Abstract
Microtubules are major constituents of the cytoskeleton in all eukaryotic cells. They are essential for chromosome segregation during cell division, for directional intracellular transport and for building specialized cellular structures such as cilia or flagella. Their assembly has to be controlled spatially and temporally. For this, the cell uses multiprotein complexes containing γ-tubulin. γ-Tubulin has been found in two different types of complexes, γ-tubulin small complexes and γ-tubulin ring complexes. Binding to adaptors and activator proteins transforms these complexes into structural templates that drive the nucleation of new microtubules in a highly controlled manner. This review discusses recent advances on the mechanisms of assembly, recruitment and activation of γ-tubulin complexes at microtubule-organizing centres.
Keywords: gamma-tubulin, gamma-tubulin complex proteins, CM1 proteins, microtubule nucleation, microtubule-organizing centres, centrosome
1. Introduction
Microtubules are tubular polymers that assemble from heterodimers of α and β-tubulin. Their formation occurs spontaneously in vitro from purified protein in the presence of GTP, if the concentration of α/β-tubulin exceeds a critical concentration. In practice, microtubules can be assembled reproducibly in vitro, at tubulin concentrations of approximately 20 µM [1]. This value matches well the concentration of tubulin that was measured in cytoplasmic extracts [2], raising the question why microtubules grow off specific organizing centres in the cell, instead of polymerizing ubiquitously in an uncontrolled manner. An answer may be that the formation of microtubules from pure tubulin is kinetically disfavoured. In vitro, microtubule assembly occurs in multiple steps: initially, a small number of tubulin dimers need to oligomerize, to form a stable nucleus with correct geometry. This is considered to be a slow process, because a dynamic equilibrium between dimers and oligomers exists, and detachment of dimers at this stage leads to immediate loss of the nucleus. However, any nucleus that has grown sufficiently large permits the longitudinal addition of new dimers, leading to rapid elongation. In cells, multiprotein complexes of γ-tubulin are used as templates for the longitudinal association with α/β-tubulin dimers, thus reducing the duration of the nucleation process [3]. These complexes are essential to permit the rapid formation of spindle microtubules at early stages of mitosis. Their absence leads to severe defects in spindle formation, cell cycle arrest and cell death [4–6]. As these γ-tubulin complexes are only active upon recruitment to specific microtubule-organizing centres (MTOCs) such as the centrosome, the cell possesses spatial and temporal control over the growth of microtubules.
2. Composition of γ-tubulin complexes
The major constituents of γ-tubulin complexes comprise γ-tubulin, a member of the tubulin family, and ‘γ-tubulin complex proteins' (GCPs). γ-Tubulin was originally discovered in the fungus Aspergillus nidulans, as a suppressor of a temperature-sensitive β-tubulin mutation [7]. Highly conserved homologues of γ-tubulin were identified soon afterwards in a variety of organisms [8,9], and it became clear that γ-tubulin would be a universal component involved in the nucleation and organization of microtubules. Two genes encoding γ-tubulin isoforms were identified in Drosophila and in vertebrates [6,10]. In mice, only one isoform, TUBG1, was found essential and ubiquitously expressed in the body [6]. GCPs were first identified in biochemical purifications of γ-tubulin-containing multiprotein complexes [11], and subsequently described in a large number of organisms. Cross-species studies revealed that Alp4/6, the homologues of GCPs 2 and 3 in fission yeast, can be replaced to a limited extent by the human proteins, or by the budding yeast homologues Spc97/98 [12]. This underlines the high degree of functional conservation of GCPs across species. Because the nomenclature for GCPs often varies for different model organisms [3], we will use here the terminology as applied for human GCPs, to facilitate the comprehension of this review article.
Sequence analysis, crystallography and structure prediction have indicated that GCPs 2, 3, 4, 5 and 6 belong to a family of structurally related proteins [13,14]. These GCPs contain two principal conserved domains, grip1 and grip2, located in the amino-terminal and carboxy-terminal halves of the GCPs, respectively (figure 1) [14]. Each grip domain contains multiple bundles of α-helices, with the grip1 domain involved in lateral contacts between GCPs and the grip2 domain mediating binding to γ-tubulin. Two different types of γ-tubulin complexes exist that are defined by size and protein composition: ‘γ-tubulin small complexes' (γTuSCs, approx. 300 kDa) and ‘γ-tubulin ring complexes' (γTuRCs, approx. 2 MDa) [15]. γTuSCs are hetero-tetramers, composed of laterally associated GCP2 and GCP3, each binding longitudinally one molecule of γ-tubulin (figure 1a). γTuRCs consist of several γTuSCs that assemble together with GCPs 4, 5 and 6 into a helical structure resembling a ‘lock washer', with the start and the end of the helix overlapping after a single turn [11,16–19]. γTuRCs thus appear like a ring when viewed from the top by electron microscopy. Whereas γTuSC components have been identified in all eukaryotes, the γTuRC-specific GCPs 4, 5 and 6 are missing in a variety of organisms, such as in budding yeast or Caenorhabditis elegans. GCPs 5 and 6 exist in single copies in the γTuRC, whereas two copies of GCP4 may be present [20,21]. GCPs 4 and 5 can bind laterally to each other independently of γTuSCs, and together with GCP6 integrate into the wall of the γTuRC helix (figure 1b), where they limit and stabilize the size of the complex [14,22,23]. γTuRCs contain several proteins in addition to GCPs, namely MOZART1, MOZART2a/b, NEDD1/GCP-WD, Cdk5rap2/Cep215 and NME7 [21,24–29]. These components are believed to be more peripheral and to have a regulatory function, taking part in assembly, recruitment or activation of the complex.
