therapy, Chemotherapy Adjuvant, Propensity Score
INTRODUCTION
The clinicopathological characteristics of gastric cancer (GC) are known to differ between East Asia patients and those from developed western countries [1]. In Korea and Japan, over half of GC patients are diagnosed at an early stage and the majority of tumors are located in the middle or lower one-third of the stomach [2,3]. Curative gastrectomy with D2 lymph node (LN) dissection is routinely performed for advanced GC in East Asia countries, and the long-term outcomes for East Asia patients are also better than those reported for non-East Asia patients. Given this, upfront surgery has been performed in East Asia countries, even though perioperative chemotherapy has been demonstrated to improve the overall survival (OS) in large-scale randomized European trials [4,5]. At present, a single or 2-drug combination adjuvant chemotherapy strategy is used in East Asia countries based on large-scale randomized trials [6,7].
However, patients with highly advanced GC still have poor prognoses [8]. Large-scale randomized-controlled trials have shown that between 26.7% and 30.6% of patients experience recurrence after curative D2 gastrectomy followed by adjuvant chemotherapy, and the 5-year disease-free survival rate was between 37.6% and 52.0% in patients with stage IIIB disease [9,10]. Thus, the need for a multidisciplinary approach for these patients is of increasing importance, and interest in neoadjuvant chemotherapy is growing in East Asia countries.
To date, only a few results of phase II trials assessing the outcomes of neoadjuvant chemotherapy have been conducted in East Asia countries [11,12,13,14,15]. In our previous phase II study of perioperative chemotherapy, S-1 combined with weekly docetaxel for patients with locally advanced gastric cancer (LAGC) showed good long-term results with manageable toxicity [12].
In this study, we aimed to evaluate the effects of perioperative chemotherapy on the surgical outcomes and long-term survival rates of East Asia patients with LAGC.
MATERIALS AND METHODS
Study population
From October 2006 through August 2008, 43 patients with LAGC were enrolled in a phase II study of perioperative S-1 combined with weekly docetaxel [12]. The inclusion criteria were as follows: 1) patients aged between 18 and 70 years with previously untreated GC, 2) the presence of histologically-confirmed adenocarcinoma of the stomach, 3) clinical stage III/IV (M0) disease according to the Japanese tumor, node, metastasis (TNM) classification system [16], 4) a performance status of 0–1 on the Eastern Cooperative Oncology Group performance status, 5) adequate organ function, and 6) measurable lesion(s) according to the Response Evaluation Criteria in Solid Tumor v 1.0 [17]. In this study, the clinical T classification was determined using endoscopic ultrasonography (EUS), and the clinical N classification was classified using abdomen/pelvic computed tomography (CT) based on the topographic location according to the Japanese classification [16]. A positive LN metastasis was confirmed when the longest diameter of the LN was more than 10 mm, or if it was between 7 and 10 mm with strong enhancement, round shape, central necrosis, or perinodal infiltration [18]. A specialist in GC examined and interpreted all CT images. All eligible patients underwent 18F-fluorodeoxyglucose (FDG)-positron emission tomography (PET) and diagnostic laparoscopy before enrollment to evaluate distant metastasis, and patients with distant metastasis, including those with positive cytology, were excluded from this study.
These patients were compared with those who had undergone gastrectomy during the same period, derived from a prospectively collected database of the surgery cohort. Esophagogastroduodenoscopy and CT were performed in all patients, but other examinations, such as EUS and diagnostic laparoscopy, were not routinely performed. The same inclusion criteria, namely, age, histological diagnosis, clinical stage, and previous history of malignancy were applied to these control patients. Moreover, patients found to have distant metastasis in the pathological report after gastrectomy, such as distant node metastasis and positive cytology, were also excluded.
This study was approved by the Institutional Review Board at the National Cancer Center (IRB No. NCC 2015-0058).
Treatment
The patients enrolled in the phase II trial each received 3 cycles of preoperative and postoperative chemotherapy that consisted of oral S-1 (40 mg/m2 twice daily) on days 1 to 14 plus intravenous docetaxel (35 mg/m2) on day 1 in a 3-week cycle. Radical gastrectomy with D2 LN dissection, according to the Japanese Gastric Cancer Association treatment guidelines, was performed within 6 weeks of starting the preoperative cycle 3 [19]. However, when the tumor was deemed unresectable during or after 3 cycles of preoperative chemotherapy, palliative chemotherapy without surgery was performed on patients who dropped out of the study. Postoperative chemotherapy was started 4 to 6 weeks after surgery.
Patients from the prospectively collected cohort database underwent the same operation as with the phase II trial. Adjuvant chemotherapy was recommended for patients with pathological stage II or greater, and the regimen consisted of 4 to 8 cycles of fluoropyrimidine-based combination therapy for 5 to 7 months.