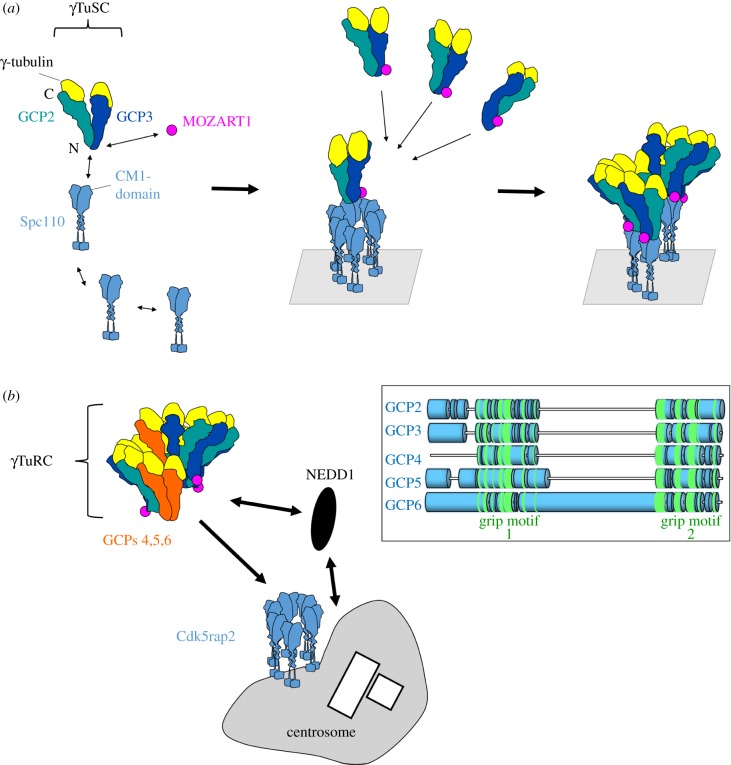
Figure 1.
Assembly and recruitment of γ-tubulin complexes. (a) GCP2 and GCP3 interact laterally, and bind longitudinally each to one molecule of γ-tubulin, to form the γTuSC. Assembly of helical complexes from γTuSCs is driven by oligomerization of proteins with a CM1 domain, such as Spc110 in S. cerevisiae. The CM1 domain binds to the amino-terminal region of GCP3, together with a small oligomerization-promoting protein, MOZART1. (b) Soluble γTuRCs are fully assembled in the cytoplasm, and are recruited to the centrosome by NEDD1 and by CM1 proteins, such as Cdk5rap2 in mammals. The inset depicts schematically sequence similarities between GCPs 2, 3, 4, 5 and 6. Conserved secondary structures are found in the amino-terminal grip1 domain, and in the carboxy-terminal grip2 domain (highlighted in green). GCPs 5 and 6 contain unique sequence extensions at their extreme amino-termini and between the grip1 and grip2 domains that are not shared with any other GCPs.
In vitro, the nucleation activity per mole of γTuRC is 150 times higher than that of a γTuSC [15]. This elevated nucleation is likely favoured by the geometry of the γTuRC, with a helical pitch and a 13-fold symmetry that matches the geometry of most microtubules in cells, containing 13 protofilaments of α/β-tubulin arranged in a cylinder with a ‘B-lattice'. For this reason, γTuRCs act as direct templates upon activation, by orienting the association of new α/β-tubulin dimers. Single γTuSCs may not nucleate microtubules efficiently unless they form oligomers. Oligomerization of seven γTuSCs into a helix is needed to acquire the geometry of a microtubule template, but this necessitates that other proteins occur efficiently [30,31].
3. Assembly of γ-tubulin complexes in fungal organisms
In several fungal organisms, oligomerization of γTuSCs is supported by a class of adaptor proteins at the spindle pole body and at cytoplasmic MTOCs. These proteins include Spc110 and Spc72 in Saccharomyces cerevisiae and Candida albicans, Pcp1 and Mto1 in Schizosaccharomyces pombe and ApsB in A. nidulans [32]. They possess a conserved sequence motif of approximately 60 amino acids in their amino-terminal region, termed CM1 (centrosomin motif 1) [33]. This motif interacts with the amino-terminal domain of GCP3 with very high affinity [34,35]. Moreover, a subgroup of CM1 proteins contains a second amino-terminal motif called SPM (Spc110/Pcp1 motif) that cooperates in γTuSC-binding [34]. CM1 proteins form coiled-coil dimers that associate laterally into higher oligomers, to build a scaffold for γTuSC oligomerization (figure 1a). In S. pombe, the CM1 protein Mto1 requires a second protein, Mto2, for efficient oligomerization [36,37]. To anchor γTuSCs to their respective MTOCs, CM1 proteins carry specific sequence motifs in their carboxy-terminal region [34]. In the final multiprotein complexes with γTuSCs, 13 copies of CM1 proteins are present at an equimolar ratio with γ-tubulin [37]. CM1 proteins can, therefore, be considered as structural templates to direct γTuSC oligomerization into nucleation-competent, helical structures [34,35,37]. MOZART1, a small protein without CM1 sequence, also interacts with the amino-terminal domain of GCP3 and cooperates with CM1 proteins to promote γTuSC oligomerization [38,39]. In models where GCPs 4, 5 and 6 are either absent (S. cerevisiae and C. albicans) or non-essential (S. pombe, A. nidulans), γTuSCs are the minimal subunits needed for microtubule nucleation.
4. Assembly of γ-tubulin complexes in other eukaryotes
In many eukaryotes, microtubule nucleation requires the presence of pre-assembled γTuRCs, comprising GCPs 4, 5 and 6. Experiments in which the expression of a single one of these GCPs is inhibited lead to the disappearance of γTuRCs in favour of smaller complexes at the size of γTuSCs, as seen by fractionation of the cytoplasm on sucrose gradients [23,40,41]. This suggests that GCPs 4, 5 and 6 are necessary either for the initial assembly of γTuRCs, for their stabilization after assembly, or both. Besides, it has been suggested that the assembly of γTuRCs in human cells also depends on MOZART1 [39], but depletion experiments in different cell lines led to conflicting results [39,41].