Outcome assessment
Patient comorbidities were assessed using the Charlson comorbidity index score as adapted by Deyo et al.; these scores were as follows: 0, 1, or ≥2 [20,21]. Histological types were classified according to the World Health Organization classification, and divided into 2 categories for this study: differentiated, including papillary, well-differentiated, and moderately-differentiated tubular adenocarcinoma; and undifferentiated, including poorly-differentiated tubular adenocarcinoma, signet ring cell carcinoma, and mucinous adenocarcinoma [16,22]. We used the 6th American Joint Committee on Cancer (AJCC) classification system to determine the pathological stage postoperatively [23].
Surgical complications were categorized as early or late based on the time of occurrence, and complications developing within 30 days of the surgery were defined as early complications. The severity of each complication was classified according to the Clavien-Dindo classification [24].
Long-term outcomes, including OS and progression-free survival (PFS), were analyzed. OS was defined as the time from the first day of neoadjuvant chemotherapy cycle 1 to death from any cause in patients who entered the phase II trial, and the time from the operative day to death from any cause in the surgery group. Survival status and the cause of death data were collected from the medical records and claims database of the Korean National Health Insurance Corporation. PFS was defined as time from the first day of treatment (neoadjuvant chemotherapy or surgery) to the date of progression, recurrence, or death. When distant metastasis was detected in the operating room or when R0 resection was not performed, this was regarded as a PFS event in the neoadjuvant group.
Postoperatively, abdomen/pelvis CT and plain chest radiographs were performed every 3 months for 3 years, then 6 months for 2 more years, and then yearly thereafter in the neoadjuvant group. These tests were performed every 6 months for 5 years in the surgery group. Esophagogastroduodenoscopy was performed annually in both groups. The last follow-up data on death and recurrence were obtained on September 1, 2015.
Statistical analysis
Continuous values are presented as the median with interquartile range (IQR), and categorical variables are shown as proportions. Distribution differences were tested using the Mann-Whitney U test for continuous variables and the Pearson χ2 or Fisher's exact tests for categorical variables.
The baseline clinicopathological characteristics were not balanced between the patients enrolled in the phase II trial and those who initially underwent surgery and had the same inclusion criteria during the same period. Thus, we applied propensity score matching analysis to minimize potential selection bias. Variables included in propensity score matching were age, sex, the Charlson/Deyo comorbidity score, body mass index (BMI), previous abdominal surgery, tumor location, gross type, histology, and clinical stage. A 1:2 nearest neighbor matching algorithm was used without replacement [25]. After propensity score matching, we tested whether the variables had been balanced through the matching procedures using standardized differences. The final sample consisted of 129 patients (43 in the perioperative chemotherapy group vs. 86 in the surgery first group).
The OS and PFS curves were graphically displayed using the Kaplan-Meier method and compared using the log-rank test. For multivariate analysis, the Cox proportional hazard regression model was used to evaluate the effects of perioperative chemotherapy on OS and PFS, and a backward selection method with a type I error criterion of 0.2 was used.
In the analysis of surgical complications, 42 patients in the perioperative chemotherapy group and 84 patients in the surgery first group were included. One patient in the neoadjuvant group was excluded because he did not undergo gastrectomy due to distant metastasis, and the matched 2 patients in the surgery group were also excluded. Multivariate logistic regression analysis was performed to ascertain whether neoadjuvant chemotherapy independently affected the surgical complications.
Data analyses were performed using SAS version 9 (SAS Institute Inc., Cary, MC, USA), R version 2.12.1 (R Project for Statistical Computing, Vienna, Austria; http://www.r-project.org/), and Stata® version 13.0 software (StataCorp LP, College Station, TX, USA). Two-sided P-values less than 0.05 were considered statistically significant.
RESULTS
Demographics and clinicopathological characteristics
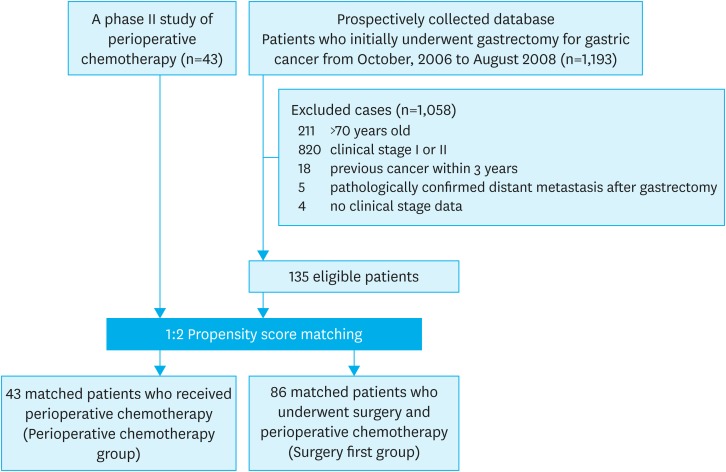
A total of 43 patients who received neoadjuvant chemotherapy and 1,193 patients who underwent upfront surgery were evaluated (Fig. 1). According to the eligibility criteria, 43 and 135 eligible patients, respectively, were included for the 2 groups. Comparing baseline characteristics, we observed significant differences in age and tumor location (P=0.048 and 0.031, respectively) (Table 1). After a 1:2 ratio of propensity score matching, 43 (perioperative chemotherapy group) and 86 (surgery first group) patients were finally included in this study, and there were no significant differences between the 2 groups.