Depletion of GCPs 4, 5 or 6 is usually accompanied by decreased recruitment of γ-tubulin complexes to the centrosome and induces defects in centriole duplication and spindle bipolarity [23,41,42]. Nevertheless, in somatic cells of Drosophila melanogaster bipolar spindles still form in the absence of GCP 4, 5 and 6, and γTuSC proteins are still recruited to mitotic centrosomes [40,43]. This suggests that local oligomerization of γTuSC into helices may occur at certain MTOCs, as described above in fungi, and that microtubules can be nucleated by alternative pathways, either from γTuRCs or from γTuSCs. In vertebrate cells, however, the γTuRC pathway seems to be prevalent.
Nevertheless, helical complexes in the form of γTuRCs or γTuSC oligomers are not sufficient to initiate microtubule nucleation in the cell, because binding to distinct effector proteins is needed for controlled activation.
5. Activation of γ-tubulin complexes
Because γTuRCs are present as full-sized complexes in the soluble fraction of the cytoplasm, the problem arises as to how the cell controls their nucleation activity, because the formation of microtubule networks is considered to be spatially restricted and cell cycle-dependent. The percentage of active γ-tubulin complexes may be as low as 1%, concentrated at MTOCs [44–46]. Thus, activation of γTuRCs should coincide with recruitment to MTOCs. γTuRC recruitment to the centrosome or to non-centrosomal MTOCs such as the Golgi apparatus or mitochondria involves proteins that carry CM1 sequence motifs [26,47–50]. The structure of these proteins is similar to their fungal orthologues, with an amino-terminal CM1 motif and a carboxy-terminal MTOC-targeting motif. Examples include Drosophila centrosomin, as well as vertebrate Cdk5rap2, myomegalin and pericentrin [26,33,34,47,48,51].
Part of the activation mechanism of γ-tubulin complexes is thought to involve a conformational change in GCP3. A swivel of the carboxy-terminal half of GCP3 results in the lateral alignment of its carboxy-terminally bound γ-tubulin molecule with neighbouring γ-tubulins, to match the geometry of the microtubule cylinder [52,53]. Likely, this activation is triggered by an allosteric mechanism, for example by binding of the CM1 domain to the amino-terminal region of GCP3. As a proof of concept, forced alignment of the γ-tubulin subunits by chemical cross-linking increases the nucleation activity of the complex [53]. Although the insight into this activation comes from studies of yeast γTuSCs, it is likely that equivalent mechanisms drive activation of γTuRCs in higher eukaryotes, given the high structural resemblance of GCPs between different species. An additional role in the activation of nucleation has been attributed to NME7 kinase activity [29]. Because NME7 associates both with soluble, inactive γTuRCs and with centrosome-bound, active γTuRCs, the question arises as to what triggers NME7 activity upon binding to the centrosome.
It has been shown that the activation of nucleation can be uncoupled experimentally from γTuRC recruitment, by expressing protein fragments containing the CM1 domain of mammalian Cdk5rap2 [21]. Cdk5rap2 binds to the pre-formed γTuRCs, and binding requires the interaction of MOZART1 with the amino-terminal domain of GCPs [21,26,39,41]. The isolated CM1 domain (also called γTuNA for ‘γTuRC nucleation activator') has the potential to induce microtubule nucleation from soluble γ-tubulin complexes in vitro or in the cytoplasm. Cdk5rap2 may thus fulfil a dual role: as an adaptor for the anchorage of γ-tubulin complexes to specific MTOCs, and as an activator of the anchored complexes. In this context, a recent study showed that knockdown of Cdk5rap2 in primary keratinocytes weakly affected recruitment of γTuRCs to centrosomes, but drastically reduced microtubule nucleation from there [54]. It was concluded that Cdk5rap2 is mainly responsible for activation but not anchorage of γTuRCs to the centrosome. Anchorage was rather attributed to NEDD1 which is not required for γTuRC assembly, but associates with the pre-formed complex in a MOZART1-dependent manner, similarly to Cdk5rap2 [41]. NEDD1 was shown to be an important recruitment factor in interphase and in mitosis [24,25,54,55]. In primary keratinocytes, knockdown of NEDD1 was recently claimed to reduce centrosomal localization of γ-tubulin without significantly affecting centrosomal microtubule nucleation [54], but this interpretation contradicts earlier studies and fails to explain how centrosomes with low levels of γ-tubulin can maintain regular rates of microtubule nucleation [24,25]. The controversy may be partly explained by the observation that different organisms, different cell types or different cellular conditions require different factors for the recruitment and activation of γ-tubulin complexes: for example, NEDD1 is downregulated during differentiation of keratinocytes [54], and multiple genes encode different CM1 proteins, of which individual ones are expressed under several splice variants in a tissue-specific manner [49,50,56,57]. Moreover, changes in expression levels or post-translational modifications may alter the interaction between γTuRCs and regulatory proteins in the same cell throughout the cell cycle, as described for the ratio of MOZART1 bound to γTuRCs in S. pombe [58].
As interactions between γTuRCs, CM1 proteins, MOZART1 and NEDD1 all depend on the amino-terminal domains of GCPs [39,41], there is a possibility of functional redundancy among γTuRC regulators. Thus, individual cell types may compensate altered protein levels of MOZART1 or NEDD1 by the expression of specific CM1 isoforms. Furthermore, individual combinations of regulators may affect the cell's capacity to build nucleation-competent complexes from γTuSCs, or to recruit ready-made γTuRCs. In addition, the presence of these regulators may affect the number and activity of γTuRCs at a given MTOC. As an example, Drosophila oocytes and sperm require full γTuRCs, although most other cells in the fly can nucleate microtubules from oligomerized γTuSCs [43]. Another notable example is cells from human patients with TUBGCP4 gene mutations, containing very low protein levels of GCP4 and consequently low cellular amounts of γTuRCs. Patients with these mutations are affected by microcephaly and retinal abnormalities, but without visible defects anywhere else in the body [59]. Similar defects could be reproduced by morpholino treatment against TUBGCP4 in zebrafish [59]. This underlines that reduced amounts of γTuRCs can be tolerated in most cells in the human body, but it remains to be determined whether this is due to a compensation by the remaining γTuRCs, by γTuSC-dependent nucleation, or by alternative nucleation mechanisms independent of γ-tubulin.