Fig. 1.
Flow diagram for the study.
Table 1. Demographics and clinicopathological characteristics in propensity score matched patients.
| Factors | All patients | Matched patients | |||||
|---|---|---|---|---|---|---|---|
| Perioperative chemotherapy (n=43) | Surgery first (n=135) | P | Perioperative chemotherapy (n=43) | Surgery first (n=86) | P | ||
| Age (yr) | 53.0 (46.0–60.0) | 59 (48.0–65.3) | 0.048 | 53.0 (46.0–60.0) | 57.0 (47.0–64.0) | 0.240 | |
| Sex | 0.563 | 0.684 | |||||
| Male | 29 (67.4) | 98 (72.6) | 29 (67.4) | 61 (70.9) | |||
| Female | 14 (32.6) | 37 (27.4) | 14 (32.6) | 25 (29.1) | |||
| Charlson/Deyo comorbidity score | 0.927 | 0.895 | |||||
| 0 | 34 (79.1) | 103 (76.3) | 34 (79.1) | 69 (80.2) | |||
| 1 | 8 (18.6) | 29 (21.5) | 8 (18.6) | 14 (16.3) | |||
| 2 | 1 (2.3) | 3 (2.2) | 1 (2.3) | 3 (3.5) | |||
| BMI (kg/m2) | 22.9 (20.3–25.0) | 22.9 (20.4–25.0) | 0.798 | 22.9 (20.3–25.0) | 22.6 (20.4–24.6) | 0.693 | |
| Previous abdominal surgery history | 0.999 | 0.999 | |||||
| Absent | 41 (95.3) | 128 (94.8) | 41 (95.3) | 81 (94.2) | |||
| Present | 2 (4.7) | 7 (5.2) | 2 (4.7) | 5 (5.8) | |||
| Location of tumor | 0.031 | 0.172 | |||||
| Lower third | 15 (34.9) | 54 (40.0) | 15 (34.9) | 33 (38.4) | |||
| Middle third | 5 (11.6) | 25 (18.5) | 5 (11.6) | 11 (12.8) | |||
| Upper third | 7 (16.3) | 5 (3.7) | 7 (16.3) | 4 (4.7) | |||
| Combined | 16 (37.2) | 51 (37.8) | 16 (37.2) | 38 (44.2) | |||
| Morphology | 0.454 | 0.530 | |||||
| Superficial | 0 (0) | 5 (3.7) | 0 (0) | 4 (4.7) | |||
| Borrmann type I | 0 (0) | 2 (1.5) | 0 (0) | 0 (0) | |||
| Borrmann type II | 5 (11.6) | 27 (20.0) | 5 (11.6) | 11 (12.8) | |||
| Borrmann type III | 36 (83.7) | 96 (71.1) | 36 (83.7) | 68 (79.1) | |||
| Borrmann type IV | 2 (4.7) | 5 (3.7) | 2 (4.7) | 3 (3.5) | |||
| Histology | 0.360 | 0.504 | |||||
| Differentiated | 12 (27.9) | 49 (36.3) | 12 (27.9) | 29 (33.7) | |||
| Undifferentiated | 31 (72.1) | 86 (63.7) | 31 (72.1) | 57 (66.3) | |||
| Clinical T classification | 0.047 | 0.291 | |||||
| T2 | 0 (0) | 14 (10.4) | 0 (0) | 5 (6.0) | |||
| T3 | 39 (70.9) | 113 (83.7) | 39 (70.9) | 75 (87.2) | |||
| T4 | 4 (9.3) | 8 (5.9) | 4 (9.3) | 6 (7.0) | |||
| Clinical N classification | 0.328 | 0.393 | |||||
| N0 | 0 (0) | 2 (1.5) | 0 (0) | 2 (2.3) | |||
| N1 | 21 (48.8) | 67 (49.6) | 21 (48.8) | 37 (43.0) | |||
| N2 | 20 (46.5) | 65 (48.1) | 20 (46.5) | 46 (53.5) | |||
| N3 | 2 (4.7) | 1 (0.7) | 2 (4.7) | 1 (1.2) | |||
| Clinical stage* | 0.211 | 0.666 | |||||
| IIIA | 20 (46.5) | 78 (57.8) | 20 (46.5) | 41 (47.7) | |||
| IIIB | 18 (41.9) | 50 (37.0) | 18 (41.9) | 39 (45.3) | |||
| IV | 6 (11.6) | 7 (5.2) | 5 (11.6) | 6 (7.0) | |||
Values are presented as median (IQR) or number of patients (%).
BMI = body mass index; IQR = interquartile range.
*The Japanese classification, 2nd English Edition [16].