6. Individual roles of GCPs 4, 5 and 6 at specific microtubule-organizing centres?
Formation of the γTuRC occurs through lateral binding of γTuSCs to the grip1 domains of GCPs 4, 5 and 6 [23]. Thus, GCPs 4, 5 and 6 have structural and functional similarities to the γTuSC components GCP2 and 3 [14]. Nevertheless, GCPs 5 and 6 possess distinct insertions between their grip1 and grip2 domains, and display unique sequence extensions at their extreme amino-terminal ends that differ from GCPs 2 and 3, whereas GCP4 is lacking any additional sequence outside the grip motifs (figure 1b) [14]. Because contacts between γ-tubulin complexes and regulatory proteins occur via the amino-terminal regions of GCPs, it is tempting to speculate whether the unique sequence features of GCPs 4, 5 and 6 enable any specific spatio-temporal regulation of γTuRCs that cannot be performed on γ-tubulin complexes composed exclusively of γTuSCs. Consistently, deletion of the genes encoding GCPs 4, 5 or 6 in S. pombe specifically weakens the activity of non-spindle pole MTOCs in interphase [22]. Moreover, individual roles for GCP5 and GCP6 have been reported in various experimental systems. GCP6 may act synergistically with MOZART1 for bipolar spindle assembly and faithful chromosome segregation in S. pombe [58]. Besides, GCPs 5 and 6 are substrates for multiple kinases that regulate γTuRC-specific functions during the cell cycle [42,60]; for example, GCP6 is phosphorylated by Plk4 at its sequence insertion between the grip1 and grip2 domains, and a non-phosphorylatable mutant specifically impairs centriole duplication, without affecting assembly or centrosomal targeting of the γTuRC [42]. Furthermore, the GCP6-specific sequence insertion has also been implicated in the recruitment of γTuRCs to keratin filaments, to create non-centrosomal MTOCs in epithelial cells [61].
In summary, GCPs 5 and 6, and possibly GCP4, may not only play a structural role in γTuRC assembly but also mediate spatio-temporal regulation of γTuRC activity, by interacting with particular regulators or adaptors, during specific phases of the cell cycle, or in specific cell types.
7. Microtubule nucleation from augmin-bound γTuRCs
In many eukaryotes, the presence of full-sized γTuRCs is essential for the nucleation of microtubules from the surface of existing ones. In Drosophila, γTuSCs can still be recruited to the centrosome in the absence of GCP4, 5 or 6, but not to the surface of spindle microtubules, where nucleation of ‘secondary microtubules' occurs, to increase the microtubule density of kinetochore fibres [40]. This ‘secondary nucleation' is driven by γTuRCs, laterally attached to the lattice of spindle microtubules (figure 2). The attachment is mediated by augmin multiprotein complexes that are conserved in animals, plants and fungi, but that have been lost during evolution of budding and fission yeast [62,63]. In humans, the augmin complex comprises 8 subunits termed HAUS1–8, and association with the γTuRC involves binding of the HAUS6 subunit to the recruitment factor NEDD1 [64,65]. In addition, augmin has been found to associate with TPX2, a spindle assembly factor that ensures high density of spindle microtubules and the correct formation of spindle poles (figure 2) [66,67]. Interestingly, a region within the TPX2 sequence has been identified that bears similarities to a combination of an SPM motif with a CM1 domain [68]. Different from ‘classic' CM1 proteins such as Spc110, this composite TPX2 motif is located in the carboxy-terminal region of the protein, and the SPM part overlaps with a sequence that resembles the first half of a regular CM1 motif, of which the last six amino acids are further separated by an unstructured stretch of 30 amino acids [68]. As deletion of this composite motif from TPX2 inhibits the branching of secondary microtubules, the mechanism of γTuRC activation by augmin/TPX2 may be comparable to centrosomal activation mechanisms, involving ‘classic' CM1 proteins.
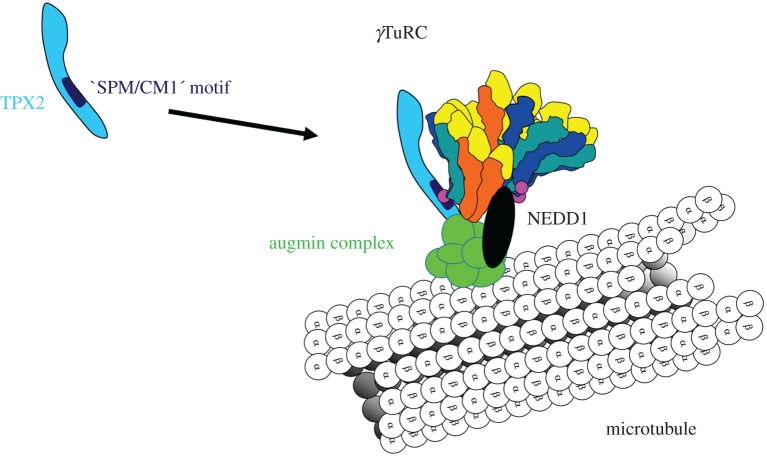
Figure 2.
Augmin complexes recruit γTuRCs to the surface of spindle microtubules, to initiate nucleation of ‘secondary microtubules'. Augmin-dependent recruitment occurs in the presence of NEDD1 and the spindle assembly factor TPX2. TPX2 interacts with the γTuRC via a composite binding sequence that bears resemblance to the SPM and CM1 motifs of yeast Spc110.