Surgical outcomes and pathological stages
Table 2 shows the surgical outcomes and pathological stages. Although the R0 resection rate was not different between the 2 groups (97.7% in both groups), the perioperative chemotherapy group had significantly lower pathologic stages than the surgery first group (stage II or less, 69.8% vs. 33.7%; P=0.001). The perioperative chemotherapy group underwent longer operating times than the surgery first group (225.0 vs. 150.5 minutes, P<0.001), and there were no differences in the postoperative hospital stay.
Table 2. Surgical outcomes.
| Factors | Matched patients | |||
|---|---|---|---|---|
| Perioperative chemtherapy (n=43) | Surgery first (n=86) | P | ||
| Extent of gastric resection | 0.208 | |||
| Subtotal | 18 (41.9) | 45 (52.3) | ||
| Total | 24 (55.8) | 39 (47.7) | ||
| Open & closure | 1 (2.3) | 0 (0) | ||
| Combined resection | 0.383 | |||
| No | 40 (93.0) | 75 (87.2) | ||
| Yes | 3 (7.0) | 11 (12.8) | ||
| Curability | 0.407 | |||
| R0 | 42 (97.7) | 84 (97.7) | ||
| R1 | 0 (0) | 2 (2.3) | ||
| R2 | 1 (2.3) | 0 (0) | ||
| Dissected LN | 45.5 (37.0–54.0) | 54.0 (39.0–63.0) | 0.075 | |
| Pathologic stage* | 0.001 | |||
| 0 | 1 (2.3)† | 0 (0) | ||
| I | 19 (44.2) | 15 (17.4) | ||
| II | 10 (23.3) | 14 (16.3) | ||
| III | 8 (18.6) | 34 (39.5) | ||
| IV | 5 (11.6) | 23 (26.7) | ||
| Operating time (min) | 225.0 (191.0–270.0) | 150.5 (115.8–231.8) | <0.001 | |
| Estimated blood loss (mL) | 300 (200–400) | 200 (200–300) | 0.094 | |
| Hospital stay (min) | 10.0 (9.0–11.0) | 11.0 (9.0–13.0) | 0.188 | |
Values are presented as median (IQR) or number of patients (%).
IQR = interquartile range; LN = lymph node; UICC = International Union Against Cancer; AJCC = American Joint Committee on Cancer; TNM = tumor, node, metastasis.
*The 6th UICC/AJCC classification, ypTNM stage in the perioperative chemotherapy group and pathological TNM stage in the surgery first group; †Pathological complete remission.
Surgical complications
The frequencies of early and late surgical complications were not different between the 2 groups (Supplementary Table 1). The incidence of Clavien-Dindo grade III or higher complications was also not different between the 2 groups. No postoperative mortality was observed in either group.
Univariate and multivariate analyses were performed to evaluate whether neoadjuvant chemotherapy affected early postoperative complications (Supplementary Table 2). The only independent factor of early complications was the BMI, with a hazard ratio (HR) of 1.20 (95% confidence interval [CI], 1.01–1.42; P=0.034). Neoadjuvant chemotherapy had no significant association with the development of postoperative complications.
Postoperative adjuvant chemotherapy
Among the 43 patients in the perioperative chemotherapy group, 40 patients completed the 3 cycles of postoperative adjuvant chemotherapy and one patient did not, due to a lack of any grade of pathological regression after the preoperative chemotherapy. In 2 patients, peritoneal seeding or liver metastasis was found during a laparotomy, and immediate palliative chemotherapy was performed.
In the surgery first group, 69 patients were pathologically diagnosed as stage II or greater. Among them, 27 (39.1%) patients received adjuvant chemotherapy, namely, capecitabine plus cisplatin (11 patients), capecitabine plus oxaliplatin (8 patients), TS-1 plus cisplatin, and (5 patients) 5-FU plus cisplatin (3 patients). Palliative chemotherapy was performed in 3 patients, including 2 patients who had an early recurrence finding on CT 1 month postoperatively, and one patient who underwent an R1 resection. The other 39 patients (56.5%) did not receive adjuvant chemotherapy for a variety of reasons, including old age, poor general condition, or patient refusal.
Long-term outcomes
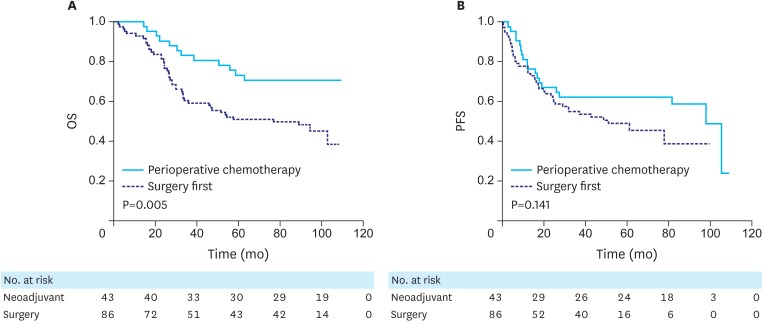
The median follow-up durations for the survivors were 103.3 (range, 90.5–108.3) and 98.4 (range, 57.3–108.2) months in the perioperative chemotherapy and the surgery first groups, respectively, and the surgery first group had a shorter follow-up duration (P=0.001). The 5-year OS rates were 73.3% (95% CI, 59.8–86.8) and 51.1% (95% CI, 40.5–61.7) in the perioperative chemotherapy and surgery first groups, respectively, and the perioperative chemotherapy group had a higher OS than did the surgery first group (P=0.005, Fig. 2A).