8. Influence of microtubule-associated proteins on the nucleation process
Compared to spontaneous microtubule nucleation from pure tubulin, the presence of γTuRCs accelerates the nucleation rate significantly [69]. Nevertheless, there remains a lag phase at the beginning of this process, indicating that γTuRCs are not the perfect templates, despite a geometry that resembles the microtubule [70]. Moreover, purified γTuRCs are poor nucleators in vitro, even if activity-enhancing conformational changes are induced experimentally [53]. This imperfection may be due to the fact that newly bound α/β-tubulin dimers have a slightly curved conformation, bending outwards and lacking lateral contacts with neighbouring dimers. Such nucleation intermediates may be unstable until lateral contacts are formed and the microtubule cylinder closes. This early, unstable phase can be shortened if nucleation intermediates are stabilized by TPX2, by direct binding to tubulin, independent of the presence of γTuRCs (figure 3) [70–72]. The presence of another microtubule-associated protein, chTOG (also known as XMAP215 or Msps in other species), has a synergistic effect in this process, because it supports polymerization (figure 3) [71]. Nucleation of microtubules in cells thus involves multiple steps: (i) the formation of a template that resembles microtubule geometry, in the form of a helical γ-tubulin complex; (ii) the activation of this template, probably by triggering a conformational change in GCP3; and (iii) stabilization of early nucleation intermediates, by favouring specific conformations of tubulin dimers, and by supporting lateral interactions between dimers, to drive closure of the microtubule cylinder.
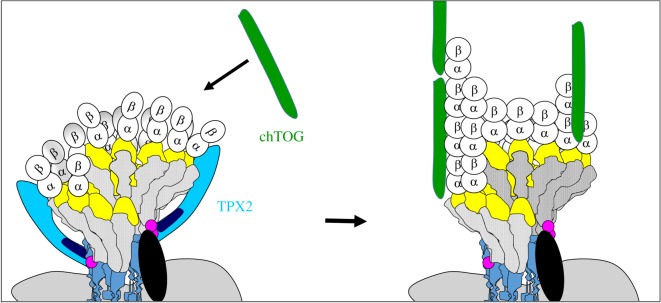
Figure 3.
Efficient formation of microtubules from γTuRCs requires additional proteins that interact with early intermediates of nucleation. At early stages of nucleation, single tubulin dimers bind to the γTuRC, some of which are lacking lateral interactions. These early nucleation intermediates are stabilized by TPX2 until a closed tube is formed, independent of the γTuRC-binding property of TPX2. In the next step, tubulin polymerization is supported by the microtubule-associated protein chTOG (= XMAP215 in Xenopus laevis).
Acknowledgements
We thank our colleagues at the Centre de Biologie du Développement, Université Paul Sabatier, Toulouse, for fruitful discussions.
Data accessibility
This article has no additional data.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by grant no.13-BSV8-0007-01 from ‘Agence Nationale de la Recherche', and by salary support from the Centre National de la Recherche, France.
References
- 1.Weisenberg RC. 1972. Microtubule formation in vitro in solutions containing low calcium concentrations. Science 177, 1104–1105. (doi:10.1126/science.177.4054.1104) [DOI] [PubMed] [Google Scholar]
- 2.Gard DL, Kirschner MW. 1987. Microtubule assembly in cytoplasmic extracts of Xenopus oocytes and eggs. J. Cell Biol. 105, 2191–2201. (doi:10.1083/jcb.105.5.2191) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kollman JM, Merdes A, Mourey L, Agard DA. 2011. Microtubule nucleation by γ-tubulin complexes. Nat. Rev. Mol. Cell Biol. 12, 709–721. (doi:10.1038/nrm3209) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strome S, Powers J, Dunn M, Reese K, Malone CJ, White J, Seydoux G, Saxton W. 2001. Spindle dynamics and the role of γ-tubulin in early Caenorhabditis elegans embryos. Mol. Biol. Cell 12, 1751–1764. (doi:10.1091/mbc.12.6.1751) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hannak E, Oegema K, Kirkham M, Gönczy P, Habermann B, Hyman AA. 2002. The kinetically dominant assembly pathway for centrosomal asters in Caenorhabditis elegans is γ-tubulin dependent. J. Cell Biol. 157, 591–602. (doi:10.1083/jcb.200202047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yuba-Kubo A, Kubo A, Hata M, Tsukita S. 2005. Gene knockout analysis of two γ-tubulin isoforms in mice. Dev. Biol. 282, 361–373. (doi:10.1016/j.ydbio.2005.03.031) [DOI] [PubMed] [Google Scholar]
- 7.Oakley CE, Oakley BR. 1989. Identification of γ-tubulin, a new member of the tubulin superfamily encoded by mipA gene of Aspergillus nidulans. Nature 338, 662–664. (doi:10.1038/338662a0) [DOI] [PubMed] [Google Scholar]
- 8.Zheng Y, Jung MK, Oakley BR. 1991. γ-Tubulin is present in Drosophila melanogaster and Homo sapiens and is associated with the centrosome. Cell 65, 817–823. (doi:10.1016/0092-8674(91)90389-G) [DOI] [PubMed] [Google Scholar]
- 9.Stearns T, Evans L, Kirschner M. 1991. γ-Tubulin is a highly conserved component of the centrosome. Cell 65, 825–836. (doi:10.1016/0092-8674(91)90390-K) [DOI] [PubMed] [Google Scholar]
- 10.Wilson PG, Zheng Y, Oakley CE, Oakley BR, Borisy GG, Fuller MT. 1997. Differential expression of two γ-tubulin isoforms during gametogenesis and development in Drosophila. Dev. Biol. 184, 207–221. (doi:10.1006/dbio.1997.8545) [DOI] [PubMed] [Google Scholar]
- 11.Zheng Y, Wong ML, Alberts B, Mitchison T. 1995. Nucleation of microtubule assembly by a γ-tubulin-containing ring complex. Nature 378, 578–583. (doi:10.1038/378578a0) [DOI] [PubMed] [Google Scholar]
- 12.Riehlman TD, Olmsted ZT, Branca CN, Winnie AM, Seo L, Cruz LO, Paluh JL. 2013. Functional replacement of fission yeast γ-tubulin small complex proteins Alp4 and Alp6 by human GCP2 and GCP3. J. Cell Sci. 126, 4406–4413. (doi:10.1242/jcs.128173) [DOI] [PubMed] [Google Scholar]
- 13.Teixidó-Travesa N, Roig J, Lüders J. 2012. The where, when and how of microtubule nucleation—one ring to rule them all. J. Cell Sci. 125, 4445–4456. (doi:10.1242/jcs.106971) [DOI] [PubMed] [Google Scholar]
- 14.Guillet V, et al. 2011. Crystal structure of γ-tubulin complex protein GCP4 provides insight into microtubule nucleation. Nat. Struct. Mol. Biol. 18, 915–919. (doi:10.1038/nsmb.2083) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oegema K, Wiese C, Martin OC, Milligan RA, Iwamatsu A, Mitchison TJ, Zheng Y. 1999. Characterization of two related Drosophila γ-tubulin complexes that differ in their ability to nucleate microtubules. J. Cell Biol. 144, 721–733. (doi:10.1083/jcb.144.4.721) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moritz M, Braunfeld MB, Sedat JW, Alberts B, Agard DA. 1995. Microtubule nucleation by γ-tubulin-containing rings in the centrosome. Nature 378, 638–640. (doi:10.1038/378638a0) [DOI] [PubMed] [Google Scholar]
- 17.Moritz M, Braunfeld MB, Guénebaut V, Heuser J, Agard DA. 2000. Structure of the γ-tubulin ring complex: a template for microtubule nucleation. Nat. Cell Biol. 2, 365–370. (doi:10.1038/35014058) [DOI] [PubMed] [Google Scholar]
- 18.Keating TJ, Borisy GG. 2000. Immunostructural evidence for the template mechanism of microtubule nucleation. Nat. Cell Biol. 2, 352–357. (doi:10.1038/35014045) [DOI] [PubMed] [Google Scholar]
- 19.Wiese C, Zheng Y. 2000. A new function for the γ-tubulin ring complex as a microtubule minus-end cap. Nat. Cell Biol. 2, 358–364. (doi:10.1038/35014051) [DOI] [PubMed] [Google Scholar]
- 20.Murphy SM, Preble AM, Patel UK, O'Connell KL, Dias DP, Moritz M, Agard D, Stults JT, Stearns T. 2001. GCP5 and GCP6: two new members of the human γ-tubulin complex. Mol. Biol. Cell 12, 3340–3352. (doi:10.1091/mbc.12.11.3340) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi YK, Liu P, Sze SK, Dai C, Qi RZ. 2010. CDK5RAP2 stimulates microtubule nucleation by the γ-tubulin ring complex. J. Cell Biol. 191, 1089–1095. (doi:10.1083/jcb.201007030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anders A, Lourenço PC, Sawin KE. 2006. Noncore components of the fission yeast γ-tubulin complex. Mol. Biol. Cell 17, 5075–5093. (doi:10.1091/mbc.E05-11-1009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farache D, Jauneau A, Chemin C, Chartrain M, Rémy MH, Merdes A, Haren L. 2016. Functional analysis of γ-tubulin complex proteins indicates specific lateral association via their N-terminal domains. J. Biol. Chem. 291, 23 112–23 125. (doi:10.1074/jbc.M116.744862) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lüders J, Patel UK, Stearns T. 2006. GCP-WD is a γ-tubulin targeting factor required for centrosomal and chromatin-mediated microtubule nucleation. Nat. Cell Biol. 8, 137–147. (doi:10.1038/ncb1349) [DOI] [PubMed] [Google Scholar]
- 25.Haren L, Remy MH, Bazin I, Callebaut I, Wright M, Merdes A. 2006. NEDD1-dependent recruitment of the γ-tubulin ring complex to the centrosome is necessary for centriole duplication and spindle assembly. J. Cell Biol. 172, 505–515. (doi:10.1083/jcb.200510028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fong KW, Choi YK, Rattner JB, Qi RZ. 2008. CDK5RAP2 is a pericentriolar protein that functions in centrosomal attachment of the γ-tubulin ring complex. Mol. Biol. Cell 19, 115–125. (doi:10.1091/mbc.E07-04-0371) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teixidó-Travesa N, Villén J, Lacasa C, Bertran MT, Archinti M, Gygi SP, Caelles C, Roig J, Lüders J. 2010. The γTuRC revisited: a comparative analysis of interphase and mitotic human γTuRC redefines the set of core components and identifies the novel subunit GCP8. Mol. Biol. Cell 21, 3963–3972. (doi:10.1091/mbc.E10-05-0408) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hutchins JR, et al. 2010. Systematic analysis of human protein complexes identifies chromosome segregation proteins. Science 328, 593–599. (doi:10.1126/science.1181348) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu P, Choi YK, Qi RZ. 2014. NME7 is a functional component of the γ-tubulin ring complex. Mol. Biol. Cell 25, 2017–2025. (doi:10.1091/mbc.E13-06-0339) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kollman JM, Polka JK, Zelter A, Davis TN, Agard DA. 2010. Microtubule nucleating γ-TuSC assembles structures with 13-fold microtubule-like symmetry. Nature 466, 879–882. (doi:10.1038/nature09207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Erlemann S, Neuner A, Gombos L, Gibeaux R, Antony C, Schiebel E. 2012. An extended γ-tubulin ring functions as a stable platform in microtubule nucleation. J. Cell Biol. 197, 59–74. (doi:10.1083/jcb.201111123) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin TC, Neuner A, Schiebel E. 2015. Targeting of γ-tubulin complexes to microtubule organizing centers: conservation and divergence. Trends Cell Biol. 25, 296–307. (doi:10.1016/j.tcb.2014.12.002) [DOI] [PubMed] [Google Scholar]