Fig. 2.
Survival curves comparing the perioperative chemotherapy and surgery first groups. (A) OS and (B) PFS are shown.
OS = overall survival; PFS = progression-free survival.
GC recurrence was observed in 18 (42.9%) patients in the perioperative chemotherapy group, and palliative chemotherapy was performed in one patient with distant metastasis at laparotomy and in 18 patients with recurrent cancer. In the surgery first group, 37 (44.0%) patients experienced recurrence, and palliative chemotherapy was performed in one of 2 patients who underwent an R1 resection and in 32 of the 37 patients with recurrent cancer. The PFS was not significantly different between the 2 groups (5-year PFS, 62.8% [95% CI, 48.3–77.3] vs. 49.9% [95% CI, 38.7–61.1], P=0.145, Fig. 2B).
Univariate and multivariate analyses were performed to evaluate the effect of perioperative chemotherapy on OS (Table 3). In multivariate analysis of the selection method, perioperative chemotherapy was an independent factor for OS in relation to sex and the Charlson/Deyo comorbidity score (HR, 0.4; 95% CI, 0.21–0.76; P=0.005). For PFS, perioperative chemotherapy had a marginal significance, with a HR of 0.56 (P=0.054) but was an independent factor in relation to sex and the clinical stage.
Table 3. Univariate and multivariate analyses for OS and PFS.
| Factors | No. | OS | PFS | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariable analysis | Multivariable adjusted (backward 0.2) | Univariable analysis | Multivariable adjusted (backward 0.2) | |||||||||||
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |||
| Treatment | ||||||||||||||
| Surgery first | 86 | 1.00 | 1.00 | 1.00 | 1.00 | |||||||||
| Perioperative chemotherapy | 43 | 0.41 | 0.22–0.78 | 0.006 | 0.40 | 0.21–0.76 | 0.005 | 0.66 | 0.37–1.16 | 0.144 | 0.56 | 0.31–1.01 | 0.054 | |
| Age (yr) | 1.01 | 0.99–1.03 | 0.460 | 1.01 | 0.99–1.04 | 0.345 | ||||||||
| Sex | ||||||||||||||
| Male | 90 | 1.00 | 1.00 | 1.00 | 1.00 | |||||||||
| Female | 39 | 1.47 | 0.86–2.51 | 0.158 | 1.92 | 1.09–3.37 | 0.024 | 1.90 | 1.13–3.18 | 0.015 | 2.07 | 1.23–3.48 | 0.007 | |
| Charlson/Deyo comorbidity score | ||||||||||||||
| 0 | 103 | 1.00 | 1.00 | 1.00 | ||||||||||
| 1 | 22 | 0.67 | 0.30–1.45 | 0.300 | 0.52 | 0.23–1.17 | 0.112 | 1.16 | 0.60–2.24 | 0.660 | ||||
| ≥2 | 4 | 3.17 | 1.14–8.84 | 0.027 | 3.50 | 1.23–9.93 | 0.019 | 1.98 | 0.61–6.39 | 0.254 | ||||
| Location of tumor | ||||||||||||||
| Lower third | 48 | 1.00 | 1.00 | |||||||||||
| Middle third | 16 | 0.62 | 0.23–1.66 | 0.342 | 0.78 | 0.32–1.95 | 0.602 | |||||||
| Upper third | 11 | 0.77 | 0.26–2.25 | 0.633 | 0.65 | 0.22–1.92 | 0.440 | |||||||
| Combined | 54 | 1.54 | 0.88–2.72 | 0.133 | 1.39 | 0.79–2.46 | 0.256 | |||||||
| Clinical stage* | ||||||||||||||
| IIIA | 61 | 1.00 | 1.00 | 1.00 | ||||||||||
| IIIB | 57 | 1.12 | 0.66–1.91 | 0.679 | 1.38 | 0.81–2.36 | 0.238 | 1.47 | 0.86–2.52 | 0.161 | ||||
| IV | 11 | 1.23 | 0.48–3.19 | 0.669 | 1.79 | 0.76–4.20 | 0.185 | 2.46 | 0.99–6.05 | 0.050 | ||||
OS = overall survival; PFS = progression-free survival; HR = hazard ratio; CI = confidence interval.
*The Japanese classification, 2nd English Edition [16].
DISCUSSION
Although survival with potentially curative surgery is better for patients living in East Asia countries than for those living in developed western countries, due to multi-factorial reasons including patient differences, tumor characteristics, and surgical techniques, patients with LAGC have a poor prognosis in East Asia countries because of the high recurrence rate [1]. Therefore, in East Asia countries, neoadjuvant chemotherapy has been investigated primarily in patients with LAGC.
To date, several phase II studies have shown promising efficacy of neoadjuvant chemotherapy in this LAGC patient population with high R0 resection rates ranging from 90.7% to 100.0% [26,27,28]. However, the results were underpowered due to small sample sizes and were controversial in terms of the survival noted [29,30]. Moreover, those studies included heterogeneous disease stages, preventing any firm conclusions from being drawn.
To evaluate the role of neoadjuvant chemotherapy in the setting of D2 lymphadenectomy and adjuvant chemotherapy, we compared patients who entered the phase II trial of perioperative chemotherapy for LAGC with propensity-score matched registry patients who underwent upfront surgery followed by the administration of adjuvant chemotherapy. Compared to the surgery first group, the perioperative chemotherapy group had a significantly lower pathologic stage and a better OS. Although the superiority of PFS in perioperative chemotherapy group compared with the surgery first group did not achieve statistical significance, multivariate analysis showed that perioperative chemotherapy had a marginally positive effect on the PFS. These results suggest that perioperative chemotherapy could provide survival benefits in the setting of D2 lymphadenectomy for LAGC.
Currently, a randomized controlled phase III study is underway in Korea (Docetaxel+Oxaliplatin+S-1 (DOS) Regimen as Neoadjuvant Chemotherapy in Advanced Gastric Cancer; PRODIGY), in which patients with resectable cT2–3/N+ or T4/any N gastric or gastroesophageal junction adenocarcinoma are being randomized to receive neoadjuvant docetaxel/oxaliplatin/S-1 followed by D2 surgery and adjuvant S-1 or upfront surgery followed by adjuvant S-1 (www.ClinicalTrials.gov, NCT01515748). This large-scale study will clarify issues regarding the efficacy and safety of neoadjuvant chemotherapy for resectable GC in the context of extensive D2 surgery and standard adjuvant chemotherapy.
Adjuvant chemotherapy has been performed for periods of 6 months or longer since the results of Adjuvant Chemotherapy Trial of S-1 for Gastric Cancer (ACTS-GC) and Capecitabine and Oxaliplatin Adjuvant Study in Stomach Cancer (CLASSIC) trials were reported in 2007 and 2012, respectively [6,7]. However, the phase II trial was started in 2006 and there was no standardized guideline for adjuvant chemotherapy at that time. Therefore, postoperative chemotherapy in 3 cycles was decided according to the findings of previous clinical trials for perioperative chemotherapy, such as the Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) trial [4].
In the pathological results, the proportion of stage IV patients differed between the perioperative chemotherapy and surgery first groups (11.6% [5 patients] vs. 26.7% [23 patients], respectively). Most stage IV patients according to the 6th AJCC were stage IIIB or IIIC patients in the 7th edition, and there was only one patient with distant metastasis in each group.
Several East Asia studies have recently evaluated the effect of neoadjuvant chemotherapy on postoperative morbidity and mortality, and found that neoadjuvant chemotherapy did not increase postoperative complications compared with surgery alone [31,32]. However, in these observational studies, the baseline characteristics were different between the neoadjuvant and surgery-only groups, which might have affected the incidence of postoperative complications. To overcome selection bias, baseline characteristics were equalized in this study using a propensity score matching method which is likely to have made our results more reliable than those of previous East Asia studies.
This study has several limitations. First, pre-operative staging methods were applied somewhat differently between the neoadjuvant and surgery groups. EUS, PET, and diagnostic laparoscopy were performed in all patients in the perioperative chemotherapy group according to the study protocol, whereas they were selectively performed in the surgery first group. PET was performed in 7 of 86 patients (8.1%) and there was no record of diagnostic laparoscopy in the surgery first group. To minimize bias towards a higher rate of undetected distant metastases preoperatively in the surgery first group, we excluded patients who were found to have distant metastases at the time of surgery in the surgery first group. Second, the regimens of postoperative chemotherapy were different between the 2 groups. While the patients in the perioperative chemotherapy group received the same adjuvant chemotherapy, various regimens were applied to the surgery first group. Third, a higher proportion of patients in the perioperative chemotherapy group received adjuvant and palliative chemotherapy than in the surgery first group (95.2% vs. 36.0% and 100.0% vs. 84.6%, respectively). Most patients in the perioperative chemotherapy group received adjuvant chemotherapy according to the phase II study protocol. However, the surgery first group had no protocol for adjuvant chemotherapy, and the randomized controlled trial (CLASSIC trial) had not been published at that time. Therefore, we suggested that a considerable number of patients in the surgery first group who were reluctant to undergo adjuvant chemotherapy not receive adjuvant chemotherapy [7]. Furthermore, survival outcomes were compared between the patients who underwent neoadjuvant and adjuvant chemotherapy (n=40) and those who underwent surgery and adjuvant chemotherapy (n=27), and similar results were observed (Supplementary Fig. 1 and Table 3). OS was marginally higher in the neoadjuvant and adjuvant chemotherapy group than in the surgery with adjuvant group (P=0.087) and neoadjuvant and adjuvant chemotherapy was an independent factor for OS in multivariate analysis (P=0.046). However, the PFS did not appear to be significantly different. Fourth, this study included a small number of patients from a single institution and therefore had limited statistical power. We believe that with a larger sample size or sufficient statistical power, statistically significant differences in PFS may have also been observed. Fifth, in the survival analysis, the initial time point was the day of initial treatment in this study, which can cause lead-time bias. However, this method was considered reasonable, because this study was a retrospective study comparing 2 different treatment modalities. Finally, despite several efforts to reduce selection bias, including propensity score matching, unadjusted bias may have been present between the 2 groups. Generally, patients in good general condition are included in clinical trials and this may affect survival.