- 33.Sawin KE, Lourenco PC, Snaith HA. 2004. Microtubule nucleation at non-spindle pole body microtubule-organizing centers requires fission yeast centrosomin-related protein mod20p. Curr. Biol. 14, 763–775. (doi:10.1016/j.cub.2004.03.042) [DOI] [PubMed] [Google Scholar]
- 34.Lin TC, Neuner A, Schlosser YT, Scharf AN, Weber L, Schiebel E. 2014. Cell-cycle dependent phosphorylation of yeast pericentrin regulates γ-TuSC-mediated microtubule nucleation. Elife 3, e02208 (doi:10.7554/eLife.02208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lyon AS, Morin G, Moritz M, Yabut KC, Vojnar T, Zelter A, Muller E, Davis TN, Agard DA. 2016. Higher-order oligomerization of Spc110p drives γ-tubulin ring complex assembly. Mol. Biol. Cell 27, 2245–2258. (doi:10.1091/mbc.E16-02-0072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Samejima I, Lourenço PC, Snaith HA, Sawin KE. 2005. Fission yeast mto2p regulates microtubule nucleation by the centrosomin-related protein mto1p. Mol. Biol. Cell 16, 3040–3051. (doi:10.1091/mbc.E04-11-1003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lynch EM, Groocock LM, Borek WE, Sawin KE. 2014. Activation of the γ-tubulin complex by the Mto1/2 complex. Curr. Biol. 24, 896–903. (doi:10.1016/j.cub.2014.03.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dhani DK, Goult BT, George GM, Rogerson DT, Bitton DA, Miller CJ, Schwabe JW, Tanaka K. 2013. Mzt1/Tam4, a fission yeast MOZART1 homologue, is an essential component of the γ-tubulin complex and directly interacts with GCP3(Alp6). Mol. Biol. Cell 24, 3337–3349. (doi:10.1091/mbc.E13-05-0253) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin TC, Neuner A, Flemming D, Liu P, Chinen T, Jäkle U, Arkowitz R, Schiebel E. 2016. MOZART1 and γ-tubulin complex receptors are both required to turn γ-TuSC into an active microtubule nucleation template. J. Cell Biol. 215, 823–840. (doi:10.1083/jcb.201606092) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vérollet C, Colombié N, Daubon T, Bourbon HM, Wright M, Raynaud-Messina B. 2006. Drosophila melanogaster γ-TuRC is dispensable for targeting γ-tubulin to the centrosome and microtubule nucleation. J. Cell Biol. 172, 517–528. (doi:10.1083/jcb.200511071) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cota RR, Teixidó-Travesa N, Ezquerra A, Eibes S, Lacasa C, Roig J, Lüders J. 2017. MZT1 regulates microtubule nucleation by linking γTuRC assembly to adapter-mediated targeting and activation. J. Cell Sci. 130, 406–419. (doi:10.1242/jcs.195321) [DOI] [PubMed] [Google Scholar]
- 42.Bahtz R, Seidler J, Arnold M, Haselmann-Weiss U, Antony C, Lehmann WD, Hoffmann I. 2012. GCP6 is a substrate of Plk4 and required for centriole duplication. J. Cell Sci. 125, 486–496. (doi:10.1242/jcs.093930) [DOI] [PubMed] [Google Scholar]
- 43.Vogt N, Koch I, Schwarz H, Schnorrer F, Nüsslein-Volhard C. 2006. The γTuRC components Grip75 and Grip128 have an essential microtubule-anchoring function in the Drosophila germline. Development 133, 3963–3972. (doi:10.1242/dev.02570) [DOI] [PubMed] [Google Scholar]
- 44.Moudjou M, Bordes N, Paintrand M, Bornens M. 1996. γ-Tubulin in mammalian cells: the centrosomal and the cytosolic forms. J. Cell Sci. 109, 875–887. [DOI] [PubMed] [Google Scholar]
- 45.Khodjakov A, Rieder CL. 1999. The sudden recruitment of γ-tubulin to the centrosome at the onset of mitosis and its dynamic exchange throughout the cell cycle, do not require microtubules. J. Cell Biol. 146, 585–596. (doi:10.1083/jcb.146.3.585) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bauer M, Cubizolles F, Schmidt A, Nigg EA. 2016. Quantitative analysis of human centrosome architecture by targeted proteomics and fluorescence imaging. EMBO J. 35, 2152–2166. (doi:10.15252/embj.201694462) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang J, Megraw TL. 2007. Proper recruitment of γ-tubulin and D-TACC/Msps to embryonic Drosophila centrosomes requires centrosomin motif 1. Mol. Biol. Cell 18, 4037–4049. (doi:10.1091/mbc.E07-05-0474) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roubin R, Acquaviva C, Chevrier V, Sedjaï F, Zyss D, Birnbaum D, Rosnet O. 2013. Myomegalin is necessary for the formation of centrosomal and Golgi-derived microtubules. Biol. Open 2, 238–250. (doi:10.1242/bio.20123392) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Z, Zhang C, Qi RZ. 2014. A newly identified myomegalin isoform functions in Golgi microtubule organization and ER–Golgi transport. J. Cell Sci. 127, 4904–4917. (doi:10.1242/jcs.155408) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen JV, Buchwalter RA, Kao LR, Megraw TL. 2017. A splice variant of centrosomin converts mitochondria to microtubule-organizing centers. Curr. Biol. 27, 1928–1940. (doi:10.1016/j.cub.2017.05.090) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zimmerman WC, Sillibourne J, Rosa J, Doxsey SJ. 2004. Mitosis-specific anchoring of γ tubulin complexes by pericentrin controls spindle organization and mitotic entry. Mol. Biol. Cell 15, 3642–3657. (doi:10.1091/mbc.E03-11-0796) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kollman JM, Zelter