In conclusion, perioperative chemotherapy was associated with better long-term survival without increasing postoperative complications in the setting of D2 surgery for LAGC. These results suggest that perioperative chemotherapy can be considered a therapeutic option for East Asia patients with LAGC as well as for patients with LAGC in other global regions.
Footnotes
Funding: This research was supported by the National Cancer Center, Republic of Korea (grant number: 1410140-3).
- Conceptualization: R.K.W., P.S.R.
- Data curation: E.B.W., K.J.Y., Y.H.M., K.M.J., K.Y.W., P.Y.I.
- Formal analysis: K.S., N.B.H.
- Funding acquisition: R.K.W.
- Writing - original draft: E.B.W.
- Writing - review & editing: E.B.W., K.S., P.S.R., R.K.W.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
SUPPLEMENTARY MATERIALS
Early and late complications
Univariate and multivariate analyses for postoperative complications
Univariate and multivariate analyses for OS and PFS in the perioperative chemotherapy and surgery first groups
Survival curves comparing patients who underwent neoadjuvant and adjuvant chemotherapy (n=40) and those who underwent surgery and adjuvant chemotherapy (n=27).
References
- 1.Strong VE, Song KY, Park CH, Jacks LM, Gonen M, Shah M, et al. Comparison of gastric cancer survival following R0 resection in the United States and Korea using an internationally validated nomogram. Ann Surg. 2010;251:640–646. doi: 10.1097/SLA.0b013e3181d3d29b. [DOI] [PubMed] [Google Scholar]
- 2.Jeong O, Park YK. Clinicopathological features and surgical treatment of gastric cancer in South Korea: the results of 2009 nationwide survey on surgically treated gastric cancer patients. J Gastric Cancer. 2011;11:69–77. doi: 10.5230/jgc.2011.11.2.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nashimoto A, Akazawa K, Isobe Y, Miyashiro I, Katai H, Kodera Y, et al. Gastric cancer treated in 2002 in Japan: 2009 annual report of the JGCA nationwide registry. Gastric Cancer. 2013;16:1–27. doi: 10.1007/s10120-012-0163-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 5.Ychou M, Boige V, Pignon JP, Conroy T, Bouche O, Lebreton G, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29:1715–1721. doi: 10.1200/JCO.2010.33.0597. [DOI] [PubMed] [Google Scholar]
- 6.Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810–1820. doi: 10.1056/NEJMoa072252. [DOI] [PubMed] [Google Scholar]
- 7.Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379:315–321. doi: 10.1016/S0140-6736(11)61873-4. [DOI] [PubMed] [Google Scholar]
- 8.Yoon HM, Ryu KW, Nam BH, Cho SJ, Park SR, Lee JY, et al. Is the new seventh AJCC/UICC staging system appropriate for patients with gastric cancer? J Am Coll Surg. 2012;214:88–96. doi: 10.1016/j.jamcollsurg.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 9.Noh SH, Park SR, Yang HK, Chung HC, Chung IJ, Kim SW, et al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:1389–1396. doi: 10.1016/S1470-2045(14)70473-5. [DOI] [PubMed] [Google Scholar]
- 10.Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29:4387–4393. doi: 10.1200/JCO.2011.36.5908. [DOI] [PubMed] [Google Scholar]
- 11.Chun J, Park S, Kim H, Kim Y, Ryu K, Lee J, et al. Randomized phase II trial of neoadjuvant vs. adjuvant docetaxel plus cisplatin in patients with locally advanced gastric carcinoma: an interim analysis. J Clin Oncol. 2006;24(suppl):abstr 4030. [Google Scholar]
- 12.Kim YW, Kim MJ, Ryu KW, Lim HS, Lee JH, Kong SY, et al. A phase II study of perioperative S-1 combined with weekly docetaxel in patients with locally advanced gastric carcinoma: clinical outcomes and clinicopathological and pharmacogenetic predictors for survival. Gastric Cancer. 2016;19:586–596. doi: 10.1007/s10120-015-0490-3. [DOI] [PubMed] [Google Scholar]
- 13.Fujitani K, Sasako M, Iwasaki Y, Yoshimura K, Sano T, Nashimoto A, et al. A phase II study of preoperative chemotherapy (CX) with S-1 and cisplatin followed by gastrectomy for clinically resectable type 4 and large type 3 gastric cancer: JCOG 0210. J Clin Oncol. 2007;25(suppl):abstr 4609. doi: 10.1002/jso.23301. [DOI] [PubMed] [Google Scholar]