A, Muller EG, Fox B, Rice LM, Davis TN, Agard DA. 2008. The structure of the γ-tubulin small complex: implications of its architecture and flexibility for microtubule nucleation. Mol. Biol. Cell 19, 207–215. (doi:10.1091/mbc.E07-09-0879) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kollman JM, et al. 2015. Ring closure activates yeast γTuRC for species-specific microtubule nucleation. Nat. Struct. Mol Biol. 22, 132–137. (doi:10.1038/nsmb.2953) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Muroyama A, Seldin L, Lechler T. 2016. Divergent regulation of functionally distinct γ-tubulin complexes during differentiation. J. Cell Biol. 213, 679–692. (doi:10.1083/jcb.201601099) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gunawardane RN, Martin OC, Zheng Y. 2003. Characterization of a new γTuRC subunit with WD repeats. Mol. Biol. Cell 14, 1017–1026. (doi:10.1091/mbc.E02-01-0034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eisman RC, Phelps MA, Kaufman TC. 2009. Centrosomin: a complex mix of long and short isoforms is required for centrosome function during early development in Drosophila melanogaster. Genetics 182, 979–997. (doi:10.1534/genetics.109.103887) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Park JS, Lee MK, Kang S, Jin Y, Fu S, Rosales JL, Lee KY. 2015. Species-specific expression of full-length and alternatively spliced variant forms of CDK5RAP2. PLoS ONE 10, e0142577 (doi:10.1371/journal.pone.0142577) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Masuda H, Toda T. 2016. Synergistic role of fission yeast Alp16GCP6 and Mzt1MOZART1 in γ-tubulin complex recruitment to mitotic spindle pole bodies and spindle assembly. Mol. Biol. Cell 27, 1753–1763. (doi:10.1091/mbc.E15-08-0577) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scheidecker S, et al. 2015. Mutations in TUBGCP4 alter microtubule organization via the γ-tubulin ring complex in autosomal-recessive microcephaly with chorioretinopathy. Am. J. Hum. Genet. 96, 666–674. (doi:10.1016/j.ajhg.2015.02.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Izumi N, Fumoto K, Izumi S, Kikuchi A. 2008. GSK-3β regulates proper mitotic spindle formation in cooperation with a component of the γ-tubulin ring complex, GCP5. J. Biol. Chem. 283, 12 981–12 991. (doi:10.1074/jbc.M710282200) [DOI] [PubMed] [Google Scholar]
- 61.Oriolo AS, Wald FA, Canessa G, Salas PJ. 2007. GCP6 binds to intermediate filaments: a novel function of keratins in the organization of microtubules in epithelial cells. Mol. Biol. Cell 18, 781–794. (doi:10.1091/mbc.E06-03-0201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goshima G, Mayer M, Zhang N, Stuurman N, Vale RD. 2008. Augmin: a protein complex required for centrosome-independent microtubule generation within the spindle. J. Cell Biol. 181, 421–429. (doi:10.1083/jcb.200711053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Edzuka T, Yamada L, Kanamaru K, Sawada H, Goshima G. 2014. Identification of the augmin complex in the filamentous fungus Aspergillus nidulans. PLoS ONE 9, e101471 (doi:10.1371/journal.pone.0101471) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhu H, Coppinger JA, Jang CY, Yates JR III, Fang G. 2008. FAM29A promotes microtubule amplification via recruitment of the NEDD1–γ-tubulin complex to the mitotic spindle. J. Cell Biol. 183, 835–848. (doi:10.1083/jcb.200807046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lawo S, et al. 2009. HAUS, the 8-subunit human augmin complex, regulates centrosome and spindle integrity. Curr. Biol. 19, 816–826. (doi:10.1016/j.cub.2009.04.033) [DOI] [PubMed] [Google Scholar]
- 66.Wittmann T, Wilm M, Karsenti E, Vernos I. 2000. TPX2, A novel Xenopus MAP involved in spindle pole organization. J. Cell Biol. 149, 1405–1418. (doi:10.1083/jcb.149.7.1405) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Petry S, Groen AC, Ishihara K, Mitchison TJ, Vale RD. 2013. Branching microtubule nucleation in Xenopus egg extracts mediated by augmin and TPX2. Cell 152, 768–777. (doi:10.1016/j.cell.2012.12.044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alfaro-Aco R, Thawani A, Petry S. 2017. Structural analysis of the role of TPX2 in branching microtubule nucleation. J. Cell Biol. 216, 983–997. (doi:10.1083/jcb.201607060) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Roostalu J, Surrey T. 2017. Microtubule nucleation: beyond the template. Nat. Rev. Mol. Cell Biol. 18, 702–710. (doi:10.1038/nrm.2017.75) [DOI] [PubMed] [Google Scholar]
- 70.Wieczorek M, Bechstedt S, Chaaban S, Brouhard GJ. 2015. Microtubule-associated proteins control the kinetics of microtubule nucleation. Nat. Cell Biol. 17, 907–916. (doi:10.1038/ncb3188) [DOI] [PubMed] [Google Scholar]
- 71.Roostalu J, Cade NI, Surrey T. 2015. Complementary activities of TPX2 and chTOG constitute an efficient importin-regulated microtubule nucleation module. Nat. Cell Biol. 17, 1422–1434. (doi:10.1038/ncb3241) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang R, Roostalu J, Surrey T, Nogales E. 2017. Structural insight into TPX2-stimulated microtubule assembly. Elife 6, e30959 (doi:10.7554/eLife.30959) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.