- 14.Yoshikawa T, Sasako M, Yamamoto S, Sano T, Imamura H, Fujitani K, et al. Phase II study of neoadjuvant chemotherapy and extended surgery for locally advanced gastric cancer. Br J Surg. 2009;96:1015–1022. doi: 10.1002/bjs.6665. [DOI] [PubMed] [Google Scholar]
- 15.Yoshikawa T, Nakamura K, Tsuburaya A, Sano T, Mizusawa J, Katai H, et al. A phase II study of preoperative chemotherapy with S-1 (S) and cisplatin (P) followed by D3 gastrectomy for gastric cancer (GC) with extensive lymph node metastasis (ELM): survival results of JCOG 0405. J Clin Oncol. 2011;29(suppl):abstr 70. [Google Scholar]
- 16.Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 2nd English edition. Gastric Cancer. 1998;1:10–24. doi: 10.1007/s101209800016. [DOI] [PubMed] [Google Scholar]
- 17.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 18.Kim SK, Kang KW, Lee JS, Kim HK, Chang HJ, Choi JY, et al. Assessment of lymph node metastases using 18F-FDG PET in patients with advanced gastric cancer. Eur J Nucl Med Mol Imaging. 2006;33:148–155. doi: 10.1007/s00259-005-1887-8. [DOI] [PubMed] [Google Scholar]
- 19.Nakajima T. Gastric cancer treatment guidelines in Japan. Gastric Cancer. 2002;5:1–5. doi: 10.1007/s101200200000. [DOI] [PubMed] [Google Scholar]
- 20.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 21.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 22.Aaltonen LA, Hamilton SR World Health Organization. Pathology and Genetics of Tumours of the Digestive System. Lyon: IARC Press; 2000. [Google Scholar]
- 23.Sobin LH. TNM Classification of Malignant Tumours. 6th ed. New York (NY): John Wiley; 2002. [Google Scholar]
- 24.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10:150–161. doi: 10.1002/pst.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park I, Ryu MH, Choi YH, Kang HJ, Yook JH, Park YS, et al. A phase II study of neoadjuvant docetaxel, oxaliplatin, and S-1 (DOS) chemotherapy followed by surgery and adjuvant S-1 chemotherapy in potentially resectable gastric or gastroesophageal junction adenocarcinoma. Cancer Chemother Pharmacol. 2013;72:815–823. doi: 10.1007/s00280-013-2257-z. [DOI] [PubMed] [Google Scholar]
- 27.Kosaka T, Akiyama H, Makino H, Takagawa R, Kimura J, Ono H, et al. Preoperative S-1 and docetaxel combination chemotherapy in patients with locally advanced gastric cancer. Cancer Chemother Pharmacol. 2014;73:281–285. doi: 10.1007/s00280-013-2350-3. [DOI] [PubMed] [Google Scholar]
- 28.Hirakawa M, Sato Y, Ohnuma H, Takayama T, Sagawa T, Nobuoka T, et al. A phase II study of neoadjuvant combination chemotherapy with docetaxel, cisplatin, and S-1 for locally advanced resectable gastric cancer: nucleotide excision repair (NER) as potential chemoresistance marker. Cancer Chemother Pharmacol. 2013;71:789–797. doi: 10.1007/s00280-013-2073-5. [DOI] [PubMed] [Google Scholar]
- 29.Takiguchi N, Nunomura M, Koda K, Oda K, Suzuki H, Miyazaki M. Neoadjuvant chemotherapy with CDDP and 5-fluorouracil for gastric cancer with serosal invasion. Oncol Rep. 2003;10:433–438. [PubMed] [Google Scholar]
- 30.Kang YK, Choi DW, Im YH, Kim CM, Lee JI, Moon NM, et al. A phase III randomized comparison of neoadjuvant chemotherapy followed by surgery versus surgery for locally advanced stomach cancer. J Clin Oncol. 1996;15(suppl):abstr 503. [Google Scholar]
- 31.Li ZY, Shan F, Zhang LH, Bu ZD, Wu AW, Wu XJ, et al. Complications after radical gastrectomy following FOLFOX7 neoadjuvant chemotherapy for gastric cancer. World J Surg Oncol. 2011;9:110. doi: 10.1186/1477-7819-9-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahn HS, Jeong SH, Son YG, Lee HJ, Im SA, Bang YJ, et al. Effect of neoadjuvant chemotherapy on postoperative morbidity and mortality in patients with locally advanced gastric cancer. Br J Surg. 2014;101:1560–1565. doi: 10.1002/bjs.9632. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Early and late complications
Univariate and multivariate analyses for postoperative complications
Univariate and multivariate analyses for OS and PFS in the perioperative chemotherapy and surgery first groups
Survival curves comparing patients who underwent neoadjuvant and adjuvant chemotherapy (n=40) and those who underwent surgery and adjuvant chemotherapy (n=27).